Abstract
Background
Moisturizers are foundational therapies for human atopic dermatitis. In veterinary medicine, the use of moisturizers has been recommended by an expert committee to alleviate skin dryness that would occur, for example, in canine atopic dermatitis (cAD). However, little is known regarding the effects of moisturizers on the skin barrier.
Hypothesis/Objectives
To investigate the effects of a moisturizer on skin barrier recovery in a canine model of chronic mechanical barrier disruption.
Animals
Six healthy beagle dogs maintained in a laboratory setting.
Methods and materials
A model of chronic skin barrier disruption was simulated by tape stripping on both sides of the thorax. The moisturizer then was applied twice daily for one week to one side of the thorax, while the other hemithorax was left untreated. The effects were evaluated by measurement of transepidermal water loss (TEWL) at various times during skin barrier recovery, and by histological assessment of the disrupted skin one week after moisturizer application.
Results
Overall, TEWL was reduced, epidermal thickness was lower, stratum corneum thickness was greater and the intensity of the dermal inflammatory infiltrate was reduced for treated sites.
Conclusions and clinical importance
These results suggest a potential benefit of the moisturizer for improving skin barrier function, which is frequently altered in chronic inflammatory dermatoses such as cAD.
Background – Moisturizers are foundational therapies for human atopic dermatitis. In veterinary medicine, the use of moisturizers has been recommended by an expert committee to alleviate skin dryness that would occur, for example, in canine atopic dermatitis (cAD). However, little is known regarding the effects of moisturizers on the skin barrier. Hypothesis/Objectives – To investigate the effects of a moisturizer on skin barrier recovery in a canine model of chronic mechanical barrier disruption. Conclusions and clinical importance – These results suggest a potential benefit of the moisturizer for improving skin barrier function, which is frequently altered in chronic inflammatory dermatoses such as cAD.

Introduction
An intact skin barrier is critical to prevent desiccation from excessive water loss and the penetration of exogenous substances detrimental to the body. This barrier is controlled mainly by the outermost layer of the epidermis, the stratum corneum (SC), composed of corneocytes surrounded by complex lipid lamellae. 1 , 2
The skin barrier has been shown to play a major role in the pathogenesis of some inflammatory dermatosis, especially canine atopic dermatitis (cAD). Several studies of cAD have shown abnormalities in SC proteins (such as filaggrin and its hygroscopic derivatives 3 , 4 ) and in SC lipids 5 (including reduction in ceramides 6 or disorganized lamellar lipids 7 , 8 ).
Moisturizers are a foundational therapy for human atopic dermatitis (AD) and for maintenance of remission from flares. When combined with topical corticosteroids, moisturizers have been shown to be more effective than topical corticosteroids alone. 9 The terms “moisturizer” and “emollient” often are used interchangeably to imply the addition of water to the skin. Moisturizers may increase SC hydration by two different mechanisms: reduction of water loss through occlusion of the skin surface by hydrophobic substances (e.g. mineral oils, petrolatum ceramide, paraffin and silicone), or hydration of the SC by humectants (e.g. propylene glycol, urea, glycerol or glycerin, panthenol) which increase the water‐binding capacity of the SC. 10
In veterinary medicine, the use of moisturizers for alleviation of skin dryness in cAD has been recommended, especially after bathing. 11 , 12 However, little is known regarding the effects of moisturizers on the canine skin barrier; most published studies have evaluated the occlusive effects of lipid‐containing topical formulations and not humectants. 13 , 14 , 15 , 16 , 17 , 18 , 19 Efficacy of moisturizers can be assessed either in clinical studies or in experimental models with artificially damaged skin. In humans, 20 laboratory animals 21 and dogs, 22 mechanical disruption of the skin barrier can be generated by removing the SC through repeated applications of adhesive tape, a procedure known as tape stripping (TS). The integrity and the restoration of the skin barrier function then can be assessed in different ways. One of the most common noninvasive methods used in human studies is the measurement of transepidermal water loss (TEWL), 23 which is well correlated with skin barrier impairment. 24 In dogs, TEWL also has been used widely for the evaluation of skin barrier integrity. 5 , 22 , 25 , 26
Ermidrà spray (ICF; Palazzo Pignano, Italy) is one of many moisturizers available for veterinary use and is indicated for xerosis in dogs. The primary components of this product are humectants including glycerol, dexpanthenol and propylene glycol, which are either freely distributed in the product or micro‐encapsulated by cyclodextrins and liposomes (which are intended to enhance penetration and provide progressive release of the moisturizing agent). The aim of this study was to investigate the in vivo effect of the moisturizer on the recovery of the skin barrier function and inflammation induced by TS, through evaluation of TEWL measurement and histopathological examination of skin biopsies.
