Abstract
In addition to the key roles of reversible acetylation of histones in chromatin in epigenetic regulation of gene expression, acetylation of nonhistone proteins by histone acetyltransferases (HATs) p300 and CBP is involved in DNA transactions, including repair of base damages and strand breaks. We characterized acetylation of human NEIL1 DNA glycosylase and AP-endonuclease 1 (APE1), which initiate repair of oxidized bases and single-strand breaks (SSBs), respectively. Acetylation induces localized conformation change because of neutralization of the positive charge of specific acetyl-acceptor Pre-proLysresidues, which are often present in clusters. Acetylation in NEIL1, APE1, and possibly other base excision repair (BER)/SSB repair (SSBR) enzymes by HATs, prebound to chromatin, induces assembly of active repair complexes on the chromatin. In this review, we discuss the roles of acetylation of NEIL1 and APE1 in modulating their activities and complex formation with other proteins for fine-tuning BER in chromatin. Further, the implications of promoter/enhancer-bound acetylated BER protein complexes in the regulation of transcriptional activation, mediated by complex interplay of acetylation and demethylation of histones are discussed.
Keywords: DNA base damage, acetylation, base excision repair (BER), APE1, NEIL1, histone acetyltransferases (HATs), BER complexes
Introduction
The genome, along with other cellular components, are continuously exposed to both endogenous and external genotoxic insults [1]. Reactive oxygen species (ROS), generated as respiration byproducts and produced by various oxidases in response to signaling, are the predominant endogenous agents, which generate multiple oxidized base lesions and DNA strand breaks containing non-ligatable termini [2]. Because such lesions could cause mutation and toxicity during transcription and genome replication, their error-free repair is essential for cell survival. Oxidized bases (and inappropriate bases) in the genome are repaired via the base excision repair (BER) process, initiated by their binding to DNA glycosylases (DGs), such as NEIL1, NEIL2, NEIL3, OGG1, and NTHI, most of which generate single-strand breaks (SSBs) with non-ligatable termini [3, 4]. ROS also induce direct SSBs, containing blocked termini. All of these damage intermediates are processed by the next enzyme in the repair pathway, APE1 in the case of OGG1-initiated base excision, or PNKP for the NEILs, before gap-filling DNA synthesis is carried out by DNA polymerases [4, 5]. Thus BER/SSBR is regulated by a DG or APE1.
Several years ago, we discovered that the enzymes and other proteins involved in the BER/SSBR process could be isolated as stable complexes from nuclear extracts of various human cells, either after size fractionation or as immunocomplexes [6]. Such complexes are competent to carry out complete repair in vitro, suggesting that BER/SSBR relies on the coordinated actions of the components assembled in repair complexes, which we named ‘BERosomes’ [6]. These complexes appear to be assembled at the sites of the DNA lesions. This discovery provided a window to explore involvement of various cellular components in BER/SSBR. Mass spectroscopic (MS) analyses of the NEIL1 and APE1 immunocomplexes identified several dozen proteins, some of which have no apparent relevance to DNA repair or nucleic acid metabolism [7]. Nevertheless, in spite of the possibility of artifacts, we did confirm the presence of known repair proteins and other nucleic acid metabolizing proteins, mostly heterogeneous nuclear ribonucleoproteins (hnRNPs) and histone chaperones in these repair complexes [8, 9]. We tested some of RNPs, histone chaperones, and Facilitates Chromatin of Transcription (FACT) complex and confirmed their involvement in BER [8–10].
