Abstract
In clinical and experimental human pancreatic islet transplantations, establishing pretransplant assessments that accurately predict transplantation outcomes is crucial. Conventional in vitro viability assessment that relies on manual counting of viable islets is a routine pretransplant assessment. However, this method does not correlate with transplantation outcomes; to improve the method, we recently introduced a semi-automated method using imaging software to objectively determine area-based viability. The goal of the present study was to correlate semi-automated viability assessment with posttransplantation outcomes of human islet transplantations in diabetic immunodeficient mice, the gold standard for in vivo functional assessment of isolated human islets. We collected data from 61 human islet isolations and 188 subsequent in vivo mouse transplantations. We assessed islet viability by fluorescein diacetate and propidium iodide staining using both the conventional and semi-automated method. Transplantations of 1,200 islet equivalents under the kidney capsule were performed in streptozotocin-induced diabetic immunodeficient mice. Among the pretransplant variables, including donor factors and post-isolation assessments, viability measured using the semi-automated method demonstrated a strong influence on in vivo islet transplantation outcomes in multivariate analysis. We calculated an optimized cutoff value (96.1%) for viability measured using the semi-automated method and showed a significant difference in diabetes reversal rate for islets with viability above this cutoff (77% reversal) vs. below this cutoff (49% reversal). We performed a detailed analysis to show that both the objective measurement and the improved area-based scoring system, which distinguished between small and large islets, were key features of the semi-automated method that allowed for precise evaluation of viability. Taken together, our results suggest that semi-automated viability assessment offers a promising alternative pretransplant assessment over conventional manual assessment to predict human islet transplantation outcomes.
Keywords: islet viability, semi-automated method, conventional manual method, islet transplantation outcomes
Introduction
Over the past four decades, advancements in the field of islet transplantation have been translated from animal models to Food and Drug Administration-approved human clinical studies for the treatment of patients with labile type 1 diabetes1. In clinical islet transplantation, evaluation of an isolated human islet product prior to transplantation is vital for predicting transplantation outcomes. Multiple factors are currently evaluated to determine suitability for transplantation, including condition of the donor-pancreas, condition of the recipient, and in vitro parameters of the isolated islets, such as islet yield, function, and viability2,3. At present, few in vitro assessments are accurate in predicting posttransplant outcomes4.
Because the islet isolation process introduces mechanical, enzymatic, and osmotic stress4, viability analysis of the isolated islets is an essential pretransplant in vitro assessment. The current method for assessing the viability of isolated islets involves concurrent application of the cell-permeable esterase substrate fluorescein diacetate (FDA) for live-cell staining and the cell-impermeant nucleic acid stain propidium iodide (PI) for dead-cell staining5,6. Islet viability is then manually evaluated based on the extent of live-cell staining present for each individual islet cluster in a given islet batch. However, this method has limitations, including its operator-biased manual count7,8. In addition, the conventional scoring system fails to consider islet size when assessing viability, creating no distinction between small and large islets, with correspondingly small or large areas of necrosis. In other words, conventional manual viability is a non-area-based method that evaluates individual islet viability, regardless of the size of islets or dead cell areas, to calculate an average viability. Consequently, the results of conventional manual viability assessments (viability-conventional) do not correlate with in vivo transplantation outcomes in mice8,9. For viability assessment to be considered an optimal pretransplant islet assessment—i.e., to facilitate appropriate selection of islets that will support successful transplantation—viability assessment must be improved.
We recently introduced a semi-automated viability assessment method that employs computer-based measurement of total viable and dead areas using the same fluorescent images as the conventional method10,11. However, to date, a correlation between viability measured using the semi-automated method (viability-semi-automated) and in vivo transplantation outcomes had not been established. Therefore, in this study, we correlated it with posttransplantation outcomes of human islets in diabetic immunodeficient mice.
Materials and Methods
Human Isolated Islet Preparations
Human islets were isolated from human pancreata obtained from deceased donors through OneLegacy, a local organ procurement organization in the greater Los Angeles area12. All islet isolations were conducted following standard operating procedures at the Southern California Islet Cell Resource (SC-ICR) Center at City of Hope. Data on human islets isolated and distributed for research purposes between March 2015 and June 2018 were retrospectively analyzed. Islets used in this study were selected using the following inclusion donor criteria: 20 to 65 yr old and glycated hemoglobin (HbA1c) less than 6.4%. Islets with >7 d post-isolation culture period before distribution were excluded from the study. In total, 61 islet batches met the inclusion criteria. Standardized characteristics, consistent with the recommendations of Diabetes and Diabetologia 13,14, are summarized for donors and islets used in this study in supplemental Table 1. After isolation, islets were cultured in PIM-R culture medium (PRODO Laboratories, Aliso Viejo, CA, USA) until they were distributed for analyses. The following measurements were collected for quality assessment of isolated islets: islet count (post-isolation islet equivalent number [IEQ] and post-isolation islet particulate number [IPN]), islet purity assessed by dithizone (DTZ) stain, viability, and in vivo function (islet transplantation using diabetic immunodeficient mice). In addition to these post-isolation assays, days of post-isolation culture (days between the completion of islet isolation and islet quality assessments) and donor/isolation factors (donor age, donor body mass index [BMI], donor HbA1c level, cold ischemia time [time between the cross-clamping for pancreas procurement to pancreas digestion for islet isolation]) were recorded.
Viability Assessment of Isolated Islets
Viability assessment of isolated islets was performed using both the conventional method and the semi-automated method using FDA (Sigma-Aldrich, St Louis, MO, USA) and PI (Sigma-Aldrich). To make the FDA/PI solution used for viability staining, 5 µl of concentrated FDA stock solution (48 µM; 2 mg FDA in 100 ml of acetone [Sigma-Aldrich]) and 5 µl PI solution (1.5 mM, the original commercially available concentration) were dissolved in 490 µl phosphate-buffered saline (PBS) in the dark at 22°C, which results in final concentrations of 0.48 µM FDA and 15 µM PI. Islet samples were prepared separately for the two viability assessment methods. In a 1.5-ml tube, 100 to 200 IEQ were incubated in FDA/PI solution in the dark at 22°C for 5 min. Islets were washed with PBS, and then transferred to the appropriate plates for subsequent viability imaging.
