ABSTRACT
Coronavirus disease 2019 (COVID-19), which causes severe acute respiratory syndrome and lung failure, is caused by the novel coronavirus, also known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Due to high transmission rates from individual to individual, it has progressed to a pandemic. However, indirect transmission from inanimate objects or surfaces that have come in contact with a patient poses an even more significant threat as it is difficult to trace the source of infection in these cases. Therefore, these surfaces and objects require disinfection with chemicals having potent viricidal activity. These include alcohols, aldehydes, quaternary ammonium compounds, chlorhexidine, and chlorine-based disinfectants, among others. They vary in their viricidal activity depending on their structure, concentrations, and mechanism of action. Several studies have looked into these agents and the transmission of the virus related to it. Moreover, certain viricides, if used as constituents of commercially available oral disinfectants, can further aid in preventing ventilator-associated pneumonia and maintain oral hygiene. However, these chemicals are not entirely free of potential hazards. In this review, we have compiled and critically appraised some commonly used viricidal agents in healthcare settings and the role they can play in the prevention of SARS-CoV-2 transmission.
KEYWORDS: COVID-19, SARS-CoV-2, viricidal, disinfectant, chlorhexidine, ethanol
1. Introduction
Human Coronavirus (HCoV) is a significant public health problem worldwide, and a substantial number of reports on nosocomial outbreaks of CoVs infections have been communicated. Coronaviruses (CoVs) are single-stranded (ss) positive-sense RNA viruses, characteristic of rapid mutation and recombination. They cause respiratory or intestinal infections in humans and animals [1]. Currently, there are 39 species in five genera and two subfamilies belonging to the family Coronaviridae, according to the Coronaviridae Study Group (CSG) [2,3]. The severe acute respiratory syndrome coronavirus (SARS-CoV) comes under the genus beta-coronavirus under the subfamily ortho-coronavirinae [2]. Comparative genomic analysis has revealed that the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), responsible for the coronavirus disease 2019 (COVID-19) pandemic, clusters in trees with the species SARS-CoV and genus beta-coronavirus, thus being assigned to the same species as mentioned earlier [4,5].
Indirect CoV transmission by contaminated objects can only be prevented through stringent infection-control measures, including the use of chemical viricides or biocides, which render the virus noninfectious. Currently, many viricidal agents are being explored for the infection control of SARS-CoV-2; however, there lacks a comprehensive review giving an update on the most potent viricidal agents that may be effective against this deadly virus. This review is a focused review of the currently available viricidal agents and their efficacy against SARS-CoV-2 infection.
1. Coronaviruses
CoVs are mainly classified by crown-like spikes on their surfaces and belong to the Coronavirinae subfamily, classified by phylogenetic clustering into four groups: the α, β, γ, and δ CoVs, of which α and β CoVs cause human infections [6]. CoVs contain four major types of structural proteins namely, the spike protein (S) (attaches to the host receptor and causes subsequent fusion of the virus and the cell membrane), nucleocapsid protein (N), membrane protein (M), and the envelope protein (E) [7]. The first HCoV was identified in the mid-1960s in human embryonic tracheal organ cultures, and until 2003, two HCoV species, HCoV-229E and HCoV-OC43 were recognized. Currently, seven different CoV strains are known to infect humans, including the important HCoV-229E, HCoV-NL63, HCoV-OC43, and HCoV-HKU1, which generally causes mild self-resolving infection. The other viruses from this group are SARS-CoV, Middle East Respiratory Syndrome coronavirus (MERS-CoV), and the novel SARS-CoV-2, which cause lethal respiratory infections in humans [8,9].
Transmissible gastroenteritis virus (TGEV), a member of the Coronaviridae family, causes severe gastroenteritis and leads to alterations of many cellular processes [10, 11].
According to current evidence, the SARS-CoV-2 virus is transmitted through respiratory droplets and contact routes. Droplet transmission occurs when a person is in close contact with a patient with respiratory symptoms and thus, is at risk of being exposed to infective respiratory droplets. It can also occur by indirect contact with surfaces infected by the patient [12]. Airborne transmission may also be possible under certain circumstances.
3. Persistence of coronavirus in different environmental conditions
HCoV (strain 229E) can remain infectious on different types of materials from two (2) hours up to nine (9) days [13,14]. High temperatures (30–40°C) have shown to decrease the time of persistence of different pathogenic viruses namely, MHV, MERS-CoV, and TGEV. However, at low temperatures, the stability of TGEV and MHV may be increased for longer durations (28 days) (Figure 1). SARS-CoV-2 may remain infectious on surfaces or objects for up to 72 hours [15], Five different experimental conditions (aerosols, plastic, stainless steel, copper, and cardboard) involving two viruses (SARS-CoV-2 and SARS-CoV) revealed that SARS-CoV-2 remained viable in aerosols for 3 hours, the infectious titer being reduced from 103.5 to 102.7 Median Tissue Culture Infectious Dose (TCID50) per milliliter of air, similar to that observed with SARS-CoV, which reduced from 104.3 to 103.5 TCID50 per milliliter. The viability of the virus was higher for plastic and stainless steel than on copper and cardboard. Viable SARS-CoV-2 was not detected after 4 hours on copper and after 24 hours on cardboard, whereas for SARS-CoV, the inactivity was detected after 8 hours on both copper and cardboard [16].
Figure 1.
