Abstract
Introduction
Deep brain stimulation (DBS) is an effective treatment for patients with Parkinson’s disease (PD). On time follow-up and timely programing of symptoms are important measures to maintain the effectiveness of DBS. Due to the highly contagious nature of 2019-nCoV, patients were quarantined. With the help of Internet technologies, we continued to provide motor and non-motor symptom assessment and remote programming services for postsurgical PD-DBS patients during this extraordinary period.
Methods
A retrospective analysis was performed on postsurgical PD-DBS patients who could not come to our hospital for programming due to the impact of the 2019-nCoV. The differences between the pre- and post-programming groups were analyzed. We designed a 5-level Likert rating scale to evaluate the effects and convenience of the remote programming and Internet self-evaluation procedures.
Results
Of the 36 patients engaged in the remote programming, 32 patients met the inclusion criteria. Four of the 32 patients set initiated stimulation parameters, and the other 28 patients had significant improvement in UPDRS-III. Nearly all the 28 patients were satisfied with the effect of the remote programming. Most of the patients were willing to use remote programming again.
Conclusion
Remote programming based on the online evaluation of patient’s symptoms can help improve motor symptoms of postsurgical DBS patients with PD during the quarantine period caused by 2019-nCoV.
Electronic supplementary material
The online version of this article (10.1007/s00415-020-10273-z) contains supplementary material, which is available to authorized users.
Keywords: Deep brain stimulation (DBS), Parkinson’s disease (PD), Remote programming, 2019-nCoV, Telemedicine
Introduction
Deep brain stimulation (DBS) has been confirmed as a long-term effective treatment for patients with certain neurologic or psychiatric disorders, such as Parkinson’s disease (PD) [1]. Successful DBS therapy requires accurate diagnosis, precise implantation of the DBS electrode, and optimal postsurgical DBS programming [2]. Several review sessions may be prescribed to attain optimal stimulation parameters. Additionally, inadvertent circumstances such as worsening of symptoms may necessitate periodic adjustment to attain optimal stimulation parameters. Therefore, on time follow-up and timely programming of symptoms is an important measure to maintain the effectiveness of DBS. Consequently, patients are required to undergo periodic clinical reviews for the much necessary follow-up and programming.
Considering the highly contagious nature 2019-nCoV [3], many cities in the People’s Republic of China were sealed off to help contain and stop the spread of the virus. During this period, travelling was severely restricted [4]. As a result, even though patients experienced aggravation of motor symptoms, they were unable to seek direct consultation and programming with their personal doctor. Fortunately, most programming centers in China have remote programming equipment produced by two DBS manufacturers (PINS and SceneRay) [5]. With the help of other Internet technologies, we continued to provide motor and non-motor symptom assessment and remote programming services for postsurgical DBS patients with Parkinson’s disease during this extraordinary period.
Methods
Patients
A retrospective analysis was conducted on postoperative DBS patients with Parkinson’s disease who could not come to our hospital for programming due to the impact of the 2019-nCoV from January 2020 to February 2020 (surgery was performed in the department of neurosurgery, Shanghai hospital). The inclusion criteria for patients who underwent STN-DBS or GPi-DBS implantations were; 1. Patient had idiopathic Parkinson's disease, 2. history of taking compound levodopa which once had a good effect, 3. Evident reduction in the therapeutic effect of anti-Parkinson's disease drugs in the form of decreased or serious motion fluctuation or dyskinesia appears, which affected the quality of life of patients. Patients that were diagnosed with dementia or severe mental illness are excluded [6]. Other notable contraindications of neurosurgery and comorbidities affecting survival were also excluded [7]. The patients chose to have pulse generator provided by two DBS manufacturers (PINS or SceneRay) implanted. Caregivers of patients were trained online and by mobile phone to operate the patient’s remote wireless DBS programming system. The patient's home had to be equipped with Wi-Fi and 4G or 5G dual networks to ensure that the communication network could be replaced, in case of communication breakdown during the process of remote programming. The remote programming was free of charge. Medical ethical committee of Changhai Hospital approved the study and all participants gave informed consent online.
