Abstract
Background:
Pyogenic liver abscess (PLA) is the end result of a number of pathologic processes that cause a suppurative infection of the liver parenchyma.
Materials and Methods:
Sixty-five patients of age more than 18 years and radiologically confirmed cases of liver abscess were included in this study. Pus and blood samples were collected. Pus was processed for microscopy of trophozoite of Entamoeba histolytica and aerobic and anaerobic bacterial culture. Blood was processed for antibody ELISA for Entamoeba histolytica and aerobic bacterial culture. Identification of aerobic and anaerobic isolates was done by Vitek2 and antibiotic sensitivity test for aerobic bacterial isolates was done by Vitek2.
Result:
Out of sixty five, twenty five were confirmed as PLA. All patients were male with mean age 37.9 years. Fever and upper abdominal pain were the most common symptoms. Right lobe comprised 80% of the abscess. Pus sample was more sensitive than blood sample for diagnosis. There were a total of 33 isolates in our study. Klebsiella pneumoniae (6/33) was the most common aerobic isolate and Clostridium spp. (7/33) was the anaerobic isolate. All gram-negative bacteria were showing good sensitivity for 3rd and 4th generation cephalosporins, fluoroquinolones, amikacin, gentamicin, piperacillin-tazobactam, imipenem and meropenem. Abscess >5 cm was treated with percutaneous drainage while abscess <5 cm was treated with antibiotics only.
Conclusion:
Diagnosis should be made with the combination of clinical suspicion, radiology, and microbiology. Empirical therapy should include anaerobic coverage too. Only antibiotic therapy can be given under consideration of size of abscess, persistence of fever after giving antibiotics, and any suspected complications.
Keywords: Anaerobe, pyogenic liver abscess, right lobe
Introduction
Liver abscess is a space-occupying lesion in liver parenchyma constituting the most common intraabdominal visceral abscess.[1] Hippocrates first described this disease around 400 BC, but Ochsner and colleagues first published a 47-cases review in 1938.[2] There are three etiologic classifications of liver abscess: amoebic liver abscess (ALA), pyogenic or bacterial liver abscess (PLA), and fungal liver abscess (FLA). Amoebic liver abscess (ALA) constitutes two-thirds of cases in developing countries while pyogenic liver abscess (PLA) constitutes three-fourths of cases in developed countries.[3] Previously, PLA was the result of intraabdominal complication such as acute appendicitis. But, nowadays biliary tract disease is the most common risk factor including 40% of cases. However, it is seen that up to 55% cases do not have any identifiable risk factor known as cryptogenic. PLA is a life-threatening infection with an incidence ranging from 8 to 22 per 1,000,000 people depending upon different geographical areas.[4] There are various poor prognostic factors like progression of sepsis, multiple abscesses, polymicrobial infection, antibiotic-resistant pathogens, age more than 70 years, abscess associated with neoplasia, and immunosuppression.[5] PLA presents through nonspecific symptoms like fever, abdominal pain, vomiting making diagnostic difficulty for clinicians. Hence, diagnosis of PLA often requires a combination of clinical features, imaging techniques like ultrasonography or CT scan, and microbiological findings.[6] Imaging techniques are helpful for diagnosing the liver abscess, but it is always essential to know about microbiological finding to plan therapeutic strategy.[7] There is considerable variation of causative agents depending upon etiology and geography. Most cases are usually polymicrobial in nature.[2] Escherichia coli, Klebsiella pneumoniae, Bacteroides, Enterococci, Streptococci, and Staphylococci are common bacteria which are isolated in PLA.[7] Escherichia coli is the common isolate in the western world while Klebsiella pneumoniae is emerging as a common isolate in Asia.[2] In fact, for the last 2 decades, Klebsiella pneumoniae has been reported as a predominant isolate in Asian countries and even in South Africa, Europe, and USA.[4]
Culture of aspirated pus is the gold standard test for the diagnosis of PLA. Blood culture can also be used as an additive test but its yield is less than pus culture. Other laboratory tests like leucocytes, C reactive protein, or liver profile get altered nonspecifically.[8] Increased alkaline phosphatase is the single most common finding observed in 90% cases.[2]
This study was aimed at understanding the various aspects of PLA like demographic profile, clinical presentations, radiological and laboratory findings, treatment modalities, and complications.
