Abstract
Context:
Primary Amenorrhea is worrisome for the adolescent as well as their guardian. It is essential to be able to identify the underlying pathology and initiate appropriate management strategies, well in time.
Aims:
To study the clinical features, with an aim to identify discriminatory clinical features to indicate a most probable diagnosis.
Design:
A prospective, observational study.
Setting:
The Gynecologic Endocrinology Clinic of Department of Obstetrics and Gynecology and Endocrinology Outpatient Department of a tertiary centre in North India.
Methodology:
In total 328 women with primary amenorrhea, registered during a duration of 10 years (January 2008 to December 2017), were enrolled for the study and a proforma was filled with their medical details.
Results:
It was observed that patients with normal stature and underdeveloped breasts were likely to be hypogonadotropic hypogonadism, whereas those with short stature and underdeveloped breasts were more likely to be gonadal failure with underlying chromosomal abnormality. The three most common causes of primary amenorrhea in the index population were hypogonadotropic hypogonadism (117/328 = 35.1%), gonadal dysgenesis (99/328 = 30.2%) and mullerian agenesis (53/328 = 16.2%).
Conclusion:
Age, height, and Tanner staging at presentation can provide a clue toward diagnosis, even before confirmatory tests are available. One of the largest studies reported on primary amenorrhea, we found hypogonadotropic hypogonadism to be the commonest cause, in contrast to previous studies.
Keywords: Amenorrhea, hypogonadotropic hypogonadism, mullerian agenesis, short stature, tanner staging
Introduction
Onset of menstruation is considered a sign of feminity in many cultures. Primary amenorrhea, defined as the absence of spontaneous menstruation by 15 years of age with normal development of secondary sexual characteristics, is a cause of significant anxiety.[1] Although the reported incidence of primary amenorrhea is <1%, but these patients pose special challenge to the physician, as the recently diagnosed adolescents have to be counseled not only about menstruation, but about coitus and fertility as well.[2] Worldwide, various studies have been conducted on etiology of primary amenorrhea, listing gonadal dysgenesis and mullerian agenesis as the most common causes.[2,3] Less common causes reported are hypogonadotropic hypogonadism, constitutional delay of puberty and anovulation.[2]
Early commencement of treatment is of paramount importance as the underlying etiology can have considerable long-term impact on biological and psychological health. Currently, a battery of tests needs to be applied to reach at a diagnosis and it may lead to significant delays. We conducted this observational study over ten years, with an aim to identify salient clinical indicators, which may help point toward the most probable diagnosis.
Material and Methods
A prospective cohort study was conducted at a tertiary center in Northern India. Patients with primary amenorrhea (excluding cryptomenorrhea) who registered in the Gynecologic Endocrinology Clinic (Department of Obstetrics and Gynecology) and the Outpatient department of Department of Endocrinology over a period of 10 years (from January 2008 to December 2017) were included. Informed consent was taken. Ethical clearance was obtained from the Institute Ethics Committee. A total of 342 patients had registered, but 14 cases were excluded due to refusal of consent or loss to follow-up. Therefore, 328 cases were analyzed. Information was recorded under history, clinical examination, laboratory investigations, imaging and special investigations on a proforma.
History included presence of stress and eating disorder, exercise patterns, weight gain or loss, presence of galactorrhea, acne, excessive hair growth, heat/cold intolerance, withdrawal bleeding to progesterone or estrogen and progesterone, any past medical or surgical history or chronic medication, history of tuberculosis, and neonatal history. Family history of similar complaints, mental retardation, and any other significant illness was also elicited.
General physical examination included height, weight, built, body mass index (BMI), palpation of thyroid, sexual maturity rating (Tanner staging of breast and pubic hairs).[4] Stigmata of Turner syndrome such as webbed neck; low hairline or cubitus valgus were looked for. Local examination included inspection and palpation of external genitalia, per rectal and gentle per vaginal examination, with patient or guardian's consent.
Then gonadotropin assays, i.e. luteinizing hormone (LH) and follicle-stimulating hormone (FSH), and other hormones prolactin (PrL), thyroid-stimulating hormone (TSH), and estradiol, were advised. Urine pregnancy test was also done.