Operation procedure
The operation procedure was as per previous report [6]. All cases underwent pre-operative MR-imaging on a 3.0 T scanner using a T1 image, T2-weighted image and fused with pre-operative CT with fame. Leksell G head frame and Surgiplan system (Elekta AB, Stockholm, Sweden) were adopted. Pre and Post-operative high-resolution CT images were acquired in all the cases. CT examination was repeated within 6 days post-operatively to exclude intracranial hemorrhage, pneumocephalus and to confirm the position of electrodes and contacts.
Electrode reconstruction technique
As previously reported [6], pre-operative MRI and post-operative CT input Lead-DBS software (https://www.lead-dbs.org/) were used [8]. Post-operative images were linearly co-registered to pre-operative images using the Statistical Parametrical Mapping software version 12 [SPM12] [9] and BRAINSFit software [10]. The three-dimensional images of the electrode and the nucleus were saved in the database after comparison with Surgiplan system (Elekta AB, Stockholm, Sweden) so that the stimulation parameters could be adjusted quickly according to the reconstruction results during remote programming [11].
Online evaluation
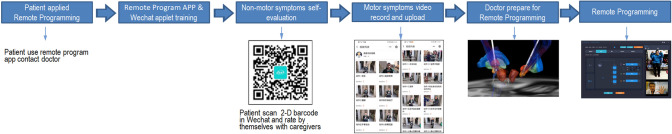
We used an applet called “PD AI Assistant” (Ningdong Medical Technology Services (Shanghai) Co. Ltd.) based on WeChat (Tencent Co., Ltd.) to help patients upload self-evaluated non-motor symptoms scales and motor videos for doctors’ evaluation. WeChat is a prevalent, user-friendly, and easily accessible social media App that most Chinese people are using. Patients apply for direct programing with their respective doctors via the application. After obtaining the patient’s personal authorization, the doctor can see the scoring results and uploaded motor symptoms videos. This data is only accessible by the programming doctor thus insuring confidentiality.
Non-motor symptoms’ self-evaluation
Each scale has instructions in the Chinese language, allowing caregivers of the patient to read and score based on the patient's response. Therefore, with the help of caregivers, patients can perform self-evaluation of Parkinson’s disease MDS Association non-motor symptoms scale (NMSS), depression scale (Hamilton depression scale, HAMD), constipation scale, King’s Parkinson’s Disease Pain Scale (KINGS), Standardized Swallowing Assessment (SSA), Parkinson’s Disease Questionnaire-39 (PDQ-39), Parkinson disease sleep scale (PDSS), Wearing-off questionnaire-9 (WOQ-9), and Unified Parkinson’s Disease Rating Scale (UPDRS-I, UPDRS-II, UPDRS-IV) etc. All patients must complete HAMD assessments. In addition, patients with complaints of pain assessed the KINGS pain scale, and for those with dysphagia evaluated the SSA scale.
Motor symptoms’ self-record and doctor evaluation online
The evaluation of motor symptoms was based on the UPDRS-III scoring standard. To make the assessment as accurate as possible, we record the Chinese version of the demo video by the Chinese staff imitating the UPDRS-III training video by MDS association (https://www.movementdisorders.org/MDS/MDS-Rating-Scales.htm). The caregivers of patients can see this video demonstration standard of each item in the “PD AI Assistant” applet. We also repeatedly explained the main points of UPDRS-III demo video to the caregivers through video calls. Making sure that the caregivers have mastered the main points of the demo video before making the recording. The time interval between drug withdrawal and recording, or medication taking, and recording was also repeatedly confirmed. Then they record the video of each item according to this standard and upload via mobile phone. To ensure the uploaded video met the recording standard, the doctors reviewed it. Then, according to those videos, patients were scored by the doctor who has been authorized by the UPDRS-III exam via the doctor’s client side of the applet.
Wireless programming technology and method
Wireless programming technology has been described in previous reports [12, 13]. The patient’s programming device was connected to the mobile smartphone via Bluetooth, on which an App was installed. Using this App, the mobile smartphone could be connected to the programming doctor’s laptop, on which the manufacturers’ software was installed. Thus, based on this remote wireless DBS programming system, the doctor could video-call the patients and see the program at the same time. And this remote programming device has the same authority as the doctor’s programming controller. All wireless transmission data could only be seen by patient-authorized programming doctors on their laptop. After patient authorization, doctors can then record communication videos on the app and save them on dedicated computers.