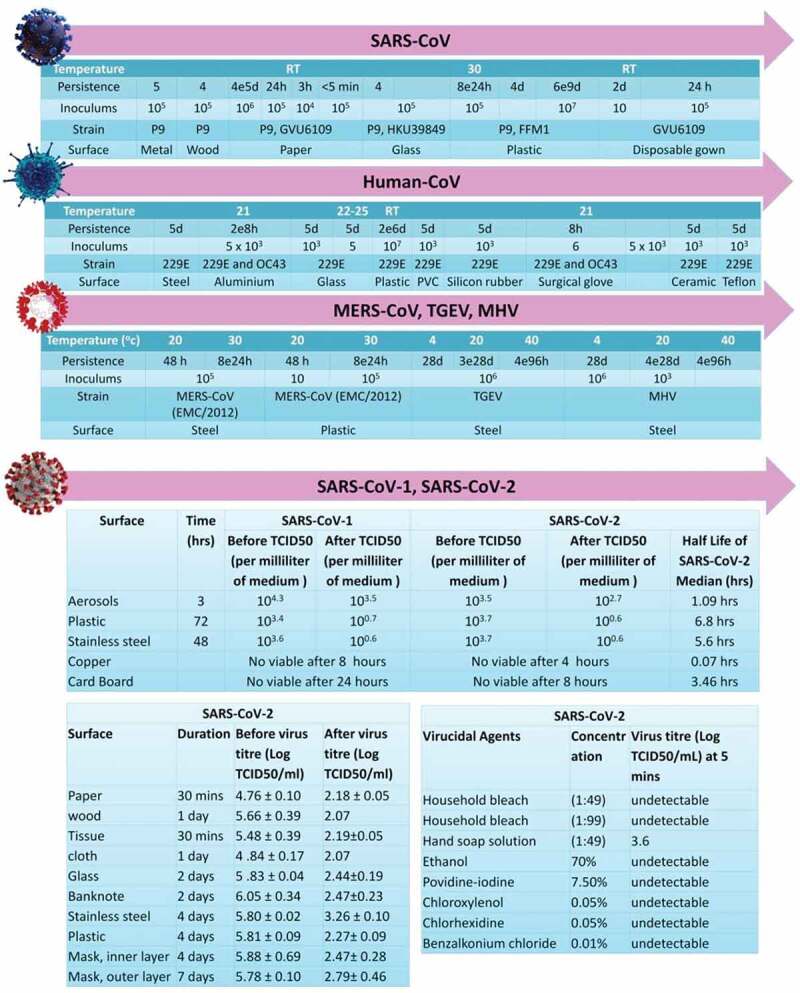
Persistence of different species of coronaviruses on various types of inanimate surfaces and viricidal agents activity against SARS-CoV-2.
Positive toilet bowl and sink samples were suggestive of viral shedding in the stool [17] and could be a potential route of transmission for SARS-CoV. Post-cleaning of the bowl and sink revealed samples were negative, suggesting that current decontamination measures were sufficient. Air samples from the patients’ room were negative, despite the extent and persistence of other environmental contamination. However, swabs taken from the air exhaust outlets tested positive, suggesting that small virus-laden droplets may be displaced by airflows and deposited on equipment such as vents. Another study [18] has shown that air-conditioned ventilation prompted droplet transmission due to recirculation of the contaminated air. The Personal Protective Equipment (PPE) samples unsurprisingly tested positive. Further, shoe covers were not part of PPE recommendations, and negative results in an anteroom and clean corridor hinted at low risk of transmission from contaminated footwear [12].
Chin et al. reported the stability of SARS-CoV-2 in different environmental conditions, including different temperatures. It is highly stable at 4°C and sensitive to heat. On increasing the incubation temperature to 70°C, the time for virus inactivation was reduced to 5 minutes. The stability of the virus on different surfaces was determined by using a 5-µL droplet of virus culture pipetted on a surface at room temperature. In contrast, no infectious virus could be recovered from tissue papers after a 3-hour incubation, and it was detected from treated wood and cloth until day 2, suggesting the enhanced viability of infectious virus for 24 hours on treated wood and cloth. SARS-CoV-2, is more stable on smooth surfaces and it was not detected from treated smooth surfaces on Day 4 (glass and banknote) or Day 7 (stainless steel and plastic). Strikingly, a significant level of the infectious virus could still be detected on the outer surface of a surgical mask, even on Day 7.
These observations show that SARS CoV-2 is a highly infectious virus that can persist in the environment and various unanimated surfaces for long durations. Nevertheless, using common household disinfectants like 0.05% triclosan, 0.12% chloroxylenol, or 79.0% ethanol provided a 3log10 reduction in viral titers with a 30-second contact time for SARS-CoV surrogate [19,20].
5. Disinfection of coronaviruses by viricidal agents
The viricidal effects of various disinfectants at different concentrations were evident, as no infectious virus could be detected after a 5-minute incubation at room temperature in SARS-CoV-2 culture [21] (Table 1 and Table 2, Figure 2). There are multiple favored disinfectants including alcohols, glutaraldehyde and ortho-phthaldehyde, chlorhexidine, hydrogen peroxide, iodine and iodophor, sodium hypochlorite and quarternary ammonium compounds.
Table 1.
Disinfection of coronaviruses by different types of viricidal agents, including their chemical structure and mechanism of action.
| Viricidal agents | Chemical Structure | Different Species of Corona Virus | Mechanism of Action | Reference |
|---|---|---|---|---|
| Ethanol | 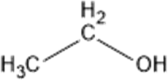 |
MERS-CoV TGEV MHV SARS-CoV-2 |
Membrane damage leads to coagulation and denaturation of protein. | [23,24,26,27] |
| 2-Propanol | 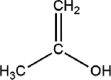 |
SARS-CoV MARS-CoV MHV CCV HCoV SARS-CoV-2 |
Membrane damage leads to coagulation and denaturation of protein. | |
| 1-propanol | 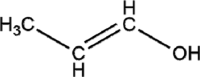 |
SARS-CoV | Membrane damage leads to coagulation and denaturation of protein. | |
| Benzalkonium chloride | 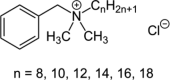 |
MHV CCV HCoV |
Damage outer membrane and envelope and leading to damage of phospholipids bi layers cause the membrane or envelop destruction leakage of genetic content (DNA or RNA) | [36] |
| Didecyldimethyl ammonium chloride | CCV SARS-CoV-2 |
Membrane damage, precipitation of protein | [A. 64, 62] [65] |
|
| Chlorhexidine digluconate |
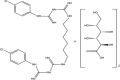 |
MHV CCV |
Membrane damage, precipitation of protein and genetic content (DNA or RNA) | [38] |
| Sodium hypochlorite | 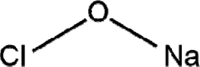 |
MHV CCV |
[19,24,36] | |
| Hydrogen peroxide | 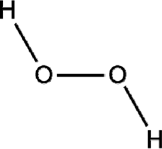 |
CCV MARS-CoV MHV |
Causes genetic content (DNA or RNA) breakage | [42,43] |
| Formaldehyde | 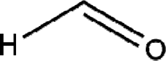 |
SARS-CoV CCV HCoV |
Damage the outer cell membrane or envelop. | [34,36] |
| Glutardialdehyde | SARS-CoV | Damage the outer cell membrane or envelop | [30,32,33] | |
| Povidone iodine | 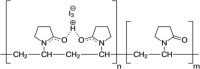 |
SARS-CoV MARS-CoV SARS-CoV-2 |
Oxidized the cell membrane and degrade the Genetic Material (RNA or DNA) | [50,52,25,54] |
CCV: Canine Coronavirus; HCoV: Human Coronavirus; MERS-CoV: Middle East Respiratory Syndrome coronavirus; MHV: Mouse Hepatitis Virus; SARS-CoV-2: Severe Acute Respiratory Syndrome Coronavirus 2
Table 2.
