Abstract
Introduction:
Obstructive sleep apnea (OSA) is one of the most common sleep-disordered breathing characterized by repeated cessation or reduction in airflow during sleep. OSA occurs in both obese and nonobese individual. This study was designed to compare the clinical and polysomnographic data between obese and nonobese patients with OSA.
Materials and Methods:
This was a retrospective study that included all the patients diagnosed as between November 2013 and December 2014. The patients were classified into nonobese (n = 23) and obese (n = 72) groups if their body mass index (BMI) was <27.5 and ≥27.5, respectively. Data were analyzed using Statistical Package for the Social Sciences (SPSS) software program, version 20.0 (SPSS, Chicago, Illinois). A value of P < 0.05 was considered statistically significant.
Results:
There were 95 patients of OSA, of which 23 (44.4%) were nonobese and 72 (75.78%) were obese with a mean BMI of 24.37 ± 3.09 and 34.27 ± 8.34 kg/m2, respectively. Characteristics, such as male predominance, higher BMI, neck circumference, and loud snoring, were significantly higher in obese group (P < 0.05) as compared to nonobese. Mild OSA (AHI 5-15) was significantly higher in nonobese patients (39.13% vs. 5.55%, P < 0.00001), whereas severe OSA (AHI >30) was higher in obese patients as compared with nonobese (66.66% vs. 30.43%, P = 0.002). When comparing comorbidities, the hypertension was significantly higher in the obese (47.22% vs. 13.04%, P = 0.003) than the nonobese patients. The incidence of diabetes (37.50% vs. 17.39%) and hypothyroidism (16.66% vs. 4.34%) was also higher in obese group as compared with nonobese. No significant difference was found for mean age, racial origin (Asian/African), and ESS score between obese and nonobese patients.
Conclusion:
Obstructive sleep apnoea is not restricted to only obese individual; rather it also occurs in nonobese. The severity of OSA in nonobese has generally less as compared with obese and its early identification required high index of suspicion.
Keywords: Apnea–hyponea index, body mass index, obesity, obstructive sleep apnea
Introduction
Obstructive sleep apnea (OSA) is a disorder that is characterized by repeated obstructive apneas, hypopneas, and/or respiratory effort-related arousals caused by repetitive collapse of the upper airway during sleep. The prevalence of OSA in the general adult population varies depending on the definition but is approximately 15%–30% in males and 5%–15% in females (when OSA is defined as an apnea–hypopnea index [AHI] greater than five events per hour of sleep).[1,2] Risk factors for OSA include older age, male gender, obesity, and craniofacial and upper airway abnormalities. Obesity is a major risk factor for OSA and well correlated with body mass index (BMI).[3,4] Moderate-to-severe OSA (AHI ≥15) was present in 11% of men who were normal weight, 21% who were overweight (BMI 25–30 kg/m2), and 63% of those who were obese (BMI >30 kg/m2) similarly, in women, OSA was present in 3% of patients who were normal weight, 9% of those who were overweight, and 22% of those who were obese.[5] However, few studies showed that clinical presentation in nonobese patient is not different as compared with obese except severity of disease, which is less this group.[6] There are few studies that characterize OSA in nonobese patients and probably require different treatment; therefore, this study was designed to compare the clinical and polysomnographic data between obese and nonobese patients with OSA.
Materials and Methods
This was a retrospective cross-sectional study conducted in Indraprasth Apollo Hospital, New Delhi. All the patients diagnosed as OSA between November 1, 2013 and December 31, 2014 included in study. OSA was considered in all patients with excessive daytime sleepiness and loud snoring with AHI >5/h. The patients were classified into nonobese (n = 23) and obese (n = 72) groups if their BMI was <27.5 and ≥27.5.[7] The detailed evaluation of patient was performed with predesigned proforma, which include age, sex, comorbid illnesses, Excessive day time sleepiness (EDS) by Epworth sleepiness scale (ESS), and anthopometric measurements (BMI, neck circumference, and Mallampati's score). Neck circumference was measured with a tape measure at the level of cricothyroid membrane. Subjects were excluded if they had any of the following: pulmonary tuberculosis, chronic obstructive pulmonary disease, bronchial asthma, interstitial lung disease, neuromuscular disorders, and history of drug abuse, chronic renal failure, congestive heart failure, pregnant, or receiving treatment for psychiatric conditions.
