Maternal death in the United States is a major public health issue that has captured the attention of the popular press, the US Congress, and the healthcare and scientific communities.1,2 A recent perspective in the New England Journal of Medicine discussed the various measures that can be implemented to reduce maternal mortality rates but did not emphasize the crucial role of research not only in understanding the breadth of the public health problem but also in identifying innovative evidence-based solutions.1 Scientists, practitioners, and policy makers have become aware of the lack of valid data, especially given that the previously identified factors that contribute to high levels of maternal complications in the United States (older maternal age, obesity, and comorbid conditions) do not completely explain recent data on severe morbidity and subsequent death (https://www.nichd.nih.gov/newsroom/news/012519-maternal-morbidity).
MacDorman et al3 brought attention to the US maternal mortality rates and the complexity of their accurate measurement. Researchers have demonstrated that the quality of data and reporting on maternal mortality rates differs substantially by state, with some states underreporting and other states overreporting the incidence. Several factors contribute to the variable quality of data on maternal mortality rates, including varied and inconsistently used terminology (eg, maternal death, late maternal deaths, pregnancy-associated death, and pregnancy-related death), delays in state adoption of the pregnancy check-box on death certificates, and reporting errors.3 Despite these data and measurement issues across numerous research projects, several findings are consistent: (1) the maternal mortality rate in the United States is currently the highest of the high-income countries; (2) clinical complications do not fully account for the maternal mortality rates; and (3) black women at all levels of income and education are more likely to experience a maternal death than are white women.1,2
Eunice Kennedy Shriver National Institute of Child Health and Human Development research on maternal morbidity/mortality