Acetylation of BER enzymes and fine-tuning of damage repair
The addition of an acetyl group to Lys (K) residues has been documented to be an important posttranslational modification for modulating protein functions by altering its conformation, stability, activity, or the ability to interact with other proteins. Over the years, our own studies and those of others have highlighted the role of acetylation of many BER proteins in the regulation and fine-tuning of DNA damage repair pathways. We discovered that the major human DGs, OGG1, NEIL1, and NEIL2, which cleave most oxidatively damaged bases, are acetylated at specific K residues in cells [11–13]. The predominant histone acetyltransferases p300, CBP, and P/CAF acetylated these proteins in vitro, and the same acceptor K residues were identified in the intracellular proteins (Fig. 1). Acetylation of most DGs increases their turnover. We showed that acetylation of OGG1 at K338 and K341 increased base excision activity by reducing the affinity of OGG1 for its AP site product [12]. OGG1 acetylation was enhanced in cells after oxidative stress, suggesting a DNA damage-dependent activation of OGG1 due to its acetylation [12]. Recently, we showed that NEIL1 is acetylated at multiple K residues (K296, K297, and K298) located in the intrinsically unstructured C-terminal domain, and acetylation of NEIL1 enhanced its glycosylase activity in vitro [13]. Furthermore, we found that the loss of acetylation of OGG1 or NEIL1 enhanced the sensitivity of cells to DNA damaging agents that produce oxidized base or SSBs in DNA [12, 13]. NEIL2 was shown to be acetylated at K49 and K153 by p300 in vitro [11]. Acetylation at K153 did not affect the activity of NEIL2 in vitro, whereas acetylation at K49 inactivated NEIL2 by inhibiting both its base excision and AP lyase activities [11].
Fig. 1.
Acetylation/deacetylation cycles of BER-initiating DGs and APE1 on chromatin by HATs, such as p300, CBP, and PCAF, and by HDACs and SIRTs. Acetyl (Ac)-acceptor K residues are shown.
We discovered that human AP-endonuclease 1 (APE1), a key 3’ exo/endo phosphodiesterase, which cleans up dirty 3’ termini after oxidative and enzymatic strand breaks, is primarily acetylated at Kn6 and K7 residues located in the unstructured N-terminal region [14]. Later, other residues K27, K31, K32, and K35 were also found to be acetylated [15]. We and others showed that acetylation of APE1 enhanced its AP-endonuclease activity in vitro [16–18]. Members of both classes of histone deacetylase (HDAC), classical HDAC1 and NAD+-dependent SIRT1 regulate APE1 acetylation/deacetylation cycles in cells [17, 18]. Interestingly, acetylation of APE1 also modulates its transcriptional coregulatory functions [14, 19]. Our studies revealed that acetylation of APE1 at K6/7 promoted its binding to negative calcium response element (nCaRE) present in the parathyroid hormone (PTH) gene promoter and regulated PTH expression [14]. Given that APE1 is also bound to such nCaRE in the human renin gene promoter, acetylation of APE1 might also negatively regulate its expression in response to calcium influx [19]. APE1's acetylation was also shown to enhance its binding to Y-box-binding protein 1 (YB-1), a transcription factor identified in the APE1 immunocomplex [19]. Acetylation of APE1 activated YB-1-mediated expression of a multidrug resistance gene MDR1 [20].
Acetylation of BER proteins affects protein-protein interactions
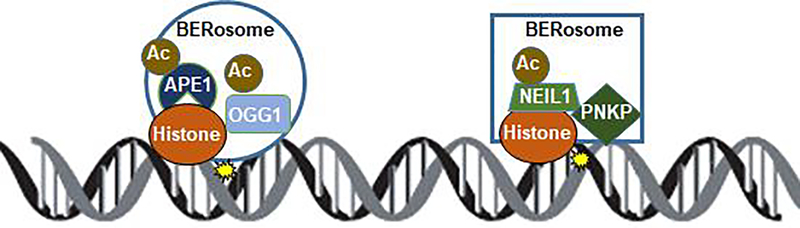
It is interesting that the specific acetyl-acceptor K residues in both NEIL1 and APE1 are present as clusters localized in their unstructured domains, suggesting acetylation induces conformation change because of neutralization of the positive charge of K. Alteration of this local conformation may affect protein-protein interaction and may induce formation of distinct BERosomes (Fig. 2). By coimmunoprecipitation (Co-IP) analysis, we showed that the immunoprecipitation (IP) complex of the non-acetylable K296–298R NEIL1 mutant contained significantly lower levels of other BER/SSBR proteins and chromatin factors (histone H3, histone chaperones CHAF1A, CHAF1B, and ASF1A), compared to that of the WT protein [13]. Such acetylable K-dependent association of APE1 with its diverse interacting partners was also observed [19].