Materials and Methods
This cross-sectional study was done in the Department of Microbiology and Department of Surgery, Maulana Azad Medical College and associated Lok Nayak hospital, New Delhi. The duration of the study was 2 years. This study was ethically approved 22 October 2017. Patients of age more than 18 years and radiologically confirmed cases of liver abscess were included in this study. Patients with immunosuppression, bleeding diatheses, and hydatid cyst were excluded from this study. Details of patients regarding physical examination, routine laboratory investigations, and radiological examinations were noted. Pus and blood samples were collected and processed as per standard procedures.
(a) Pus
Pus was collected ultrasonographically, taken with all precautionary steps, by a spinal needle fitted with 10–20 mL syringe. Some quantity of the sample was immediately inoculated in laboratory prepared Cooked meat broth media while some was used for making Gram's stain, culture for bacteria, and examination of trophozoite of Entamoeba histolytica. Samples were cultured both aerobically and anaerobically. For aerobic culture, Blood agar and MacConkey's agar (Himedia, Mumbai) were used and incubated at 37°C for 24 h. For anaerobic culture, Schaedler agar or Brucella blood agar with hemin and vitamin K1 (Himedia, Mumbai) was used and incubated at 37°C maximally up to 1 week. Anaerobiosis was made by using Anoxomat AN2OP system. Those showing no growth either aerobically or anaerobically on plates were subcultured from Cooked meat broth. Both types of isolates were identified by VITEK2 (Biomereux, France). Antibiotic sensitivity test for aerobic isolates was done by VITEK2 (Biomereux, France) also.
(b) Blood
Five milliliter of blood collected under aseptic conditions was inoculated into the Brain heart infusion broth (laboratory prepared, Himedia) and incubated at 37°C for 24 h. Subcultures were made on Blood agar and MacConkey's agar and plates were incubated at 37°C for 24 h. Organisms were identified and also checked for antibiotic susceptibility by VITEK2 (Biomereux, France). Blood culture bottle was subcultured every day up to 7 days in case no growth on plates.
Blood samples were also processed for antibody detection of E. histolytica by ELISA method (IVD Research Inc., Carlsbad, California).
Statistical analysis
Data were expressed as proportion and percentage.
Result
A total of sixty-five clinically and ultrasonographically confirmed cases of liver abscess were included in the study, out of which thirty nine were identified as ALA, twenty-five cases as PLA, and one as FLA. PLA was diagnosed on the basis of (a) positive pus or blood culture for bacteria and (b) negative pus and blood culture with negative serum IgG ELISA for Entamoeba histolytica.
Demographic profile, symptoms, and signs
The age of the patients ranged from 18 to 60 years. Maximum number of patients were in the 31–40 years of age group and the mean age was 37.9 year. All patients were male. Eighty-four percent of cases were addicted to regular intake of alcohol. Twenty patients (80%) had an acute presentation (within 14 days), three patients (12%) with subacute (14–30 days), and two patients (8%) with chronic presentation (>30 days). Fever and upper abdominal pain were the most common symptoms while fever ≥38°C, abdominal tenderness, and hepatomegaly were the most common signs noted [Table 1].
Table 1.