Disinfection of coronaviruses by various types of viricidal agents in suspension tests.
| Virus | Strain/isolate | Exposure time | Virucidal agent | Concentration | Reduction of viral infectivity (log10) | Reference |
|---|---|---|---|---|---|---|
| SARS-CoV-2 | München1.1/2020/929 | 30 s | Ethanol | ≥30% | ≥5.9 | [27, 54,66] |
| 2-Propanol | ≥30% | 4.8 and ≥5.9 | ||||
| USA-WA1/2020 | 1 min | ethanol | 61% | ≥4.2 | ||
| 1 min | Salicylic Acid | 0.10% | ≥3.1 | |||
| 30 s | Iodine | 90% | 3.0 ± 4 | |||
| 2 min | 50% | 1.1 ± 0.3 | ||||
| 5 min | Parachloro-metaxylenol | 0.094% | ≥4.7 | |||
| 1 min | 0.018% | ≥3.0 | ||||
| 5 min | Quaternary ammonium compound | 0.19% | ≥4.1 | |||
| 2 min | ≥3.5 | |||||
| SARS-CoV/SARS-CoV-1 | Isolate FFM-1 | 30 s | Ethanol | 95% | 5.5 | [34,26,25,53,67] |
| 85% | 5.5 | |||||
| 80% | 4.3 | |||||
| 78% | 5 | |||||
| 2-Propanol | 100% | 3.3 | ||||
| 75% | 4 | |||||
| 70% | 3.3 | |||||
| 1-& 2-Propanol | 45% and 30% | 4.3 | ||||
| 2.8 | ||||||
| 2 min | Formaldehyde | 1% | > 3.0 | |||
| 0.70% | > 3.0 | |||||
| Hanoi strain | 5 min | Glutaraldehyde | 2.50% | > 4.0 | ||
| Isolate FFM-1 | 2 min | 0.50% | > 4.0 | |||
| Hanoi strain | 1 min | 1% | > 4.0 | |||
| 0.47% | 3.8 | |||||
| 0.25% | > 4.0 | |||||
| 0.23% | > 4.0 | |||||
| Isolate FFM-1 | 15 s | 0.23% | 4.4 | |||
| MERS-CoV | Strain EMC | 30 s | Ethanol | 80% | > 4.0 | [67,52,53] |
| 2-Propanol | 75% | 4 | ||||
| Isolate HCoV-EMC/2012 | 15 s | Povidone iodine | 7.50% | 4.6 | ||
| 4% | 5 | |||||
| 1% | 4.3 | |||||
| 0.23% | 4.4 | |||||
| CCV | Strain I-71 | 10 min | Ethanol | 70% | > 3.3 | [36] [64] [38] |
| 2-Propanol | 50% | > 3.7 | ||||
| Benzalkonium chloride | 0.05% | > 3.7 | ||||
| Strain S378 | 3 d | 0.00% | 3 | |||
| Didecyldimethyl ammonium chloride | 0.00% | > 4.0 | ||||
| Strain I-71 | 10 min | Chlorhexidine digluconate | 0.02% | 0.3 | ||
| Sodium hypochlorite | 0.01% | 1.1 | ||||
| 0.00% | 0.9 | |||||
| Formaldehyde | 0.70% | > 3.7 | ||||
| 24 h | 0.01% | > 4.0 | ||||
| MHV | Strains MHV-2 and MHV-N | 10 min | Ethanol | 70% | > 3.9 | [36,19] |
| 2-Propanol | 50% | > 3.7 | ||||
| Benzalkonium chloride | 0.05% | > 3.7 | ||||
| Chlorhexidine digluconate | 0.02% | 0.7e0.8 | ||||
| Strain MHV-1 | 30 s | Sodium hypochlorite | 0.21% | 4 | ||
| Strains MHV-2 and MHV-N | 10 min | 0.01% | 2.3e2.8 | |||
| 0.00% | 0.3e0.6 | |||||
| 10 min | Formaldehyde | 0.70% | > 3.5 |
CCV: Canine Coronavirus; HCoV: Human Coronavirus; MERS-CoV: Middle East Respiratory Syndrome coronavirus; MHV: Mouse Hepatitis Virus; SARS-CoV-2: Severe Acute Respiratory Syndrome Coronavirus 2
Figure 2.
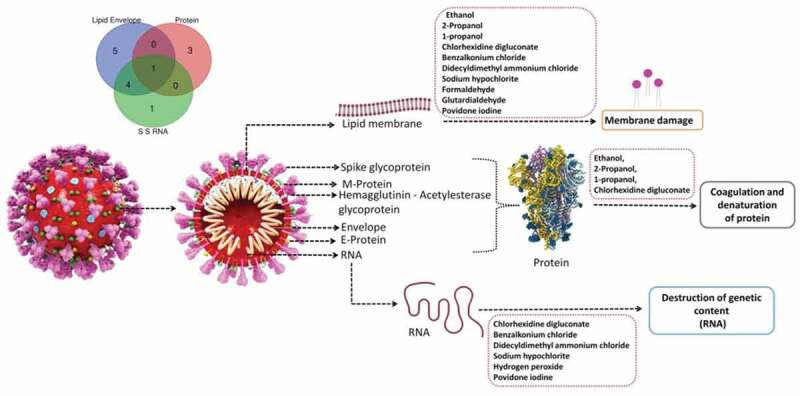
Mechanism of disinfection of coronaviruses by various types of viricidal agents.