All patients with OSA underwent a level 1 sleep study consisting of an overnight polysomnographic examination, which included an Electroencephalography (EEG) (C3-A2, C4-A1, O2-A1, and O3-A2), bilateral Electrooculogram (EOG), chin and lower leg Electromyography (EMG), nasal and mouth airflow, thoracic and abdominal respiratory movements, Electrocardiogram (ECG), oxygen saturation measured by pulse oximetry, and body position. The episodes of apnea were defined as complete cessation of airflow for ≥10 s, and hypopnea consisted of a ≥50% reduction in oronasal airflow accompanied by a reduction in oxygen saturation measured by pulse oximetry of at least 4%. Apnea events were classified as obstructive, mixed, or central, according to the presence or absence of breathing efforts with thoracoabdominal paradox. AHI was determined by the frequency of these events per hour during sleep time based on the results of the overnight polysomnography. RERAs (Respiratory effort related arousal) defined by a series of respiratory cycles of increasing/decreasing effort or flattening lasted for ≥10 s, leading to an arousal that cannot be defined as apnea or hypopnea.
Statistical analysis
Numerical and categorical data were compared between groups using the Student's t test and Chi-square test as appropriate. Data were analyzed using Statistical Package for the Social Sciences (SPSS) software program, version 20.0 (SPSS, Chicago, Illinois). A value of P < 0.05 was considered statistically significant.
Results
There were 95 patients of OSA, of which 23 (44.4%) were nonobese and 72 (75.78%) were obese with a mean BMI of 24.37 ± 3.09 and 34.27 ± 8.34 kg/m2, respectively. Characteristics, such as male predominance, higher BMI, neck circumference, and loud snoring, were significantly higher in obese group (P < 0.05) as compared with nonobese [Table 1]. No significant difference was found for mean age, racial origin (Asian/African), and ESS score between obese and nonobese patients. The significantly higher proportion of nonobese patients have Mallampati grade 1 and 2 (P < 0.005), whereas grades 3 and 4 were found higher in obese group. When comparing comorbidities, the hypertension was significantly higher in the obese (47.22% vs. 13.04%, P = 0.003) than the nonobese patients. The incidence of diabetes (37.50% vs. 17.39%) and hypothyroidism (16.66% vs. 4.34%) was also higher in obese group as compared with nonobese [Table 2 and Figure 1]. Mild OSA (AHI 5-15) was significantly higher in nonobese patients (39.13% vs. 5.55%, P < 0.00001), whereas severe OSA (AHI >30) was higher in obese patients as compared with nonobese (66.66% vs. 30.43%, P = 0.002) [Table 2 and Figure 1]. Most of the study patients maintain their baseline oxygen saturation >90% in both nonobese and obese groups (100% and 95.83%). There was significantly higher proportion of nonobese patients who maintained their minimum oxygen saturation >90% as compared with nonobese (34.78% vs. 15.27%, P-0.041). Respiratory effort-related arousal (>5/h) was significantly higher in nonobese patients as compared with obese (52.12% vs. 29.16%, P-0.043). No significant difference was found for paradoxical breathing, periodic leg movement, atrial/ventricular arrhythmia, and frequency of ectopic.
Table 1.