Methods and materials
All procedures were approved by the Institutional Animal Care and Use Committee of our institute. (Ethics committee reference number 1812).
Animals
Six healthy, male, 2‐year‐old beagle dogs weighing 7 to 12 kg were used for these studies. None of the dogs had a history or current evidence of skin lesions, and had not received any systemic or topical therapies within the three months preceding the study. The dogs belonged to a research colony housed indoors in a temperature‐ and humidity‐controlled facility (25–28°C, relative humidity 40–60%) and were housed in individual cement runs which were cleaned twice daily. Twelve weeks before and throughout the course of the study the dogs were fed the same maintenance dry food regimen (Specific Adult medium breed, Dechra Veterinary Products; Montigny le Bretonneux, France) and tap water ad libitum. One week before the beginning of the study, the dogs were acclimatized to their environment.
Tape stripping
The TS of the SC for barrier disruption was performed using a commercial adhesive tape (19 mm Scotch Transparent Tape, 3M; Cergy, France). At each test site, a new piece of tape was applied, pressed on with finger pressure for 10 s, then removed in one swift motion. 22
Experimental procedure
The lateral thorax was selected as the test site because it provided adequate surface area for TS, skin biopsy and a horizontal orientation for the evaporimeter probe. In addition, TS in this area triggered little response from the dog and was less likely to be licked. The hair was gently clipped using an electric hair clipper (Favorita II GT 104, Aesculap; Sulh, Germany) 24 h before the start of the study in order to minimize any effect of recent hair clipping. The same investigator performed all procedures and measurements in order to minimize variability. Six sites were evaluated on each hemithorax (12 sites in total). Sites were approximately 4 cm2 and spaced 3 cm apart. Each site was stripped until TEWL reached 50 g/m2/h. This procedure was carried out once a day for seven consecutive days.
From Day 7 to Day 13, 1 mL of Ermidrà was applied on the six sites located on the right hemithorax every 12 h. On the left hemithorax, sites were left untreated as a control.
TEWL measurements
The TEWL was measured four times at each of the 12 sites on Day 7: just before the first application of the moisturizer (T0) and 2, 4 and 8 h after application (T2, T4 and T8). TEWL then was measured once daily until Day 15 (T24 to T192). Measurements were made using a portable, battery operated, closed, unventilated chamber evaporimeter (VapoMeter SWL4001TJ, Delfin Technologies Ltd; Kuopio, Finland). The evaporimeter was activated by pressing a single button, after which its probe placed directly onto the skin, perpendicular to the surface. The 1 cm diameter of probe was positioned in the centre of the 2 cm diameter (4 cm2) test area for the measurement. The pressure applied to the probe was sufficient to prevent air leakage between the probe and the skin. The device automatically determined the measurement duration and signalled completion with an audible tone. 22 Measurements were repeated consecutively three times and the mean of the three measurements was used as a representative value. 14 , 16 , 18 , 22 When there was a variation >15% between the three measurements, they were repeated.
All measurements were performed in an air‐conditioned room where the temperature and humidity were identical to those in the dog’s living area. Before each measurement, the dogs were acclimatized to experimental conditions for 15–20 min and they were not allowed to exercise during the hour before the measurements.
Histological examination
For five of the six dogs, one skin biopsy was collected from each hemithorax after the final TS and repeated on days 7 and 15 (end of study). Skin biopsy specimens were collected using a 6 mm skin punch under local anaesthesia induced by subcutaneous injection of lidocaine (Xylovet, Ceva; La Ballastière, France). Specimens were fixed in 10% neutral buffered formalin for 24 h, embedded in paraffin, routinely processed in the laboratory, sectioned at 4 μm thickness and stained with Haematoxylin & Eosin. All skin biopsy specimens were examined in a blinded fashion by two of the authors. One section of tissue was examined per slide. The following histopathological changes weresevaluated and scored: thickness and nature of the SC, thickness of the epidermis and intensity of the dermal inflammatory infiltrate (Table 1). Epidermal and SC thicknesses were determined using imagej software https://imagej.nih.gov/ij/. Three measurements were performed randomly through the epidermis present on the section and the mean of the three values was taken as representative.