Improving the quality and availability of maternal mortality data is a national priority. The Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) is prioritizing research that improves maternal health. In 2017, the National Institutes of Health (NIH) created an official reporting category for maternal health that encompasses both maternal morbidity and death. These data and their trends over the past 2 fiscal years are publicly available via the NIH Reporter (https://report.nih.gov/categorical_spending.aspx). In fiscal year 2018, NIH-funded maternal health projects totaled $302.6 million dollars, of which 57% was funded by NICHD (Figure 1). Research by the NICHD-supported investigators addresses scientific gaps such as risk prediction, severe morbidity, optimal timing for delivery, maternal long-term outcomes, and data collection (https://doi.org/10.1016/j.ajog.2019.02.055).
FIGURE 1. National Institutes of Health Funding on Maternal Health by institute, center, and office for fiscal year 2018.
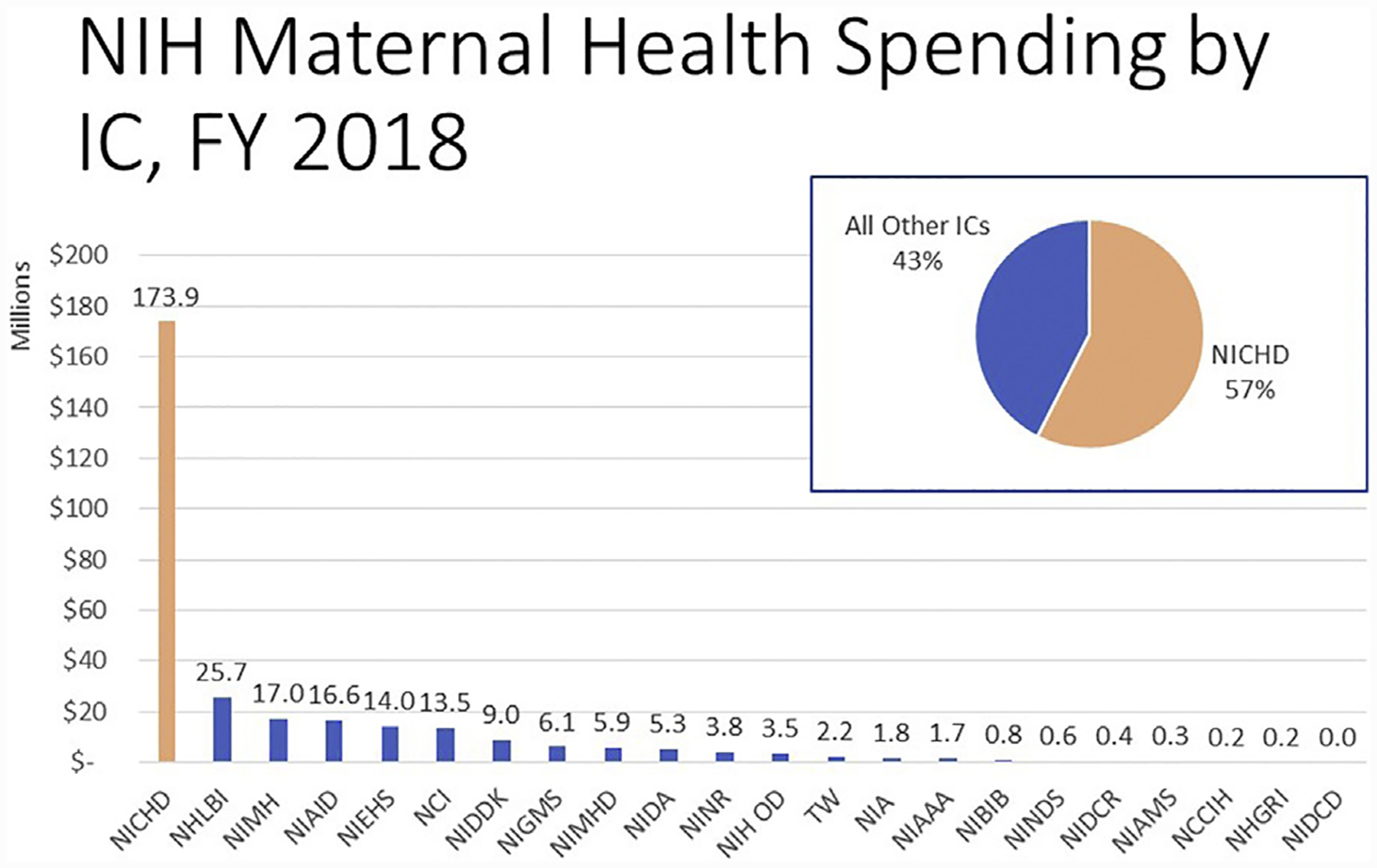
This figure represents the funded research in the category of Maternal Health by 22 institutes, centers, and offices at the National Institutes of Health for fiscal year 2018. Note: The Eunice Kennedy Shriver National Institute of Child Health and Human Development funds almost 7 times the amount of maternal health research as the next highest institute and center.
FY, fiscal year; IC, institute and center; NCCIH, National Center on Complementary and Integrative Medicine; NCI, National Cancer Institute; NHGRI, National Human Genome Research Center; NHLBI, National Heart, Lung, and Blood Institute; NIA, National Institute on Aging; NIAAA, National Institute on Alcohol Abuse and Alcoholism; NIAID, National Institute of Allergy and Infectious Diseases; NIAMS, National Institute of Arthritis and Musculoskeletal and Skin Diseases; NIBIB, National Institute of Biomedical Imaging and Bioengineering; NICHD, Eunice Kennedy Shriver National Institute of Child Health and Human Development; NIDA, National Institute on Drug Abuse; NIDCD, National Institute on Deafness and Other Communication Disorders; NIDCR, National Institute of Dental and Craniofacial Research; NIDDK, National Institute of Diabetes and Digestive and Kidney Diseases; NIEHS, National Institute of Environmental Health Sciences; NIGMS, National Institute of General Medical Sciences; NIH, National Institutes of Health; NIH OD, Office of the Director (includes research supported by the Office of Research on Women’s Health); NIMH, National Institute of Mental Health; NIMHD, National Institute on Minority Health and Health Disparities; NINDS, National Institute on Neurological Disorders and Stroke; NINR, National Institute of Nursing Research; TW, Fogarty.