4.1. Alcohols
Alcohols have been widely used for disinfection of both skin and inanimate surfaces [22]. The primary mode of action of alcohol-based disinfectants is coagulation or denaturation of proteins and solubility of the lipids in alcohols, which is achieved by increased membrane permeability and subsequently, membrane-disruption, as shown by Kamm and Rutala in two separate studies [23,24]. At concentrations of 75% to 95%, methanol inactivated enveloped viruses like SARS-CoV (Isolate FFM-1) and MERS-CoV (Strain EMC) (24, 25), while at 70% concentrations, it inactivated MHV (Strains MHV-2 and MHV-N) in 30 seconds. The propanols were more active for SARS-CoV (Isolate FFM-1), MERS-CoV (Strain EMC) and at concentrations of 70% to 100%, they inactivated the viruses in 30 seconds. Hence, propanols required lower concentrations to have the same effect with similar exposure times (14 seconds and 28 seconds, respectively). Similarly, Kariwa et al. and Rabenau et al. showed that the combination of 2-propanol (45%) and 1-propanol (30%) was found to be effective for SARS-CoV (isolate FFM-1) [25, 26] (Table 3). Kratzel et al. showed that both ethanol and propanol were efficient in virus inactivation at >30% (v/v) concentration in 30 seconds. It is worth mentioning that this is the recommended time for practical purposes but not usually followed. Hence, following these WHO-recommended formulations can be crucial in minimizing the transmission and maximizing the viral inactivation of SARS-CoV-2 [27]. Varied types and concentrations of alcohols thus enable effective disinfection. However, alcohols being flammable substances should be used with caution. It is also worth noting that anionic additives in hand disinfectants containing alcohol may negate the efficacy of chlorhexidine gluconate persistence [28].
Table 3.
Action of active viricidal agents on different species of coronaviruses.
| Viricidal agent | SARS CoV-2 | SARS CoV-1 | MERS CoV | MHV | CCV | HCoV | Reference |
|---|---|---|---|---|---|---|---|
| Ethanol | + | + | + | + | + | + | [34, 26, 36, 67] [27] |
| 2-Propanol | + | + | + | + | + | - | [27,34,36,67] |
| 1-Propanol | - | + | - | - | - | - | [34] |
| Benzalkonium chloride | - | - | - | + | + | + | [60, 36, 68] |
| Didecyldimethyl ammonium chloride | + | - | - | - | + | - | [60][59,66] |
| Chlorhexidine digluconate | - | - | + | + | + | - | [36] |
| Sodium hypochlorite | + | - | + | + | + | - | [19,21,36] |
| Hydrogen peroxide | + | - | + | - | - | - | [46,69] |
| Formaldehyde | - | + | - | + | + | - | [35, 34, 36] |
| Glutaraldehyde | - | + | - | - | - | - | [25,34] |
| Povidone iodine | + | + | + | - | - | - | [28,54,55,56] |
CCV: Canine Coronavirus; HCoV: Human Coronavirus; MERS-CoV: Middle East Respiratory Syndrome coronavirus; MHV: Mouse Hepatitis Virus; SARS-CoV-2: Severe Acute Respiratory Syndrome Coronavirus 2; SARS-CoV-1: Severe Acute Respiratory Syndrome Coronavirus-1. ‘+’ denotes that the viricidal agent is effective against the respective virus. ‘-‘ studies have not been carried out on the efficacy of these agents yet
4.2. Glutaraldehyde and ortho-phthalaldehyde
The biocidal activity of aldehydes is based on the reactivity of the aldehyde group [29] and its ability to undergo alkylation reactions. Formaldehyde and glutaraldehyde have been in use for sterilization and disinfection of medical devices for a long time. Formaldehyde is an excellent biocide, but high toxicity limits its use. Glutaraldehyde is widely used for routine disinfection of medical devices, like flexible fiber optic endoscopes and heat-sensitive medical devices. Ortho-Phthalaldehyde (OPA) is a high-level disinfectant.
Glutaraldehyde is a monomeric-free aldehyde molecule. Reactivity of glutaraldehyde with protein, being a condensation reaction, increases as the pH rises from 4 to 9 [30]. It interacts with sulfhydryl moieties of membrane proteins and compromises cellular function by disrupting DNA and RNA [31]. A 1997 study on poliovirus type 1 and echovirus type 25 showed that glutaraldehyde disrupts the protein capsid by reacting with the lysine residues, disrupting the viral integrity [32]. When the virus was exposed to glutaraldehyde, enzymatic function via DNA polymerase was also affected [33]. Further, glutaraldehyde has proven viricidal activity against SARS-CoV (Hanoi strain) at 2.5% concentration, and SARS-CoV (Isolate FFM-1) at 0.5% concentration, as it inactivated the respective viruses in 5 and 2 minutes [25,34]. Other studies have demonstrated the viricidal activity of formaldehyde against SARS-CoV (Hanoi strain), CCV (I-71), and MHV [38, 37, 39]. The European Center for Disease Prevention and Control has reported in an interim document released in February 2020, that 2% glutaraldehyde is effective against HCoV-229E. Thus, the viricidal activity of glutaraldehyde and phthalaldehyde is proven in SARS-CoV strains, but its effect on SARS-CoV-2 is explicitly lacking and seems an attractive area to be explored further [37].
4.3. Chlorhexidine
Chlorhexidine is a substituted biguanide, which contains the C2H5N7 component, and has highly potent antimicrobial activity, low toxicity, and binds to the stratum corneum of the skin and mucous membranes [38]. It is one of the most common components of alcohol-based sanitizers, as the addition of chlorhexidine in low concentrations (0.5–1.0%) to alcohol-based preparations has higher residual activity than alcohol alone [39]. Antiviral effects of chlorhexidine are due to its interaction with the viral membrane lipids in enveloped viruses. Due to higher solubility of the lipids in chlorhexidine, it is more active against enveloped viruses with a lipid membrane, such as HIV [40,41]. Also, its antimicrobial activity is minimally affected by the presence of organic material, including blood. Coronaviruses, MHV (MHV-2 and MHV-N) and CCV (I-71), were inactivated within 10 min by 0.02% chlorhexidine. Aqueous formulations (0.50–0.75%) were found to be more effective than plain soap.