Table 1.
Histopathological changes evaluated before and after stratum corneum (SC) barrier disruption.
| Grade | Definition |
|---|---|
| SC thickness | Epidermal thickness (µm): mean of three measurements for one section |
| Nature of the SC | Basket weave or compact |
| Epidermal thickness | Epidermal thickness (µm): mean of three measurements for one section |
| Intensity of the dermal inflammatory infiltrate | 0: No infiltrate |
| 1: Mild infiltrate | |
| 2: Moderate infiltrate | |
| 3: Severe infiltrate |
Statistical analysis
All analyses were performed using R Development Core Team (2008) R: A language and environment for statistical computing (R Foundation for Statistical Computing; Vienna, Austria). To test the effect of the moisturizer on the three quantitative variables TEWL, epidermal thickness and SC thickness, we used three generalized mixed models (linear, GLMM; additive, GAMM) using the lmer() function of the R/lme4 package and the gamm() function of the R/mgcv package, respectively. We chose GLMM and GAMM for two main reasons. First, mixed models were implemented because two grouping variables (“Dog” and “Site” on each dog) needed to be specified with a random effect to take into account the nonindependence between measures made at the same site and on the same dog. Second, we selected a GAMM over a GLMM for the first model because one explanatory variable (“Time”) was expected to have a nonlinear effect on the response variable (log‐transformation of the TEWL). Furthermore, GLMM and GAMM also allowed use of a continuous autoregressive correlation structure for the “Time” covariate.
In the TEWL GAMM, the log‐transformation of the TEWL was used as the response variable with a normal distribution because it was normally distributed for nine of 12 groups of measures (T0 to T192, Shapiro–Wilk normality test), whereas the TEWL was normally distributed for only three of 12 groups of measurements. The treatment (moisturizer or control) was used as an explanatory variable with a fixed effect in order to test the impact of the moisturizer on the TEWL. “Time” was used as an explanatory variable with a nonlinear fixed effect because TEWL was expected to decrease faster in the first days than in the later days when the skin barrier was closer to full recovery. “Site” and “Dog” were used as random effects to take into account nonindependence between measures made at the same site and on the same dog. We used a continuous autoregressive correlation structure for the “Time” covariate in order to take into account the correlation between the successive measures of TEWL made at the same site of the same dog.
Epidermal thickness was used as the response variable with a normal distribution in the GLMM model (Shapiro–Wilk normality test, P = 0.4553). The treatment (moisturizer or control) was used as an explanatory variable with a fixed effect in order to test the impact of the moisturizer on epidermal thickness. “Dog” was used as a random effect to take into account the nonindependence between measures made on the same dog.
The SC thickness was used as response variable in the GLMM model, with a normal distribution (Shapiro–Wilk normality test, P = 0.6911). The treatment (moisturizer or control) was used as an explanatory variable with a fixed effect in order to test the impact of Ermidrà on the SC thickness. “Dog” was used as a random effect to take into account the nonindependence between measures made on the same dog. All equations are provided in the Supporting Information.
Finally, to test the impact of the moisturizer on the categorical variable “Intensity” of the dermal inflammatory infiltrate (including categories 0 to 3) we used a Kruskal–Wallis rank sum test.
Results
TEWL measurement
Before disruption of the skin barrier, the mean TEWL of the six dogs was 11 g/m2/h. Overall, TEWL was lower for moisturizer‐treated sites than control sites (Figure 1a). Results from the TEWL GAMM showed this difference was significant for the quantitative variable tested (TEWL; Table 2). Moreover, a faster decrease in TEWL was observed during the first 24 h for moisturizer‐treated sites (Figure 2a, b). Interestingly, a short rise of the TEWL was noticed, based on visual inspection, at T8 on the control sites, yet this rise was not observed on moisturizer treated sites. On Day 15, TEWL values had returned to normal on both moisturizer treated and control sites. For the TEWL GAMM, “Time” also had a significant effect, where TEWL decreased with time (Figure S1).