Fig. 2.
Formation of distinct BER complexes, “BERosomes”, for the BER-initiating DGs, OGG1 and NEIL1, with distinct partners and histones, which are probably also acetylated, at damage sites on chromatin.
Acetylation of APE1 and NEIL1 confers protection Pofthe cell genome from oxidative stress and toxicity to DNA damaging drugs [13, 16, 17]. It is also important to examine whether a defect in regulation of BER via acetylation is associated with drug resistance in tumor. Indeed, we have shown elevated levels of acetylated APE1 in diverse cancer tissues and its association with drug resistance [17]. It is thus clear that enzymes controlling BER posttranslational modifications may be novel targets for therapy by using drugs or small-molecule inhibitors that, when combined with radiotherapy or chemotherapy (alkylating agents), may improve treatment.
Acetylated NEIL1 and APE1 are exclusively chromatin-bound proteins: Spatio-temporal regulation of acetylation of BER proteins
We discovered that while unmodified NEIL1 and APE1 are present both in cytosol and nucleus, acetylated NEIL1 and APE1 are exclusively bound to chromatin [13, 16]. Using biochemical analysis and high-resolution microscopy, we provided evidence that acetylation most likely occurs once these proteins bind to DNA damage sites in chromatin, suggesting that spatiotemporal acetylation plays a regulatory role in modulating their damage repair efficiency (Fig. 1). We have recently shown that APE1 interacted with a nucleosome remodeling histone chaperone FACT complex, and FACT facilitated the binding and acetylation of APE1 at AP sites or SSBs in cells [10]. On the other hand, we showed that CHAF1A, the largest subunit of chromatin assembly factor 1 (CAF-1), and its partner ASF1A affected NEIL1-initiated single-nucleotide BER, where these chaperones temporally dissociated from BER complexes after oxidative stress [9]. Recently, we have shown that the acetylation-negative NEIL1 mutant was not inhibited by CHAF1A, probably due to the absence of direct interaction, unlike the WT enzyme [13]. However, because this acetylation-negative mutant, compared to WT NEIL1, formed less stable complexes with BER partners, complete BER carried out by NEIL1 IP was more proficient with the WT than that with the mutant [13]. Therefore, acetylation regulates the formation of chromatin-bound multiprotein repair complexes with other proteins, thus affecting BER.
Thus, although acetylation of these proteins is dispensable for their activities in vitro, it fine-tunes its consequences in the cellular environment by affecting protein-protein interaction, stability of chromatin-bound repair complexes, overall repair efficiency, and finally resistance of cells to oxidative stress and diverse DNA-damaging agents.
Interplay between covalent modification of histones and BER proteins and transcription regulation
Epigenetic modifications, usually reversible, play a profound role in spatio-temporal regulation of gene expression. Chromatin unfolding in euchromatin regions is required for accessibility of the transcription machinery to the promoters followed by transcription initiation. Transcription initiation is regulated by reversible, covalent modifications in histones and promoter/enhancer sequences. While 5-methylation of Cs in CpG sequences in the promoter represses promoter function, typical activation of genes is mediated by reversible, covalent modification of histones via phosphorylation, acetylation, and methylation at unique acceptor sites [21]. Acetylation is limited to specific K residues, predominantly localized in the tails of histones H3 and H4 [22]. Often the same K residues are targeted for both acetylation and methylation. However, unlike acetylation, methylation may cause more complex change in the ionic environment. The existence of multiple enzymes for both modification and demodification suggests additional regulatory complexities, which are yet to be unraveled.