Pelvic ultrasonography (Trans abdominal or transvaginal) was done to evaluate mullerian anomalies and ovaries. Magnetic resonance imaging (MRI) pelvis was further done in ambiguous cases.
Specific investigations
Diagnostic hysteroscopy was done and endometrial aspirate was sent for histopathology and MGIT culture (Mycobacterial Growth in tube) in suspected cases. Karyotyping was done in cases suggestive of gonadal failure and mullerian agenesis. MRI brain was done in hypogonadotropic hypogonadism cases. If chronic illness was suspected, investigations like complete blood count, erythrocyte sedimentation rate (ESR), Mantoux test, chest X-ray were advised, as indicated. Other tests like 17 hydroxyprogesterone, serum testosterone were performed, if required. Patients with provisional diagnosis of delayed puberty had undergone LH response to GnRH stimulation. After giving subcutaneous leuprolide 500 μg, LH response >5mIU/mL at 3 h was considered optimal.
Tanner stage B3 was assigned when breast tissue extended past the areola causing the elevation of the breast and areola, with the contour of the areola being same as the rest of the breast. Short stature was defined as height less then third centile for the given age, according to the Indian academy of pediatrics (IAP) charts for those >18 years, while height <146.6 cm was considered short stature for those 18 years and above.[5] Patients were categorized into 4 groups based on Tanner stage of breast development and short stature as follows:
Tanner stage ≥ B3 and normal stature
Tanner stage ≥ B3 and short stature
Tanner stage ≤ B2 and normal stature
Tanner stage ≤ B2 and short stature
Patients with obvious etiology at presentation (e.g. Chemotherapy or autoimmune disorder related premature ovarian insufficiency (PoI), chronic illness) or with features of virilization/genital ambiguity (partial Androgen Insensitivity Syndrome (PAIS), Congenital Adrenal Hyperplasia (CAH), Disorder of Sexual Differentiation (DSD)) suggesting a diagnosis were not included in this scheme.
All the patients were also broadly classified into 4 broad categories of Hypogonadotropic hypogonadism (low FSH and estradiol), Hypergonadotropic hypogonadism (high FSH), Eugonadotropic/estrogen sufficient (normal FSH and estradiol) and miscellaneous and sub classified into the actual etiology, based on other available investigations.
Results
Analysis of 328 cases of primary amenorrhea was done, after excluding 14 cases with incomplete evaluation. Majority of the patients had normal height at the time of first contact with hospital. Sub-classifying those with normal stature, according to breast development, it was noted that mullerian agenesis (33.6%) and hypogonadotropic hypogonadism (32.8%) were the commonest etiologies in patients presenting with normal breast development, whereas hypogonadotropic hypogonadism (60.2%) was the commonest cause in those presenting with underdeveloped breasts. Among patients presenting with short stature, Turner syndrome (60.3%) was commonest cause for patients with combination of underdeveloped breasts and short stature. Only 10 patients presented with combination of short stature with normal breast development and were mostly found to be variants of mullerian agenesis (80%). Detailed classification of patients (excluding those with obvious etiologies or virilization/genital ambiguity at presentation) into 4 groups based on Tanner staging and height, along with percentage contribution from various etiologies is given in table 1. This information was further used to design a flowchart for approach to diagnosis, using clinical features as indicators of most probable etiology [Figure 1].
Table 1.