Figure 1.
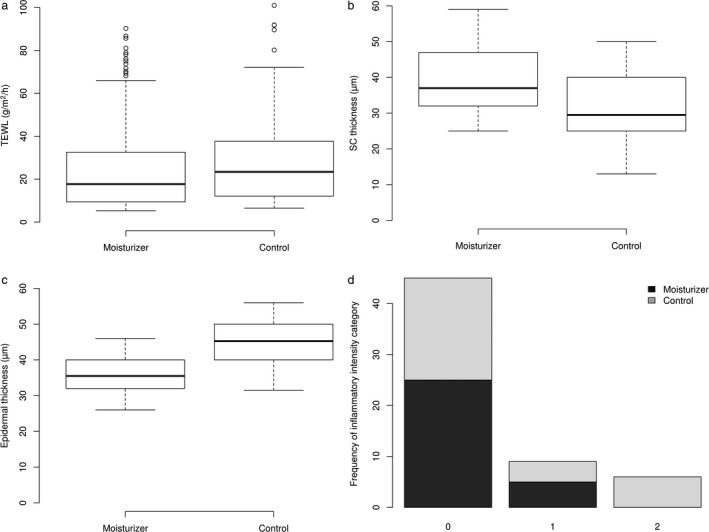
(a–c) Boxplots of treated and control sites: (a) for transepidermal water loss (TEWL), (b) for stratum corneum thickness and (c) for epidermis thickness.
For each boxplot the median (line within the box), first and third quartiles (box), nonoutlier range (whiskers) and outliers (dot) are shown. (d) Classification of inflammatory cells at treated and control sites as assessed by histopathological investigation.
The intensity of the dermal inflammatory infiltrate was categorized as: 0, no infiltrate; 1, mild infitrate; 2, moderate infiltrate; and 3, severe infiltrate.
Table 2.
Results from the three generalized mixed models to evaluate the effects of stratum corneum barrier disruption and application of a moisturizing agent.
| Model type | Response variable | Explanatory variable | Model coefficient | 95% confidence interval | P‐value |
|---|---|---|---|---|---|
| GAMM 1 | log(TEWL) | Treatment = Ermidrà | –0.081 | [–0.0978; –0.0643] | < 2x10‐16 |
| Time (s) | n.a. | n.a. | < 2x10‐16 * | ||
| GLMM 2 | Epidermal thickness | Treatment = Ermidrà | –8.767 | [–10.53; 7.008] | 7.31e‐14 |
| GLMM 3 | SC thickness | Treatment = Ermidrà | 7.95 | [5.176; 10.72] | 5.77e‐07 |
n.a., not assessed; TEWL, transepidermal water loss.
Generalized additive mixed model (GAMM): logTEWL ~ s(Time) + Treatment.
Generalized linear mixed model (GLMM): Epidermal thickness ~ Treatment.
Generalized linear mixed model: SC thickness ~ Treatment.
Approximate significance of smooth terms given by the gamm() function.
Figure 2.
