Abstract
Anti-CD154 blockade-based regimens remain unequaled in prolonging graft survival in various organ transplantation models. Several studies have focused on transplantation tolerance with the anti-CD154 blockade, but none of these studies has investigated the mechanisms associated with its use as the sole treatment in animal models, delaying our understanding of anti-CD154 blockade-mediated immune tolerance. The purpose of this study was to investigate the mechanism underlying the anti-CD154 monoclonal antibody (mAb) blockade in inducing immune tolerance using an intrahepatic murine allogeneic islet transplantation model. Allogeneic BALB/c AnHsd (BALB/c) islets were infused into the liver of diabetic C57BL/6 (B6) mice via the cecal vein. Anti-CD154 mAb (MR1) was administered on −1, 0, 1, 3, 5, and 7 d posttransplantation at 0.5 mg per mouse. We showed that short-term MR1 monotherapy could prolong the allogeneic islet grafts to more than 250 d in the murine intrahepatic islet transplantation model. The second islet grafts transplanted under the kidney capsule of the recipients were protected from rejection. We also found that rejection of same-donor skin grafts transplanted to the tolerant mice was modestly delayed. Using a DEREG mouse model, FoxP3+ regulatory T (Treg) cells were shown to play important roles in transplantation tolerance. In mixed lymphocyte reactions, Treg cells from the tolerant mice showed more potency in suppressing BALB/c splenocyte-stimulated Teff cell proliferation than those from naïve mice. In this study, we demonstrated for the first time that a short-term anti-CD154 mAb single treatment could induce FoxP3+ Treg cell-mediated immune tolerance in the intrahepatic murine allogeneic islet transplantation model.
Keywords: anti-CD154 mAb (MR1), immune tolerance, regulatory T cell, islet transplantation, liver
Introduction
Allogeneic islet transplantation is a promising therapy for patients with type I diabetes1–3. To date, more than 1,500 patients have undergone β-cell replacement therapy at 40 different international centers, and 50% to 70% of them have shown insulin independence at 5 yr4. However, patients receiving life-long immunosuppressive (IS) drugs to minimize donor-specific immune responses are susceptible to several adverse effects, such as infections, malignancies, and organ toxicities5,6. Immune tolerance induction is therefore attractive as a major strategy to enable acceptance of histocompatibility complex (MHC)-mismatched allografts without compromising the host’s resistance to infections or risking other complications. Several strategies have been established to induce immune tolerance in various rodent models of transplantation using costimulatory signal blockades, induction and expansion of regulatory T (Treg) cells, peripheral T cell deletion, and mixed hematopoietic chimerism7,8. Also, the role of novel immunomodulatory cell groups, such as mesenchymal stromal cells and regulatory macrophages, in response to tolerogenic therapies is currently under investigation9,10. However, five decades of preclinical and clinical research outcomes in solid organ transplantation have demonstrated that, unlike the studies conducted using rodent models, immune tolerance induction in nonhuman primates (NHPs) and humans is extremely difficult to achieve11,12 and may only be applicable in a limited subset of patients13–15. This concern is in line with our previous work, in which we found that porcine xeno-islets transplanted into the liver of NHPs survived only when the recipients were under regular administration of IS therapies, implying a failure of immune tolerance induction in our preclinical studies16. Although there are several possible explanations for these inconsistent outcomes between rodents and NHPs, a lack of definite therapies demonstrating the clear success of immune tolerance in the rodent model has delayed our understanding of in vivo tolerance, hampering the progress of research to the next level.
CD154 (CD40 L) is a type II transmembrane protein belonging to the tumor necrosis factor subfamily17. CD154 is a costimulatory molecule mainly expressed on the surface of activated T cells; its expression is tightly regulated to maintain the activation of T cells17. Regarding islet transplantation, most studies have demonstrated the superiority of adopting the anti-CD154 blockade in combination therapy regimens since the long-term survival of allo- and xenografts has been achieved in various rodent models18–22. Among several studies showing the potency of anti-CD154 monoclonal antibody (mAb), one study has shown that the administration of anti-CD154 mAb with anti-ICOS mAb achieved transplantation tolerance in a murine islet allo-transplantation model where retransplantation of donor islets was accepted by the same recipients, without the need to administer IS drugs23. Mice treated with anti-CD154 mAb and anti-LFA-1 mAb have also been shown to achieve long-term survival of xeno-islets with selective immunomodulatory activities over donor islets, but not over third-party antigens24. These studies have emphasized the role of FoxP3+ Treg cells in achieving transplantation tolerance, but the combined regimens used make it difficult to establish whether the anti-CD154 blockade truly induces immune tolerance. There have been reports on the effect of anti-CD154 monotherapy in islet transplantation in rodent and NHP models25–27; however, none of these studies investigated the mechanisms associated with Treg cells, which are known to play a critical role in tolerance induction. This omission makes it difficult to determine the efficacy of anti-CD154 mAb therapy for tolerance induction.
The kidney subcapsule has been adopted as a site for islet transplantation in an experimental rodent model due to the advantage of graft retrieval for histological and functional analysis of the islet grafts28. However, since the success of the Edmonton protocol29, hepatic infusion via the portal vein is currently accepted as a clinical site for islet transplantation4 despite the potential risks, including thrombosis, hepatic ischemia, and an instant blood-mediated inflammatory reaction30,31. Although bone marrow cavities and brachioradialis muscles have been suggested as alternative sites for clinical islet transplantation32,33, recent improvements in islet purification and IS therapies with their minimal invasive approach in surgery make intrahepatic islet transplantation a feasible option for most patients2,3. The liver also offers anatomical advantages as a result of its first-pass exposure to both nutrients and insulin, sensing the blood glucose level to regulate it immediately without the delay of insulin secretion34. Hence, in order to minimize the interspecies variation, a rodent model of intrahepatic islet transplantation, which mimics the clinical islet transplantation, seemed more appropriate to expand current understanding of immune tolerance across species. In addition, we previously established a novel technique of transplanting islets via the cecal vein, which can lead to more effective control of bleeding-related death, compared to portal vein infusion35. Using newly developed techniques, we chose to transplant MHC-mismatched islet allografts into the liver of rodents, aiming to investigate the mechanism behind anti-CD154 mAb on its own in inducing immune tolerance.
In this study, we demonstrated that, following CD154 blockade, augmented graft-protective FoxP3+ Treg cells play a critical role in the induction of transplantation tolerance, preventing allograft rejection in the intrahepatic murine islet transplantation model.
Materials and Methods
Animals
All experimental procedures were approved by the Seoul National University Institutional Animal Care and Use Committee and were conducted according to the animal experiment ethical guidelines and regulations. All animals were aged 8 wk. Female C57BL/6 (B6, H-2b), BALB/c AnHsd (BALB/c, H-2d), and C3H/HeJ (C3H, H-2 K) inbred mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). Female knock-in B6 (DEREG) mice expressing Forkhead box P3 (FoxP3) together with diphtheria receptor and enhanced green fluorescent protein (eGFP) were also purchased from the Jackson Laboratory. FoxP3 tagged with eGFP reporter mice were kindly provided by Alexander Y. Rudensky (Memorial Sloan Kettering Cancer Center, New York, NY, USA).
Monoclonal Antibodies and Diphtheria Toxin Treatment Protocols
Anti-CD154 mAbs (MR1; Bio X Cell, West Lebanon, NH, USA) were administered at 0.5 mg per mouse on days −1, 0, 1, 3, 5, and 7 posttransplantation. Diphtheria toxin (DT was injected on days 28, 29, 31, and 32 posttransplantation at 1.5 µg per mouse. The concentration of DT was titrated for every experiment, as described elsewhere36.