Classification of patients with primary amenorrhea using Tanner staging and Stature
| Clinical features | Tanner stage ≥B3 AND Normal Stature | Tanner stage ≥B3 AND Short Stature | Tanner stage ≤B2 AND Normal Stature | Tanner stage ≤B2 AND Short Stature |
|---|---|---|---|---|
| Hypogonadotropic Hypogonadism (n=117) | 41 (32.8%) | 0 | 71 (60.2%) | 5 |
| XX Gonadal Dysgenesis (n=51) | 14 | 0 | 29 (24.6%) | 8 |
| Turner Syndrome (n=46) | 1 | 2 | 8 | 35 (60.3%) |
| Swyer Syndrome (n=2) | 0 | 0 | 0 | 2 |
| Mullerian agenesis (n=53) | 42 (33.6%) | 8 (80%) | 3 | 0 |
| PCOS (n=13) | 13 | 0 | 0 | 0 |
| CAIS (n=2) | 2 | 0 | 0 | 0 |
| Asherman syndrome (n=4) | 4 | 0 | 0 | 0 |
| Delayed Puberty (n=19) | 4 | 0 | 7 | 8 |
| Hyperprolactinemia (n=4) | 4 | 0 | 0 | 0 |
| Total (n=311a) | 125 | 10 | 118 | 58 |
aExcluding 17 patients who presented with known etiology or exhibited virilization/ambiguous genitalia at presentation suggesting an etiology: Partial AIS (5), Chronic illness (5), CAH (3), DSD (2), Chemotherapy induced PoI (1), Autoimmune PoI (1)
Figure 1.
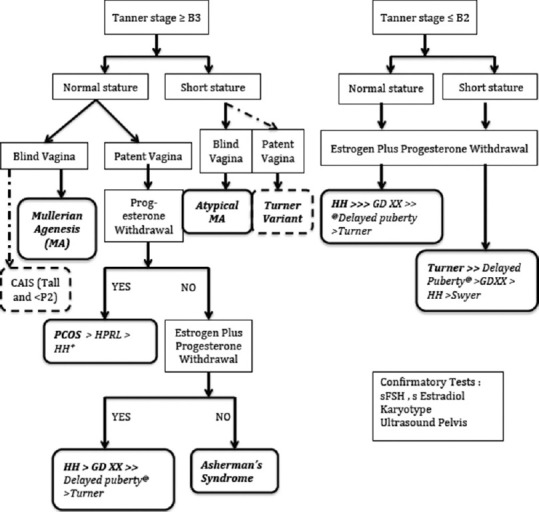
Discriminatory clinical features indicating the most probable etiology in patients with primary amenorrhea. aExcluding patients with obvious etiology at presentation (e.g. Chemotherapy or autoimmune disorder related premature ovarian insufficiency, Chronic illness) or with features of virilization/genital ambiguity (partial AIS, CAH, DSDs). Abbreviations: CAIS Complete Androgen Insensitivity Syndrome; B3 Breast Tanner stage 3; P2 Pubic hair Tanner stage 2; PCOS Polycystic Ovarian Syndrome; HPrl Hyperprolactinemia; HH Hypogonadotropic Hypogonadism; GDXX XX Gonadal Dysgenesis, bFew HH patients may have progesterone withdrawal cPatients with delayed puberty have early presentation (Mean age: 15.5 y) and LH response to GnRH stimulation is optimal
The three most common causes of primary amenorrhea in the index population were hypogonadotropic hypogonadism (35.1%), gonadal failure (30.2%) and MayerRokitansyKuster Hauser Syndrome (16.2%). Summary of etiological classification and clinical features are presented in Table 2.
Table 2.