Conventional Manual Method
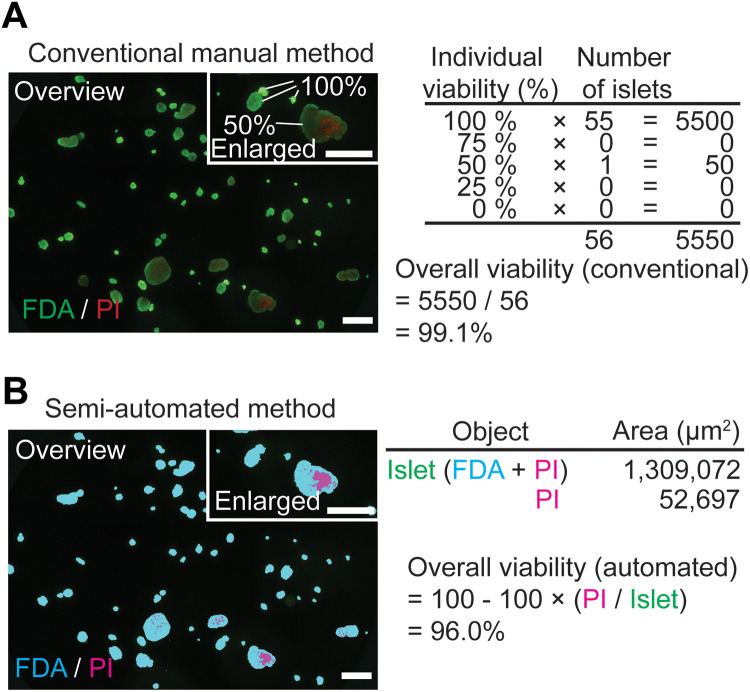
Viability was assessed manually by trained personnel following standard operating procedures at the SC-ICR Center. The FDA/PI-stained islet suspension was transferred to a six-well plate. Assessment of the entire well was performed under a fluorescence microscope (IX50, Olympus, Tokyo, Japan) using a 10× objective lens. Each islet cluster was categorized into one of five “estimated % viability” categories (individual viability), according to the percentage of FDA-positive (green/alive) staining (Table 1). The percent viability of the islet sample was calculated as follows: viability-conventional (%) = ([(1.00 × number of islets scored as 100%) + (0.75 × number of islets scored as 75%) + (0.5 × number of islets scored as 50%) + (0.25 × number of islets scored as 25%)]/total islet number scored) × 100 (Fig. 1A). Conventional viability was performed using a single sample per batch of human islets (n = 61).
Table 1.
Scoring of Islet Viability in the Conventional Manual Method Using FDA/PI Staining.
| Description | Estimated % viability |
|---|---|
| Almost all cells stained green (FDA-positive); few to no cells stained red (PI-positive) | 100 |
| ≥65% islet cells stained green | 75 |
| 35%-65% islet cells stained green | 50 |
| ≤35% islet cells stained green | 25 |
| Few to no cells stained green | 0 |
FDA: fluorescein diacetate; PI: propidium iodide.
Fig. 1.
Viability assessment using conventional and semi-automated methods. (A) An example of viability calculation using the conventional manual method (green: FDA-positive area; red: PI-positive area). Overall viability is assessed based on estimated viability in individual islets; islets are assessed in the enlarged figure (upper right). Calculation according to the conventional assessment is described. (B) An example of viability calculation using the semi-automated method (blue: FDA-positive area; pink: PI-positive area). Overall viability is assessed based on the total islet area and total dead area of all islets in the micrograph image (not based on individual islet viability). Scale bar: 500 µm.
FDA: fluorescein diacetate; PI: propidium iodide.
Semi-Automated Method
Islet viability was assessed using the semi-automated method as previously described11. An FDA/PI-stained islet suspension was applied to a 96-well plate. Micrographs were captured using a 4× objective lens (IX50, Olympus). For evaluation, multiple images of a total well were captured and assembled into a single image covering the entire well. By setting a threshold for green (for FDA; i.e., live cells) and red (for PI; i.e., dead cells), FDA-positive or PI-positive areas were automatically calculated by the imaging software (cellSens, Olympus). FDA-positive area and PI-positive area were mutually exclusive in the islet structure for the analysis (as colored by the software: blue for FDA-positive area and pink for PI-positive area; Fig. 1B), and islet area was defined as the sum of FDA-positive and PI-positive areas. The percent viability of an islet sample was calculated as follows: viability-semi-automated (%) = 100 – ([PI-positive area/islet area] × 100). Unlike the conventional method, the semi-automated method does not measure the viability of individual islets but calculates overall viability using data from all areas including PI-positive areas and total islet area.
Size Assessment of Isolated Islets
The size of isolated islets was analyzed using data from the viability assay images. Individual islet area in each viability assay image was calculated using imaging software (cellSens, Olympus). Islet area data were converted to a two-dimensional (2D) circular model to calculate islet diameter15. Average islet diameter in each islet preparation was calculated.
In Vivo Islet Transplantation
Nonobese diabetic, severe combined immunodeficiency mice (Charles River Laboratories, Wilmington, MA, USA) were used as recipients for islet transplantations, as previously described16. Mice were rendered diabetic by streptozotocin injections (50 mg/kg, intraperitoneal injections in consecutive 3 d). Mice with a blood glucose level >400 mg/dl for two consecutive measurements were considered diabetic. For every islet isolation that met the inclusion criteria described above (human isolated islet preparations), 2 to 8 mice (3.7 mice per islet batch on average) were transplanted with 1,200 IEQ under the renal capsule. Mice were closely monitored for more than 30 d and blood glucose levels were measured twice a week using a glucose meter (LifeScan, Inc., Milpitas, CA, USA). Diabetes reversal was defined as having blood glucose below 200 mg/dl for more than two consecutive measurements. To analyze posttransplant blood glucose control quantitatively, the area under the curve (AUC) for days 0 to 28 was calculated as the sum of blood glucose measurements from days 0 to 28, which reflects glucose change for 4 wk in an individual mouse (supplemental Figure 1). The use of animals and animal procedures in this study was approved by the City of Hope/Beckman Research Institute Institutional Animal Care and Use Committee.