Demographic profile, symptoms, and signs of patients
| Variables | Number (n=25) | Percentage |
|---|---|---|
| Age (in years) | ||
| 18-20 | 2 | 8 |
| 21-30 | 5 | 20 |
| 31-40 | 11 | 44 |
| 41-50 | 2 | 8 |
| 51-60 | 5 | 20 |
| >60 | 0 | 0 |
| Mean age | 37.9 years | |
| Sex | ||
| Male | 25 | 100 |
| Female | 0 | 0 |
| Alcohol intake | ||
| Regular (≥3 times/week) | 21 | 84 |
| Occasional (≤3 times/week) | 0 | 0 |
| Never | 4 | 16 |
| Symptoms | ||
| Fever | 23 | 92 |
| Upper abdominal pain | 23 | 92 |
| Nausea/Vomiting | 10 | 40 |
| Anorexia | 10 | 40 |
| Diarrhea | 4 | 16 |
| Signs | ||
| Fever (>38°C) | 23 | 92 |
| Abdominal tenderness | 23 | 92 |
| Hepatomegaly (>14 cm) | 22 | 88 |
| Jaundice | 3 | 12 |
| Ascites | 4 | 16 |
Laboratory and radiological investigations
Leucocytosis and anemia were the common laboratory findings followed by increased C- reactive protein and hypoalbuminemia. Alkaline phosphatase was increased in 68% cases. Eighty percent abscess was seen in right lobe ultrasonographically. Single abscess was seen in 76% cases while multiple abscesses were seen in 24% cases. Pleural effusion (24%) and intraperitoneal rupture (4%) were two complications noted in our study [Table 2].
Table 2.
Laboratory investigations and radiological findings
| Parameters | Number (n=25) | Percentage (%) |
|---|---|---|
| Blood Pictures | ||
| Leukocytosis | 21 | 84 |
| Anemia | 19 | 76 |
| Thrombocytopenia | 5 | 20 |
| ↑ C reactive protein | 18 | 72 |
| Alkaline phosphatase | 17 | 68 |
| Aspartate transaminase | 15 | 60 |
| Alanine transaminase | 15 | 60 |
| Hypoalbuminemia | 18 | 72 |
| ↑ Total bilirubin | 4 | 16 |
| Ultrasonographic findings | ||
| Right lobe | 20 | 80 |
| Left lobe | 1 | 4 |
| Both lobe | 4 | 16 |
| Single | 19 | 76 |
| Right | 18 | 72 |
| Left | 1 | 4 |
| Multiple | 6 | 24 |
| Right | 2 | 8 |
| Both | 4 | 16 |
| Size | ||
| ≤5 cm | 12 | 48 |
| 6-10 cm | 3 | 12 |
| >10 cm | 10 | 40 |
| Complications | ||
| Pleural effusion | 6 | 24 |
| Intraperitoneal rupture | 1 | 4 |
Microbiology and antibiotic susceptibility profile
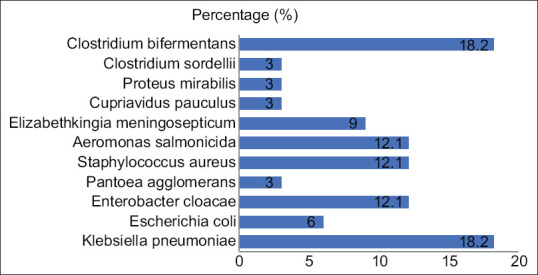
Out of twenty-five cases, twenty four (96%) were positive for bacteria either by pus or blood culture. Pus sample was positive in twenty cases (80%), being monomicrobial in thirteen cases (52%) and polymicrobial in seven cases (28%). Blood sample was positive in six cases (24%), being monomicrobial in five cases (20%) and polymicrobial in one case (4%). Aerobic isolates were found in twenty cases (80%) and anaerobic isolates were found in seven cases (28%). There were a total of 33 aerobic isolates in our study [Figure 1]. Klebsiella pneumoniae (6/33) was the most common aerobic isolate. Next common isolates were Enterobacter cloacae (4/33), Aeromonas salmonicida (4/33), and Staphylococcus aureus (4/33). Other aerobic isolates were Escherichia coli (2/33), Pantoea agglomerans (1/33), Elizabethkingia meningosepticum (3/33), Cupriavidus pauculus (1/33), and Proteus mirabilis (1/33). Clostridium bifermentans (6/33) and Clostridium sordellii (1/33) were the anaerobic isolates found in our study.
Figure 1.