Diabetic Induction
One hundred twenty milligrams per kilogram of streptozotocin (STZ; Sigma-Aldrich, St. Louis, MO, USA) was intraperitoneally injected twice (one time a day) and only the mice with more than 16.8 mmol/l (=302.67 mg/dl) for three consecutive days were considered to have diabetic status37,38. After diabetic induction, mice were caged for the next 5 to 6 d prior to islet transplantation to excrete the remaining STZ from the body. The mouse tail was snipped to obtain blood, and the blood glucose level was measured with an OneTouch Ultra device kit (Lifescan, Inc., Chesterbrook, PA, USA).
Isolation and Transplantation of Pancreatic Islets
For intrahepatic islet transplantation, 700 IEQ of BALB/c islets were infused. For renal subcapsular islet transplantation, 400 IEQ or 500 IEQ of BALB/c islets with or without corresponding amounts of C3H/HeJ (C3H) islets were implanted. The technical details of the surgery have previously been described35,39. Once allogeneic islets were transplanted, all mice remained euglycemic without exogenous insulin for 34 to 250 d until they were euthanized for histological examination.
Skin Transplantation
Syngeneic B6 and allogeneic BALB/c skin grafts were transplanted onto the left flanks of B6 mice. The technical details of the surgery have previously been described39.
Immunohistochemistry
Immunohistochemical staining was carried out as described previously16. Paraffin-embedded liver, kidney, and pancreatic tissue sections were triple stained. For primary antibody staining, anti-insulin guinea pig immunoglobulin G (IgG; Genetex, Irvine, CA, USA), anti-CD3 rabbit IgG (Dako Laboratories, Santa Clara, CA, USA), and anti-FoxP3 rat IgG (eBioscience, San Diego, CA, USA) antibodies were used. For secondary antibody staining, goat anti-guinea pig IgG-AP (Abcam, Cambridge, UK), goat anti-rabbit IgG-HRP (Abcam), and goat anti-rat IgG-AP (Abcam) were used. The precipitates, Fast Red (Zytomed System GmbH, Berlin, Germany), DAB (GBI Labs, Bothell, WA, USA), and LV Blue (Vector Laboratories, Burlingame, CA, USA) were applied for color development. The sections were visualized with imaging microscopy (Axio Imager A1; Carl Zeiss, Heidenheim, Germany).
Mixed Lymphocyte Reaction
For stimulators, a BALB/c mouse was killed to obtain the spleen, and isolated splenocytes were irradiated with 25 Gy of γ-ray. For responders, a naïve FoxP3-eGFP transgenic B6 mouse was sacrificed to obtain the splenocytes, which were further purified into Thy1.2+ T cells using a Thy1.2 magnetic bead separation (Magnetic-Activated Cell Sorting; Miltenyi, Bergisch Gladbach, Germany). Using a fluorescence-activated cell sorting (FACS) Aria sorter III (BD Biosciences, Franklin Lakes, NJ, USA), Thy1.2+ T cells were divided into the GFP+ Treg cells and GFP− Teff cells. With 1 μM of CFSE (Thermo Fisher Scientific, Waltham, MA, USA) 5 × 105 GFP− Teff cells were labeled, and cocultured with irradiated 5 × 105 BALB/c splenocytes in 96-well round-bottom plate for 5 d. In this coculture, GFP+ Treg cells isolated from tolerant and naïve FoxP3-eGFP transgenic mice were added at a ratio of 2:1 (2.5 × 105), 8:1 (6.25 × 104), and 32:1 (1.56 × 104), respectively. GFP− Teff cells stimulated with anti-CD3 (eBioscience) and CD28 Abs (eBioscience) were used as a positive control. After 5 d of incubation, the cells were harvested and stained with PerCP-Cy5.5-anti-mouse H2-Kb Ab (Biolegend, San Diego, CA, USA), fixable viability dye eFluor660 (Invitrogen, Carlsbad, CA, USA), and PE-anti-mouse FoxP3 (eBioscience) to analyze only the proliferation of viable B6 Teff cells. The analysis was conducted using a FACS Canto II flow cytometer (BD Biosciences).
Interferon gamma (IFN-γ) ELISpot Assay
Splenocytes of tolerant recipients were stained with APC-Cy7-anti-mouse CD8 Ab (eBioscience), and CD8+ T cells were subsequently isolated using a FACS Aria sorter III (BD Biosciences). Microtitre plates (EMD Millipore Corporation, Billerica, MA, USA) were coated overnight at 4 °C with anti-mouse IFN-γ mAb (15 μg/ml; eBioscience) and then blocked for 2 h with 10% fetal bovine serum (FBS)-supplemented RPMI1640 media (Hyclone Laboratories, Inc., Logan, UT, USA) at 37 °C in a 5% CO2 incubator. After removing the media, 7 × 104 CD8+ T cells were seeded with 5 × 105 of 20 Gy irradiated BALB/c splenocytes in 10% FBS-supplemented RPMI1640 media for 24 h at 37 °C in a 5% CO2 incubator. After incubation, cells were washed off, and the plates were then washed three times with PBST (0.1% TWEEN20). After washing the plates a further three times with sterile PBS, biotinylated anti-mouse IFN-γ detection antibodies diluted (3 μg/ml, eBioscience) in PBS (1% bovine serum albumin [BSA]) were added and incubated overnight at 4 °C. After washing, 1 µg of streptavidin-alkaline phosphatase (eBioscience) diluted at a ratio of 1:100 in ELISA diluent buffer (eBioscience) was added in 100 µl for 2 h at room temperature. Then, color was developed by adding 100 µl of AEC substrates (BD Biosciences) and stopped by washing off using tap water. The spots were analyzed using an ELISpot reader system (AID, Strassberg, Germany).
Statistical Analysis
All statistical analyses were performed using GraphPad Prism 5 (GraphPad Software, La Jolla, CA, USA). Statistical significance was determined by paired or unpaired two-tailed Student’s t test. All P values were defined as * level of significance, P < 0.05.
Results
Short-Term Anti-CD154 mAb Treatment Alone Significantly Prolonged the Survival of BALB/c Islets Transplanted into the Liver of Diabetic B6 Mice
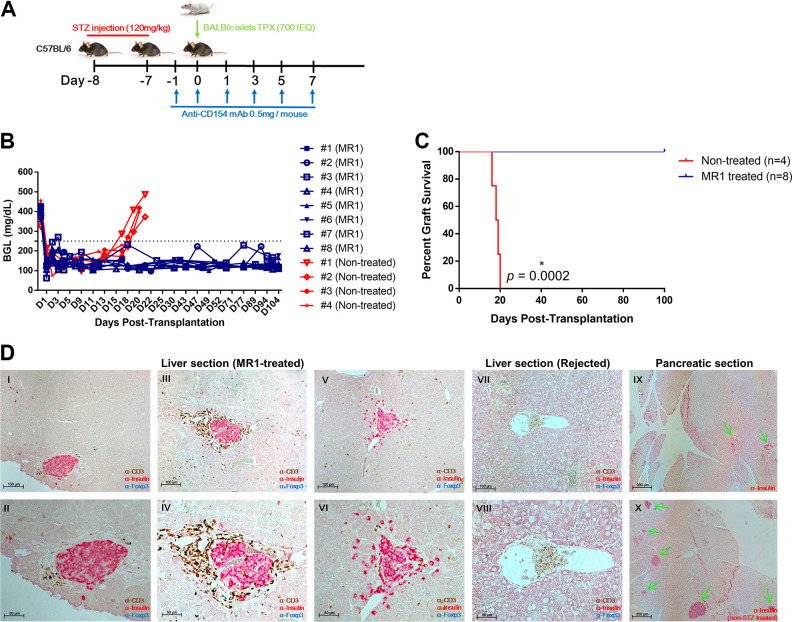
To determine the effect of the CD154 blockade alone, we administered MR1 for a short time to diabetic B6 mice transplanted with BALB/c islets (Fig. 1A). It was of note that all mice injected with MR1 maintained normoglycemia for more than 100 d, whereas the untreated mice rejected the islet allografts within 20 d (Fig. 1B, C). All mice treated with MR1 were normoglycemic until they were euthanized. The immunostaining of the liver showed that islets located near the liver sinusoids were positive for insulin and remained intact for more than 250 d in MR1-treated mice only and not in untreated mice (Fig. 1D). Also, the MR1-treated mice still showed a complete lack of β-cells in the pancreas, whereas these cells were present in wild-type mice (Fig. 1D). These findings proved that the recovered normoglycemia was solely controlled by the engrafted allo-islets in the liver, and not by pancreatic regeneration. Altogether, these results indicate that MR1 treatment alone results in indefinite islet allograft survival in the liver.