Baseline characteristics of study patients
| Characteristics | Nonobese (n=23) | Obese (n=72) | P |
|---|---|---|---|
| Age | 44.82±12.47 | 48.88±13.73 | 0.210 |
| Male% | 21/23 (91.30) | 51/72 (70.83) | 0.048 |
| Asian/African | 22/1 | 58/14 | 0.058 |
| BMI | 24.37±3.09 | 34.27±8.34 | 0.0001 |
| Neck circumference | 36.10±3.62 | 39.21±4.18 | 0.001 |
| Mallampati score | |||
| 1 | 02 (8.69) | 05 (6.94) | 0.779 |
| 2 | 09 (39.13) | 11 (15.27) | 0.014 |
| 3 | 07 (30.43) | 27 (37.50) | 0.535 |
| 4 | 05 (17.39) | 29 (40.27) | 0.107 |
| ESS score | 9.86±4.59 | 10.6±4.47 | 0.493 |
| Loud snoring | 04 (17.39) | 42 (58.33) | 0.0006 |
| DM | 04 (17.39) | 27 (37.50) | 0.073 |
| Hypertension | 03 (13.04) | 34 (47.22) | 0.003 |
| Hypothyroidism | 01 (4.34) | 12 (16.66) | 0.133 |
Number in bracket shows proportion. P value is significant indicates bold values
Table 2.
Comparison of polysomnographic characteristics between obese and nonobese
| Characteristics | Nonobese (n=23) | Obese (n=72) | P |
|---|---|---|---|
| AHI | |||
| 5-15 | 09 (39.13) | 04 (5.55) | 0.00001 |
| 16-30 | 07 (30.43) | 20 (27.77) | 0.802 |
| >30 | 07 (30.43) | 48 (66.66) | 0.002 |
| Baseline oxygen saturation | |||
| >90% | 23 (100) | 69 (95.83) | 0.322 |
| <90% | 00 | 03 (4.16) | NA |
| Lowest oxygen saturation(%) | |||
| >90 | 08 (34.78) | 11 (15.27) | 0.041 |
| 75-90 | 11 (47.82) | 36 (50.00) | 0.857 |
| <75 | 4 (17.39) | 25 (34.72) | 0.116 |
| RERA | |||
| >5/h | 12 (52.12) | 21 (29.16) | 0.043 |
| Paradoxical breathing | 13 (56.52) | 52 (72.22) | 0.158 |
| Ectopics | 03 (13.04) | 05 (6.94) | 0.357 |
| Atria/ventricular arrthymia | 02 (8.69) | 06 (8.33) | 0.960 |
| PLM | 01 (4.34) | 07 (9.72) | 0.417 |
Number in bracket shows proportion. P value is significant indicates bold values
Figure 1.
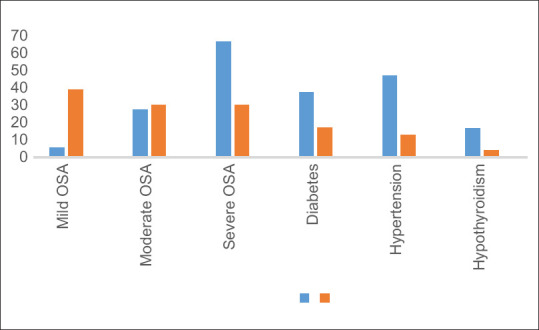
Difference in severity of OSA and comorbidities between obese and nonobese OSA. Number on the Y-axis represent proportion
Discussion
OSA is characterized by recurrent episodes of cessation and or reduction of respiratory airflow due to upper airway collapse during sleep, with a consequent decrease in oxygen saturation. Obesity is one of the most important risk factors and there are many cross-sectional studies which showed a strong association between increased body weight and the risk of OSA.[8] Primary care and family physician generally aware about this association but very few know that OSA can also occur in nonobese individual. This will lead to identification of disease in early stage and been put on specific treatment. This nonobese phenotype is challenging to treat as patient are less adherent to treatment and positive pressure therapy such as continuous positive airway pressure (CPAP) may not be effective[9,10]
Our study showed that nonobese patients with OSA tend to have significantly milder OSA compared with obese patients regarding parameter such as apnea–hyponea index (AHI), which are consistent with previous studies (P < 0.05).[11,12,13] There was significantly higher proportion of nonobese patients who maintained their minimum oxygen saturation above 90% as compared with nonobese during sleep study (P < 0.05), which is in line with other study.[14] The upper airway soft-tissue enlargement playa an important role in pathogenesis of obese patients with OSA, whereas bony structure discrepancies may be the dominant contributing factors among nonobese patients.[15] This study shows higher Mallampati grade (3–4) and higher neck circumference in nonobese patients as compared with obese, which is in line with other study.[14]
The incidence of hypertension was observed significantly higher in obese OSA patients than the nonobese patients. This finding has been supported by several studies (P < 0.05).[16,17,18] OSA is independently associated with hypertension, independent of obesity. Approximately 14% of study patients have hypothyroidism, which is higher than older study and slightly more in obese group as compared with nonobese.[19,20] OSA is a well-recognized risk factor for insulin resistance and type 2 diabetes mellitus (T2DM), independently of BMI[21] and this study shows higher incidence of diabetes in obese group with OSA than nonobese patients but statistically not significant (P > 0.05).