Statistical Analysis
All data points were plotted to illustrate the correlation between viability (assessed using conventional or semi-automated methods) and other factors. Correlations were analyzed using fitted regression lines and coefficient of correlation (R) and coefficient of determination (R) values, and statistical significance was calculated using F-tests. A fitted regression line is shown in a figure when statistical significance was detected. Multivariate analysis was performed to extract the factors that statistically impacted upon in vivo islet transplantation outcomes. Comparisons between two factors were analyzed using a Student’s t-test. Receiver operating characteristic (ROC) curves were used to calculate the optimal cutoff value to distinguish two groups among the data set. Comparisons between two groups in the cumulative diabetes reversal assessment were analyzed using a log-rank test. All statistical analyses were performed using JMP 13.0.0 (SAS Institute, Cary, NC, USA). P <0.05 was considered significant.
Results
Results Using Two Viability Assessment Methods Do Not Correlate and the Semi-Automated Method Shows a More Dynamic Range of Values Than the Conventional Method
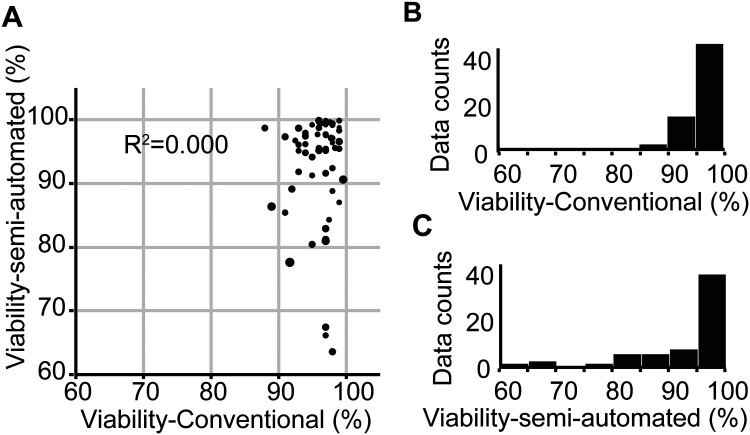
As described in Materials and Methods, we assessed human islet viability using both conventional and semi-automated methods. Manual viability assessment evaluates individual islet viability regardless of dead-cell area, to calculate the average viability as an overall viability (Fig. 1A, right). In contrast, semi-automated assessment uses total islet area and total dead islet area among all the islets analyzed (Fig. 1B, right). We correlated viability-conventional with viability-semi-automated in each of 61 islet batches. We found no statistical relationship between the results of the two methods (R 2 = 0.000, P = 0.9435; Fig. 2A). Percent viability calculated using the conventional method ranged from 88.0% to 99.6% (mean = 95.9%, standard deviation = 2.6, Fig. 2B), whereas viability calculated using the semi-automated method showed a wider range of 63.5% to 99.9% (mean = 92.9%, standard deviation = 8.5, Fig. 2C).
Fig. 2.
Lack of correlation between viability data obtained using the conventional method and the semi-automated method. (A) Islet samples from 61 islet batches were analyzed using both conventional and semi-automated viability methods. No correlation was demonstrated between methods (R 2 = 0.000). (B, C) The distribution of viability data collected using the conventional method (B) showed a narrower range compared to viability data collected using the semi-automated method (C).
Blood Glucose AUC Is a Sensitive and Quantitative Indicator of In Vivo Transplantation Outcomes
Diabetes reversal in mice receiving islet transplantations is commonly used to evaluate in vivo transplantation outcomes. Although this is a convenient assessment, the results are qualitative (categorized only into yes or no). To determine whether a quantitative measurement, blood glucose change, is an appropriate in vivo assessment of transplantation outcomes, we determined whether blood glucose AUC is associated with diabetes reversal in mice receiving islet transplantations (1,200 IEQ). To do this, we calculated AUC for blood glucose after transplantation (0 to 28 d [AUC_0-28], days × mg/dl) in individual mice (supplemental Figure 1); in this analysis, a smaller AUC indicates better posttransplant glycemic control. We then compared AUC data between mice that experienced diabetes reversal (n = 112) and mice that did not (n = 63). We showed a distinct distribution between diabetes-reversed mice and nonreversed mice; the average AUC_0-28 was significantly lower for diabetes-reversed mice compared to nonreversed mice (P < 0.0001, supplemental Figure 2A). We used ROC curves to calculate an optimal cutoff AUC value for diabetes reversal of 7,928 d mg/dL (sensitivity = 0.9035, specificity = 0.8481, supplemental Figure 2B). Although the cutoff AUC value distinguished two groups (reversed and not reversed), both groups demonstrated a wide range of values; i.e., blood glucose controls of individual mice were different even when they were categorized in the same group. This suggests that blood glucose AUC is an appropriate quantitative indicator for in vivo transplantation outcomes.
Viability Measured Using the Semi-Automated Method Strongly Influences In Vivo Islet Transplantation Outcomes in Multivariate Analysis
We used multivariate analysis to assess the relative impact of 10 pretransplant variables, including donor/isolation-related characteristics, on predicting in vivo transplantation outcomes. We used AUC_0-28 as a quantitative transplantation outcome representative of posttransplant glycemic control. We showed that four variables, viability-semi-automated, donor BMI, average islet area, and post-isolation IPN, significantly correlated with AUC_0-28 (Table 2; the intercorrelation matrix is shown separately in supplemental Table 2). Among these, viability-semi-automated demonstrated the strongest correlation; higher viability-semi-automated was statistically associated with lower AUC_0-28, i.e., better posttransplant glycemic control. Viability-conventional did not correlate with AUC_0-28.
Table 2.
Variables Influencing In Vivo Islet Transplantation Outcomes.
| Variables | Category | R (AUC_0-28) | P-value | Notes |
|---|---|---|---|---|
| Viability-semi-automated (%) | Post-isolation assay | −0.299 | 0.0001 | Correlated with good glycemic control |
| Donor BMI | Donor/Isolation | 0.2239 | 0.0048 | Correlated with poor glycemic control |
| Average islet area (µm2) | Post-isolation assay | 0.2204 | 0.0055 | Correlated with poor glycemic control |
| Post-isolation IPN | Post-isolation assay | −0.2068 | 0.0094 | Correlated with good glycemic control |
Pretransplant variables are listed according to the order of statistical relationship to AUC_0-28. Coefficient of correlation (R) is shown instead of coefficient of determination (R 2); negative R value indicates that the factor has a positive correlation with favorable transplantation outcomes (i.e., low AUC_0-28). Only variables with statistically significant correlations with transplantation outcome are listed. An intercorrelation matrix of all variables is shown separately in supplemental Table 2.AUC: area under the curve; BMI: body mass index; IPN: islet particulate number.