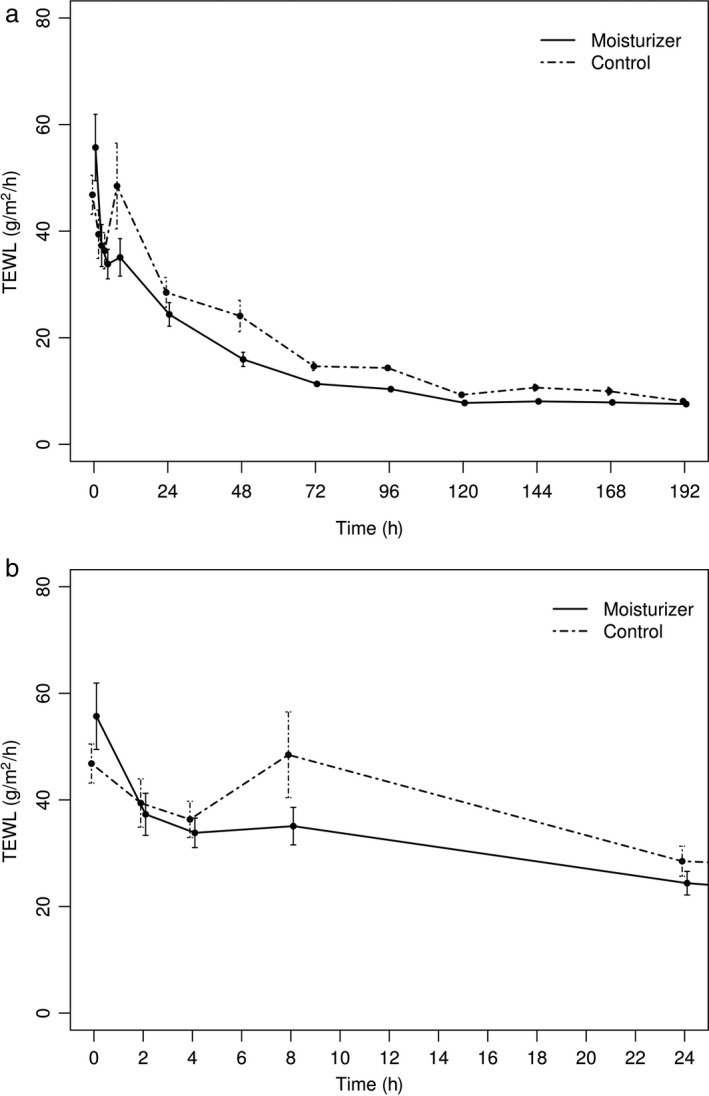
Transepidermal water loss (TEWL) values for skin treated with control and moisturizer agent.
(a) Effect of treatment on the recovery of the skin permeability barrier over time. The data represent the mean values ± 95% confidence interval from the six dogs. (b) Close‐up on the first 24 h.
Histopathological results
The application of the moisturizer decreased the intensity of epidermal hyperplasia. Overall, the epidermal thickness was thinner for moisturizer‐treated sites (Figure 1c). Results from the epidermal thickness GLMM showed this difference was significant for the quantitative variable tested (Table 2). In the control sites, the epidermis retained a more proliferative appearance with the presence of small rete ridges (Figure 3b).
Figure 3.
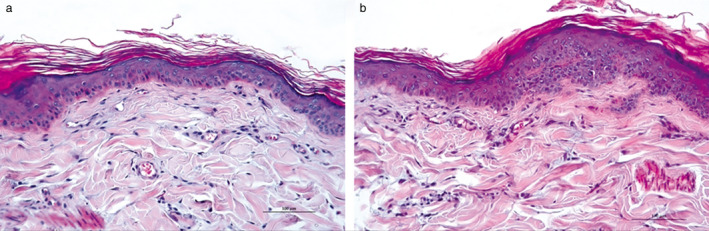
Histopathological changes in the skin of Dog 3 after treatment with control or moisturizer.
(a) After seven days of moisturizer application, the epidermis thickness was near normal, and the stratum corneum (SC) had more layers and had a basket weave appearance closer to normal. Haematoxylin & Eosin. Scale bar, 100 µm. (b) After seven days of treatment for the contralateral side, the epidermis in the control (untreated) site was moderately acanthotic with small rete ridges, and the SC was thinner and more compact than in moisturizer‐treated sites. H&E. Scale bar, 100 µm.
The application of the moisturizer also increased the restoration of the SC. Overall, SC thickness was greater for moisturizer‐treated sites (Figure 1b). Results from the SC thickness GLMM showed that these differences were significant for the quantitative variable tested (Table 2). In the moisturizer‐treated sites, the SC had more layers and had a basket weave appearance closer to normal (Figure 3a). However, in the control sites, the SC was thinner and more compact (Figure 3b). Finally, the application of the moisturizer was associated with a significant decrease in the intensity of the dermal inflammation (Kruskal–Wallis χ2 = 10.5281, d.f. 1, P= 0.001176) (Figure 1d). The inflammatory infiltrate induced by chronic barrier disruption was almost absent in Ermidrà‐treated sites, whereas it persisted, with moderate intensity, in control sites (Figure 3a, b).
Discussion
This canine model of chronic mechanical skin barrier disruption allowed us to investigate the effect of the moisturizer Ermidrà on both the restoration of the skin barrier, assessed by TEWL measurements, and the inflammatory modifications induced by chronic disruption, assessed by histopathological examination.