Fig. 1.
Effect of short-term MR1 single treatment on intrahepatic islet survival in vivo. (A) Schematic illustration of the experimental setup. Diabetic C57BL/6 mice were transplanted with BALB/c allogenic islets (700 IEQ) through the cecal vein route. Anti-CD154 mAbs (MR1) were intraperitoneally administered on days −1, 0, 1, 3, 5, and 7 (n = 8). Nontreated mice were used as controls (n = 4). (B) blood glucose level (BGL) was measured with a OneTouch Ultra device from day 0. The blood was obtained from snipped tail. (C) The survival graph was plotted from B. Statistical significance was determined by the Mantel–Cox (log-rank) test. Asterisk (*) indicates statistical significance (P < 0.05). All normoglycemic mice were sacrificed at 257 DPT (1 to 4) or 105 DPT (#5 to 8) for histologic analysis. (D) Immunohistochemical stain of paraffin-embedded islet-transplanted liver and pancreatic tissues. Section slides were triple-stained with anti-CD3 (brown), anti-insulin (red), and anti-FoxP3 (blue) or mono-stained with anti-insulin (red). I to II (105 DPT), III to VI (257 DPT): liver section of islet-transplanted mouse. VII to VIII: liver section of graft-rejected mouse. IX: pancreatic section of islet-transplanted mouse. X: pancreatic section of non-STZ-treated mouse. Original magnification 200, 100, and 50 μm.
DPT: days posttransplantation; mAb: monoclonal antibody; STZ: streptozotocin.
Secondly Transplanted BALB/c Islets Under the Kidney Capsule of the B6 Mice Engrafted Formerly with BALB/c Islet in the Liver Were Permanently Accepted
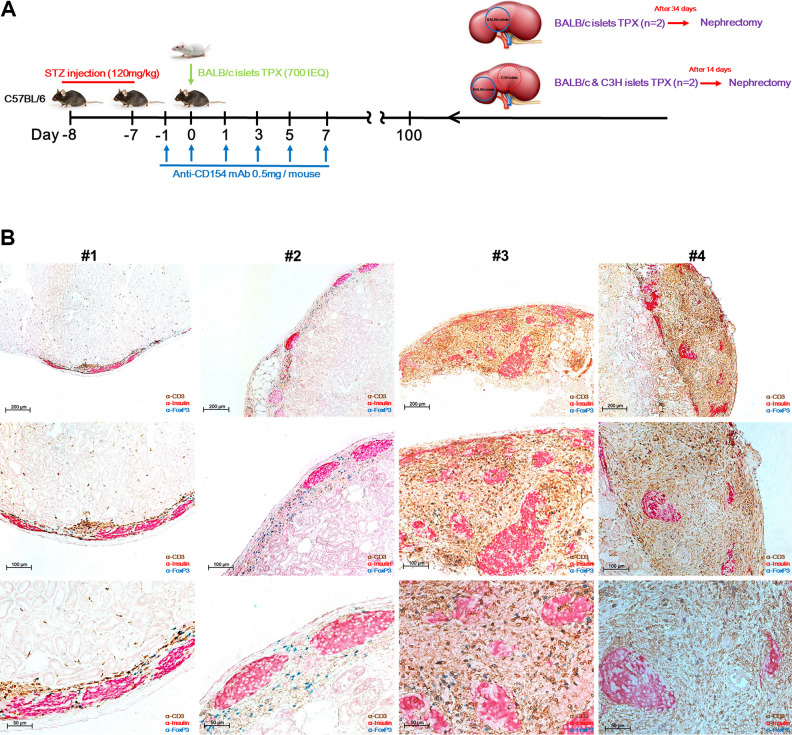
We investigated whether MR1 alone could induce immune tolerance in our model. Thus, without administering additional IS therapy, we transplanted second allo-islets under the kidney capsule of B6 recipients that had formerly been transplanted with BALB/c islets into the liver. Among those mice that were maintaining normoglycemia for more than 100 d, one group was transplanted with second-party (donor-specific) BALB/c islets, and the other group was transplanted with both BALB/c and C3H islets (third-party) beneath the left kidney capsule (Fig. 2A). On day 14 or 34 posttransplantation, islet-bearing kidneys were removed by nephrectomy. Surprisingly, we found that second-party BALB/c islets survived for periods up to kidney removal in all mice, whereas third-party C3H islets were completely rejected at 14 days posttransplantation (DPT) (S1). Immunostaining analysis of the surviving graft-bearing kidneys revealed that donor islets transplanted under the kidney capsule remained almost intact in all mice, while heavy infiltration of CD3+FoxP3+ Treg cells was mostly observed in the perigraft sites (Fig. 2B). Considering the fact that CD3+FoxP3+ Treg cells were consistently found around the surviving allo-islets, these immune-regulatory cells might contribute to immune tolerance in our model. Overall, these results may indicate that long-term graft survival is due to the immune tolerance induced by MR1 in the intrahepatic islet allo-transplantation model.
Fig. 2.
Second transplantation of BALB/c and C3H islets to confirm tolerance. (A) Schematic illustration of the experimental setup. After recovery to normoglycemia, second allo-islets were transplanted under the left kidney capsule of the recipients without administration of immunosuppressive drugs. #1 and 2 mice were transplanted with single BALB/c islets (blue line circle), and #3 and 4 mice were transplanted with BALB/c islets (blue line circle) and C3H islets (red dot circle) at different sites of the same kidney. After 34 or 14 d, the islet-bearing kidneys were removed (nephrectomy) and fixed with 4% paraformaldehyde (PFA) for subsequent immunostaining. (B) Immunohistochemical stain of the surviving islets under the kidney capsule. Section slides were triple-stained with anti-CD3 (brown), anti-insulin (red), and anti-FoxP3 (blue). Original magnification 200, 100, and 50 μm.