Experimental and population-based data identify OSA as a significant risk factor for cardiovascular disease and support its association with increased cardiovascular morbidity and mortality.[22,23,24] It is associated with nocturnal cardiac arrhythmias. The bradycardia-tachycardia phenomenon may be observed with respiratory events (apneas and hypopneas).[25] This study did not show significant difference for frequency of atrial or ventricular arrhythmia, and ectopic in two study groups but definitely more frequent in obese patients. There are no studies that look on incidence of arrhythmia between obese and nonobese OSA but study shows that sleep-related breathing disorder group had a higher prevalence of nocturnal atrial fibrillation (AF; 4.8% vs. 0.9%), nonsustained ventricular tachycardia (5.3% vs. 1.2%), and complex ventricular ectopy (25% vs. 14.5%).[26]
There are few limitations to this study. We have assessed only neck circumference and Mallampati grading for upper airway soft-tissue enlargement as they are easy to do at outpatient level and can correlate with severity of OSA. There are several imaging modality to evaluate the upper airway in patients with OSA such as cephalometric radiography, computed tomography, magnetic resonance imaging, fluoroscopy, and somnofluoroscopy but these methods are not easy to do and are expensive. Another limitation is that the study performed in corporate hospital where many foreigners were also included in study and so results cannot be generalized for our country.
Conclusion
Obstructive sleep apnoea is not restricted to only obese individuals; rather it also occurs in nonobese. The severity of OSA in nonobese has generally less as compared with obese and its early identification required high index of suspicion. No significant difference was found in prevalence of hypothyroidism between obese and nonobese OSA.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men: I. Prevalence and severity. Am J Respir Crit Care Med. 1998;157:144–8. doi: 10.1164/ajrccm.157.1.9706079. [DOI] [PubMed] [Google Scholar]
- 2.Young T, Palta M, Dempsey J, Peppard PE, Nieto FJ, Hla KM, et al. Burden of sleep apnea: Rationale, design, and major findings of the Wisconsin Sleep Cohort study. WMJ. 2009;108:246–9. [PMC free article] [PubMed] [Google Scholar]
- 3.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177:1006–14. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young T, Skatrud J, Peppard PE. Risk factors for obstructive sleep apnea in adults. JAMA. 2004;291:2013–6. doi: 10.1001/jama.291.16.2013. [DOI] [PubMed] [Google Scholar]
- 5.Tufik S, Santos-Silva R, Taddei JA, Bittencourt LR. Obstructive sleep apnea syndrome in the Sao Paulo Epidemiologic Sleep Study. Sleep Med. 2010;11:441–6. doi: 10.1016/j.sleep.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Dacal Quintas R, Tumbeiro Novoa M, Alves Perez MT, Santalla Martinez ML, Acuria Fernandez A, Marcos Velazquez P. Obstructive sleep apnea in normal weight patients: Characteristics and comparison with overweight and obese patients. Arch Bronconeumol. 2013;49:513–7. doi: 10.1016/j.arbres.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Nishida C. Appropriate body mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–63. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 8.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: A population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 9.Gray EL, McKenzie DK, Eckert DJ. Obstructive sleep apnea without obesity is common and difficult to treat: Evidence for a distinct pathophysiological phenotype. J Clin Sleep Med. 2017;13:81–8. doi: 10.5664/jcsm.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zinchuk A, Yaggi HK. Phenotypic subtypes of OSA: A challenge and opportunity for precision medicine. Chest. 2020;157:403–20. doi: 10.1016/j.chest.2019.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghanem A, Mahmood S. Is obstructive sleep apnoea in non-obese patients a less serious disease than in obese patients? Chest. 2005;128:231s–a. [Google Scholar]