Optimized Semi-Automated Viability Cutoff Value Predicts Diabetes Reversal Rate
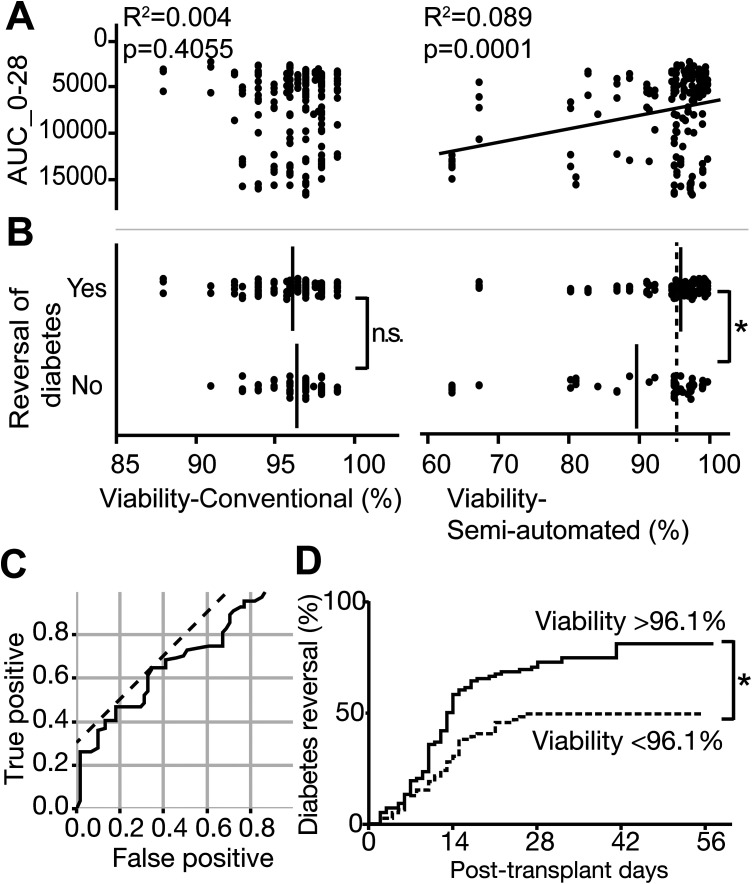
Because viability-semi-automated correlated with AUC_0-28 in the multivariate analysis, we further evaluated the application of islet viability as a useful pretransplant assessment for the prediction of transplantation outcomes. We plotted glycemic control (AUC_0-28) data from all mice against islet viability measured using each method (viability-conventional or viability-semi-automated). Consistent with the data shown in Table 2, AUC_0-28 did not correlate with viability-conventional (R 2 = 0.004, P = 0.4055, Fig. 3A left panel), but did correlate with viability-semi-automated (R 2 = 0.089, P = 0.0001, Fig. 3A right panel). The average viability-conventional in mice with diabetes reversal (96.2% ± 0.2%) was not significantly different from that in mice with no diabetes reversal (96.4% ± 0.2%) (P = 0.4197, Fig. 3B left panel). In contrast, for viability-semi-automated, the average viability in mice with diabetes reversal (96.2% ± 2.3%) was significantly greater than the average viability in mice with no diabetes reversal (89.8% ± 1.5%) (P < 0.0001, Fig. 3A right panel). To establish the optimal cutoff value for semi-automated viability that may predict diabetes reversal, we used an ROC curve (sensitivity = 0.6486, specificity = 0.6557, Fig. 3C) to calculate this cutoff value as 96.1% (dotted line in Fig. 3C). Indeed, the cumulative curves of diabetes reversal in mice that received islets with >96.1% viability vs. islets with <96.1% viability were significantly different, with overall reversal rates of 77.4% and 49.4%, respectively (P = 0.0005, Fig. 3D). This suggests that viability-semi-automated can be used to predict diabetes reversal rate.
Fig. 3.
Correlation of in vivo islet transplantation outcomes with viability. In vivo transplantation outcomes data from individual mice (n = 188) that received 1,200 islet equivalent number of human islets were plotted against viability measured using both methods. In vivo transplantation outcomes were measured by AUC_0-28 as quantitative data, and by diabetes reversal (<200 mg/dl glycemic control) as qualitative data. (A) AUC_0-28 correlated with viability measured using the semi-automated (R2 = 0.089) but not conventional method (R 2 = 0.004). (B) Average islet viability (vertical bars in plots) in mice with diabetes reversal vs. mice without diabetes reversal did not differ for viability measured manually (96.2% vs. 96.4%), but demonstrated a significant difference for viability measured using the semi-automated method (96.2% vs. 89.8%, *P < 0.0001). Dotted line in viability-semi-automated plot shows the cutoff line for diabetes reversal calculated in the following ROC curve assessment. (C) ROC curve to calculate optimal cutoff line (96.1%) for the prediction of diabetes reversal using viability-semi-automated. Dotted line is the reference line to calculate the optimal cutoff compromising between sensitivity (0.6486) and specificity (0.6557). (D) Cumulative curves of diabetes reversal in mice. Two groups (29 batches in >96.1% viability group, and 30 batches in <96.1% viability group) demonstrated a significant difference in diabetes reversal rate (77.4% vs. 49.4%). *P = 0.0005.
AUC: area under the curve; ROC: receiver operating characteristic.
The Semi-Automated Method Is an Islet-Size-Sensitive Assessment that Distinguishes Between Small and Large Islets and Enables Precise Viability Evaluation
Given that viability measured using the semi-automated method, but not the conventional method, correlated with islet transplantation outcomes, we examined possible reasons for this. For example, the semi-automated method is advantageous over the conventional method in terms of a more precise area calculation. In contrast, the conventional manual scoring system is a non-area-based method that evaluates individual islet viability regardless of size. Using a similar graph to that shown in Fig. 2A, in which we plotted islet viability data collected using conventional vs. semi-automated methods, we further assessed conventional vs. semi-automated methods relative to average islet size within islet batches. To do this, we first colored data plots according to the average islet size for visualization. We then categorized islet batches into two groups based on the relative estimation of viability by each method to compare islet size between groups: for Group 1, viability-conventional < viability-semi-automated, n = 28; for Group 2, viability-semi-automated < viability-conventional, n = 33 (Fig. 4A). Group 2, in which the conventional method overestimated viability compared to the semi-automated method, contained large-sized islets. Figure 4B shows the representative appearance of an islet batch in each group. To assess this difference statistically, we compared average islet size between the two groups. Group 2 (viability-semi-automated < viability-conventional) exhibited a significantly greater average islet area compared to Group 1 (viability-conventional < viability-semi-automated) (Fig. 4C, P = 0.0071).