There were two groups of patients in need of programming. The first group of patients needed parameter adjustment for symptomatic alleviation (i.e. in those presenting with chorea dyskinesia, dystonia, psychiatric symptoms, dysphagia, pain, gait disorders, etc.) [14–17]. The side effects of programming were defined in existence of the following; obvious pyramid-tract stimulation symptoms (including eyes gaze at the same direction, muscle twitch in limbs face and trunk, dysarthria or tongue stiffness caused by electrical stimulation), dysarthria, dysphagia, numbness, dizziness, palpitations, sweating, eye movement disorders, stimulus-induced dyskinesia or dystonia, and balance disorders caused by electrical stimulation. We could classify the symptoms as “Worsening” in event electrical stimulation aggravated already existing ones. The second group of patients required optimal postsurgical DBS programming. In this group of patients, initial parameters were set according to the method of fast locating stimulation parameters as in previous reports [15]. (Fig. 1) The initial stimulation parameters for the patient (in “med off state”) were placed 4 weeks after the surgery. Considering that patients can have microlesion effect [18] and impedance changes immediately after surgery [19], our center usually set initiated stimulation parameters 4 weeks after surgery, which is consistent with the consensus of Chinese experts in programming after deep brain stimulation for Parkinson's disease [20].According to the anatomical relationship between the electrode and STN reconstructed three-dimensionally, we selected two contacts closest to the dorsolateral part of STN. With a frequency of 130 Hz and a pulse width of 60 μs, we gradually increased the voltage from 1.5 V to find the treatment threshold and the side effect threshold. If the tremor and motor inflexibility could not be well relieved at the above frequency and pulse width, we would then gradually increase the frequency to 160 Hz with a step of 5 Hz, and also increase the pulse width by 10–20 μs as appropriate. If the symptom improvement of the two contacts was similar, the contact closer to the dorsal side was chosen to reduce the occurrence of electrical stimulation-induced dyskinesia. After the electrical stimulation parameters were set, patients were given Parkinson's drugs. Furthermore, patients were ordered to stop taking benzhexol and amantadine. Levodopa drugs were given about the original maximum single dose and gradually decrease doses with doctor’s guide. If the patients did not have dyskinesia within 2 h, the initial parameters setting were done. If the patient developed dyskinesia or other electrical stimulation-induced symptoms later, then they could contact us to adjust the stimulation parameters by remote programming again (Fig. 2).
Fig. 1.
Remote programming technology and method. The procedure of remote programming of patients
Fig. 2.
The Initial Programming Process of This Study
Statistical analysis
The means and SDs of all parameters were calculated. Data were expressed as mean ± SD. The differences between the pre- and post-programming groups were analyzed by a paired T-test. A P value < 0.05 was regarded as statistically significant, and all tests were 2-sided. We designed a 5-level Likert rating scale, asking patients' caregivers to evaluate the convenience effect of the remote programming and self-evaluation procedures respectively (1, very unsatisfied; 2, unsatisfied; 3, neutral; 4, satisfied; 5, very satisfied). And evaluate whether you are willing to use remote programming again (1, strongly disagree; 2, disagree; 3, no opinion; 4, agree; 5, strongly agree). Statistical analyses were performed using Microsoft Excel 2003, Matlab 7.0 (Math Works, Natick, Massachusetts) and SPSS17.0 (SPSS Inc., Chicago, Illinois).
Results
Patient clinical data
A total of 36 patients requested remote programming, of which 32 patients (14 women) met the inclusion criteria. The patients' baseline clinical characters were in Table 1.The average age was 62.53 ± 7.71 years old, the mean course of disease at the time of surgery was 11.06 ± 4.43 years, the average LEDD was 747.29 ± 314.49 mg, the average preoperative UPDRS-III (med off) was 54.63 ± 16.05, and the preoperative UPDRS-III (med on) was 28.00 ± 10.04 on average. The average preoperative LEDD of 28 patients was 755.38 ± 324.77, and the average LEDD before applying for remote programming of 28 patients was 321.43 ± 205.56 (two patients did not take any anti-Parkinson's drugs at all). Of the 32 patients, 30 received bilateral STN-DBS (7 IPG for PINS G102RZ, 22 IPG for PINS G102R, 1 IPG for SceneRay 1180), and two received bilateral GPi-DBS (PINS G102R). In total, they received 49 times of remote programming, of which four patients had stimulation-induced dyskinesia on the second day after remote programming, and one patient had difficulty speaking on the second day, so they went through remote programming multiple times..