Bacteriological isolates
All gram-negative bacteria excluding E. meningosepticum and C. pauculus were sensitive to 3rd and 4th generation cephalosporins, fluoroquinolones, amikacin, gentamicin, piperacillin-tazobactam, imipenem and meropenem, but all were resistant to amoxicillin-clavulanic acid. Oxidase positive bacteria (three E. meningoseptica and one C. pauculus) were showing nil sensitivity to amoxicillin-clavulanic acid, 50% sensitivity to ceftazidime, amikacin, gentamicin, tobramycin, and 100% sensitivity to fluoroquinolones and piperacillin-tazobactam. C. pauculus was sensitive to imipenem and meropenem (E. meningoseptica shows intrinsic resistance to carbapenems). All Staphylococcus aureus were sensitive to vancomycin, teicoplanin, and linezolid; only two (50%) were sensitive to other antimicrobial agents like cefoxitin, amikacin, gentamicin, erythromycin, and clindamycin and all (100%) were resistant to penicillin.
Treatment
Forty percent of patients were treated with pigtail drainage and drug, 12% patients with needle aspiration, and 48% patients with drug therapy alone [Table 3].
Table 3.
Treatment of patients
| Treatment given | Number (n=25) | Percentage (%) |
|---|---|---|
| Only drug therapy | 12 | 48 |
| Needle aspiration and drug therapy | 3 | 12 |
| Pigtail drainage and drug therapy | 10 | 40 |
Discussion
PLA accounts for 48% of visceral abscess.[9] It has still a great impact on health care facility and delay or improper management may lead to significant loss of life. In our study, the mean age of patients was 37.9 years with maximum cases (44%) between 31 and 40 years age group. Our study is in coherence with some studies.[3,9] But, this is in contrast to several studies which found a mean age in sixth and seventh decades.[4,10,11,12,13] Most of the studies found both male and female as patients and male outnumbered over female.[10,13,14,15,16] But, we found only male patients in our study. Probably, female sex hormones play a protective role in inflammatory responses.[14] It may be due to alcohol addiction more common in males.[1] All patients usually presented with nonspecific symptoms; thus, a high suspicion of clinical pictures leads to diagnosis easily. Fever and abdominal pain were the most common symptoms noted in our study. This is in coherence with many studies.[1,7,10,12,17] Hepatomegaly was found in 88% cases in our study. Jaundice was also seen in 12% of cases in our study which is similar to other study.[4]
Leukocytosis and anemia were the two common blood pictures found in our study. C reactive protein (CRP) was increased in 72% cases. There are some studies which found increased CRP in more than 95% cases.[4,15] We found hypoalbuminemia (72%) as the most common altered liver profile. Alkaline phosphatase was increased in 68% of cases having similarity with other study.[4] But, this is in contrast to several studies which found increased alkaline phosphatase in more than 90% cases.[13,15]
Ultrasonography and CT scan are the imaging techniques which are used for diagnosis of liver abscess. The sensitivity of ultrasonography and CT scan is reported 80%–95% and 95%–98%, respectively.[5] But, ultrasonography is cheaper; hence, it is more commonly used for diagnosis.[1] On imaging techniques, PLA is found as a solitary abscess in 65%–85% of cases having more affinity for right lobe of liver.[2] In the majority of our cases, abscess was solitary and confined to the right lobe of liver as seen in other works of literature too.[4,10,13,18,19] The possible explanation is that right liver lobe receives most of the portal blood flow making more prone for infection.[19]
There are many predisposing factors for PLA such as biliary tract disease, portal pyemia, malignancy, or contiguous spread of infection from intraabdominal organs.[3,9] Biliary tract diseases account for 50%–60% of all cases.[9] But, newer studies suggest that there is an increasing trend of cryptogenic PLA.[3,20,21,22] All twenty-five cases (100%) in our study were cryptogenic in nature which was in confirmation with these studies. In our study, we found 24/25 (96%) culture-positive cases which proves culture to be a sensitive method for diagnosis of PLA. This is in harmony with some studies which also found culture as a sensitive method for diagnosis of PLA.