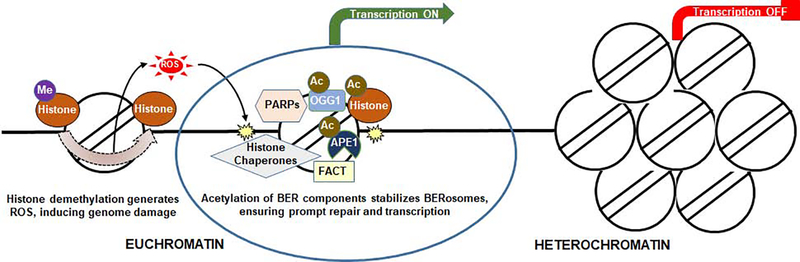
Recent findings suggest complex, unanticipated interplays between transcription and DNA repair. Accumulating evidence points to a role of eliciting DNA damage at gene promoters, which can influence transcriptional activation. Active demethylation of histones plays a key role in transcriptional control and resetting of epigenetic memory during embryonic development and cellular reprogramming [23]. K-specific demethylase 1 (LSD1) demethylates histone H3 at K9 via oxidative reaction, which releases hydrogen peroxide, which in turn oxidizes proximal Gs to 8-oxo-Gs and activates recruitment of BER enzymes [24]. Recently, using unbiased genome-wide mapping of AP sites and binding of APE1 and OGG1, we have documented that endogenous oxidative base damages are not randomly located [25]. Indeed, a distinct distribution of oxidative damage and enrichment of acetylated OGG1 or acetylated APE1 predominately in the promoter/enhancer regions of the transcribed genes suggest a connection of DNA base damage or assembly of repair complexes with regulation of gene expression (Fig. 3). Perillo et al. demonstrated earlier that LSD1-mediated oxidation and OGG1 recruitment on the BCL-2 promoter was essential for BCL-2 gene activation [24]. Pan et al. also showed that oxidation of G in NF-kB binding sites promoted NF-kB binding and stimulated transcription [26]. Furthermore, recently, we have shown that endogenous oxidized DNA bases and APE1 regulated the formation of G-quadruplex (G4) structures in the genome to regulate G4-mediated oncogene expression [25]. Therefore, involvement of DNA repair factors in transcriptional control might have originated as an adaptive measure by cells to preserve genetic information, which later have evolved to take on additional roles in transcriptional regulation. After all, both processes could generate damage-induced assembly of DNA repair complexes, which are highly similar, if not identical to each other.
Fig. 3.
BERosomes coordinate transcription and damage Prepair.A schematic of coordinated histone demethylation and BER in transcriptionally active chromatin domains. ROS, generated as byproducts of demethylation of histones in promoter/enhancer regions, induce base oxidation, and subsequent acetylation-dependent stabilization of BERosomes, containing chromatin factors, such as acetylated histones, histone chaperones, FACT, PARPs, ensures prompt repair and transcriptional activation.
Future perspective
Since the discovery of epigenetic regulation of cell growth, differentiation, and development via transcriptional programming, which is dependent on covalent modifications of histones and DNA in chromatin, it is now evident that in various cellular functions, particularly, in DNA transactions, reversible covalent modifications of diverse participating proteins occur extensively in a well-controlled manner. At the same time, such modifications have moderate impact on the reaction processes indicating that covalent modifications are intended for the fine-tuning of the cellular processes. In any event, our understanding of the role of acetylation in cellular functions is still in its infancy. Few obvious questions warranting answers are as follows. (a) What are the structural bases for the specificity of acetylation sites in repair enzymes? (b) Do various HATS and HDACs have specialized roles in regulating BER/SSBR? (c) Are acetylation of repair proteins and histones coordinately regulated? (d) Although there is only preliminary evidence for additional covalent modification (methylation) of BER enzymes, are these modifications coregulated? (e) How an indiscriminate oxidant like hydrogen peroxide generated during LSD1-mediated histone demethylation induces specific G oxidation in promoter/enhancer sequences to regulate gene expression? (f) Do DNA repair factors, in complexes, are often required for transcription of developmentally regulated and activator-dependent genes, but less so for constitutive housekeeping transcription? Addressing these questions would help illuminate the complex parameters affecting genome functions and stability.