- 12.Young T, Peppard PE, Taheri S. Excess weight and sleep-disordered breathing. J Appl Physiol. 2005;99:1592–9. doi: 10.1152/japplphysiol.00587.2005. [DOI] [PubMed] [Google Scholar]
- 13.Sergi M, Rizzi M, Comi AL, Resta O, Palma P, De Stefano A, et al. Sleep apnea in moderate-severe obese patients. Sleep Breath. 1999;3:47–52. doi: 10.1007/s11325-999-0047-y. [DOI] [PubMed] [Google Scholar]
- 14.Garg R, Singh A, Prasad R, Saheer S, Jabeed P, Verma R. A comparative study on the clinical and polysomnographic pattern of obstructive sleep apnea among obese and non-obese subjects. Ann Thorac Med. 2012;7:26–30. doi: 10.4103/1817-1737.91561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakakibara H, Tong M, Matsushita K, Hirata M, Konishi Y, Suetsugu S. Cephalometric abnormalities in non-obese and obese patients with obstructive sleep apnoea. Eur Respir J. 1999;13:403–10. doi: 10.1183/09031936.99.13240399. [DOI] [PubMed] [Google Scholar]
- 16.Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study: Sleep heart health study. JAMA. 2000;283:1829–36. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 17.Peker Y, Hedner J, Norum J, Kraiczi H, Carlson J. Increased incidence of cardiovascular disease in middle-aged men with obstructive sleep apnea: A 7-year follow-up. Am J Respir Crit Care Med. 2002;166:159–65. doi: 10.1164/rccm.2105124. [DOI] [PubMed] [Google Scholar]
- 18.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 19.Mete T, Yalcin Y, Berker D, Ciftci B, Guven Firat S, Topaloglu O, et al. Relationship between obstructive sleep apnea syndrome and thyroid diseases. Endocrine. 2013;44:723–8. doi: 10.1007/s12020-013-9927-9. [DOI] [PubMed] [Google Scholar]
- 20.Popovici I, Khawaja I. Efficacy of thyroid function tests in patients suspected of having obstructive sleep apnea. Chest. 1997;112:149. [Google Scholar]
- 21.Reutrakul S, Mokhlesi B. Obstructive sleep apnea and diabetes: A state of the art review. Chest. 2017;152:1070–86. doi: 10.1016/j.chest.2017.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 23.Punjabi NM, Caffo BS, Goodwin JL, Gottlieb DJ, Newman AB, O'Connor GT, et al. Sleep-disordered breathing and mortality: A prospective cohort study. PLoS Med. 2009;6:e1000132. doi: 10.1371/journal.pmed.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gottlieb DJ, Yenokyan G, Newman AB, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: The sleep heart health study. Circulation. 2010;122:352–60. doi: 10.1161/CIRCULATIONAHA.109.901801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monahan K, Storfer-Isser A, Meha R, Shahar E, Mittleman M, Rottman J, et al. Triggering of nocturnal arrhythmias by sleep-disordered breathing events. J Am Coll Cardiol. 2009;54:1797–804. doi: 10.1016/j.jacc.2009.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meha R, Stone KL, Varosy PD, Hoffman AR, Marcus GM, Blackwell T, et al. Nocturnal arrhythmias across a spectrum of obstructive and central sleep-disordered breathing in older men: Outcomes of sleep disorders in older men (MrOS sleep) study. Arch Intern Med. 2009;169:1147–55. doi: 10.1001/archinternmed.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]