mAb: monoclonal antibody; STZ: streptozotocin, TPX: transplantation
Modest Delay in BALB/c Skin Graft Rejection in Tolerant B6 Mice
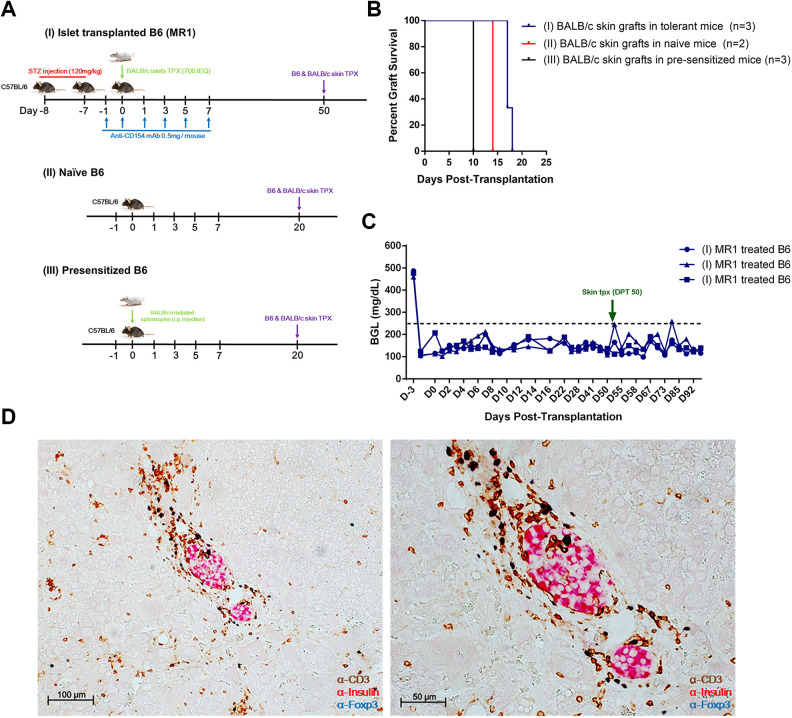
Next, in order to investigate whether transplantation tolerance to islet allografts could be extended to allogeneic skin grafts in our model, we prepared three groups: tolerant B6 mice with engrafted intrahepatic BALB/c islets by MR1 monotherapy, naïve B6 mice, and presensitized B6 mice previously injected intraperitoneally with irradiated BALB/c splenocytes (Fig. 3A). All groups were then transplanted with syngeneic (B6) and allogeneic (BALB/c) skin grafts on the left flank of the body, and the skin grafts were inspected daily until they were fully engrafted (S2). We found that both tolerant and naïve B6 mice accepted syngeneic skin grafts at 10 DPT. It is of note that, unlike naïve B6 mice, which rejected BALB/c skin allografts at 14 DPT, tolerant B6 mice showed a modest prolongation of skin allograft survival for up to 16 to 17 DPT (Fig. 3B, S2). Meanwhile, presensitized B6 mice rejected the BALB/c skin graft at 9 to 10 DPT (Fig. 3B, S2). Interestingly, tolerant B6 mice still maintained normoglycemia (Fig. 3C) throughout the observation period even after the rejection of BALB/c skin, implicating that the immune response of skin allograft rejection does not induce the rejection of engrafted intrahepatic allo-islets. Subsequent immunostaining of the liver showed undamaged allo-islets, with infiltration of CD3+FoxP3+ T cells in the perigraft sites (Fig. 3D).
Fig. 3.
Second transplantation of skin grafts to confirm tolerance. (A) Schematic illustration of the experimental setup. Tail skin grafts were obtained from naïve B6 and BALB/c mice and transplanted into the left flank of (I) tolerant, (II) naïve, and (III) presensitized B6 mice. Presensitized B6 mice were prepared by intraperitoneally injecting irradiated BALB/c splenocytes (7 × 105 cells/mouse). (B) Survival graph of BALB/c skin grafts was plotted. (C) BGL was measured with a OneTouch Ultra device from day 0. The blood was obtained from snipped tail. (D) Immunohistochemical stain of liver tissues of tolerant B6 mice. Section slides were triple-stained with anti-CD3 (brown), anti-insulin (red), and anti-FoxP3 (blue). Original magnification 100 and 50 μm.
STZ: streptozotocin.
CD4+FoxP3+ Treg Cells in Tolerant B6 Mice Play a Key Role in Protecting Islet Allografts from Rejection
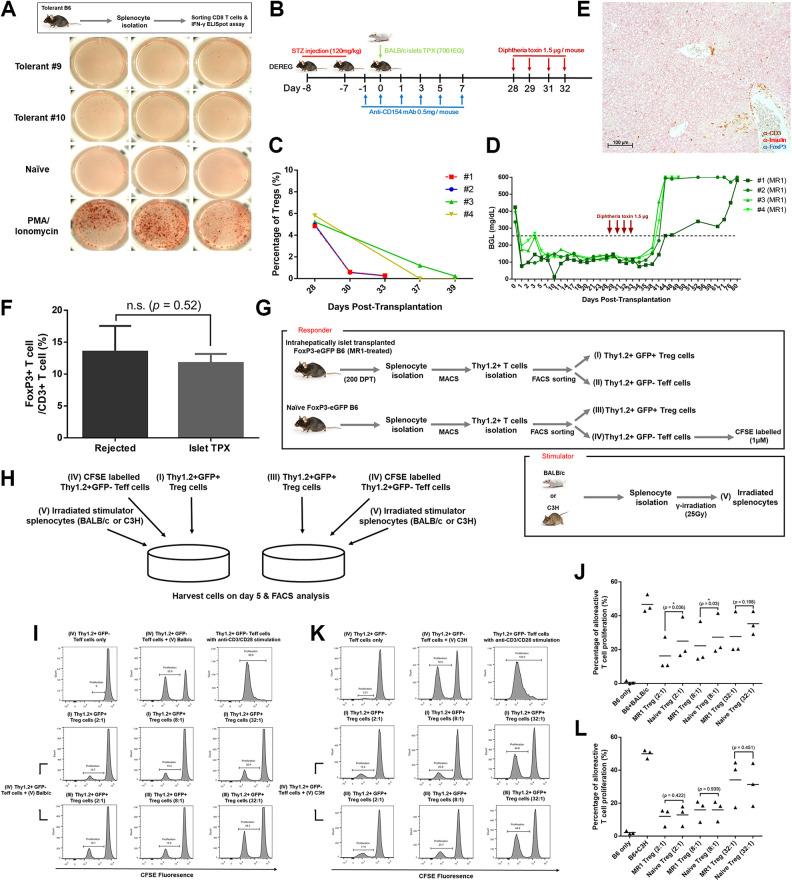
To examine the underlying mechanisms of immune tolerance exerted by MR1, we first performed an IFN-γ ELISpot assay using CD8+ T cells isolated from tolerant B6 mice. CD8+ T cells stimulated with irradiated BALB/c splenocytes secreted comparable amounts of IFN-γ compared to the control, indicating that alloantigen recognizing CD8+ T cells were neither anergized nor deleted (Fig. 4A). To evaluate the role of Treg cells, we exploited B6 DEREG mice in which FoxP3+ Treg cells can be selectively depleted in vivo upon administration of DT. Diabetic B6 DEREG mice were transplanted with BALB/c islets via the cecal vein under short-term MR1 monotherapy. When normoglycemia was achieved, DT was intraperitoneally injected at 28, 29, 31, and 32 DPT (Fig. 4B). The complete ablation of CD4+CD25+FoxP3+ Treg cells was confirmed in peripheral blood by FACS analysis (Fig. 4C). Within 2 wk of DT administration, hyperglycemia (≥600 mg/dl) recurred, indicating that the islet allograft was being rejected in the liver (Fig. 4D). Immunostaining analysis of the whole liver of these mice showed the total absence of islets, with heavy infiltration of CD3+ T cells near the sinusoids, confirming the complete rejection of engrafted intrahepatic allo-islets (Fig. 4E). Collectively, these data suggest that FoxP3+ Treg cells play a critical role in protecting the allo-islets from rejection. We then assessed the ratio of FoxP3+ Treg cells from the tissues to those from the recipients that rejected the allo-islets. We could not observe any significant difference between those two groups (Fig. 4F, S3). Thus, by conducting mixed lymphocyte reaction analysis, we investigated whether Treg cells in tolerant mice would be qualitatively different compared with those from the naïve mice, with stronger suppressive capacity against alloantigens. The diabetic FoxP3-eGFP mice were infused with BALB/c islets via the cecal vein with short-term MR1 monotherapy. When normoglycemia was achieved, FoxP3+ Treg cells and FoxP3− Teff cells were separately obtained from the splenocytes of tolerant and naïve B6 mice (Fig. 4G). CFSE-labeled FoxP3− Teff cells from a naïve B6 mouse were adopted as a universal responder, and irradiated BALB/c or C3H APCs were cocultured to stimulate the proliferation of responder cells (Fig. 4G–H). FoxP3+ Treg cells isolated from tolerant and naïve B6 mice were added at ratios of 2:1, 8:1, and 32:1, respectively, to the coculture of stimulator and responder cells (Fig. 4H). After 5 d of incubation, cells were harvested and analyzed to examine the proliferation of viable B6 Teff cells. We found that almost one-half of the naïve Teff cells proliferated when cocultured with irradiated BALB/c or C3H splenocytes (Fig. 4I–L). Surprisingly, in the coculture of Teff cells with BALB/c splenocytes, Treg cells from tolerant mice exhibited better suppressive capacity over Teff cell proliferation compared to the Treg cells obtained from the naïve mice (Fig. 4I–J). However, in the coculture with C3H splenocytes, Treg cells from both tolerant and naïve mice showed comparable suppressive capacity over Teff cell proliferation (Fig. 4K–L). Taken together, MR1 monotherapy induced donor-specific Treg cells to protect allogeneic islet grafts in the liver.