Acknowledgement
This work was supported by the NIH grants: R03 CA235214 (KKB), R01 GM105090 (SM), P01 CA092584 (SM), and the training fellowship (SS) from the Cancer Prevention & Research Institute of Texas (CPRIT No. RP101489).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].De Bont R, van Larebeke N, Endogenous DNA damage in humans: a review of quantitative data, Mutagenesis, 19 (2004) 169–185. [DOI] [PubMed] [Google Scholar]
- [2].Çağlayan M, Wilson SH, Reprint of “Oxidant and environmental toxicant-induced effects compromise DNA ligation during base excision DNA repair”, DNA repair, 36 (2015) 86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hegde ML, Hazra TK, Mitra S, Early steps in the DNA base excision/single-strand interruption repair pathway in mammalian cells, Cell Res, 18 (2008) 27–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Beard WA, Horton JK, Prasad R, Wilson SH, Eukaryotic Base Excision Repair: New Approaches Shine Light on Mechanism, Annual review of biochemistry, 88 (2019) 137–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wiederhold L, Leppard JB, Kedar P, Karimi-Busheri F, Rasouli-Nia A, Weinfeld M, Tomkinson AE, Izumi T, Prasad R, Wilson SH, Mitra S, Hazra TK, AP endonuclease-independent DNA base excision repair in human cells, Mol Cell, 15 (2004) 209–220. [DOI] [PubMed] [Google Scholar]
- [6].Hegde PM, Dutta A, Sengupta S, Mitra J, Adhikari S, Tomkinson AE, Li GM, Boldogh I, Hazra TK, Mitra S, Hegde ML, The C-terminal Domain (CTD) of Human DNA Glycosylase NEIL1 Is Required for Forming BERosome Repair Complex with DNA Replication Proteins at the Replicating Genome: DOMINANT NEGATIVE FUNCTION OF THE CTD, J Biol Chem, 290 (2015) 20919–20933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Dutta A, Yang C, Sengupta S, Mitra S, Hegde ML, New paradigms in the repair of oxidative damage in human genome: mechanisms ensuring repair of mutagenic base lesions during replication and involvement of accessory proteins, Cell Mol Life Sci, 72 (2015) 1679–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hegde ML, Banerjee S, Hegde PM, Bellot LJ, Hazra TK, Boldogh I, Mitra S, Enhancement of NEIL1 protein-initiated oxidized DNA base excision repair by heterogeneous nuclear ribonucleoprotein U (hnRNP-U) via direct interaction, J Biol Chem, 287 (2012) 34202–34211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yang C, Sengupta S, Hegde PM, Mitra J, Jiang S, Holey B, Sarker AH, Tsai MS, Hegde ML, Mitra S, Regulation of oxidized base damage repair by chromatin assembly factor 1 subunit A, Nucleic Acids Res, 45 (2017) 739–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Song H, Zeng J, Roychoudhury S, Biswas P, Mohapatra B, Ray S, Dowlatshahi K, Wang J, Band V, Talmon G, Bhakat KK, Targeting Histone Chaperone FACT Complex Overcomes 5-Fluorouracil Resistance in Colon Cancer, Molecular cancer therapeutics, 19 (2020) 258–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bhakat KK, Hazra TK, Mitra S, Acetylation of the human DNA glycosylase NEIL2 and inhibition of its activity, Nucleic Acids Res, 32 (2004) 3033–3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bhakat KK, Mokkapati SK, Boldogh I, Hazra TK, Mitra S, Acetylation of human 8-oxoguanine-DNA glycosylase by p300 and its role in 8-oxoguanine repair in vivo, Mol Cell Biol, 26 (2006) 1654–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sengupta S, Yang C, Hegde ML, Hegde PM, Mitra J, Pandey A, Dutta A, Datarwala AT, Bhakat KK, Mitra S, Acetylation of oxidized base repair-initiating NEIL1 DNA glycosylase required for chromatin-bound repair complex formation in the human genome increases