As a common side-effect to chlorhexidine, concentration-dependent skin irritation can occur. Chlorhexidine in >1% concentration can cause conjunctivitis and corneal damage, so care must be taken to avoid such exposure. Further, it should not be used in middle and inner ear surgery as it is ototoxic.
4.4. Hydrogen peroxide (H2O2)
Hydrogen peroxide has been used for its antiseptic properties since the 1800s [42]. Initially, H2O2 was used as a disinfectant, and it is used at various concentrations to disinfect materials such as drinking water, medical equipment, and septic tank waste. Free hydroxyl radicals react with lipids, proteins, and nucleic acids, cleavage of the RNA and DNA backbone, oxidation, causing denaturation of proteins, disruption of biological membranes and sulfhydryl bonds in proteins and enzymes [43]. High concentrations of H2O2 can counter the decrease in efficacy when it gets degraded in water and oxygen with the presence of catalase. High temperatures are also needed as a crucial factor with increased H2O2 concentrations [44,45]. Recently, in a study conducted on 73 N95 masks from five different models, Cramer et al. have shown that ionized H2O2 technology Stera-Mist is effective in sterilizing N95 masks [46].
4.5. Iodine and iodophor
Iodine in the form of a tincture has been used since the early 1900s as preoperative skin preparation, and iodophors are classified as low- or intermediate-level disinfectants [24] because they are not sufficiently sporicidal in a short application time. The destructive effect of iodine and iodophors is related to the concentration of the free molecular iodine, which correlates with its antimicrobial activity [47,48]. N-iodo compounds act by destroying the protein structure by reacting with the -NH group of amino acids and irreversible oxidation of -SH (thiol) groups of cysteine, ultimately leading to loss of protein disulfide linkages [24,31].
Iodination of phenolic and imidazole groups of the amino-acids tyrosine and histidine and pyrimidine derivatives of cytosine and uracil lead to steric hindrances in hydrogen bonds and denaturation of DNA. Iodine binding to unsaturated fatty acids has been shown to alter the physical properties of lipids and hence lipid-containing membranes [49].
Iodine and iodophors have been effective against a wide range of viruses, including CoVs and enteroviruses, polio, herpes, vaccinia, rabies, and tobacco mosaic viruses [49–51]. Povidone-iodine, a complex of polyvinylpyrrolidone and iodine, is routinely used in surgical procedures, and numerous studies have validated its safety. It showed rapid and effective viricidal activity against different types of HCoVs like SARS-CoVs (Isolate FFM-1 & Hanoi strain), and MERS-CoV (HCoV-EMC/2012), at concentrations of 0.23% to 7.5% with 15- and 60-second exposures, respectively [25,52,53]. Liang et al. characterized the viricidal activity of long-acting povidone-iodine gel formulations in the inactivation of SARS-CoV-2 in VERO76 cells in a time- and dose-dependent manner. Further, no toxicity was observed [54]. Povidone-iodine has also been recommended by UK investigators as nasal spray and mouthwash in health workers to prevent infection of the airways [55].
Due to increased free iodine concentrations, lower concentration iodophor preparations have better antimicrobial activity but are more prone to cause skin irritation and contact dermatitis, therefore must be used with caution.
4.6. Sodium hypochlorite
Sodium hypochlorite in solution shows a broad-spectrum antimicrobial activity and is generally used in sterilization of healthcare facilities [24]. ‘Strong chlorine solution’ is a 0.5% solution of hypochlorite (containing approximately 5000 ppm free chlorine) used for disinfecting areas contaminated with body fluids, including large blood spills [24,56].
Sodium hypochlorite damages membrane envelopes by the oxidation of proteins and lipids and nucleic acid degradation [31]. Sakjnimit et al. showed its viricidal activity against MHV (MHV-2 and MHV-N) and CCV (I-71) at 0.001% to 0.01% concentrations, respectively, and the viruses were inactivated in 10 minutes [36], while MHV (MHV-1) was inactivated in 30 seconds at 0.21% concentration [19].
4.7. Quaternary ammonium compounds
Quaternary ammonium compounds (QACs) are widely used as disinfectants, and alkyl benzalkonium chlorides are one of the most familiar examples [39,57]. The antimicrobial activity of these compounds can be attributed to the adsorption to cytoplasmic membrane and leakage of cellular constituents. Chemically, the quaternaries are organically substituted ammonium compounds in which the nitrogen atom has a valence of five. Four of the substituted radicals (R1–R4) are alkyl or heterocyclic radicals of a given size or chain length, and the fifth (X−) is a halide, sulfate, or similar radical. The chemical names of QACs used in hospitals include alkyl dimethyl benzyl ammonium chloride, alkyl di-decyl dimethyl ammonium chloride, and di-alkyl dimethyl ammonium chloride. The mechanisms by which these chemicals act are denaturation of essential cell proteins, disruption of the lipid membrane, and damage to proteins and nucleic acid [58]. Known QAC having anti-coronaviral activity are ammonium chloride, cetylpyridinium chloride, and miramistin. Among these, cetylpyridinium chloride has demonstrated its antiviral activity against an array of coronaviruses and is cheap and widely accessible to be used in hospital settings [59]. Di-decyl dimethyl ammonium chloride has proven viricidal activity against CCV (Strain S378) at 0.0025% concentration and inactivates viruses in 3 days [60]. Hence, this compound may potentially be useful in disinfecting surgical masks and N95 masks.
However, having weak activity against gram-negative bacteria, these compounds are prone to contamination, and have been traced to outbreaks in the past. Therefore, they must be used judiciously.