Fig. 4.
Analyzing the role of Treg cells in tolerant B6 mice. (A) Analysis of IFN-γ-secreting CD8+ T cells in tolerant mice using an ELISpot assay. CD8+ T cells stimulated with PMA (phorbol myristate acetate) (50 ng/ml) and ionomycin (1 μg/ml) were used as a positive control. The absolute number of IFN-γ-secreting CD8+ T cells was counted using an ELISpot Reader. (B) Schematic illustration of the experimental setup. Diphtheria toxin was given on days 28, 29, 31, and 32 posttransplantation at 1.5 µg per dosage. (C) Percentage of Treg cells in peripheral blood before and after diphtheria toxin treatment. (D) BGL was measured with a OneTouch Ultra device from day 0. The blood was obtained from snipped tail. (E) Immunohistochemical stain of liver tissues of hyperglycemic B6 mice. Section slides were triple-stained with anti-CD3 (brown), anti-insulin (red), and anti-FoxP3 (blue). Original magnification 100 μm. (F) The ratio of FoxP3+ Treg cells to CD3+ T cells near the graft sites was analyzed using Cell Counter Image J software. The ratio was obtained from three different areas, and each group expressed as mean ± SD (Fig. S3). (G) Schematic illustration of mixed lymphocyte reaction. Stimulator and responder cells were prepared as described in Materials and Methods. (H) Schematic illustration of cell coculture. In a coculture of CFSE-labeled naïve effector T cells with irradiated BALB/c or C3H splenocytes, Treg cells isolated from tolerant and naïve mice were added at a ratio of 2:1, 8:1, and 32:1, respectively. Cells were incubated in a 96-well round-bottom plate for 5 d. (I, J). The suppressive ability of Treg cells against the proliferation of naïve effector T cells in the coculture with irradiated BALB/c splenocytes was evaluated through FACS analysis. (K, L) The suppressive ability of Treg cells against the proliferation of naïve effector T cells in the coculture with irradiated C3H splenocytes was evaluated through FACS analysis. Naïve effector T cells stimulated with anti-CD3 and CD28 Abs were used as a positive control. The proliferation of each group was expressed as mean ± SD (n = 3). Statistical significance was determined by paired Student’s t test. Asterisk (*) indicates statistical significance (P < 0.05).
FACS: fluorescence-activated cell sorting; IFN-γ: interferon gamma; STZ: streptozotocin; Treg cell: regulatory T cell.
Discussion
We confirmed that short-term MR1 monotherapy could achieve normoglycemia for more than 250 d in intrahepatic-islet-transplanted diabetic mice. To evaluate whether immune tolerance was achieved in the mice, the second graft, along with third-party graft (islets from C3H mice), were transplanted underneath the kidney capsule. Rechallenging allo-islets into the liver was not possible since readministration of STZ could affect the established immune system of the recipients with its cytotoxicity40. Hepatic lobectomy was not available because it is not a life-sustaining surgical method. By conducting nephrectomy, islet-bearing kidneys showed total acceptance of second-party islets but not of third-party islets. Successful acceptance of second allo-islets into the nonliver solid organ emphasized the apparent ability of MR1 to induce immune tolerance, regardless of the microenvironment of anatomic sites for the islet transplantation. Since we found the infiltration of Treg cells in the perigraft sites (Fig. 2B), it is conceivable that graft-protective Treg cells induced by MR1 treatment might have migrated into the kidney capsule, creating the immunologically privileged site where Treg cells may prevent immune-mediated graft damage, considering that Treg cells are key regulators of dominant tolerance for graft protection41–43. The mechanisms underpinning the inhibitory functions of Treg cells and their migration to perigraft sites would be of interest for further investigation.
Next, we verified whether transplantation tolerance could be extended to organs other than islets by exploiting allogeneic skin transplantation. Among transplantation models, skin transplantation has been noted to be extremely challenging for achieving tolerance induction44. As expected, BALB/c skin transplanted to the tolerant B6 was all rejected, but graft survival was modestly prolonged compared to the same grafts in the control group. Most interestingly, tolerant B6 mice still maintained normoglycemia during and after the rejection phase of skin allografts. Similar results were reported in a recent study that in diabetic B6 mice treated with anti-LFA-1 and anti-CD154 mAb, intact neonatal porcine islets (NPIs) were maintained beneath the kidney capsule with normoglycemic control, even after the rejection of retransplanted porcine skin xenografts24. These mice became diabetic when the NPI xenograft-bearing kidney was removed, and abundant FoxP3+ Treg cells were observed near the perigraft sites24. We also confirmed the existence of Treg cells near the perigraft sites in the liver after the rejection of skin allografts, and the islets could be protected from potentially fatal immune responses during skin rejection by the Treg cells, as shown by Arefanian et al.24. The rejection of same-donor skin grafts in tolerant mice could be explained by two reasons. First, since the primary target of allogeneic immune responses is the MHC molecule45, allogeneic MHC-specific tolerance mediated by Treg cells could, in some way, delay the rejection of skin allografts in tolerant mice. However, strong T cell-mediated immune responses mounted by abundant epidermal and dermal dendritic cells (DCs)46–48 may exceed a threshold of Treg-mediated immunoregulation, leading to the eventual failure of allogeneic skin grafts in all mice. Second, another possibility is the occurrence of split tolerance49–52. Although the mechanism of split tolerance in accordance with anti-CD154 blockade has barely been studied, strong immunity against skin-specific antigens53–55 could have been formed to break allogeneic MHC-specific tolerance, resulting in skin graft rejection.
Using the intrahepatic islet allo-transplantation DEREG mouse model, we showed that immune tolerance could be mediated by Treg cells. Whole liver sections showed complete destruction of the entire islets with the depletion of Treg cells. A limitation of this experiment was that upon DT treatment, all FoxP3+ Treg cells were eradicated without selectively depleting the Treg cells responsible for graft protection. An in-depth study characterizing the distinctive markers of graft-protective Treg cells should be conducted to target them selectively, which would lead to more precise interpretation of the role of the Treg cells among the FoxP3+ heterogeneous population. Although CD154 blockade was reported as generating inducible Treg (iTreg) cells in OT-I and OT-II transgenic mouse transplanted with ovalbumin-expressing skin grafts56, it still remains controversial to define the lineage of Treg cells in the polyclonal T cell population in our model due to the lack of universal markers to distinguish natural Treg cells from iTreg cells57.
Since the ratio of Treg to CD3+ T cells in the perigraft sites was not significantly different from that in the rejected control, further experiments were conducted to investigate whether these Treg cells contained qualitatively different characteristics. Indeed, the Treg cells in tolerant mice showed higher suppressive capacity on effector T cell proliferation after stimulation with the same allogeneic donor of BALB/c splenocytes, than with the third-party donor of C3H splenocytes. It is evident that MR1 enriched a lineage of donor-specific Treg cells in our model, but the underlying molecular mechanisms for the generation of the Treg cells have not yet been identified56. Previous work has implicated the tolerogenic plasmacytoid DCs in lymph nodes in generating donor-specific peripheral FoxP3+ Treg cells in an allo-cardiac transplantation mouse model treated with anti-CD154 mAb and donor-specific transfusion (DST)58. Moreover, immunogenic DCs, which are triggered by CD40 signaling, have been shown to convert into tolerogenic DCs when JAK3, a downstream molecule of CD40, is inhibited59,60. We assume that the production of costimulatory molecules and inflammatory cytokines for immunogenic DC activation may be hampered by inhibited CD40 signaling61,62, thus driving them into tolerogenic DCs. The Notch ligand Jagged-1, which is expressed on the surface of DCs, has been shown to contribute to the induction and expansion of alloantigen-specific Treg cells63–65. Although the common signaling network between Jagged-1 and CD40 is yet fully understood, overexpression of Jagged-1 and blockade of CD40 signaling seems to be effective in prolonging allograft survival in transplantation models66. Therefore, JAK3 and Jagged-1 might be the key factors for further investigation of the specific molecular mechanism involved in the generation of donor-specific Treg cells by anti-CD154 mAb treatment.