[7] But, some studies report a low yield of culture result probably due to the early use of antibiotics.[4] Bacteria are isolated more commonly with culture of liver aspirate (70%–80%) rather than the blood culture (30%–60%).[2] In our study, pus culture (80%) was high yielding as compared to blood culture (24%) which is in confirmation with other studies.[8,10,14,16] Monomicrobial culture was more common as seen in other study too.[7] Asian works of literature reveal Klebsiella as maximum isolates with cryptogenic origin.[12,13,23] Our study is also in agreement with this fact as we found Klebsiella pneumoniae (18.2%) as maximum aerobic isolate. Several studies are also in coherence with this fact.[5,10] We also found oxidase-positive bacteria like Elizabethkingea meningosepticum (9%) and Cupriavidus pauculus (3%), which we did not find in any literature. Elizabethkingea meningosepticum is an opportunistic pathogen which is predisposed by diabetes mellitus, steroid use, organ transplantation, neutropenia, prolonged hospitalization, prior administration of multiple antibiotics, and chronic hemodialysis.[24] Cupriavidus pauculus is an environmental bacterium which usually causes superficial site infection and bacteremia.[25] Patients infected with E. meningosepticum were diabetic for 3 years and they were taking insulin regularly. Diabetic or immunosuppressed patients have risk factors for other bacterial infections like Acinetobacter spp.; thus, these types of patients should be handled with a new insight.[26] Anaerobic bacteria have been reported in recent studies in the range of 9%–46%.[27,28] We identified anaerobes in 28% cases (21.2% of total isolates) which is in coherence with this fact. Bacteroides, Fusobacterium, Peptococcus, Prevotella, and Clostridium are reported in various studies.[14,16,18,28] We found Clostridium spp. (Clostridium bifermentans and Clostridium sordellii) in our study [Figure 1].
All gram-negative bacteria were showing good sensitivity to antibiotics which are commonly used like in hospitals. This finding shows that empirical therapy is useful for therapeutic purposes. This is in coherence with some studies which show very much sensitivity to antibiotics.[7,11]
Interventional radiology has changed the management of PLA over the past three decades. Now, antibiotic with percutaneous drainage (needle aspiration and pigtail drainage) is used as a preferred method of management. There are certain criteria for percutaneous drainage like- abscess size >5 cm, ongoing pyrexia even after 48 to 72 h of targeted antibiotic administration, and clinical or imaging features suggesting impending perforation.[2] Many newer studies have used percutaneous drainage in case abscess size >5 cm.[15,18,22] In our study, ten cases (40%) were with very large abscess (>10 cm) and treated with pigtail drainage, while three cases (12%) were with large (5–10 cm) abscess and treated with needle aspiration. Twelve cases (48%) were treated with only drug therapy alone. Some authors suggest that only antibiotic therapy can be started if abscess size less than 3 cm.[29,30] Some additional points should be considered while giving only antibiotic therapy like prolonged pyrexia after 48–72 h of proper antibiotic treatment and clinical or ultrasonographic findings suggesting a tendency of perforation.[31] There was no mortality in our study.
Conclusion
Pyogenic liver abscess presents through nonspecific symptoms and signs; hence, high clinical suspicion plays a pivotal role in early diagnosis. Risk factors like sex, alcoholism, or other factors with clinical symptoms and signs may be a clinching point for making an early diagnosis. Once the diagnosis confirmed, bacterial culture should always be performed to know the isolate and antibiotic sensitivity pattern. Diabetic or immunosuppressed patients may have a possibility of infection with some rare isolate like E. meningosepticum; hence, these facts should be kept in mind. Anaerobic bacteria constitute a major part; thus, empirical therapy should cover these bacteria too. Empirical therapy is very effective in combination with radiological interventions. Only antibiotic therapy can be given with evaluation of some factors like size of abscess, duration of fever after giving antibiotics, and any suspected complications.