5. SARS-CoV-2 and oral hygiene
High viral loads in the oropharynx of infected patients of COVID-19, as well as an increased risk of ventilator-associated pneumonia in critically ill patients, beg the consideration of proper oral hygiene. Ingredients such as chlorhexidine are part of mouth rinses available on the market, and hence can be used to reduce oral transmission of SARS-CoV-2. It is indicated in gingivitis and post-surgery periodontal disease and has been reported to be able to penetrate oral biofilms [61,62]. Flavonoids have also shown anti-coronaviral activity due to the inhibitory effect of 3 C protease type [63]. Compounds such as herbacetin and 3-β-d-glucoside can inhibit the enzyme activity of MERS-CoV/3 CLpro [63]. Citrox, a combination of natural bioflavonoids, which has a broad spectrum of antimicrobial effects, is also useful. It is an oxidizing agent, making it potentially useful in reducing the salivary viral load, including that of SARS-CoV-2 [64]. Cyclodextrins, modified with mercapto-undecane sulfonic acids, are capable of destroying outer shells of the virus. Hence, these agents, alone or in combinations, can also be used to reduce oral viral load [64], which can prove to be extremely beneficial in containing and reducing any viral spread through speech or coughing.
7. Conclusion
Coronaviruses are highly infectious, and novel coronavirus SARS CoV-2 has the menacing feature of longer persistence in the environment and various inanimate surfaces. Additionally, persistent lack of specific antiviral treatments makes it a challenging entity for the development of efficacious means of prevention. Under such alarming conditions, disinfection for personal hygiene as well as disinfection of various hospital areas, medical devices, and medical personnel protection is a primary modality of controlling the spread of this virus. Available antiseptic-disinfectants should be fundamentally and rigorously evaluated in this health crisis. However, few chemicals are efficient enough within a specific contact time and without toxicity. Formulations having the presence of multiple disinfectants may be considered. Among the major chemical formulations that can be useful, alcohol and chlorhexidine-based disinfectants and sodium hypochlorite and benzalkonium chloride solutions are primary choices. These are tried and tested in various hospital settings and medical personnel use have shown robustness in viricidal action. An effective disinfection strategy used by healthcare service providers and by individuals has the potential to go a long way in fighting this global pandemic.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Cheng VCC, Lau SKP, Woo PCY, et al. Severe acute respiratory syndrome coronavirus as an agent of emerging and reemerging infection. Clin Microbiol Rev. 2007;20(4):660–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Coronaviridae Study Group of the International Committee on Taxonomy of Viruses . The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5(4): 536–544. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lefkowitz EJ, Dempsey DM, Hendrickson RC, et al. Virus taxonomy: the database of the International committee on taxonomy of viruses (ICTV). Nucleic Acids Res. 2018;46(D1):D708–D717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Fauci AS, Lane HC, Redfield RR.. Covid-19—Navigating the Uncharted. N Engl J Med. 2020;382(13):1268–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Guo Y-R, Cao Q-D, Hong Z-S, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak – an update on the status. Mil Med Res. 2020;7(1). DOI: 10.1186/s40779-020-00240-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zhang S, Tuo J, Huang X, et al. Epidemiology characteristics of human coronaviruses in patients with respiratory infection symptoms and phylogenetic analysis of HCoV-OC43 during 2010-2015 in Guangzhou. Plos One. 2018;13(1):e0191789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Millet JK, Nal B. Investigation of the functional roles of host cell proteins involved in coronavirus infection using highly specific and scalable RNA Interference (RNAi) approach. Maier HJ, Bickerton E, Britton P, editors.Coronaviruses; 2015. p. 231–240. Springer Science+Business Media New York. 10.1007/978-1-4939-2438-7_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020;395(10223):507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Li W, Hulswit RJG, Kenney SP, et al. Broad receptor engagement of an emerging global coronavirus may potentiate its diverse cross-species transmissibility. Proc Nat Acad Sci. 2018;115(22):E5135–E5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chae C, Kim O, Choi C, et al. Prevalence of porcine epidemic diarrhoea virus and transmissible gastroenteritis virus infection in Korean pigs. Vet Rec. 2000;147(21):606–608. [DOI] [PubMed] [Google Scholar]
- [11].Song X, Zhao X, Huang Y, et al. Transmissible Gastroenteritis Virus (TGEV) Infection Alters the Expression of Cellular MicroRNA Species That Affect Transcription of TGEV Gene 7. Int J Biol Sci. 2015;11(8):913–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ong SWX, Tan YK, Chia PY, et al. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA. 2020;323(16):1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ijaz MK, Brunner AH, Sattar SA, et al. Survival characteristics of airborne human coronavirus 229E. J Gen Virol. 1985;66(12):2743–2748. [DOI] [PubMed] [Google Scholar]
- [14].Kampf G, Todt D, Pfaender S, et al. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect. 2020;104(3):246–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Desai AN, Aronoff DM. Food Safety and COVID-19. JAMA. 2020;323(19):1982. [DOI] [PubMed] [Google Scholar]