Etiological classification and clinical features of patients presenting with primary amenorrhea
| Etiology | n (%) (Total=328) | Mean age (±SD) [Range] | Mean height (±SD) [Range] |
|---|---|---|---|
| Category 1: Hypogonadotropic hypogonadism | |||
| Idiopathic | 117 (35.5%) | 19 (±3.6) [14-34] | 155 (±1.4) [135-173] |
| Category 2: Hypergonadotropic hypogonadism | |||
| XX Gonadal Dysgenesis | 51 (15.5%) | 19.7 (±4.9) [15-35] | 152 (±11.3) [131-168] |
| Turner/Variant/Mosaic | 46 (14%) | 17.5 (±3.5) [13-29] | 136.9 (±9.8) [120-163] |
| Swyer Syndrome | 2 | *17, 23 | *152, 152 |
| Autoimmune POF | 1 | 19 | 162 |
| Chemotherapy induced POF | 1 | 14 | 143 |
| Category 3: Eugonadotropic, Estrogen sufficient | |||
| Mullerian Agenesis | 53 (16.2%) | 21 (±4.4) [14-32] | 153.4 (±6.3) [140-167] |
| Asherman’s Syndrome | 4 | *20.5 [18-29] | *154 [152-160] |
| Polycystic ovaries | 13 | *18 [116-26] | *156 [148-163] |
| Miscellaneous | |||
| Constitutionally Delayed Puberty | 19 (5.8%) | 15.5 (±1.38) [13-18] | 146.6 (±8.8) [135-160] |
| Hyperprolactinemia | 4 | *19 [15-25] | *153.5 [148-164] |
| Androgen Insensitivity Syndrome (Complete=2, partial=5) | 7 | *18 [16-20] | *163 [158-168] |
| Others (Chronic disease, DSD, etc ) | 10 |
*Median [Range] has been presented for data which did not follow normal distribution
Among the patients with hypergonadotropic hypogonadism, there were 46 cases (45.5%) of Turner Syndrome (TS) (Monosomic or mosaic or variant), two cases of Swyer syndrome (46 × Y), one each of autoimmune premature ovarian failure (systemic lupus erythematosus) and chemotherapy induced gonadal failure in a patient who had received cisplatin and etoposide for posterior fossa primitive neuroectodermal tumor, while rest of 51 patients (50.5%) with normal karyotype were classified as XX gonadal dysgenesis. Among these patients, it was noticed that patients presenting at later age (*mean age 19.7 years) and with normal stature (#mean height = 152 cm) had normal karyotype, whereas those who presented earlier (*mean age = 17.5 years) and with associated short stature (#mean height = 136.9) had underlying karyotypic abnormality and the difference was statistically significant (*p = 0.013, #p < 0.001).
Stigmata of Turner (webbed neck, high arched palate, low hair line, multiple pigmented nevi, shield chest, cubitus valgus) were recorded in 18 (39.1%) patients with Turner Syndrome. Twelve patients (22.6%) with MRKH Syndrome had associated renal anomalies (left absent/ectopic kidney = 6, right absent/ectopic kidney = 4, B/L ectopic kidneys = 1, malrotated right kidney = 1). Conductive hearing loss was noted in four, whereas cardiac anomalies were seen in seven Turner's patients (Two cases each of mild-moderate tricuspid regurgitation and aberrant right subclavian artery, while one each of bicuspid aorta, dilated aortic root, and ostium secundum atrial septal defect).
Discussion
Clinical features can help in pointing toward a probable diagnosis underlying primary amenorrhea, even before confirmation can be obtained from diagnostic tests. The resultant decrease in diagnostic delay helps in ameliorating the psychological stress of waiting period and early institution of appropriate counseling and treatment. A high index of suspicion, for associated clinical features, may help in early detection of these cases of abnormal pubertal development. Thus family physicians have a major role to play. By employing simple measures like incorporating menstrual history for all adolescents and measurement of height as part of routine workup, family physicians can pick up such cases even before they alarm the patient or guardian.
Our study revealed that when patient presents with normal stature and normal development of breasts, mullerian agenesis and hypogonadotropic hypogonadism are the most likely differential diagnoses and these can be further differentiated based on patency of vagina. Among those with short stature and underdeveloped breasts, Turner syndrome was the most probable etiology. Our results are consistent with that of a large study in diverse populations, suggesting that ~ 86% (63/73) Turner syndrome patients presented with short stature.[6]
Looking at this data from a different angle, we found that most common form of presentation of hypogonadotropic hypogonadism patients is with normal stature and underdeveloped breast (60.6%), although 35% had adequate breast development at presentation. But short stature is a rarity in these patients (4.2%). This explains the fact that due to normal development of breast and adequate height, patients with hypogonadotropic hypogonadism tend to present at a later age as compared to those with gonadal failure [Table 1]. The patients with gonadal failure due to underlying karyotypic abnormality like Turner Syndrome seek medical advice at relatively early age due to the added concern of short stature, while subjects with normal stature and delayed presentation are likely to lack karyotypic abnormalities. It is beneficial as the workup for associated comorbidities in patients with Turner Syndrome, like echocardiography for cardiac anomalies, auditory evaluation, screening ultrasound for renal anomalies, autoimmune hepatitis, thyroid disorders can commence early.[7] In a previously published clinical review, by Samar, et al. height has been included in the workup of patients presenting with primary amenorrhea and underdeveloped secondary sex characters.[8]
Complete androgen insensitivity syndrome (CAIS) is the likely diagnosis in patients presenting with normal breast development and blind vagina, but relatively tall stature (more than 50th centile for age) and Tanner staging of pubic hair ≤ P2, whereas, patients with MRKH present with normal stature and pubic hair development. The median (range) height for four cases of CAIS, reported from a referral hospital in South India was 165 (152-190), reiterating the fact that relative tallness and discrepant breast and pubic hair staging are strong clinical predictors for likelihood of CAIS.[9] The need for confirmatory karyotype can thus be eliminated in the latter.