The key points in our single-drug therapy enabling long-term control of blood glucose levels would be partially explained by the difference in MR1 dosage, which was higher and more frequently given than those in previous studies. Ferrer and colleagues revealed that MR1 monotherapy could decrease the frequency of CD44high CD8+ T cells but elevate the frequency of KLRG-1high CD8+ T cells, leading to a delay in the expansion of antigen-specific CD8+ T cells56,67. The differentiation into antigen-specific CD8+ T cells was also delayed by depleted cytokine production at an early stage56. Previous studies have also shown that the potent immunoregulatory function of anti-CD154 mAb is not simply restricted to blockade of the signal pathway of CD154-CD40; elimination of CD154-expressing immune cells by recruiting complement-mediated mechanisms is also important in avoiding islet allograft rejection19. Although the exact role of anti-CD154 mAb on CD154-expressing effector T cells was not the main focus of our study, antibody-based therapy for CD154-CD40 blockade could provide a beneficial effect in regulating immune responses with their additional unknown effects. Therefore, considering the effect of anti-CD154 mAb, increased dosage of MR1 administration could be effective by taking advantage of sparing time to develop donor-specific Treg cells in weakened alloimmune responses at the early time point after transplantation.
In conclusion, we demonstrated for the first time that short-term MR1 monotherapy could achieve transplantation tolerance, which is critical to protecting allo-islets in recipients. In addition, we found that transplantation tolerance is mediated by donor-specific FoxP3+ Treg cells. We expect that our model could provide concrete evidence for anti-CD154 mAb-mediated immune tolerance, securing a beachhead to unveil the molecular mechanism of anti-CD154 mAb-mediated Treg cell induction in the allo-islet transplantation.
Supplemental Material
Supplemental Material, Fig_S1 for Donor-Specific Regulatory T Cell-Mediated Immune Tolerance in an Intrahepatic Murine Allogeneic Islet Transplantation Model with Short-Term Anti-CD154 mAb Single Treatment by Seok-Joo Lee, Hyun-Je Kim, Na-ri Byun and Chung-Gyu Park in Cell Transplantation
Supplemental Material, Fig_S2 for Donor-Specific Regulatory T Cell-Mediated Immune Tolerance in an Intrahepatic Murine Allogeneic Islet Transplantation Model with Short-Term Anti-CD154 mAb Single Treatment by Seok-Joo Lee, Hyun-Je Kim, Na-ri Byun and Chung-Gyu Park in Cell Transplantation
Supplemental Material, Fig_S3 for Donor-Specific Regulatory T Cell-Mediated Immune Tolerance in an Intrahepatic Murine Allogeneic Islet Transplantation Model with Short-Term Anti-CD154 mAb Single Treatment by Seok-Joo Lee, Hyun-Je Kim, Na-ri Byun and Chung-Gyu Park in Cell Transplantation
Footnotes
Author Note: Seok-Joo Lee and Hyun-Je Kim equally contributed to this work.
Author Contributions: Seok-Joo Lee participated in research design, performed the experiments, analyzed and interpreted all data, and wrote and edited the manuscript. Hyun-Je Kim participated in design of the study, performed experiments, and interpreted the data. Na-ri Byun performed the experiments and interpreted the data. Chung-Gyu Park designed the study, interpreted the data, and wrote and edited the manuscript.
Data Accessibility Statement: All relevant data in the article and its supplementary information files can be accessed on request.
Ethical Approval: This study was approved by the Institutional Animal Care and Use Committee of Seoul National University, Korea.
Statement of Human and Animal Rights: All operations were performed according to international guidelines concerning the care and treatment of experimental animals.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by a grant from the Korea Healthcare Technology R&D project, Ministry of Health & Welfare, Republic of Korea (Project No. HI13C0954) and partly by a grant from Seoul National University Hospital (2020).
ORCID iD: Chung-Gyu Park  https://orcid.org/0000-0003-4083-8791
https://orcid.org/0000-0003-4083-8791
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Hering BJ, Clarke WR, Bridges ND, Eggerman TL, Alejandro R, Bellin MD, Chaloner K, Czarniecki CW, Goldstein JS, Hunsicker LG, Kaufman DB, et al. Phase 3 trial of transplantation of human islets in type 1 diabetes complicated by severe hypoglycemia. Diabetes Care. 2016;39(7):1230–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bottino R, Knoll MF, Knoll CA, Bertera S, Trucco MM. The future of islet transplantation is now. Front Med (Lausanne). 2018;5:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anazawa T, Okajima H, Masui T, Uemoto S. Current state and future evolution of pancreatic islet transplantation. Ann Gastroenterol Surg. 2019;3(1):34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shapiro AM, Pokrywczynska M, Ricordi C. Clinical pancreatic islet transplantation. Nat Rev Endocrinol. 2017;13(5):268–277. [DOI] [PubMed] [Google Scholar]
- 5. Rother KI, Harlan DM. Challenges facing islet transplantation for the treatment of type 1 diabetes mellitus. J Clin Invest. 2004;114(7):877–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Naesens M, Kuypers DR, Sarwal M. Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol. 2009;4(2):481–508. [DOI] [PubMed] [Google Scholar]
- 7. Bhatt S, Fung JJ, Lu L, Qian S. Tolerance-inducing strategies in islet transplantation. Int J Endocrinol. 2012;2012:396524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alpdogan O, van den Brink MR. Immune tolerance and transplantation. Semin Oncol. 2012;39(6):629–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hyvarinen K, Holopainen M, Skirdenko V, Ruhanen H, Lehenkari P, Korhonen M, Kakela R, Laitinen S, Kerkela E. Mesenchymal stromal cells and their extracellular vesicles enhance the anti-inflammatory phenotype of regulatory macrophages by downregulating the production of interleukin (IL)-23 and IL-22. Front Immunol. 2018;9:771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Galipeau J, Sensebe L. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell. 2018;22(6):824–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Oluwole SF, Oluwole OO, Adeyeri AO, DePaz HA. New strategies in immune tolerance induction. Cell Biochem Biophys. 2004;40(3 Suppl):27–48. [DOI] [PubMed] [Google Scholar]
- 12. Fehr T, Sykes M. Tolerance induction in clinical transplantation. Transpl Immunol. 2004;13(2):117–130. [DOI] [PubMed] [Google Scholar]