The study showed that the moisturizer tested could be indicated for promoting skin barrier restoration and for alleviating cutaneous inflammation. All parameters evaluated, including TEWL, epidermal thickness, SC thickness and dermal inflammatory intensity scores, improved in treated sites compared to control sites. Interestingly, a short duration peak in TEWL was noticed, based on visual inspection at 8 h on the control sites; this may have represented a measurement error.
In murine models, three phases of barrier recovery with distinct metabolic activities occur after acute barrier disruption. The first phase is initiated by secretion of a preformed pool of lamellar bodies which leads to a rapid decrease of TEWL values. 29 In the second phase, increased lipid synthesis and accelerated lamellar body formation and secretion lead to a slower decrease of TEWL values. 30 During the final phase, increased keratinocyte proliferation 31 and differentiation complete the skin barrier recovery and the TEWL value returns to baseline levels. 32 However, in chronic barrier disruption the second phase could be stronger than in the acute situation, leading to a small increase in TEWL values rather than a slower decrease. The use of moisturizers might avoid this TEWL increase during the restoration of the skin barrier.
Few clinical and experimental studies have been conducted using moisturizers on dogs and even fewer have used TEWL measurements to evaluate the skin barrier integrity. Results have been inconsistent. Randomized, double‐blinded, placebo‐controlled studies have evaluated the effects of topical blackcurrant emulsion (enriched in essential fatty acids, ceramides and 18‐beta glycyrrhetinic acid), 16 a topical lipid complex therapy, 13 and topical demethicone 14 on barrier function of dogs with cAD. All failed to demonstrate significant improvement in skin barrier function as measured by TEWL. By contrast, TEWL values were significantly decreased in two open clinical studies evaluating a ceramide‐based moisturizer in dogs with cAD, 15 and a spray containing unsaturated fatty acids and essential oils. 19 One study demonstrated a positive impact on skin inflammation after application of an emulsion in a canine model of chronic barrier disruption, and failed to show any impact on TEWL measurements. 18
Humectants, by absorbing water, can stimulate a water flux which creates a stimulus for barrier repair. 10 One of the main hygroscopic components of the moisturizer is glycerol. Glycerol interacts with the SC lipid structures or with proteins, altering their own water‐bonding and hydrophilic properties. 27 It also promotes lamellar body secretion, modifies the plasticity of SC lipids, and has keratolytic effects. 27 The moisturizer also contains propylene glycol which is known to have a partial occlusive effect. 33 This combination allows for the creation of an artificial film on the skin surface until the defective barrier is repaired in order to maintain adequate stratum corneum hydration. Moreover, it is possible that some components of the moisturizer also could directly stimulate lipid metabolism. Dexpanthenol, for example, is a precursor of vitamin B5, a component of coenzyme A that catalyzes early steps in the synthesis of fatty acids and sphingolipids which are of critical importance for SC lipid bilayers. 28 Lastly, based on the results of the present study, the moisturizer could act on the changes induced by the chronic skin barrier disruption, namely the epidermal hyperplasia and dermal inflammation, which also promote SC restoration. Numerous epidermal cytokines probably are involved in the inflammatory changes observed in chronic barrier disruption. Some components of the moisturizer, such as dexpanthenol 28 and zinc gluconate 34 , have anti‐inflammatory properties that could contribute to the decrease of dermal inflammation.
In conclusion, the application of the moisturizer Ermidrà both improved the restoration of the skin permeability barrier and decreased the inflammatory changes noted in a model of chronic SC barrier disruption. Because similar lesions and functional changes are observed in inflammatory dermatoses, the results of this study are useful in understanding the effects of moisturizers on the skin barrier and their significant role in the management of inflammatory dermatoses, particularly cAD.
Supporting information
Figure S1. Marginal effect of time on TEWL.
Document S1. Marginal effect of time on TEWL.
Sources of Funding: The ICF laboratory has partially funded the study.
Conflict of Interest: No conflicts of interest have been declared.
This study was presented at the European Society of Veterinary Dermatology‐European College of Veterinary Dermatology annual congress, 2011, Brussels, Belgium. Vet Dermatol 2011; 22: 462 (Abstract).