Table 1.
Patients' Baseline Clinical Characters
| Age (year) | Gender | Target (bilateral) | Course (year) | LEDD (mg) | Preoperative UPDRS-III | Preoperative MMSE | Preoperative HAMD | The Months Post-operation | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | STN | GPi | Med-off | Med-on | ||||||
| 62.53 ± 7.71 | 18 | 14 | 30 | 2 | 11.06 ± 4.43 | 747.29 ± 314.49 | 54.63 ± 16.05 | 28.00 ± 10.04 | 26.50 ± 2.87 | 18.47 ± 8.93 | 20.91 ± 11.45 |
Remote programming demands from patients
Four of the 32 patients set the initial parameters of stimulation by remote programming. Of the remaining 28 patients, nine complained of gait problems, seven complained of speech disability and/or dysphagia, and seven complained of increased pain. Two of them complained of bradykinesia aggravation of the whole body, two of them complained of recurrence of tremor symptoms, and one of them complained of dyskinesia after self-adjusting stimulation parameters. (We set the limitations for self-programming by the patients. The threshold for voltage is ± 0.5 V, the threshold for pulse width is ± 20 μs, and the threshold for frequency permission is ± 20 Hz. If the frequency of patients with cross-electrical pulse is set at 125 Hz, thus the frequency permission is − 20 Hz.)
Results of remote programming and patient satisfaction with remote programming
Four patients with newly set stimulation parameters achieved improvement in UPDRS-III (pre-operative med off 65.75 ± 21.48 vs. post-operative med off IPG on 24.00 ± 6.73). The remaining 28 patients also achieved significant improvement in UPDRS-III after remote programming (pre-programming med off IPG on 34.79 ± 11.34 vs. post-programming med off IPG on 30.36 ± 10.26, P < 0.001). Except for one of the 28 patients who complained of drooling due to dysphagia, the other 27 patients reported improvement in varying degrees. Seven patients with dysphagia had significant improvements in SSA scores (pre-programming 24.29 ± 2.50 vs. post-programming 22.57 ± 2.44, P = 0.003). The Kings pain scale of seven patients, whose main complaint was pain, showed significant improvement in pain symptoms (pre-programming 13.43 ± 5.62 vs. post-programming 6.29 ± 3.15, P = 0.002). In the nine patients with gait disorder as the main complaint, Gait disorder scores (UPDRS-III, items 9–11) improved significantly (pre-programming 4.78 ± 1.72 vs. post-programming 2.33 ± 2.35, P < 0.001).
Most of the 28 patients were satisfied or very satisfied with the effect of the remote programming (10.71% neutral, 39.29% satisfied, 50% very satisfied). All patients with newly set stimulation parameters were very satisfied with the effect of remote programming.
Patients' self-evaluation of symptoms and evaluation of remote programming
Although remote programming eliminates the need of patients travelling to and from the hospital, it also requires patients or caregivers to have certain smartphone operating application capabilities. In this study, it took a lot of time to train patients and their caregivers to learn the self-evaluation and remote programming procedures. Moreover, although most patients know that programming needs to be conducted during the med-off time, there are still a few patients who are in the med-on time when they contact us, and it took a long time to wait for the effect of the drug to fade. The average time from the request to the operation of remote programming of the remaining 28 patients was 10.11 ± 11.36 h, except for 4 patients who setup the initial parameters received remote programming at the scheduled time. In this study, 46.875% of the patients were satisfied with the convenience of using WeChat applet to self-evaluate non-motor symptoms, and 53.125% were very satisfied. The patient's evaluation of the convenience of self-video uploading of motor symptoms was 3.125% neutral, 18.75% satisfied, and 78.125% very satisfied. 6.25% of the patients were satisfied with the procedure convenience of remote programming and 93.75% were very satisfied. When asked if the patient would like to continue using remote programming next time, 6.25% of the patients indicated disagree (both patients were mainly complained of dysphagia), 40.625% agreed, and 53.125% strongly agreed.
Safety
We had no network disconnection during the 49 times of remote programming. The mobile smartphone of patient's caregiver was out of power twice with long programming time, the Bluetooth binding between the mobile smartphone and the patient's programming device was interrupted twice (the patient's programming device needed to be restarted). For once, the battery of the patient's programming device needed to be replaced because of low power. Two patients' caregivers mistakenly shut down the patient’s programming device during the remote programming process, and the results of the remote programming were not affected after restarting. None of them affected the setting of programming parameters.