- [16].van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N Engl J Med. 2020;382(16):1564–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Young BE, Ong SWX, Kalimuddin S, et al., for the Singapore 2019 Novel Coronavirus Outbreak Research Team . 2020. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA. 323(15): 1488. 10.1001/jama.2020.3204 10.1001/jama.2020.3204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lu J, Gu J, Li K, et al. COVID-19 outbreak associated with air conditioning in restaurant, Guangzhou, China, 2020. Emerg Infect Dis. 2020. July;26(7): 1628–1631. 10.3201/eid2607.200764 10.3201/eid2607.200764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Dellanno C, Vega Q, Boesenberg D. The antiviral action of common household disinfectants and antiseptics against murine hepatitis virus, a potential surrogate for SARS coronavirus. Am J Infect Control. 2009;37(8):649–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Geller C, Varbanov M, Duval RE. Human coronaviruses: insights into environmental resistance and its influence on the development of new antiseptic strategies. Viruses. 2012;4(11):3044–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chin AW, Chu JT, Perera MR, et al. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe. 2020. DOI: 10.1016/S2666-5247(20)30003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ascenzi JM, Ed. Handbook of disinfectants and antiseptics. New York: M. Dekker; 1996. [Google Scholar]
- [23].Kamm O. The relation between structure and physiological action of the alcohols**read before the detroit branch of the american pharmaceutical association decemher 1920. J Am Pharm Assoc. 1921;10(2):87–89. [Google Scholar]
- [24].Rutala WA. Guideline for Disinfection and Sterilization in Healthcare Facilities. 2008;2008:163. https://www.cdc.gov/infectioncontrol/guidelines/disinfection/ [Google Scholar]
- [25].Kariwa H, Fujii N, Takashima I. Inactivation of SARS coronavirus by means of povidone-iodine, physical conditions and chemical reagents. Dermatology. 2006;212(1):119–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Rabenau HF, Kampf G, Cinatl J, et al. Efficacy of various disinfectants against SARS coronavirus. J Hosp Infect. 2005;61(2):107–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kratzel A, Todt D, V’kovski P, et al. Inactivation of severe acute respiratory syndrome coronavirus 2 by WHO-ols. Emerg Infect Dis. 2020;26(7):1592–1595. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kaiser N, Klein D, Karanja P, et al. Inactivation of chlorhexidine gluconate on skin by incompatible alcohol hand sanitizing gels. Am J Infect Control. 2009;37(7):569–573. [DOI] [PubMed] [Google Scholar]
- [29].C-Praktikum IO. Oxidations- und Reduktions-Reaktionen. n.d.. p. 38. Lehman Media LOB. de. [Google Scholar]
- [30].Payne JW. Polymerization of proteins with glutaraldehyde. Soluble molecular-weight markers. Biochem J. 1973;135(4):867–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Amsterdam D, Ed. Antibiotics in laboratory medicine (Sixth edition). Philadelphia: Wolters Kluwer; 2015. [Google Scholar]
- [32].Chambon M, Jallat-Archimbaud C, Bailly JL, et al. Comparative sensitivities of Sabin and Mahoney poliovirus type 1 prototype strains and two recent isolates to low concentrations of glutaraldehyde. Appl Environ Microbiol. 1997;63(8):3199–3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Howard CR, Dixon J, Young P, et al. Chemical inactivation of hepatitis B virus: the effect of disinfectants on virus-associated DNA polymerase activity, morphology and infectivity. J Virol Methods. 1983;7(3):135–148. [DOI] [PubMed] [Google Scholar]
- [34].Rabenau HF, Cinatl J, Morgenstern B, et al. Stability and inactivation of SARS coronavirus. Med Microbiol Immunol. 2005;194(1–2):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Pratelli A. Canine coronavirus inactivation with physical and chemical agents. Vet J. 2008;177(1):71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Saknimit M, Inatsuki I, Sugiyama Y, et al. Virucidal Efficacy of Physico-chemical Treatments Against Coronaviruses and Parvoviruses of Laboratory Animals. Exp Anim. 1988;37(3):341–345. [DOI] [PubMed] [Google Scholar]
- [37].European Centre for Disease Prevention and Control. Interim guidance for environmental cleaning in non-healthcare facilities exposed to SARS-CoV-2. Stockholm: ECDC; 2020. [Google Scholar]
- [38].Eslami ARD. Antimicrobial assay of chlorhexidine-wetted textile napkins for surgical site disinfection in ocular surgery. Int J Clin Med. 2013;04(12):577–581. [Google Scholar]
- [39].Boyce JM, Pittet D, Healthcare Infection Control Practices Advisory Committee, & HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force . Guideline for hand hygiene in health-care settings. recommendations of the healthcare infection control practices advisory committee and the HICPAC/SHEA/APIC/IDSA hand hygiene task force. Society for healthcare epidemiology of America/Association for Professionals in Infection Control/Infectious Diseases Society of America. MMWR. Recommendations and Reports: morbidity and Mortality Weekly Report. Recommendations Rep. 2002;51(RR–16):1–45. (quiz CE1-4). [PubMed] [Google Scholar]
- [40].Harbison MA, Hammer SM. Inactivation of human immunodeficiency virus by Betadine products and chlorhexidine. J Acquired Immune Deficiency Syndromes. 1989;2(1):16–20. [PubMed] [Google Scholar]
- [41].Montefiori D. Effective inactivation of human immunodeficiency virus with chlorhexidine antiseptics containing detergents and alcohol. J Hosp Infect. 1990;15(3):279–282. [DOI] [PubMed] [Google Scholar]
- [42].Linley E, Denyer SP, McDonnell G, et al. Use of hydrogen peroxide as a biocide: new consideration of its mechanisms of biocidal action. J Antimicrob Chemother. 2012;67(7):1589–1596. [DOI] [PubMed] [Google Scholar]
- [43].Russell AD. Similarities and differences in the responses of microorganisms to biocides. J Antimicrob Chemother. 2003;52(5):750–763. [DOI] [PubMed] [Google Scholar]
- [44].Ortega KL, Oliveira Rech B, De, Ferreira Costa AL, et al. Is 0.5% Hydrogen Peroxide Effective against SARS‐CoV‐2? Oral Dis Odi. 2020;13503. doi: 10.1111/odi.13503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Rutala WA, Gergen MF, Weber DJ. Impact of an Oil-Based Lubricant on the Effectiveness of the Sterilization Processes. Infect Control Hosp Epidemiol. 2008;29(1):69–72. [DOI] [PubMed] [Google Scholar]
- [46].Cramer A, Plana D, Yang HL, et al. Analysis of SteraMist ionized hydrogen peroxide technology in the sterilization of N95 respirators and other PPE: a quality improvement study (Occupational and Environmental Health). 2020. doi: 10.1101/2020.04.19.20069997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Berkelman RL, Holland BW, Anderson RL. Increased bactericidal activity of dilute preparations of povidone-iodine solutions. J Clin Microbiol. 1982;15(4):635–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Gottardi W, Puritscher M. [Germicidal experiments with aqueous PVP-iodine-containing disinfecting solutions: effect of the content of free iodine on the bactericidal action against Staphylococcus aureus]. Zentralblatt Fur Bakteriologie, Mikrobiologie Und Hygiene. Serie B, Umwelthygiene, Krankenhaushygiene, Arbeitshygiene, Praventive Medizin. 1986;182(4):372–380. [PubMed] [Google Scholar]