- 13. Tzakis AG, Reyes J, Zeevi A, Ramos H, Nour B, Reinsmoen N, Todo S, Starzl TE. Early tolerance in pediatric liver allograft recipients. J Pediatr Surg. 1994;29(6):754–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mazariegos GV, Sindhi R, Thomson AW, Marcos A. Clinical tolerance following liver transplantation: long term results and future prospects. Transpl Immunol. 2007;17(2):114–119. [DOI] [PubMed] [Google Scholar]
- 15. Orlando G, Soker S, Wood K. Operational tolerance after liver transplantation. J Hepatol. 2009;50(6):1247–1257. [DOI] [PubMed] [Google Scholar]
- 16. Shin JS, Kim JM, Kim JS, Min BH, Kim YH, Kim HJ, Jang JY, Yoon IH, Kang HJ, Kim J, Hwang ES, et al. Long-term control of diabetes in immunosuppressed nonhuman primates (NHP) by the transplantation of adult porcine islets. Am J Transplant. 2015;15(11):2837–2850. [DOI] [PubMed] [Google Scholar]
- 17. van Kooten C, Banchereau J. CD40-CD40 ligand. J Leukoc Biol. 2000;67(1):2–17. [DOI] [PubMed] [Google Scholar]
- 18. Nicolls MR, Coulombe M, Beilke J, Gelhaus HC, Gill RG. CD4-dependent generation of dominant transplantation tolerance induced by simultaneous perturbation of CD154 and LFA-1 pathways. J Immunol. 2002;169(9):4831–4839. [DOI] [PubMed] [Google Scholar]
- 19. Mai G, Bucher P, Morel P, Mei J, Bosco D, Andres A, Mathe Z, Wekerle T, Berney T, Buhler LH. Anti-CD154 mAb treatment but not recipient CD154 deficiency leads to long-term survival of xenogeneic islet grafts. Am J Transplant. 2005;5(5):1021–1031. [DOI] [PubMed] [Google Scholar]
- 20. Mai G, del Rio ML, Tian J, Ramirez P, Buhler L, Rodriguez-Barbosa JI. Blockade of the PD-1/PD-1 L pathway reverses the protective effect of anti-CD40 L therapy in a rat to mouse concordant islet xenotransplantation model. Xenotransplantation. 2007;14(3):243–248. [DOI] [PubMed] [Google Scholar]
- 21. Jung DY, Kim EY, Joo SY, Park JB, Moon C, Kim SH, Sim EY, Joh JW, Kwon CH, Kwon GY, Kim SJ. Prolonged survival of islet allografts in mice treated with rosmarinic acid and anti-CD154 antibody. Exp Mol Med. 2008;40(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Samy KP, Butler JR, Li P, Cooper DKC, Ekser B. The role of costimulation blockade in solid organ and islet xenotransplantation. J Immunol Res. 2017;2017:8415205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nanji SA, Hancock WW, Luo B, Schur CD, Pawlick RL, Zhu LF, Anderson CC, Shapiro AM. Costimulation blockade of both inducible costimulator and CD40 ligand induces dominant tolerance to islet allografts and prevents spontaneous autoimmune diabetes in the NOD mouse. Diabetes. 2006;55(1):27–33. [PubMed] [Google Scholar]
- 24. Arefanian H, Tredget EB, Mok DC, Ramji Q, Rafati S, Rodriguez-Barbosa J, Korbutt GS, Rajotte RV, Gill RG, Rayat GR. Porcine islet-specific tolerance induced by the combination of anti-LFA-1 and anti-CD154 mAbs is dependent on PD-1. Cell Transplant. 2016;25(2):327–342. [DOI] [PubMed] [Google Scholar]
- 25. Molano RD, Berney T, Li H, Cattan P, Pileggi A, Vizzardelli C, Kenyon NS, Ricordi C, Burkly LC, Inverardi L. Prolonged islet graft survival in NOD mice by blockade of the CD40-CD154 pathway of T-cell costimulation. Diabetes. 2001;50(2):270–276. [DOI] [PubMed] [Google Scholar]
- 26. Kenyon NS, Fernandez LA, Lehmann R, Masetti M, Ranuncoli A, Chatzipetrou M, Iaria G, Han D, Wagner JL, Ruiz P, Berho M, et al. Long-term survival and function of intrahepatic islet allografts in baboons treated with humanized anti-CD154. Diabetes. 1999;48(7):1473–1481. [DOI] [PubMed] [Google Scholar]
- 27. Kenyon NS, Chatzipetrou M, Masetti M, Ranuncoli A, Oliveira M, Wagner JL, Kirk AD, Harlan DM, Burkly LC, Ricordi C. Long-term survival and function of intrahepatic islet allografts in rhesus monkeys treated with humanized anti-CD154. Proc Natl Acad Sci USA. 1999;96(14):8132–8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rajab A. Islet transplantation: alternative sites. Curr Diab Rep. 2010;10(5):332–337. [DOI] [PubMed] [Google Scholar]
- 29. Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343(4):230–238. [DOI] [PubMed] [Google Scholar]
- 30. Ryan EA, Lakey JR, Paty BW, Imes S, Korbutt GS, Kneteman NM, Bigam D, Rajotte RV, Shapiro AM. Successful islet transplantation: continued insulin reserve provides long-term glycemic control. Diabetes. 2002;51(7):2148–2157. [DOI] [PubMed] [Google Scholar]
- 31. Rafael E, Ryan EA, Paty BW, Oberholzer J, Imes S, Senior P, McDonald C, Lakey JR, Shapiro AM. Changes in liver enzymes after clinical islet transplantation. Transplantation. 2003;76(9):1280–1284. [DOI] [PubMed] [Google Scholar]
- 32. Rafael E, Tibell A, Ryden M, Lundgren T, Savendahl L, Borgstrom B, Arnelo U, Isaksson B, Nilsson B, Korsgren O, Permert J. Intramuscular autotransplantation of pancreatic islets in a 7-year-old child: a 2-year follow-up. Am J Transplant. 2008;8(2):458–462. [DOI] [PubMed] [Google Scholar]
- 33. Wang C, Du X, He S, Yuan Y, Han P, Wang D, Chen Y, Liu J, Tian B, Yang G, Yi S, et al. A preclinical evaluation of alternative site for islet allotransplantation. PLoS One. 2017;12(3):e0174505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, Secchi A, Brendel MD, Berney T, Brennan DC, Cagliero E, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355(13):1318–1330. [DOI] [PubMed] [Google Scholar]
- 35. Byun N, Kim HJ, Min BH, Shin JS, Yoon IH, Kim JM, Kim YH, Park CG. A novel method for murine intrahepatic islet transplantation via cecal vein. J Immunol Methods. 2015;427:122–125. [DOI] [PubMed] [Google Scholar]
- 36. Lahl K, Sparwasser T. In vivo depletion of FoxP3+ Tregs using the DEREG mouse model. Methods Mol Biol. 2011;707:157–172. [DOI] [PubMed] [Google Scholar]
- 37. Pan X, Xue W, Li Y, Feng X, Tian X, Ding C. Islet graft survival and function: concomitant culture and transplantation with vascular endothelial cells in diabetic rats. Transplantation. 2011;92(11):1208–1214. [DOI] [PubMed] [Google Scholar]
- 38. Estil Les E, Tellez N, Nacher M, Montanya E. A model for human islet transplantation to immunodeficient streptozotocin-induced diabetic mice [published online ahead of print October 1, 2018]. Cell Transplant. 2018:963689718801006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yoon IH, Choi SE, Kim YH, Yang SH, Park JH, Park CS, Kim Y, Kim JS, Kim SJ, Simpson E, Park CG. Pancreatic islets induce CD4(+) [corrected] CD25(-)Foxp3(+) [corrected] T-cell regulated tolerance to HY-mismatched skin grafts. Transplantation. 2008;86(10):1352–1360. [DOI] [PubMed] [Google Scholar]
- 40. Diab RA, Fares M, Abedi-Valugerdi M, Kumagai-Braesch M, Holgersson J, Hassan M. Immunotoxicological effects of streptozotocin and alloxan: in vitro and in vivo studies. Immunol Lett. 2015;163(2):193–198. [DOI] [PubMed] [Google Scholar]
- 41. Rudensky A. Foxp3 and dominant tolerance. Philos Trans R Soc Lond B Biol Sci. 2005;360(1461):1645–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133(5):775–787. [DOI] [PubMed] [Google Scholar]