There are certain limitations to the simplified view presented above, as there are rare causes like Asherman Syndrome and hyperprolactinemia. These diagnoses can also be clinically suspected and further differentiated based on presence or absence of withdrawal bleeding after progesterone only or combined estrogen and progesterone [Figure 1]
In our study, the prevalence of various etiologies of primary amenorrhea was different from that reported by other studies, with hypogonadotropic hypogonadism (35.1%) being the commonest. In Western literature, gonadal dysfunction has been reported as the commonest factor for primary amenorrhea.[10] In contrast, in the study conducted by Tanmahasamut in Thailand, mullerian agenesis was found to be the most frequent cause.[11] In a study from a tertiary center in northern India, hypogonadotropic hypogonadism was the third commonest cause, with a percentage representation of 19.5%, while mullerian agenesis and gonadal dysgenesis were the commonest.[12] Although in a previous study of 48 patients from the same center, commonest causes were mullerian agenesis (54.2%) followed by hypogonadotropic hypogonadism (22.4%) and gonadal dysgenesis (16.6%).[13] Comparison with other studies describing the etiology of primary amenorrhea is summarized in Table 3. Ours is a tertiary referral center. Considering that diagnosis of hypergonadotrophic hypogonadism is relatively straightforward, with the high FSH values, with or without stigmata of Turner disease, such patients are likely to receive treatment at local hospitals. Diagnosis of hypogonadotropic hypogonadism can be a challenge, with interpretation of low or normal FSH and correlation of these with estradiol levels differentiating from conditions like PCOS. Thus such patients are more likely to be referred to academic centers and consequently referral bias may be a cause for the above results, in addition to genetic, environmental or racial factors, which cannot be ruled out.
Table 3.
Comparison with other studies describing etiology of primary amenorrhea
| Etiology | Reindollar[10] 1960-80 | Tanmahasamut[11] 1992-2009 | Amin SV[14] 2006-12 | Kriplani[12] 2012-15 | Present study 2008-2017 |
|---|---|---|---|---|---|
| Region | USA | Thailand | India (South) | India (North) | India (North) |
| Sample size | 252 | 295 | 91 | 102 | 328 |
| Most common etiology | Gonadal dysgenesis (43%) | Mullerian agenesis (39.7%) | Mullerian agenesis (37.4%) | Mullerian agenesis (30.4%) | Hypogonado-tropic hypogonadism (35.5%) |
| Hypogonado-tropic hypogonadism | 9% | 9.2% | 9.9% | 14.7% | 35.5% |
| Physiologic delay | 14% | 7.7% | 5.8% | ||
| Crypto-menorrhea | 20.8% | 16.6% |
Our study is the largest, from a single tertiary center, till date on the etiology and management of primary amenorrhea. It has few limitations like omission of patients with cryptomenorrhea; as such patients are not referred to the endocrinology clinics.