Limitations
We did not perform a molecular analysis of the pathogens. Therefore, we were unable to find out any relation between the virulence of the pathogens and clinical manifestations. As we have less sample size, we cannot comment on complications.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Ali WM, Ali I, Rizvi SAA, Rab AZ, Ahmed M. Recent trends in the epidemiology of liver abscess in western region of Uttar Pradesh: A retrospective study. J Surg Anesth. 2018;2:117. [Google Scholar]
- 2.Longworth S, Han J. Pyogenic liver abscess. Clin Liver Dis (Hoboken) 2015;6:51–4. doi: 10.1002/cld.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghosh S, Sharma S, Gadpayle AK, Gupta HK, Mahajan RK, Sahoo R, et al. Clinical, laboratory, and management profile in patients of liver abscess from northern India? J Trop Med. 2014;2014:142382. doi: 10.1155/2014/142382. doi: 10.1155/2014/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serraino C, Elia C, Bracco C, Rinaldi G, Pomero F, Silvestri A, et al. Characteristics and management of pyogenic liver abscess: A European experience. Medicine (Baltimore) 2018;97:e0628. doi: 10.1097/MD.0000000000010628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castillo S, Manterola C. Morphological characteristics of liver abscesses according its etiology. Int J Morphol. 2020;38:406–14. [Google Scholar]
- 6.Luo M, Yang XX, Tan B, Zhou XP, Xia HM, Xue J, et al. Distribution of common pathogens in patients with pyogenic liver abscess in China: A meta-analysis. Eur J Clin Microbiol Infect Dis. 2016;35:1557–65. doi: 10.1007/s10096-016-2712-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kong H, Yu F, Zhang W, Li X. Clinical and microbiological characteristics of pyogenic liver abscess in a tertiary hospital in East China. Medicine (Baltimore) 2017;96:e8050. doi: 10.1097/MD.0000000000008050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khim G, Em S, Mo S, Townell N. Liver abscess: diagnostic and management issues found in the low resource setting. Br Med Bull. 2019;132:45–52. doi: 10.1093/bmb/ldz032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chadwick M, Shamban L, Neumann M. Pyogenic liver abscess with no predisposing risk factors? Case Rep Gastrointest Med. 2018;2018:9509356. doi: 10.1155/2018/9509356. doi: 10.1155/2018/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li W, Chen H, Wu S, Peng J. A comparison of pyogenic liver abscess in patients with or without diabetes: A retrospective study of 246 cases. BMC Gastroenterol. 2018;18:144. doi: 10.1186/s12876-018-0875-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang WJ, Tao Z, Wu HL. Etiology and clinical manifestations of bacterial liver abscess: A study of 102 cases. Medicine. 2018;97:e12326. doi: 10.1097/MD.0000000000012326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shelat VG, Chia CL, Yeo CS, Qiao W, Woon W, Junnarkar SP. Pyogenic liver abscess: Does Escherichia coli cause more adverse outcomes than Klebsiella pneumoniae? World J Surg. 2015;39:2535–42. doi: 10.1007/s00268-015-3126-1. [DOI] [PubMed] [Google Scholar]
- 13.Santos-Rosa OM, Lunardelli HS, Ribeiro-Junior MA. Pyogenic liver abscess: Diagnostic and therapeutic management. Arq Bras Cir Dig. 2016;29:194–7. doi: 10.1590/0102-6720201600030015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J, Du Z, Bi J, Wu Z, Lv Y, Zhang X, et al. Comparison of clinical characteristics and outcomes of pyogenic liver abscess patientset al. Modern management of pyogenic hepatic abscess: A case series and review of the literature. BMC Res Notes. 2011;4:80. doi: 10.1186/1756-0500-4-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heneghan HM, Healy NA, Martin ST, Ryan RS, Nolan N, Traynor O, et al. Modern management of pyogenic hepatic abscess: A case series and review of the literature. BMC Res Notes. 2011;4:80. doi: 10.1186/1756-0500-4-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Du ZQ, Zhang LN, Lu Q, Ren YF, Lv Y, Liu XM, et al. Clinical charateristics and outcome of pyogenic liver abscess with different size: 15-year experience from a single center. Sci Rep. 2016;6:35890. doi: 10.1038/srep35890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Law ST, Li KK. Is pyogenic liver abscess associated with recurrent pyogenic cholangitis a distinct clinical entity? A retrospective analysis over a 10-year period in a regional hospital. Eur J Gastroenterol Hepatol. 2011;23:770–7. doi: 10.1097/MEG.0b013e328348cb9c. [DOI] [PubMed] [Google Scholar]