cellular resistance to oxidative stress, DNA Repair (Amst), 66–67 (2018) 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bhakat KK, Izumi T, Yang SH, Hazra TK, Mitra S, Role of acetylated human AP-endonuclease (APE1/Ref-1) in regulation of the parathyroid hormone gene, Embo J, 22 (2003) 6299–6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lirussi L, Antoniali G, Vascotto C, D'Ambrosio C, Poletto M, Romanello M, Marasco D, Leone M, Quadrifoglio F, Bhakat KK, Scaloni A, Tell G, Nucleolar accumulation of APE1 depends on charged lysine residues that undergo acetylation upon genotoxic stress and modulate its BER activity in cells, Mol Biol Cell, 23 (2012) 4079–4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Roychoudhury S, Nath S, Song H, Hegde ML, Bellot LJ, Mantha AK, Sengupta S, Ray S, Natarajan A, Bhakat KK, Human Apurinic/Apyrimidinic Endonuclease (APE1) Is Acetylated at DNA Damage Sites in Chromatin, and Acetylation Modulates Its DNA Repair Activity, Mol Cell Biol, 37 (2017) 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sengupta S, Mantha AK, Song H, Roychoudhury S, Nath S, Ray S, Bhakat KK, Elevated level of acetylation of APE1 in tumor cells modulates DNA damage repair, Oncotarget, 7 (2016) 75197–75209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Yamamori T, DeRicco J, Naqvi A, Hoffman TA, Mattagajasingh I, Kasuno K, Jung SB, Kim CS, Irani K, SIRT1 deacetylates APE1 and regulates cellular base excision repair, Nucleic Acids Res, 38 (2010) 832–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bhakat KK, Mantha AK, Mitra S, Transcriptional regulatory functions of mammalian AP-endonuclease (APE1/Ref-1), an essential multifunctional protein, Antioxidants & redox signaling, 11 (2009) 621–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sengupta S, Mantha AK, Mitra S, Bhakat KK, Human AP endonuclease (APE1/Ref-1) and its acetylation regulate YB-1-p300 recruitment and RNA polymerase II loading in the drug-induced activation of multidrug resistance gene MDR1, Oncogene, 30 (2011) 482–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Marmorstein R, Trievel RC, Histone modifying enzymes: structures, mechanisms, and specificities, Biochim Biophys Acta, 1789 (2009) 58–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Marmorstein R, Zhou MM, Writers and readers of histone acetylation: structure, mechanism, and inhibition, Cold Spring Harbor perspectives in biology, 6 (2014) a018762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hosseini A, Minucci S, A comprehensive review of lysine-specific demethylase 1 and its roles in cancer, Epigenomics, 9 (2017) 1123–1142. [DOI] [PubMed] [Google Scholar]
- [24].Perillo B, Ombra MN, Bertoni A, Cuozzo C, Sacchetti S, Sasso A, Chiariotti L, Malorni A, Abbondanza C, Avvedimento EV, DNA oxidation as triggered by H3K9me2 demethylation drives estrogen-induced gene expression, Science, 319 (2008) 202–206. [DOI] [PubMed] [Google Scholar]
- [25].Roychoudhury S, Pramanik S, Harris HL, Tarpley M, Sarkar A, Spagnol G, Sorgen PL, Chowdhury D, Band V, Klinkebiel D, Bhakat KK, Endogenous oxidized DNA bases and APE1 regulate the formation of G-quadruplex structures in the genome, Proc Natl Acad Sci U S A, 117 (2020) 11409–11420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Pan L, Zhu B, Hao W, Zeng X, Vlahopoulos SA, Hazra TK, Hegde ML, Radak Z, Bacsi A, Brasier AR, Ba X, Boldogh I, Oxidized Guanine Base Lesions Function in 8-Oxoguanine DNA Glycosylase-1-mediated Epigenetic Regulation of Nuclear Factor kappaB-driven Gene Expression, J Biol Chem, 291 (2016) 25553–25566. [DOI] [PMC free article] [PubMed] [Google Scholar]