Fig. 4.
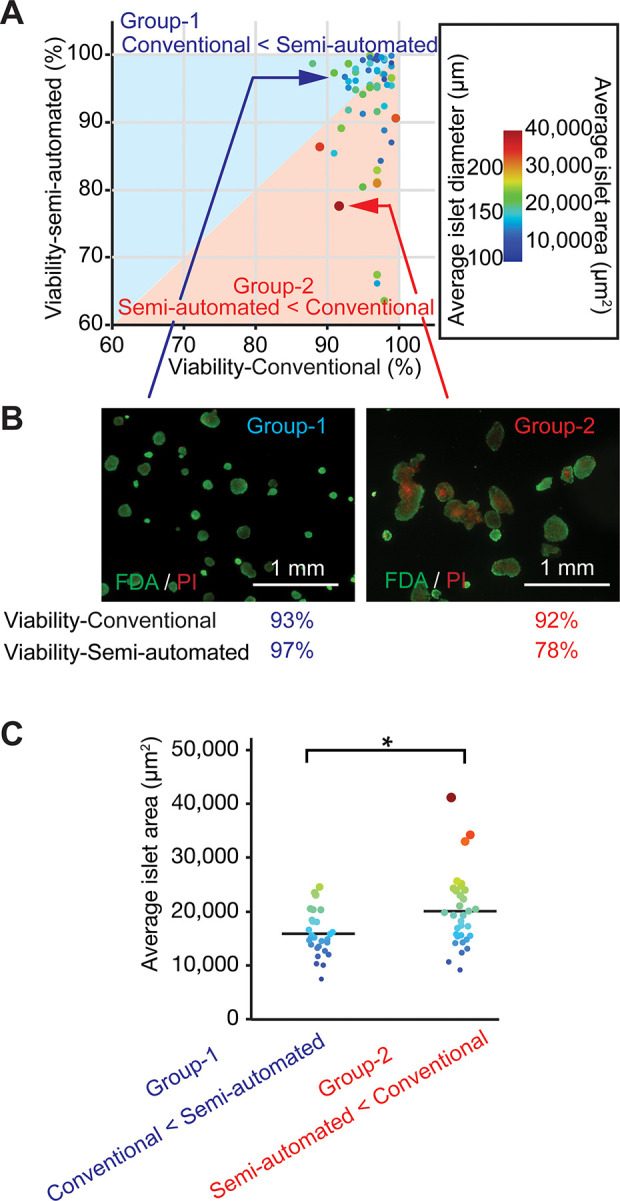
The semi-automated method allows islet-size-sensitive analysis with distinction between small and large islets and enables precise viability evaluation. (A) Islet batches are colored based on average islet size. Islet batches were also classified into two groups: Group 1 (upper left, hatched in blue, n = 28) demonstrating viability-conventional < viability-semi-automated and Group 2 (lower right, hatched in orange, n = 33) demonstrating viability-semi-automated < viability-conventional. Group 2 contained batches of large-sized islets. (B) Representative islet appearance in the two indicated groups, exemplifying the difference in islet size. (C) Average islet size analysis demonstrated a significant difference in islet area between the two groups. *P = 0.0071.
In islet batches containing some relative proportion of large islets, the conventional viability assay fails to calculate a precise percentage of the area of dead (or viable) cells among the entire islet preparation. This is because the conventional assay assesses individual islet viability, then averages those viabilities, without considering islet size at any point. Notably, large islets typically develop large areas of central necrosis that greatly impact the total dead area among the entire islet preparation15. Indeed, in our data set, viability-semi-automated is negatively correlated with average islet size (P < 0.0001, supplemental Figure 3); i.e., islet preparations containing larger islets exhibit lower viability. We present schematics representing two distinct islet batches, one that does not include large islets (supplemental Figure 4A) and one that includes large islets (supplemental Figure 4B), with data from both methods of viability assessment. Viability calculated based on these schematics demonstrates an overestimation of viability using the conventional vs. semi-automated method in the islet batch containing large islets but not in the batch without large islets. Note that in the conventional viability calculation, a large, 75%-viable islet is regarded as equivalent to a small, 75%-viable islet, although the areas of necrosis differ considerably between those islets (i.e., conventional method is non-islet-size-sensitive). This is consistent with the less precise, narrower viability range we measured using the conventional method (88.0% to 99.6%) compared to the semi-automated method (63.5% to 99.9%) in the empirical data set (Fig. 2), as well as with the consequent inability of the conventional method to predict transplantation outcomes. The more precise, islet-size-sensitive, semi-automated method overcame this scoring drawback by estimating total dead and viable areas within islets, which likely contributed to its successful prediction of transplantation outcomes.
Discussion
The conventional manual method used to assess the viability of isolated human islets prior to transplantation is limited by inherent drawbacks, such as operator bias and reliance on a non-islet-size-sensitive scoring system, which can affect assay integrity. We previously introduced a semi-automated method of calculating islet viability based on relative live and dead cell area to eliminate such drawbacks11. In this study, we found that viability measured using the semi-automated method demonstrated a correlation with islet transplantation outcomes in mice, but viability measured using the conventional method did not.
Several factors have been reported to affect transplantation outcomes of isolated islets, including donor age, isolation techniques, and islet dose2,17. Among in vitro assays used for quality assessment, several groups have reported that the glucose-stimulated insulin secretion assay and conventional viability assay are ineffective at predicting posttransplant outcomes3–5,8,18,19, despite being routinely performed. Recent studies introduced the concept of using the oxygen consumption rate of islets or the adenosine diphosphate/adenosine triphosphate ratio as a measurement of mitochondrial activity as possible predictive factors that correlate with mouse transplantation outcomes4,20,21. Accordingly, there are some in vitro prediction factors widely accepted today, which predict transplantation success in clinical and experimental settings. Human islet transplantation to diabetic immunodeficient mice is the only in vivo test widely utilized8,22, although the lengthy duration of the assay renders only retrospective results.