During the process of programming, 4 patients developed dyskinesia or dystonia-like symptoms on the second day after remote programming. The dyskinesia was considered induced by electrical stimulation and was relieved after multiple remote programming. One patient had difficulty speaking on the second day after remote programming and was improved during the second time of remote programming. One patient's caregivers reported that their patient felt slightly emotionally excited after getting up in the morning, and then the symptoms returned to normal without re-program.
Discussion
Legal issues
Because of the ethical and legal uncertainties associated with Telemedicine compliance, it is important to clarify that China has been encouraging and has high safety regulations for Telemedicine technology [21–23].China has further encouraged the use of Telemedicine by doctors for patients quarantined at home during this extraordinary period of the 2019-nCoV. Therefore, this study conforms to Chinese laws and regulations.
Security issues
Security issues of Telemedicine come from four main areas:
Stability of wireless network and Bluetooth connection: the security hurdle lies in the information delay and disconnection during real-time programming. We adopt dual network guarantee, giving priority to WI-FI and connecting to 4G or 5G if necessary. We did not observe significant information delays and data connection disruptions. There were two times when the Bluetooth binding between the mobile smartphone and the patient’s programming device was interrupted, but the network video call was not interrupted. After instructing the patient to restart the programming device, the patient was programmed normally again.
Safety issues of programming stimulation parameter setting: such as stimulation parameter settings. Since all voltage increase amplitudes in the programming process are 0.05 V, the doctor will be reminded to confirm when the stimulation parameters may be too high. Therefore, there is no problem with the safety of stimulation parameters different from that of face-to-face programming. The temporary numbness and pyramidal tract irritation symptoms during the programming process were quickly adjusted and relieved. The postoperative dyskinesia and difficulty speaking of the patient were side effects of the electrical stimulation parameters. It was improved after remote programming again, and the stimulation parameters did not appear abnormal considering the common programming side effects [14].
Mistakes of operation from patients and their caregivers: For remote programming, patients’ caregivers only need to establish a remote connection and do not participate in operating the stimulation parameters. Two patients' caregivers mistakenly operated the programming device, which resulted in the withdrawal of the remote programming mode in the process of remote programming, but the wireless video did not interrupt. After real-time guidance to re-enter the remote programming mode, we continued the remote programming.
Unexpected situations such as falls of patients: when the patients were remotely programmed with standing and gait functions, we would first ask the patients to practice the right and left foot raising and kicking on the ground to ensure that they could stand on both legs, and then evaluate their standing time. Before asking the patient to walk, we would assess the time spent standing on one leg. If the patient could stand on one leg for more than 1 s, then we ask the patient to walk. Otherwise, the patient was required to use a walker or walk with the help of the caregivers. None of the patients in this study had accidents such as falls.
The accuracy of the self-evaluation scale
Previous studies have shown that with the software, patients can complete a variety of self-evaluation scales at home [24, 25]. The self-evaluation scale software used in this study was similar to the previous works [24, 25]. The self-evaluation scale mainly focuses on non-motor symptoms. This study focuses on whether the chief complaint of motor symptoms can be improved. The indicators involved are UPDRS-III and the patient's self-evaluation scale. Among them, UPDRS-III is evaluated by doctors with the certification of UPDRS-III score based on videos on the app, which has high credibility. The autonomous video recording method for motor symptoms evaluation was used for the first time. After training, caregivers could record motor videos according to the requirements of the UPDRS-III at the appropriate time (med-on and med-off time). The self-evaluation scale of patients' symptoms after programming can reflect the effect of programming to some extent.
Convenience of Internet and remote programming operation
Our Likert scale showed that all patients who have received remote programming and self-evaluation were appreciative of Internet telemedical technology. However, based on the inclusion and exclusion criteria, 4 patients who were over 70 years old and had no one (neither the patients themselves nor their caregivers) who could operate the smartphone were excluded. But in general, most patients and their caregivers in China could meet such requirements.
Treatment effect of remote programming
By virtue of remote programming is based on the video, physician physical examination cannot be performed. For this reason, it was difficult to accurately evaluate the changes of muscle tone in patients. The muscle tone indicator was hence assessed indirectly from the flexibility of movement.