Social determinants, data, and measurement
According to an NICHD-supported analysis of nearly 20 years of California hospital records (https://www.nichd.nih.gov/newsroom/news/030519-maternal-morbidity-disparities), racial and ethnic disparities in severe maternal morbidity parallel those in maternal death. NICHD-supported investigators are analyzing extensive US birth and mortality records from 2005 to the present to ascertain the impact of state policies on women’s health, with a special focus on racial disparities in maternal death. Other researchers are analyzing cause of death literal data (ie, the actual written words on the death certificate) to identify and correct problems in data collection and coding of maternal deaths.
Data from the Consortium on Safe Labor (https://www.nichd.nih.gov/about/org/diphr/officebranch/eb/safe-labor), an observational study that explored the underlying causes of the high cesarean rate in the US population, have also been used to investigate other potential factors (such as environment, air pollution, comorbid conditions, risk prediction, and even weather) that may increase the risk of pregnancy complications and racial differences in outcomes. The Consortium on Safe Labor data are publicly available in NICHD’s Data and Specimen Hub (https://dash.nichd.nih.gov/study/2331). As of March 2019, 57 data-use agreements have been approved that have resulted in 12 publications. The findings of these studies, ongoing research, and future research will continue to add to our understanding of the complexities of the social determinants of maternal morbidity and death and inform the development of new equitable approaches to improve maternal health.
Pregnancy complications and risk factors
Among the leading causes of maternal morbidity and death in the United States are cardiovascular disease, hypertensive disorders, thromboembolism, hemorrhage, and infections.2 Research into the clinical causes of maternal morbidity and death has led to some improvements in the care of women during the antepartum period and labor and delivery. Given that >50% of maternal deaths in the United States are preventable,1,2 continued research investments are needed to provide safe and effective prevention and treatment options for women.
The NICHD supports a broad range of research on maternal health in both the extramural and intramural divisions. The Perinatology Research Branch, in the Division of Intramural Research, has a large program that evaluates high-risk pregnancy conditions. The Division of Intramural Population Health has programs on maternal nutrition and gestational diabetes mellitus among other programs. The NICHD also collaborates with other NIH institutes and federal agencies to support research that is related to the leading causes of maternal morbidity and death (Figure 2).