Our semi-automated method is a computer-based assessment that automatically measures the total FDA-positive (i.e., live) area and total PI-positive (i.e., dead) area to calculate the percent viability of the entire islet sample11. This method contributes to the objective evaluation of islet viability by limiting operator bias; however, it still has the potential to involve operator bias in the step of setting a threshold for live vs. dead cells. Additionally, an area-based analysis using 2D images has the potential to underestimate viability compared to a volume-based estimation of viability15. With respect to a more precise estimation of viability, conversion to volume-based viability from 2D images may be an ideal option, although some technical limitations may affect conversion; e.g., the irregular shape of the islets makes conversion difficult, as islets are not always spherical23. Another advantage of the semi-automated method over the conventional manual method is the improved scoring system. The conventional manual method measures the viability of individual islets to average the overall percent viability, in which neither the islet size nor the corresponding size of any dead areas are taken into account. For example, using the conventional method, a large, 75%-viable islet is regarded as equivalent to a small, 75%-viable islet, although the dead volume between the islets is considerably different, which only the semi-automated method can accurately account for. This explains why viability-conventional is overestimated compared to viability-semi-automated in islet batches containing large islets. Automated viability determination has recently been applied with success in various studies10,16. In addition to being a resourceful tool for assessing islet viability, a similar automated method has been developed to evaluate islet purity using zinc-chelating DTZ staining24,25. The high-throughput capability of the DTZ method allows for high-volume applications, which is attractive. With regard to the practicality of the assay, our semi-automated method for measuring viability is feasibly equivalent to conventional viability assessment; both methods require similar staining preparations and the duration of each procedure is similar. Conventional viability assessment requires consistent and proficient training of appropriate personnel to perform viability evaluation with precision and diligence. The semi-automated method requires only basic software operation, to set the threshold of green (for FDA) and red (for PI). To make the semi-automated method a widely accepted and uniformly applied practice across multiple centers and operators, the ultimate development of a fully automated instrument is ideal.
Diabetes reversal has frequently been used to evaluate islet transplantation outcomes in diabetic mouse models26,27. Diabetes reversal is a qualitative assessment (i.e., yes or no). To calculate the % diabetes reversal, transplantations using multiple mice from the same islet batch are performed in order to evaluate islet potential. However, because the number of recipient mice is generally limited, due to small numbers of islets allocated from the isolation and the availability of same-day surgical procedures, results are not continuous data but stepwise data. For example, four mice were transplanted from the same donor islet in most of the cases in our study (3.7 mice per islet batch on average); subsequent % diabetes reversal falls into one of five categories (0%, 25%, 50%, 75%, or 100%), which makes precise quantitative analysis difficult. To overcome this issue in this study, we quantitated AUC_0-28 in individual mice as a transplantation outcome that reflects posttransplant glucose control in a more quantitative manner. The resultant values allowed us to precisely evaluate the correlation between viability and transplantation outcomes, which revealed that viability measured using the semi-automated method was significantly correlated with AUC_0-28.
Because the conventional viability assay using FDA/PI staining does not correlate with transplantation outcomes, some have criticized the staining dyes; i.e., FDA is not specific for beta cells, FDA remains positive in dying cells with intact intracellular hydrolysis activity, and PI cannot detect damaged cells with intact cell membranes. To address these issues, different fluorescent dyes and cytometry-based analyses have been introduced for more precise detection of viable beta cells in islets5,11,28–30. Although our study suggests that conventional FDA/PI staining works well when analyzed using the semi-automated method, combining our method with a beta cell-specific dye could further refine the method for improved prediction of transplantation outcomes. For example, we previously introduced a beta cell-specific fluorescent dye, Newport Green11, which has great potential to further improve accuracy in transplantation prediction.
In summary, we demonstrated that semi-automated viability assessment of human islets is a promising alternative approach over conventional manual assessment, and that it correlates with transplantation outcomes in diabetic mice. Further validation of this computational assessment is still necessary, including use of clinical islet transplantation data, before it can be universally implemented as a replacement for the conventional manual assessment of islet viability.
Supplemental Material
Supplemental_Table_1 for Semi-Automated Assessment of Human Islet Viability Predicts Transplantation Outcomes in a Diabetic Mouse Model by Mayra Salgado, Nelson Gonzalez, Leonard Medrano, Jeffrey Rawson, Keiko Omori, Meirigeng Qi, Ismail Al-Abdullah, Fouad Kandeel, Yoko Mullen and Hirotake Komatsu in Cell Transplantation
Supplemental_Table_2 for Semi-Automated Assessment of Human Islet Viability Predicts Transplantation Outcomes in a Diabetic Mouse Model by Mayra Salgado, Nelson Gonzalez, Leonard Medrano, Jeffrey Rawson, Keiko Omori, Meirigeng Qi, Ismail Al-Abdullah, Fouad Kandeel, Yoko Mullen and Hirotake Komatsu in Cell Transplantation
Supplemental Material, SupplFig1_81mm_02032020-01 for Semi-Automated Assessment of Human Islet Viability Predicts Transplantation Outcomes in a Diabetic Mouse Model by Mayra Salgado, Nelson Gonzalez, Leonard Medrano, Jeffrey Rawson, Keiko Omori, Meirigeng Qi, Ismail Al-Abdullah, Fouad Kandeel, Yoko Mullen and Hirotake Komatsu in Cell Transplantation
Supplemental Material, SupplFig2_81mm_02032020-01 for Semi-Automated Assessment of Human Islet Viability Predicts Transplantation Outcomes in a Diabetic Mouse Model by Mayra Salgado, Nelson Gonzalez, Leonard Medrano, Jeffrey Rawson, Keiko Omori, Meirigeng Qi, Ismail Al-Abdullah, Fouad Kandeel, Yoko Mullen and Hirotake Komatsu in Cell Transplantation
Supplemental Material, SupplFig3_81mm_02172020-01 for Semi-Automated Assessment of Human Islet Viability Predicts Transplantation Outcomes in a Diabetic Mouse Model by Mayra Salgado, Nelson Gonzalez, Leonard Medrano, Jeffrey Rawson, Keiko Omori, Meirigeng Qi, Ismail Al-Abdullah, Fouad Kandeel, Yoko Mullen and Hirotake Komatsu in Cell Transplantation
Supplemental Material, CT-2466.R1_Suppl_Figure_4 for Semi-Automated Assessment of Human Islet Viability Predicts Transplantation Outcomes in a Diabetic Mouse Model by Mayra Salgado, Nelson Gonzalez, Leonard Medrano, Jeffrey Rawson, Keiko Omori, Meirigeng Qi, Ismail Al-Abdullah, Fouad Kandeel, Yoko Mullen and Hirotake Komatsu in Cell Transplantation
Acknowledgments
We acknowledge the Manufacturing Team led by Dr Ismail Al-Abdullah at the Southern California Islet Cell Resources Center for preparation of human islets. We also thank Sarah T. Wilkinson, PhD for critical reading and editing of the manuscript.