The main complaints from the patients were tremor or bradykinesia, difficulty speaking or dysphagia, walking disorder, limb pain, and dyskinesia. Remote programming provided mild symptomatic relief to most of the patients. Similar to previous studies, DBS can improve patients' tremor, stiffness, and dyskinesia in the long term [1, 26–34], gait [26, 27, 29, 30, 34] and axial symptoms to a certain extent [32, 33]. Improvement of axial symptoms such as speaking or swallowing may not always be satisfactory to patients [1, 26, 28–31, 34]. Other patients expressed willingness to continue to receive remote programming in the future. The effectiveness of programming depends on whether the patient's symptom type is suitable for programming and the programming doctor’s experience. Therefore, whether remote programming can be more widely accepted or not is a multi-factor problem, which needs to be verified by multi-center research.
We did not see any similar reports about using remote programming to set the initial stimulation parameters for IPG. Combined with the 3D image data of the electrodes reviewed after the operation, the activation contacts were quickly selected, and the stimulation parameters were set in 30 min. After rapid test and recheck 1 h after the patients were asked to take anti-parkinsonism medication, all four patients achieved good clinical efficacy.
Limitation
This study is a single-center retrospective study, which led to sourcing of data from a one source. Due to the inability to attain multi-center data, it led to difficulties in making satisfactory unbiased conclusion. However, it is in our view that this model is a very good option for less critical patients, and those who have logistical challenges getting to a health institution. Since patients' caregivers lack of experience to evaluate muscle tension like movement disorders experts, the assessment based on video UPDRS-III in this study do exist bias, and it is hard to be as accurate as usual. But during this pandemic, it is an expedient way to understand patients' motor symptoms. And patient satisfaction may be due to lockdown during the epidemic, and more studies are needed to demonstrate this effect in the future.
Conclusion
Remote programming based on the online evaluation of patient’s symptoms can help improve motor symptoms of postsurgical DBS patients with PD during the quarantine time caused by 2019-nCoV.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work was supported by the National Key Research and Development Program of China (No. 2016YFC0105900) and Climax Project of Changhai Hospital (No.2019YXK049).
Funding
This work was supported by the National Key Research and Development Program of China (No. 2016YFC0105900) and Climax Project of Changhai Hospital (No.2019YXK049). And the study sponsors had no such involvement in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Data availability
All data have already been included in the manuscript.
Compliance with ethical standards
Conflicts of interest
The authors declare that there is no conflict of interest.
Footnotes
Jinyu Xu and Jiali Wang are co-first authors.
References
- 1.Castrioto A, Lozano AM, Poon YY, Lang AE, Fallis M, Moro E. Ten-year outcome of subthalamic stimulation in Parkinson disease: a blinded evaluation. Arch Neurol. 2011;68:1550–1556. doi: 10.1001/archneurol.2011.182. [DOI] [PubMed] [Google Scholar]
- 2.Ramirez-Zamora A, Ostrem JL. Globus pallidus interna or subthalamic nucleus deep brain stimulation for parkinson disease: a review. JAMA Neurol. 2018;75:367–372. doi: 10.1001/jamaneurol.2017.4321. [DOI] [PubMed] [Google Scholar]
- 3.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Announcement from the Headquarter for novel coronavirus pneumonia prevention and control (No 1). Beijing: China National Health Commission. http://www.gov.cn/xinwen/2020-01/23/content_5471751.html (2020)
- 5.Zhang C, Zhang Y, Zhan S, Li D, Jin H, Denys D, Sun B. Telemedical deep brain stimulation: merits and limitations. Stereotact Funct Neurosurg. 2018;96:272–273. doi: 10.1159/000491603. [DOI] [PubMed] [Google Scholar]