- 43. Leguern C. Regulatory T cells for tolerance therapy: revisiting the concept. Crit Rev Immunol. 2011;31(3):189–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhou J, He W, Luo G, Wu J. Fundamental immunology of skin transplantation and key strategies for tolerance induction. Arch Immunol Ther Exp (Warsz). 2013;61(5):397–405. [DOI] [PubMed] [Google Scholar]
- 45. Rogers NJ, Lechler RI. Allorecognition. Am J Transplant. 2001;1(2):97–102. [PubMed] [Google Scholar]
- 46. Larsen CP, Steinman RM, Witmer-Pack M, Hankins DF, Morris PJ, Austyn JM. Migration and maturation of Langerhans cells in skin transplants and explants. J Exp Med. 1990;172(5):1483–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Richters CD, van Gelderop E, du Pont JS, Hoekstra MJ, Kreis RW, Kamperdijk EW. Migration of dendritic cells to the draining lymph node after allogeneic or congeneic rat skin transplantation. Transplantation. 1999;67(6):828–832. [DOI] [PubMed] [Google Scholar]
- 48. Benichou G, Yamada Y, Yun SH, Lin C, Fray M, Tocco G. Immune recognition and rejection of allogeneic skin grafts. Immunotherapy. 2011;3(6):757–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Qian S, Lu L, Li Y, Fu F, Li W, Starzl TE, Thomson AW, Fung JJ. Apoptosis of graft-infiltrating cytotoxic T cells: a mechanism underlying “split tolerance” in mouse liver transplantation. Transplant Proc. 1997;29(1-2):1168–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mathes DW, Randolph MA, Solari MG, Nazzal JA, Nielsen GP, Arn JS, Sachs DH, Lee WP. Split tolerance to a composite tissue allograft in a swine model. Transplantation. 2003;75(1):25–31. [DOI] [PubMed] [Google Scholar]
- 51. Chung Y, Ko SY, Ko HJ, Kang CY. Split peripheral tolerance: CD40 ligation blocks tolerance induction for CD8 T cells but not for CD4 T cells in response to intestinal antigens. Eur J Immunol. 2005;35(5):1381–1390. [DOI] [PubMed] [Google Scholar]
- 52. de Mestre A, Noronha L, Wagner B, Antczak DF. Split immunological tolerance to trophoblast. Int J Dev Biol. 2010;54(2-3):445–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Luo B, Chan WF, Shapiro AM, Anderson CC. Non-myeloablative mixed chimerism approaches and tolerance, a split decision. Eur J Immunol. 2007;37(5):1233–1242. [DOI] [PubMed] [Google Scholar]
- 54. Chan WF, Razavy H, Luo B, Shapiro AM, Anderson CC. Development of either split tolerance or robust tolerance along with humoral tolerance to donor and third-party alloantigens in nonmyeloablative mixed chimeras. J Immunol. 2008;180(8):5177–5186. [DOI] [PubMed] [Google Scholar]
- 55. Fuchimoto Y, Gleit ZL, Huang CA, Kitamura H, Schwarze ML, Menard MT, Mawulawde K, Madsen JC, Sachs DH. Skin-specific alloantigens in miniature swine. Transplantation. 2001;72(1):122–126. [DOI] [PubMed] [Google Scholar]
- 56. Ferrer IR, Wagener ME, Song M, Kirk AD, Larsen CP, Ford ML. Antigen-specific induced Foxp3+ regulatory T cells are generated following CD40/CD154 blockade. Proc Natl Acad Sci USA. 2011;108(51):20701–20706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lin X, Chen M, Liu Y, Guo Z, He X, Brand D, Zheng SG. Advances in distinguishing natural from induced Foxp3(+) regulatory T cells. Int J Clin Exp Pathol. 2013;6(2):116–123. [PMC free article] [PubMed] [Google Scholar]
- 58. Ochando JC, Homma C, Yang Y, Hidalgo A, Garin A, Tacke F, Angeli V, Li Y, Boros P, Ding Y, Jessberger R, et al. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat Immunol. 2006;7(6):652–662. [DOI] [PubMed] [Google Scholar]
- 59. Saemann MD, Kelemen P, Zeyda M, Bohmig G, Staffler G, Zlabinger GJ. CD40 triggered human monocyte-derived dendritic cells convert to tolerogenic dendritic cells when JAK3 activity is inhibited. Transplant Proc. 2002;34(5):1407–1408. [DOI] [PubMed] [Google Scholar]
- 60. Zhou Y, Leng X, Li H, Yang S, Yang T, Li L, Xiong Y, Zou Q, Liu Y, Wang Y. Tolerogenic dendritic cells induced by BD750 ameliorate proinflammatory T cell responses and experimental autoimmune encephalitis in mice. Mol Med. 2017;23:204–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhang X, Kedl RM, Xiang J. CD40 ligation converts TGF-beta-secreting tolerogenic CD4-8- dendritic cells into IL-12-secreting immunogenic ones. Biochem Biophys Res Commun. 2009;379(4):954–958. [DOI] [PubMed] [Google Scholar]
- 62. Jiang XF, Cui ZM, Zhu L, Guo DW, Sun WY, Lin L, Wang XF, Tang YF, Liang J. CD40-CD40 L costimulation blockade induced the tolerogenic dendritic cells in mouse cardiac transplant. Int Surg. 2010;95(2):135–141. [PubMed] [Google Scholar]
- 63. Yvon ES, Vigouroux S, Rousseau RF, Biagi E, Amrolia P, Dotti G, Wagner HJ, Brenner MK. Overexpression of the Notch ligand, Jagged-1, induces alloantigen-specific human regulatory T cells. Blood. 2003;102(10):3815–3821. [DOI] [PubMed] [Google Scholar]
- 64. Cahill EF, Tobin LM, Carty F, Mahon BP, English K. Jagged-1 is required for the expansion of CD4+ CD25+ FoxP3+ regulatory T cells and tolerogenic dendritic cells by murine mesenchymal stromal cells. Stem Cell Res Ther. 2015;6:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Xu LL, Fu HX, Zhang JM, Feng FE, Wang QM, Zhu XL, Xue J, Wang CC, Chen Q, Liu X, Wang YZ, et al. Impaired function of bone marrow mesenchymal stem cells from immune thrombocytopenia patients in inducing regulatory dendritic cell differentiation through the notch-1/jagged-1 signaling pathway. Stem Cells Dev. 2017;26(22):1648–1661. [DOI] [PubMed] [Google Scholar]
- 66. Lin Y, Chen W, Li J, Yan G, Li C, Jin N, Chen J, Gao C, Ma P, Xu S, Qi Z. Overexpression of Jagged-1 combined with blockade of CD40 pathway prolongs allograft survival. Immunol Cell Biol. 2015;93(2):213–217. [DOI] [PubMed] [Google Scholar]
- 67. Ferrer IR, Wagener ME, Song M, Ford ML. CD154 blockade alters innate immune cell recruitment and programs alloreactive CD8+ T cells into KLRG-1(high) short-lived effector T cells. PLoS One. 2012;7(7):e40559. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, Fig_S1 for Donor-Specific Regulatory T Cell-Mediated Immune Tolerance in an Intrahepatic Murine Allogeneic Islet Transplantation Model with Short-Term Anti-CD154 mAb Single Treatment by Seok-Joo Lee, Hyun-Je Kim, Na-ri Byun and Chung-Gyu Park in Cell Transplantation
Supplemental Material, Fig_S2 for Donor-Specific Regulatory T Cell-Mediated Immune Tolerance in an Intrahepatic Murine Allogeneic Islet Transplantation Model with Short-Term Anti-CD154 mAb Single Treatment by Seok-Joo Lee, Hyun-Je Kim, Na-ri Byun and Chung-Gyu Park in Cell Transplantation
Supplemental Material, Fig_S3 for Donor-Specific Regulatory T Cell-Mediated Immune Tolerance in an Intrahepatic Murine Allogeneic Islet Transplantation Model with Short-Term Anti-CD154 mAb Single Treatment by Seok-Joo Lee, Hyun-Je Kim, Na-ri Byun and Chung-Gyu Park in Cell Transplantation