References
- 1. Madison KC. Barrier function of the skin: “La Raison d’Être” of the epidermis. J Invest Dermatol 2003; 121: 231–241. [DOI] [PubMed] [Google Scholar]
- 2. Proksch E, Brandner JM, Jensen J‐M. The skin: an indispensable barrier. Exp Dermatol 2008; 17: 1,063–1,072. [DOI] [PubMed] [Google Scholar]
- 3. Olivry T. Is the skin barrier abnormal in dogs with atopic dermatitis? Vet Immunol Immunopathol 2011; 144: 11–16. [DOI] [PubMed] [Google Scholar]
- 4. Chervet L, Galichet A, McLean WHI, et al. Missing C‐terminal filaggrin expression, NFkappaB activation and hyperproliferation identify the dog as a putative model to study epidermal dysfunction in atopic dermatitis. Exp Dermatol 2010; 19: e343–e346 (Letter). [DOI] [PubMed] [Google Scholar]
- 5. Shimada K, Yoshihara T, Yamamoto M, et al. Transepidermal water loss (TEWL) reflects skin barrier function of dog. J Vet Med Sci 2008; 70: 841–843. [DOI] [PubMed] [Google Scholar]
- 6. Reiter LV, Torres SMF, Wertz PW. Characterization and quantification of ceramides in the nonlesional skin of canine patients with atopic dermatitis compared with controls. Vet Dermatol 2009; 20: 260–266. [DOI] [PubMed] [Google Scholar]
- 7. Inman AO, Olivry T, Dunston SM, et al. Electron microscopic observations of stratum corneum intercellular lipids in normal and atopic dogs . Vet Pathol 2001; 38: 720–723. [DOI] [PubMed] [Google Scholar]
- 8. Piekutowska A, Pin D, Rème CA, et al. Effects of a topically applied preparation of epidermal lipids on the stratum corneum barrier of atopic dogs. J Comp Pathol 2008; 138: 197–203. [DOI] [PubMed] [Google Scholar]
- 9. van Zuuren EJ, Fedorowicz Z, Arents BWM. Emollients and moisturizers for eczema: Abridged Cochrane systematic review including GRADE assessments. Br J Dermatol 2017; 177: 1,256–1,271. [DOI] [PubMed] [Google Scholar]
- 10. Lodén M. The clinical benefit of moisturizers. J Eur Acad Dermatol Venereol 2005; 19: 672–688. [DOI] [PubMed] [Google Scholar]
- 11. Olivry T, DeBoer DJ, Favrot C, et al. Treatment of canine atopic dermatitis: 2015 updated guidelines from the International Committee on Allergic Diseases of Animals (ICADA). BMC Vet Res 2015; 11: 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Olivry T, DeBoer DJ, Favrot C, et al. Treatment of canine atopic dermatitis: 2010 clinical practice guidelines from the International Task Force on Canine Atopic Dermatitis. Vet Dermatol 2010; 21: 233–248. [DOI] [PubMed] [Google Scholar]
- 13. Hobi S, Klinger C, Classen J, et al. The effects of a topical lipid complex therapy on dogs with atopic dermatitis: a double blind, randomized, placebo‐controlled study. Vet Dermatol 2017; 28: 369–e84. [DOI] [PubMed] [Google Scholar]
- 14. Pellicoro C, Marsella R, Ahrens K. Pilot study to evaluate the effect of topical dimethicone on clinical signs and skin barrier function in dogs with naturally occurring atopic dermatitis. Vet Med Int 2013; 2013: 239186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jung J, Nam E, Park S, et al. Clinical use of a ceramide‐based moisturizer for treating dogs with atopic dermatitis. J Vet Sci 2013; 14: 199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marsella R, Cornegliani L, Ozmen I, et al. Randomized, double‐blinded, placebo‐controlled pilot study on the effects of topical blackcurrant emulsion enriched in essential fatty acids, ceramides and 18‐beta glycyrrhetinic acid on clinical signs and skin barrier function in dogs with atopic dermatitis. Vet Dermatol 2017; 28: 577–e140. [DOI] [PubMed] [Google Scholar]