Footnotes
Ethical Approval: This study was approved by our institutional review board.
Statement of Animal Rights: All of the experimental animal procedures in this study were approved by the City of Hope/Beckman Research Institute Institutional Animal Care and Use Committee.
Statement of Informed Consent: Human islets were isolated from human pancreata of deceased donors with a research consent.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Nora Eccles Treadwell Foundation (Title of Grant: CURE OF DIABETES, Grant Period: July 1, 2012–June 30, 2020, P.I.: Yoko Mullen, MD, PhD).
ORCID iD: Hirotake Komatsu  https://orcid.org/0000-0003-0876-4809
https://orcid.org/0000-0003-0876-4809
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Farney AC, Sutherland DE, Opara EC. Evolution of islet transplantation for the last 30 Years. Pancreas. 2016;45(1):8–20. [DOI] [PubMed] [Google Scholar]
- 2. Hanley SC, Paraskevas S, Rosenberg L. Donor and isolation variables predicting human islet isolation success. Transplantation. 2008;85(7):950–955. [DOI] [PubMed] [Google Scholar]
- 3. Eckhard M, Brandhorst D, Winter D, Jaeger C, Jahr H, Bretzel RG, Brendel MD. The role of current product release criteria for identification of human islet preparations suitable for clinical transplantation. Transplant Proc. 2004;36(5):1528–1531. [DOI] [PubMed] [Google Scholar]
- 4. Pepper AR, Hasilo CP, Melling CW, Mazzuca DM, Vilk G, Zou G, White DJ. The islet size to oxygen consumption ratio reliably predicts reversal of diabetes posttransplant. Cell Transplant. 2012;21(12):2797–2804. [DOI] [PubMed] [Google Scholar]
- 5. Boyd V, Cholewa OM, Papas KK. Limitations in the use of fluorescein diacetate/propidium iodide (fda/pi) and cell permeable nucleic acid stains for viability measurements of isolated islets of langerhans. Curr Trends Biotechnol Pharm. 2008;2(2):66–84. [PMC free article] [PubMed] [Google Scholar]
- 6. Jones KH, Senft JA. An improved method to determine cell viability by simultaneous staining with fluorescein diacetate-propidium iodide. J Histochem Cytochem. 1985;33(1):77–79. [DOI] [PubMed] [Google Scholar]
- 7. Hanson MS, Park EE, Sears ML, Greenwood KK, Danobeitia JS, Hullett DA, Fernandez LA. A simplified approach to human islet quality assessment. Transplantation. 2010;89(10):1178–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Papas KK, Suszynski TM, Colton CK. Islet assessment for transplantation. Curr Opin Organ Transplant. 2009;14(6):674–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Papas KK, Colton CK, Nelson RA, Rozak PR, Avgoustiniatos ES, Scott WE, Wildey GM, Pisania A, Weir GC, Hering BJ. Human islet oxygen consumption rate and DNA measurements predict diabetes reversal in nude mice. Am J Transplant. 2007;7(3):707–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Komatsu H, Omori K, Kandeel F, Mullen Y. Surfactants improve live cell imaging of human pancreatic islets. Pancreas. 2018;47(9):1093–1100. [DOI] [PubMed] [Google Scholar]
- 11. Komatsu H, Omori K, Parimi M, Rawson J, Kandeel F, Mullen Y. Determination of islet viability using a zinc-specific fluorescent dye and a semi-automated assessment method. Cell Transplant. 2016;25(10):1777–1786. [DOI] [PubMed] [Google Scholar]
- 12. Qi M, Bilbao S, Forouhar E, Kandeel F, Al-Abdullah IH. Encompassing ATP, DNA, insulin, and protein content for quantification and assessment of human pancreatic islets. Cell Tissue Bank. 2018;19(1):77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Poitout V, Satin LS, Kahn SE, Stoffers DA, Marchetti P, Gannon M, Verchere CB, Herold KC, Myers MG, Jr, Marshall SM. A call for improved reporting of human islet characteristics in research articles. Diabetes. 2019;68(2):239–240. [DOI] [PubMed] [Google Scholar]
- 14. Poitout V, Satin LS, Kahn SE, Stoffers DA, Marchetti P, Gannon M, Verchere CB, Herold KC, Myers MG, Jr, Marshall SM. A call for improved reporting of human islet characteristics in research articles. Diabetologia. 2019;62(2):209–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Komatsu H, Cook C, Wang CH, Medrano L, Lin H, Kandeel F, Tai YC, Mullen Y. Oxygen environment and islet size are the primary limiting factors of isolated pancreatic islet survival. PLoS One. 2017;12(8):e0183780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Komatsu H, Rawson J, Medrano L, Cook CA, Barriga A, Gonzalez N, Salgado M, Omori K, Kandeel F, Tai YC, Mullen Y. Optimizing temperature and oxygen supports long-term culture of human islets. Transplantation. 2019;103(2):299–306. [DOI] [PubMed] [Google Scholar]
- 17. Sabek OM, Cowan P, Fraga DW, Gaber AO. The effect of isolation methods and the use of different enzymes on islet yield and in vivo function. Cell Transplant. 2008;17(7):785–792. [DOI] [PubMed] [Google Scholar]
- 18. Bertuzzi F, Ricordi C. Prediction of clinical outcome in islet allotransplantation. Diabetes Care. 2007;30(2):410–417. [DOI] [PubMed] [Google Scholar]
- 19. Ricordi C, Lakey JR, Hering BJ. Challenges toward standardization of islet isolation technology. Transplant Proc. 2001;33(1-2):1709. [DOI] [PubMed] [Google Scholar]