- 6.Yang C, Qiu Y, Wu X, Wang J, Wu Y, Hu X (2019) Analysis of contact position for subthalamic nucleus deep brain stimulation-induced hyperhidrosis. Parkinson's disease 2019:8180123. 10.1155/2019/8180123 [DOI] [PMC free article] [PubMed]
- 7.Chinese expert committee on Deep Brain Stimulation for Parkinson's Disease, Chinese expert consensus on deep brain stimulation for patients with Parkinson's disease (2012), Chin J Neurosurg 28:855–857
- 8.Horn A, Kuhn AA. Lead-DBS: a toolbox for deep brain stimulation electrode localizations and visualizations. Neuroimage. 2015;107:127–135. doi: 10.1016/j.neuroimage.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Friston KJ, Ashburner JT, Kiebel SJ, Nichols TE, Penny WD. Statistical parametric mapping: the analysis of functional brain images. New York: Academic Press; 2011. [Google Scholar]
- 10.Johnson HHG, Williams K. BRAINSFit: mutual information rigid registrations of whole-brain 3D images, using the insight toolkit. Insight J. 2007;57:51. [Google Scholar]
- 11.Pourfar MH, Mogilner AY, Farris S, Giroux M, Gillego M, Zhao Y, Blum D, Bokil H, Pierre MC. Model-based deep brain stimulation programming for Parkinson's disease: the GUIDE pilot study. Stereotact Funct Neurosurg. 2015;93:231–239. doi: 10.1159/000375172. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y, Hao H, Chen H, Li L (2015) The study on a telemedicine interaction mode for Deep Brain Stimulation postoperative follow-up. Conference proceedings : Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Annual Conference 2015:186-189 [DOI] [PubMed]
- 13.Zhang C, Li D, Zeljic K, Tan H, Ning Y, Sun B. A remote and wireless deep brain stimulation programming system. Neuromodulation. 2016;19:437–439. doi: 10.1111/ner.12448. [DOI] [PubMed] [Google Scholar]
- 14.Baizabal-Carvallo JF, Jankovic J. Movement disorders induced by deep brain stimulation. Parkinsonism Relat Disord. 2016;25:1–9. doi: 10.1016/j.parkreldis.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 15.Picillo M, Lozano AM, Kou N, Puppi Munhoz R, Fasano A. Programming deep brain stimulation for Parkinson's disease: the toronto western hospital algorithms. Brain Stimul. 2016;9:425–437. doi: 10.1016/j.brs.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Ramirez-Zamora A, Kahn M, Campbell J, DeLaCruz P, Pilitsis JG. Interleaved programming of subthalamic deep brain stimulation to avoid adverse effects and preserve motor benefit in Parkinson's disease. J Neurol. 2015;262:578–584. doi: 10.1007/s00415-014-7605-3. [DOI] [PubMed] [Google Scholar]
- 17.Weiss D, Walach M, Meisner C, Fritz M, Scholten M, Breit S, Plewnia C, Bender B, Gharabaghi A, Wachter T, Kruger R. Nigral stimulation for resistant axial motor impairment in Parkinson's disease? A randomized controlled trial. Brain. 2013;136:2098–2108. doi: 10.1093/brain/awt122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tykocki T, Nauman P, Koziara H, Mandat T. Microlesion effect as a predictor of the effectiveness of subthalamic deep brain stimulation for Parkinson's disease. Stereotact Funct Neurosurg. 2013;91:12–17. doi: 10.1159/000342161. [DOI] [PubMed] [Google Scholar]
- 19.Wei XF, Grill WM. Impedance characteristics of deep brain stimulation electrodes in vitro and in vivo. J Neural Eng. 2009;6:046008. doi: 10.1088/1741-2560/6/4/046008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chinese expert committee on Deep Brain Stimulation programming (2016) Chinese expert consensus on deep brain stimulation programming for patients with Parkinson's disease. Chin J Neurosurg 32:1192–1198
- 21.(2015) The Guiding Opinions of the State Council on Actively Promoting the “Internet Plus” Action (No.40 [2015], the State Council): promote the new model of online medical and health care. http://www.gov.cn/zhengce/zhengceku/2015-07/04/content_10002.htm
- 22.(2018) Opinions of the General Office of the State Council on Promoting the Development of “Internet Plus Medical Health” (No. 26 [2018], the General Office of the State Council). http://www.gov.cn/zhengce/content/2018-04/28/content_5286645.htm