FIGURE 2. Major programs, projects, studies and networks that support maternal health research.
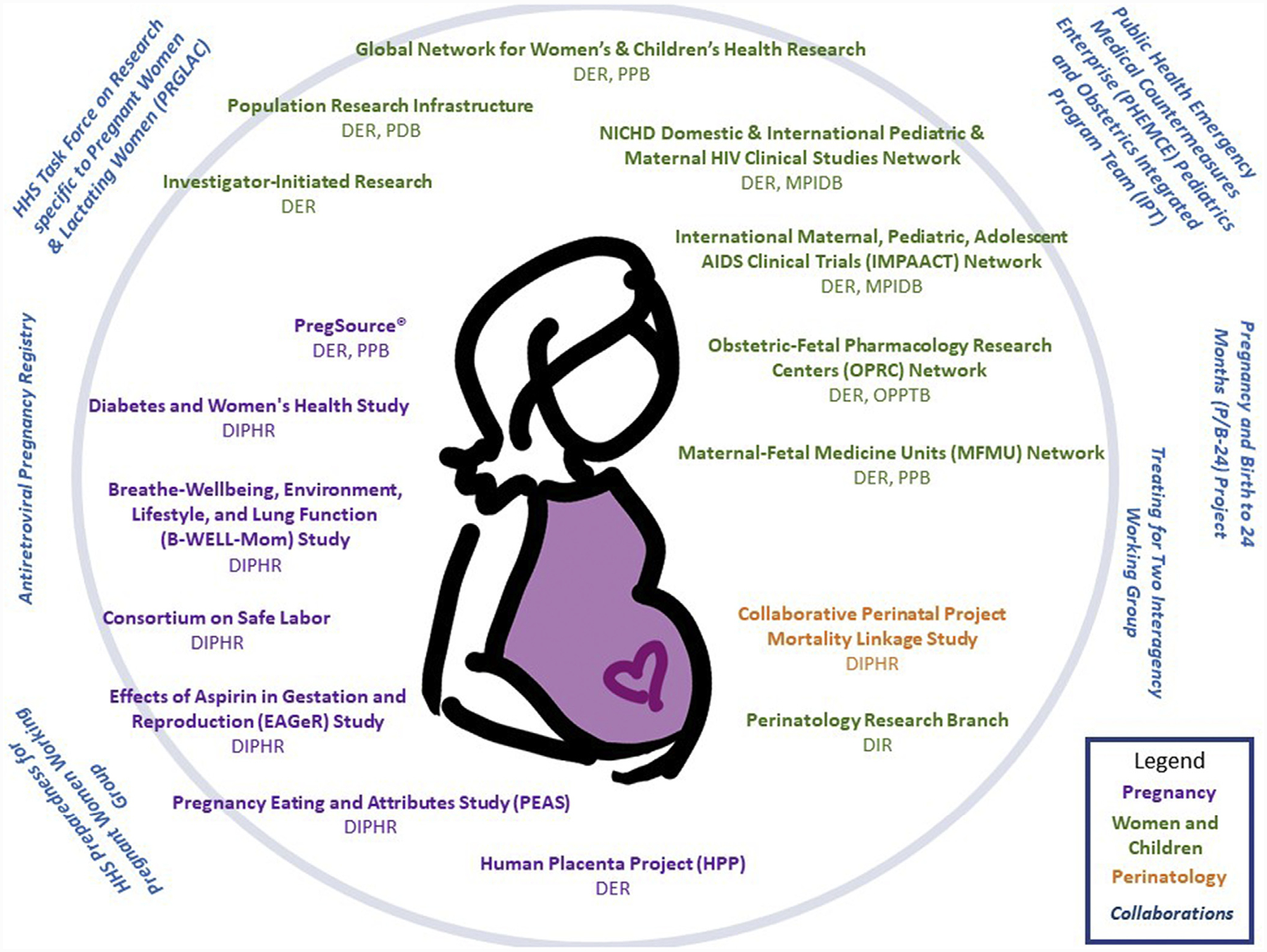
Inside the circle are efforts supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development. Outside of the circle are trans-National Institutes of Health and trans-federal efforts.
DER, Division of Extramural Research, NICHD; DIPHR, Division of Intramural Population Health Research, NICHD; DIR, Division of Intramural Research, NICHD; HHS, Department of Health and Human Services; MPIDB, Maternal Pediatric Infectious Disease Branch, DER, NICHD; NICHD, Eunice Kennedy Shriver National Institute of Child Health and Human Development; OPPTB, Obstetric and Pediatric Pharmacology and Therapeutics Branch, DER, NICHD; PDB, population dynamics branch; PPB, Pregnancy and Perinatology Branch, DER, NICHD.
For cardiovascular disease, hypertension, and thromboembolism as leading causes of maternal morbidity and death, studies supported by the NIH and other funding agencies have contributed evidence to support the updated recommendations for the administration of low-dose aspirin early in pregnancy and continuation until delivery for the prevention of preeclampsia in pregnant women with a history of preeclampsia, a multifetal gestation, and/or chronic hypertension (www.acog.org). Past and ongoing studies that have used transcriptomics and proteomics have focused on developing prediction markers for early- and late-onset preeclampsia. Such studies provide insights into the mechanisms of disease and identify patients who may benefit from interventions (ie, placental growth factor and antiangiogenic factors). Studies that assess the safety and efficacy of therapeutic drugs in women with preeclampsia during pregnancy and lactation and of treatment options, such as statins, are ongoing currently by the NICHD network investigators. Research on genetic variation, systems biology approaches, basic research on placental pathophysiology, and the effects of social factors on the risk for preeclampsia is also underway. The NICHD researchers are also investigating the mechanisms for thrombotic disorders in pregnancy and postpartum cardiomyopathy to potentially identify molecular pathways that could be targeted for therapeutic interventions. The NICHD also supports research on the development and treatment of mental health conditions and the impact on pregnancy, which includes research on appropriate medication dosing during pregnancy and after delivery.