- 17. Marsella R, Genovese D, Gilmer L, et al. Investigations on the effects of a topical ceramides‐containing emulsion (Allerderm Spot on) on clinical signs and skin barrier function in dogs with topic dermatitis: a double‐blinded, randomized, controlled study. J Appl Res Vet Med 2013; 11: 110–116. [Google Scholar]
- 18. Pin D, Bekrich M, Fantini O, et al. An emulsion restores the skin barrier by decreasing the skin pH and inflammation in a canine experimental model. J Comp Pathol 2014; 151: 244–254. [DOI] [PubMed] [Google Scholar]
- 19. Tretter S, Mueller RS. The influence of topical unsaturated fatty acids and essential oils on normal and atopic dogs. J Am Anim Hosp Assoc 2011; 47: 236–240. [DOI] [PubMed] [Google Scholar]
- 20. Pinkus H. Examination of the epidermis by the strip method of removing horny layers. I. Observations on thickness of the horny layer, and on mitotic activity after stripping. J Invest Dermatol 1951; 16: 383–386. [DOI] [PubMed] [Google Scholar]
- 21. Hennings H, Elgjo K. Epidermal regeneration after cellophane tape stripping of hairless mouse skin. Cell Prolif 1970; 3: 243–252. [DOI] [PubMed] [Google Scholar]
- 22. Vidémont E, Mariani C, Vidal S, et al. Characterization of the canine skin barrier restoration following acute disruption by tape stripping. Vet Dermatol 2012; 23: 103–109. [DOI] [PubMed] [Google Scholar]
- 23. Pinnagoda J, Tupkek RA, Agner T, et al. Guidelines for transepidermal water loss (TEWL) measurement. A report from the Standardization Group of the European Society of Contact Dermatitis. Contact Dermatitis 1990; 22: 164–178. [DOI] [PubMed] [Google Scholar]
- 24. Levin J, Maibach H. The correlation between transepidermal water loss and percutaneous absorption: an overview. J Control Release 2005; 103: 291–299. [DOI] [PubMed] [Google Scholar]
- 25. Cornegliani L, Vercelli A, Sala E, et al. Transepidermal water loss in healthy and atopic dogs, treated and untreated: a comparative preliminary study. Vet Dermatol 2012; 23: 41–44. [DOI] [PubMed] [Google Scholar]
- 26. Yoshihara T, Shimada K, Momoi Y, et al. A new method of measuring the transepidermal water loss (TEWL) of dog skin. J Vet Med Sci 2007; 69: 289–292. [DOI] [PubMed] [Google Scholar]
- 27. Fluhr JW, Darlenski R, Surber C. Glycerol and the skin: holistic approach to its origin and functions. Br J Dermatol 2008; 159: 23–34. [DOI] [PubMed] [Google Scholar]
- 28. Ebner F, Heller A, Rippke F, et al. Topical use of dexpanthenol in skin disorders. Am J Clin Dermatol 2002; 3: 427–433. [DOI] [PubMed] [Google Scholar]
- 29. Menon GK, Feingold KR, Elias PM. Lamellar body secretory response to barrier disruption. J Invest Dermatol 1992; 98: 279–289. [DOI] [PubMed] [Google Scholar]
- 30. Grubauer G, Feingold KR, Elias PM. Relationship of epidermal lipogenesis to cutaneous barrier function. J Lipid Res 1987; 28: 746–752. [PubMed] [Google Scholar]
- 31. Barthel D, Matthé B, Potten CS, et al. Proliferation in murine epidermis after minor mechanical stimulation. Part 2. Alterations in keratinocyte cell cycle fluxes. Cell Prolif 2000; 33: 247–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Elias PM. The epidermal permeability barrier: from Saran Wrap to Biosensor In: Elias PM, Feingold KR, eds. Skin Barrier. New York, NY: HD Taylor & Francis Group, 2006; 25–31. [Google Scholar]
- 33. Baumann LS. The Baumann skin typing system In: Farage MA, Miller KW, Maibach HI, eds. Textbook of Aging Skin. Berlin: Springer, 2010; 929–943. [Google Scholar]
- 34. Schwartz JR. Zinc and skin health: overview of physiology and pharmacology. Dermatol Surg 2005; 31: 837–847. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Marginal effect of time on TEWL.
Document S1. Marginal effect of time on TEWL.