- 23.(2016) The Outline of the Plan for “Healthy China 2030”: Developing Internet-based health care services. http://www.gov.cn/gongbao/content/2016/content_5133024.htm
- 24.Antonini A, Gentile G, Giglio M, Marcante A, Gage H, Touray MML, Fotiadis DI, Gatsios D, Konitsiotis S, Timotijevic L, Egan B, Hodgkins C, Biundo R, Pellicano C, consortium PDM Acceptability to patients, carers and clinicians of an mHealth platform for the management of Parkinson's disease (PD_Manager): study protocol for a pilot randomised controlled trial. Trials. 2018;19:492. doi: 10.1186/s13063-018-2767-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Terroba-Chambi C, Bruno V, Medina-Escobar A, Nanni F, Cerquetti D, Rossi M, Merello M. Open-access electronic diary for motor fluctuation and dyskinesia evaluation in Parkinson disease: comparison with paper diary. Clin Neuropharmacol. 2018;41:20–22. doi: 10.1097/WNF.0000000000000264. [DOI] [PubMed] [Google Scholar]
- 26.Fasano A, Romito LM, Daniele A, Piano C, Zinno M, Bentivoglio AR, Albanese A. Motor and cognitive outcome in patients with Parkinson's disease 8 years after subthalamic implants. Brain. 2010;133:2664–2676. doi: 10.1093/brain/awq221. [DOI] [PubMed] [Google Scholar]
- 27.Gervais-Bernard H, Xie-Brustolin J, Mertens P, Polo G, Klinger H, Adamec D, Broussolle E, Thobois S. Bilateral subthalamic nucleus stimulation in advanced Parkinson's disease: five year follow-up. J Neurol. 2009;256:225–233. doi: 10.1007/s00415-009-0076-2. [DOI] [PubMed] [Google Scholar]
- 28.Jiang LL, Liu JL, Fu XL, Xian WB, Gu J, Liu YM, Ye J, Chen J, Qian H, Xu SH, Pei Z, Chen L. Long-term efficacy of subthalamic nucleus deep brain stimulation in Parkinson's disease: a 5-year follow-up study in China. Chin Med J (Engl) 2015;128:2433–2438. doi: 10.4103/0366-6999.164925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krack P, Batir A, Van Blercom N, Chabardes S, Fraix V, Ardouin C, Koudsie A, Limousin PD, Benazzouz A, LeBas JF, Benabid AL, Pollak P. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson's disease. N Engl J Med. 2003;349:1925–1934. doi: 10.1056/NEJMoa035275. [DOI] [PubMed] [Google Scholar]
- 30.Moro E, Lozano AM, Pollak P, Agid Y, Rehncrona S, Volkmann J, Kulisevsky J, Obeso JA, Albanese A, Hariz MI, Quinn NP, Speelman JD, Benabid AL, Fraix V, Mendes A, Welter ML, Houeto JL, Cornu P, Dormont D, Tornqvist AL, Ekberg R, Schnitzler A, Timmermann L, Wojtecki L, Gironell A, Rodriguez-Oroz MC, Guridi J, Bentivoglio AR, Contarino MF, Romito L, Scerrati M, Janssens M, Lang AE. Long-term results of a multicenter study on subthalamic and pallidal stimulation in Parkinson's disease. Mov Disord. 2010;25:578–586. doi: 10.1002/mds.22735. [DOI] [PubMed] [Google Scholar]
- 31.Rizzone MG, Fasano A, Daniele A, Zibetti M, Merola A, Rizzi L, Piano C, Piccininni C, Romito LM, Lopiano L, Albanese A. Long-term outcome of subthalamic nucleus DBS in Parkinson's disease: from the advanced phase towards the late stage of the disease? Parkinsonism Relat Disord. 2014;20:376–381. doi: 10.1016/j.parkreldis.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 32.Romito LM, Contarino MF, Vanacore N, Bentivoglio AR, Scerrati M, Albanese A. Replacement of dopaminergic medication with subthalamic nucleus stimulation in Parkinson's disease: long-term observation. Mov Disord. 2009;24:557–563. doi: 10.1002/mds.22390. [DOI] [PubMed] [Google Scholar]
- 33.Wider C, Pollo C, Bloch J, Burkhard PR, Vingerhoets FJ. Long-term outcome of 50 consecutive Parkinson's disease patients treated with subthalamic deep brain stimulation. Parkinsonism Relat Disord. 2008;14:114–119. doi: 10.1016/j.parkreldis.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 34.Zibetti M, Merola A, Rizzi L, Ricchi V, Angrisano S, Azzaro C, Artusi CA, Arduino N, Marchisio A, Lanotte M, Rizzone M, Lopiano L. Beyond nine years of continuous subthalamic nucleus deep brain stimulation in Parkinson's disease. Mov Disord. 2011;26:2327–2334. doi: 10.1002/mds.23903. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data have already been included in the manuscript.