Conclusion
Clinical pointers like age, height and Tanner staging at presentation, can help indicate underlying etiology in patients with primary amenorrhea. Normal stature and breast development at presentation point towards hypogonadotropic hypogonadism and mullerian agenesis as the most likely causes, although majority of hypogonadotropic hypogonadism patients present with underdeveloped breasts and normal stature. At the other end of spectrum, most common etiology underlying primary amenorrhea with short stature and underdeveloped breasts is Turner syndrome or its variant. Index study is one of the largest studies reporting on primary amenorrhea, finding hypogonadotropic hypogonadism to be the commonest cause, in contrast to previous studies.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Baker V, Schillings WJ, McClamrock HD. Amenorrhea. In: Berek JS, editor. Berek and Novac's Gynecology. 15th Ed. New Delhi: Wolters Kluver, Lippincott Williams and Wilkins; 2012. p. 1035. [Google Scholar]
- 2.Timmreck LS, Reindollar RH. Contemporary issues in primary amenorrhea. Obstet Gynecol Clin North Am. 2003;30:287–302. doi: 10.1016/s0889-8545(03)00027-5. [DOI] [PubMed] [Google Scholar]
- 3.Schorge JO, Schaffer JI, Halvorson LM, Hoffman BL, Bradshaw KD, Cunningham FG. Amenorrhea. In: Schorge JO, Schaffer JI, editors. Williams Gynecology. New York, NY: McGraw Hill; 2008. pp. 1112–28. [Google Scholar]
- 4.Tanner JM, Whitehouse RH. Variations of growth and development at puberty Atlas of children's growth, normal variation and growth disorders. 1982:122–7. [Google Scholar]
- 5.Khadilkar V, Yadav S, Agrawal KK, Tamboli S, Banerjee M, Cherian A, et al. Revised IAP growth charts for height, weight and body mass index for 5-to 18-year-old Indian children. Indian Pediatr. 2015;52:47–55. doi: 10.1007/s13312-015-0566-5. [DOI] [PubMed] [Google Scholar]
- 6.Kruszka P, Addissie YA, Tekendo-Ngongang C, Jones KL, Savage SK, Gupta N, et al. Turner syndrome in diverse populations. Am J Med Genet A. 2020;182:303–13. doi: 10.1002/ajmg.a.61461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shankar Kikkeri N, Nagalli S. StatPearls [Internet] Treasure Island (FL): StatPearls Publishing; 2020. Jan- Turner Syndrome. Available from: https://www.ncbi.nlm.nih.gov/books/NBK554621/ Updated on 2020 Jan 22. [PubMed] [Google Scholar]
- 8.Samal R, Habeebullah S. Primary amenorrhea: A clinical review. Int J Reprod Contracept Obstet Gynecol. 2017;6:4748–53. [Google Scholar]
- 9.Kanagal D, Anitha C. Case series of primary amenorrhoea. Int J Sci Res. 2020;8:52–5. [Google Scholar]
- 10.Reindollar RH, Tho SPT, McDonough PG. Delayed puberty: An update study of 326 patients. Trans Am GynecolObstetSoc. 1989;8:146–62. [Google Scholar]
- 11.Tanmahasamut P, Rattanachaiyanont M, Dangrat C, Indhavivadhana S, Angsuwattana S, Techatraisak K. Causes of primary amenorrhea: A report of295 cases in Thailand. J ObstetGynaecol Res. 2012;38:297–301. doi: 10.1111/j.1447-0756.2011.01677.x. [DOI] [PubMed] [Google Scholar]
- 12.Kriplani A, Goyal M, Kachhawa G, Mahey R, Kulshrestha V. Etiology and management of primary amenorrhea: A study of 102 cases at tertiary centre. Taiwan J Obstet Gynecol. 2017;56:761–4. doi: 10.1016/j.tjog.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 13.Kumar A, Mittal S. Primary amenorrhea: Analysis of 48 cases. J Indian Med Assoc. 1998;96:119–20. [PubMed] [Google Scholar]
- 14.Amin SV, Rai L, Palpandi P, Kumaran A. Ever intriguing 'Primary amenorrhea'-an audit. Int J Reprod Contracept Obstet Gynecol. 2017;3:1090–6. [Google Scholar]