For hemorrhage, tranexamic acid is a promising new effective treatment, because it has been shown to reduce death attributed to hemorrhage after vaginal delivery in low-to-middle income countries. In high-income countries, however, it was not effective as a prophylactic agent for vaginal deliveries.4,5 Given the conflicting data on the benefits of tranexamic acid, particularly in high-income countries, the NICHD’s Maternal-Fetal Medicine Network initiated a randomized placebo-controlled trial of 11,000 women to assess whether the preemptive use of tranexamic acid can lower the risk of postpartum hemorrhage in women who undergo a cesarean delivery. Additional research is also needed to identify better options for the management of obstetric hemorrhage.
As for maternal infections, the NICHD supports research investigators on the diagnosis, treatment, and prevention of chorioamnionitis, endometritis, and sepsis. For example, an NICHD-supported study that examined whether pretreatment with azithromycin lowered the rates of infection after cesarean delivery found significantly lower rates of endometritis, wound infection, and serious maternal adverse outcomes in the group that received azithromycin, compared with the placebo group.6 Plans are underway to extend this research globally.
Comment
The prevention of maternal morbidity and death and the promotion of maternal health are integral to the NICHD’s core mission of assuring that women experience no harm from reproductive processes. Although NICHD-sponsored research projects have led to significant scientific advances, we recognize that more must be done to reduce severe maternal morbidity and mortality rates, especially among black women and those of advanced maternal age in the United States.
A coordinated research agenda is needed to understand disease processes and clinical interventions, demographic and socioeconomic risk factors, racial and ethnic disparities, and health system factors that result in adverse pregnancy outcomes. Multidisciplinary research teams would be valuable in the investigation of the effect of pregnancy and the post-partum period on overall health both short-term and over the lifespan of women.
To address these needs, a trans-NICHD Maternal Health Coordinating Committee was established in 2018, coordinated by the Office of Health Equity with direct reporting to the NICHD director, to develop a research agenda that will be informed by collaborating NIH institutes (in particular, the National Heart, Lung and Blood Institute and the National Institute on Minority Health and Health Disparities), federal agencies, community advocates, researchers, and other stakeholders. In the spring of 2019, the NICHD organized and convened 2 meetings. One meeting was a “Community Engagement Forum on Improving Maternal Health” (https://videocast.nih.gov/summary.asp?Live=31665&bhcp=1), and the other meeting was a scientific workshop on “Maternal Mortality in the United States: Future Research Directions” (https://videocast.nih.gov/summary.asp?live=31709&bhcp=1). Furthermore, the NICHD has commissioned the National Academy of Sciences to undertake a birth settings study with a focus on how the birth setting impacts maternal outcomes.
Proceedings from these meetings, in conjunction with an ongoing strategic planning process that will be completed in the fall of 2019, will be used to focus the NICHD’s research agenda on the prevention of severe maternal morbidity and death. ■
Footnotes
The authors report no conflict of interest.
REFERENCES
- 1.Mann S, Hollier LM, McKay K, Brown H. What we can do about maternal mortality-and how to do it quickly. N Engl J Med 2018;379(18):1689–91. [DOI] [PubMed] [Google Scholar]
- 2.Main E, Menard K. Maternal mortality: time for national action. Obstet Gynecol 2013;122:735–6. [DOI] [PubMed] [Google Scholar]
- 3.MacDorman MF, Declercq E, Cabral H, Morton C. Recent increases in the US maternal mortality rate: disentangling trends from measurement issues. Obstet Gynecol 2016;128:447–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum hemorrhage (WOMAN): an international, randomized, double-blind, placebo-controlled trial. Lancet 2017;389:2105–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sentilhes L, Winer N, Azria E, et al. Tranexamic acid for the prevention of blood loss after vaginal delivery. N Engl J Med 2018;379:731–42. [DOI] [PubMed] [Google Scholar]
- 6.Tita AT, Szychowski JM, Boggess K, et al. Adjunctive azithromycin prophylaxis for cesarean delivery. N Engl J Med 2016;375:1231–41. [DOI] [PMC free article] [PubMed] [Google Scholar]