- 20. Papas KK, Bellin MD, Sutherland DE, Suszynski TM, Kitzmann JP, Avgoustiniatos ES, Gruessner AC, Mueller KR, Beilman GJ, Balamurugan AN, Loganathan G. et al. Islet oxygen consumption rate (ocr) dose predicts insulin independence in clinical islet autotransplantation. PLoS One. 2015;10(8):e0134428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goto M, Holgersson J, Kumagai-Braesch M, Korsgren O. The ADP/ATP ratio: a novel predictive assay for quality assessment of isolated pancreatic islets. Am J Transplant. 2006;6(10):2483–2487. [DOI] [PubMed] [Google Scholar]
- 22. Ricordi C, Scharp DW, Lacy PE. Reversal of diabetes in nude mice after transplantation of fresh and 7-day-cultured (24 degrees C) human pancreatic islets. Transplantation. 1988;45(5):994–996. [DOI] [PubMed] [Google Scholar]
- 23. Gonzalez N, Salgado M, Medrano L, Mullen Y, Komatsu H. Isolated pancreatic islet yield and quality is inversely related to organ donor age in rats. Exp Gerontol. 2019;128:110739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Habart D, Svihlik J, Schier J, Cahova M, Girman P, Zacharovova K, Berkova Z, Kriz J, Fabryova E, Kosinova L, Papackova Z. et al. Automated analysis of microscopic images of isolated pancreatic islets. Cell Transplant. 2016;25(12):2145–2156. [DOI] [PubMed] [Google Scholar]
- 25. Gmyr V, Bonner C, Lukowiak B, Pawlowski V, Dellaleau N, Belaich S, Aluka I, Moermann E, Thevenet J, Ezzouaoui R, Queniat G. et al. Automated digital image analysis of islet cell mass using Nikon’s inverted eclipse Ti microscope and software to improve engraftment may help to advance the therapeutic efficacy and accessibility of islet transplantation across centers. Cell Transplant. 2015;24(1):1–9. [DOI] [PubMed] [Google Scholar]
- 26. Qi M, McFadden B, Valiente L, Omori K, Bilbao S, Juan J, Rawson J, Oancea AR, Scott S, Nair I, Ferreri K. et al. Human pancreatic islets isolated from donors with elevated HbA1c levels: islet yield and graft efficacy. Cell Transplant. 2015;24(9):1879–1886. [DOI] [PubMed] [Google Scholar]
- 27. Qi M, Luis V, Bilbao S, Omori K, Rawson J, McFadden B, Juan J, Nair I, Mullen Y, El-Shahawy M, Dafoe D. et al. Sodium levels of human pancreatic donors are a critical factor for determination of islet efficacy and survival. Am J Physiol Endocrinol Metab. 2015;308(5):E362–E369. [DOI] [PubMed] [Google Scholar]
- 28. Jayaraman S. Assessment of beta cell viability. Curr Protoc Cytom. 2011:Chapter 6 55(1):1–16 [DOI] [PubMed] [Google Scholar]
- 29. Ichii H, Inverardi L, Pileggi A, Molano RD, Cabrera O, Caicedo A, Messinger S, Kuroda Y, Berggren PO, Ricordi C. A novel method for the assessment of cellular composition and beta-cell viability in human islet preparations. Am J Transplant. 2005;5(7):1635–1645. [DOI] [PubMed] [Google Scholar]
- 30. Lukowiak B, Vandewalle B, Riachy R, Kerr-Conte J, Gmyr V, Belaich S, Lefebvre J, Pattou F. Identification and purification of functional human beta-cells by a new specific zinc-fluorescent probe. J Histochem Cytochem. 2001;49(4):519–528. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental_Table_1 for Semi-Automated Assessment of Human Islet Viability Predicts Transplantation Outcomes in a Diabetic Mouse Model by Mayra Salgado, Nelson Gonzalez, Leonard Medrano, Jeffrey Rawson, Keiko Omori, Meirigeng Qi, Ismail Al-Abdullah, Fouad Kandeel, Yoko Mullen and Hirotake Komatsu in Cell Transplantation
Supplemental_Table_2 for Semi-Automated Assessment of Human Islet Viability Predicts Transplantation Outcomes in a Diabetic Mouse Model by Mayra Salgado, Nelson Gonzalez, Leonard Medrano, Jeffrey Rawson, Keiko Omori, Meirigeng Qi, Ismail Al-Abdullah, Fouad Kandeel, Yoko Mullen and Hirotake Komatsu in Cell Transplantation
Supplemental Material, SupplFig1_81mm_02032020-01 for Semi-Automated Assessment of Human Islet Viability Predicts Transplantation Outcomes in a Diabetic Mouse Model by Mayra Salgado, Nelson Gonzalez, Leonard Medrano, Jeffrey Rawson, Keiko Omori, Meirigeng Qi, Ismail Al-Abdullah, Fouad Kandeel, Yoko Mullen and Hirotake Komatsu in Cell Transplantation
Supplemental Material, SupplFig2_81mm_02032020-01 for Semi-Automated Assessment of Human Islet Viability Predicts Transplantation Outcomes in a Diabetic Mouse Model by Mayra Salgado, Nelson Gonzalez, Leonard Medrano, Jeffrey Rawson, Keiko Omori, Meirigeng Qi, Ismail Al-Abdullah, Fouad Kandeel, Yoko Mullen and Hirotake Komatsu in Cell Transplantation
Supplemental Material, SupplFig3_81mm_02172020-01 for Semi-Automated Assessment of Human Islet Viability Predicts Transplantation Outcomes in a Diabetic Mouse Model by Mayra Salgado, Nelson Gonzalez, Leonard Medrano, Jeffrey Rawson, Keiko Omori, Meirigeng Qi, Ismail Al-Abdullah, Fouad Kandeel, Yoko Mullen and Hirotake Komatsu in Cell Transplantation
Supplemental Material, CT-2466.R1_Suppl_Figure_4 for Semi-Automated Assessment of Human Islet Viability Predicts Transplantation Outcomes in a Diabetic Mouse Model by Mayra Salgado, Nelson Gonzalez, Leonard Medrano, Jeffrey Rawson, Keiko Omori, Meirigeng Qi, Ismail Al-Abdullah, Fouad Kandeel, Yoko Mullen and Hirotake Komatsu in Cell Transplantation