- [49].Block SS, Ed. Disinfection, sterilization, and preservation (5th ed). Philadelphia, PA: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- [50].Berg G, Chang SL, Harris EK. Devitalization of microorganisms by iodine. Virology. 1964;22(4):469–481. [DOI] [PubMed] [Google Scholar]
- [51].Gershenfeld L. Iodine as a Virucidal Agent. J Am Pharm Assoc (Scientific Ed). 1955;44(3):177–182. [DOI] [PubMed] [Google Scholar]
- [52].Eggers M, Eickmann M, Zorn J. Rapid and effective virucidal activity of povidone-iodine products against middle east respiratory syndrome coronavirus (MERS-CoV) and Modified vaccinia virus ankara (MVA). Infect Dis Ther. 2015;4(4):491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Eggers M, Koburger-Janssen T, Eickmann M, et al. In vitro bactericidal and virucidal efficacy of povidone-iodine gargle/mouthwash against respiratory and oral tract pathogens. Infect Dis Ther. 2018;7(2):249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Liang B, Yuan X, Wei G, et al. In-vivo toxicity studies and in-vitro inactivation of SARS-CoV-2 by povidone-iodine in-situ gel forming formulations (Microbiology). 2020. doi: 10.1101/2020.05.18.103184 [DOI] [Google Scholar]
- [55].Kirk-Bayley J, Challacombe S, Sunkaraneni V, et al. The use of povidone iodine nasal spray and mouthwash during the current COVID-19 pandemic may protect healthcare workers and reduce cross infection. SSRN J; 2020. 10.2139/ssrn.3563092 [DOI] [Google Scholar]
- [56].Sattar SA, Zargar B, Wright KE, et al. Airborne pathogens inside automobiles for domestic use: assessing in-car air decontamination devices using staphylococcus aureus as the challenge bacterium. Appl Environ Microbiol. 2017;83(10). DOI: 10.1128/AEM.00258-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Simmons BP. Anticipatics, handwashing, and handwashing facililties. Am J Infect Control. 1983;11(3):97–103. [DOI] [PubMed] [Google Scholar]
- [58].Tischer M, Pradel G, Ohlsen K, et al. Quaternary ammonium salts and their antimicrobial potential: targets or nonspecific interactions? ChemMedChem. 2012;7(1):22–31. [DOI] [PubMed] [Google Scholar]
- [59].Baker N, Williams AJ, Tropsha A, et al. Repurposing quaternary ammonium compounds as potential treatments for COVID-19. Pharm Res. 2020;37(6):104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Pratelli A. Action of disinfectants on canine coronavirus replication in vitro. Zoonoses Public Health. 2007;54(9–10):383–386. [DOI] [PubMed] [Google Scholar]
- [61].Gartenmann SJ, Dörig I, Sahrmann P, et al. Influence of different post-interventional maintenance concepts on periodontal outcomes: an evaluation of three systematic reviews. BMC Oral Health. 2017;17(1). DOI: 10.1186/s12903-016-0244-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Shapiro S, Giertsen E, Guggenheim B. An in vitro oral biofilm model for comparing the efficacy of antimicrobial mouthrinses. Caries Res. 2002;36(2):93–100. [DOI] [PubMed] [Google Scholar]
- [63].Jo S, Kim H, Kim S, et al. Characteristics of flavonoids as potent MERS‐CoV 3C‐like protease inhibitors. Chem Biol Drug Des. 2019;94(6):2023–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Carrouel F, Conte MP, Fisher J, et al. COVID-19: a recommendation to examine the effect of mouthrinses with β-cyclodextrin combined with citrox in preventing infection and progression. J Clin Med. 2020;9(4):1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Schrank CL, Minbiole KPC, Wuest WM. Are quaternary ammonium compounds, the workhorse disinfectants, effective against severe acute respiratory syndrome-coronavirus-2?. ACS Infect Dis Acsinfecdis 2020. 0c00265. 10.1021/acsinfecdis.0c00265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Ijaz MK, Whitehead K, Srinivasan V, et al. Microbicidal actives with virucidal efficacy against SARS-CoV-2. Am J Infect Control. 2020;48(8):972–973. S0196655320303138. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Siddharta A, Pfaender S, Vielle NJ, et al. Virucidal activity of world health organization–recommended formulations against enveloped viruses, including zika, ebola, and emerging coronaviruses. J Infect Dis. 2017;215(6):902–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Wood A, Payne D. The action of three antiseptics/disinfectants against enveloped and non-enveloped viruses. J Hosp Infect. 1998;38(4):283–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Caruso AA, Del Prete A, Lazzarino AI. Hydrogen peroxide and viral infections: A literature review with research hypothesis definition in relation to the current covid-19 pandemic. Med Hypotheses. 2020;144:109910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Casanova LM, Jeon S, Rutala WA, et al. Effects of Air Temperature and Relative Humidity on Coronavirus Survival on Surfaces. Appl Environ Microbiol. 2010;76(9):2712–2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Chan KH, Peiris JSM, Lam SY, et al. The effects of temperature and relative humidity on the viability of the SARS coronavirus. Adv Virol. 2011;2011:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Duan S-M, Zhao X-S, Wen R-F, et al. Stability of SARS coronavirus in human specimens and environment and its sensitivity to heating and UV irradiation. BES. 2003;16(3):246–255. [PubMed] [Google Scholar]
- [73].Lai MYY, Cheng PKC, Lim WWL. Survival of severe acute respiratory syndrome coronavirus. Clinl Infect Dis. 2005;41(7):e67–e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Sizun J, Yu MWN, Talbot PJ. Survival of human coronaviruses 229E and OC43 in suspension and after drying onsurfaces: A possible source ofhospital-acquired infections. J Hosp Infect. 2000;46(1):55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].van Doremalen N, Bushmaker T, Munster V. Stability of Middle East respiratory syndrome coronavirus (MERS-CoV) under different environmental conditions. Eurosurveillance. 2013;18(38):20590. [DOI] [PubMed] [Google Scholar]
- [76].Warnes SL, Little ZR, Keevil CW. Human Coronavirus 229E remains infectious on common touch surface materials. MBio. 2015;6(6):e01697–01615. [DOI] [PMC free article] [PubMed] [Google Scholar]