- 18.Mezhir JJ, Fong Y, Jacks LM, Getrajdman GI, Brody LA, Covey AM, et al. Current management of pyogenic liver abscess: Surgery is now second-line treatment. J Am Coll Surg. 2010;210:975–83. doi: 10.1016/j.jamcollsurg.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Foo NP, Chen KT, Lin HJ, Guo HR. Characteristics of pyogenic liver abscess patients with and without diabetes mellitus. Am J Gastroenterol. 2010;105:328–35. doi: 10.1038/ajg.2009.586. [DOI] [PubMed] [Google Scholar]
- 20.Chen J, Zhang M, Chen J, Ning Y, Cai X, Zhang L, et al. Cryptogenic and non-cryptogenic liver abscess: A retrospective analysis of 178 cases revealed distinct characteristics. J Int Med Res. 2018;46:3824–36. doi: 10.1177/0300060518781256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pang TC, Fung T, Samra J, Hugh TJ, Smith RC. Pyogenic liver abscess: An audit of 10 years' experience. World J Gastroenterol. 2011;17:1622–30. doi: 10.3748/wjg.v17.i12.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mangukiya DO, Darshan JR, Kanani VK, Gupta ST. A prospective series case study of pyogenic liver abscess: Recent trands in etiology and management. Indian J Surg. 2012;74:385–90. doi: 10.1007/s12262-011-0397-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee JH, Jang YR, Ahn SJ, Choi SJ, Kim HS. A retrospective study of pyogenic liver abscess caused primarily by Klebsiella pneumoniae vs non-Klebsiella pneumoniae: CT and clinical differentiation. Abdom Radiol (NY) 2020 doi: 10.1007/s00261-019-02389-2. doi: 101007/s00261-019-02389. [DOI] [PubMed] [Google Scholar]
- 24.Govindaswamy A, Bajpai V, Trikha V, Mittal S, Malhotra R, Mathur P. Multidrug resistant Elizabethkingia meningoseptica bacteremia–Experience from a level 1 trauma centre in India. Intractable Rare Dis Res. 2018;7:172–6. doi: 10.5582/irdr.2018.01077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duggal S, Gur R, Nayar R, Rongpharpi SR, Jain D, Gupta RK. Cupriavidus pauculus (Ralstonia paucula) concomitant meningitis and septicemia in a neonate: First case report from India. Indian J Med Microbiol. 2013;31:405–9. doi: 10.4103/0255-0857.118871. [DOI] [PubMed] [Google Scholar]
- 26.Mohanty A, Kabi A, Mohanty A. Acinetobacter lwoffii-Emerging pathogen causing liver abscess: A case report. Natl J Integr Res Med. 2018;9:53–4. [Google Scholar]
- 27.Chen SC, Wu WY, Yeh CH, Lai KC, Cheng KS, Jeng LB, et al. Comparison of Escherichia coli and Klebsiella pneumoniae liver abscesses. Am J Med Sci. 2007;334:97–105. doi: 10.1097/MAJ.0b013e31812f59c7. [DOI] [PubMed] [Google Scholar]
- 28.Singh A, Banerjee T, Kumar R, Shukla SK. Prevalence of cases of amebic liver abscess in a tertiary care centre in India: A study on risk factors, associated microflora and strain variation of Entamoeba histolytica. PLoS One. 2019;14:e0214880. doi: 10.1371/journal.pone.0214880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chung YF, Tan YM, Lui HF, Tay KH, Lo RH, Kurup A, et al. Management of pyogenic liver abscesses-percutaneous or open drainage? Singapore Med J. 2007;48:1158–65. [PubMed] [Google Scholar]
- 30.Hope WW, Vrochides DV, Newcomb WL, Mayo-Smith WW, Iannitti DA. Optimal treatment of hepatic abscess. Am Surg. 2008;74:178–82. [PubMed] [Google Scholar]
- 31.Malik AA, Bari SU, Rouf KA, Wani KA. Pyogenic liver abscess: Changing patterns in approach. World J Gastrointest Surg. 2010;2:395–401. doi: 10.4240/wjgs.v2.i12.395. [DOI] [PMC free article] [PubMed] [Google Scholar]


