Abstract
Trehalose plays important roles in plant growth and stress responses and is synthesized from trehalose‐6‐phosphate by trehalose‐6‐phosphate phosphatase (TPP). Here, we show that trehalose and abscisic acid (ABA) have synergistic effects on root growth and stomatal closure. The Arabidopsis thaliana genome contains ten genes encoding TPPs and the expression level of one, TPPE, and trehalose contents increased in response to ABA. In the presence of ABA, the ABA‐responsive transcription factor ABA RESPONSE ELEMENT BINDING FACTOR2 (ABF2) directly binds to the TPPE promoter to activate its expression. Genetic analysis revealed that TPPE acts downstream of ABF2, which is supported by the findings that TPPE expression and trehalose content are reduced in the abf2 mutant and that a mutation in TPPE abolished the ABA‐sensitive root elongation phenotype of 35S:ABF2 plants. Reactive oxygen species (ROS) accumulation in response to ABA failed to occur in tppe mutant plants, suggesting that TPPE is involved in ABA‐controlled root elongation and stomatal movement by inducing ROS accumulation. This study uncovers a new branch of the ABA signaling pathway and provides a molecular basis for the role of trehalose in plant responses to abiotic stress.
Keywords: ABA, ROS, root elongation, stomatal movement, trehalose
Trehalose and ABA are known for their roles in plant stress response. In this study, we demonstrate a synergistic effect of trehalose and ABA on root elongation and stomatal closure. Trehalose‐6‐phosphate phosphatase E (TPPE) controls trehalose accumulation and modulates ABA‐controlled root growth and stomatal movement in Arabidopsis.
INTRODUCTION
The non‐reducing disaccharide trehalose is composed of two glucose molecules linked by α,α‐1,1‐glycoside bond. In bacteria and yeast, trehalose plays roles in resistance to environmental stresses such as dehydration and heat (Hounsa et al. 1998). In insects, trehalose serves as a major source of sugar, participating in growth, development, molting, and metamorphosis (Elbein et al. 2003). In plants, trehalose plays an important role in regulating gene expression, thus tuning plant metabolism, growth, development, and stress responses (Paul et al. 2008). Expressing a bacterial or yeast trehalose biosynthesis gene to alter trehalose content significantly affected abiotic stress tolerance in tobacco (Nicotiana tabacum), Arabidopsis thaliana, rice (Oryza sativa), potato (Solanum tuberosum), and other plants (Miranda et al. 2007; Kondrak et al. 2011; Kondrak et al. 2012).
In eukaryotes, trehalose‐6‐phosphate synthase (TPS) catalyzes the transfer of glucose from UDP‐glucose to glucose‐6‐phosphate (G6P) to produce trehalose‐6‐phosphate (T6P), which is dephosphorylated by trehalose‐6‐phosphate phosphatase (TPP) to produce trehalose (Avonce et al. 2006). Trehalose‐6‐phosphate, the precursor of trehalose, inhibits the activity of the protein kinase SnRK1 and is involved in regulating plant respiration, starch synthesis, and sucrose metabolism (O'Hara et al. 2013; Figueroa 2016; Paul and Gonzalez‐Uriarte 2018). The A. thaliana genome contains 11 TPS genes (TPS1–TPS11). The absence of TPS1 leads to an embryo lethal phenotype (Ramon et al. 2009; Vandesteene et al. 2010). In addition, germination and stomatal movement of tps1 mutants are hypersensitive to abscisic acid (ABA) compared to the wild type (WT) (Gomez et al. 2010). Overexpressing OsTPS1 in rice seedlings enhanced tolerance to a variety of abiotic stresses by increasing trehalose levels and upregulating the expression of several abiotic stress‐related genes (Li et al. 2011; Yang et al. 2012; Han et al. 2016).
By contrast, the roles of TPPs in trehalose production, as well as their biological functions, are poorly understood. Analysis of ten Arabidopsis TPPs (TPPA–TPPJ) revealed that these genes exhibit different tissue‐specific expression patterns, pointing to their possible functional diversity (Vandesteene et al. 2012; Van Houtte et al. 2013). However, there is some overlap in their expression patterns, suggesting that their functions might be partially redundant. Moreover, phylogenetic analysis clustered each TPP and related TPPs from other species into separate groups (Ma et al. 2007; Li et al. 2008). TPP expression is induced by different hormone and abiotic stress treatments and TPPs function in plant stress responses. For example, AtTPPF plays an active role in protecting cells from reactive oxygen species (ROS) damage by increasing the levels of soluble sugars in plants under drought stress (Lin et al. 2019). AtTPPD overexpression lines exhibited significantly enhanced salt tolerance due to hypersensitivity to redox changes in two cysteine residues of TPPD. The activity increased rapidly under salt stress, which led to trehalose accumulation (Krasensky et al. 2014). Overexpressing OsTPS1 and OsTPP1 in rice increased trehalose contents and improved plant survival under low‐temperature stress (Ge et al. 2008). However, the specific mechanism underlying the activities of other TPPs remains to be explored.
Abscisic acid regulates many processes in plants, including seed germination and dormancy, stomatal movement, and adaptation to stress (Cutler et al. 2010). In Arabidopsis, the ABA signaling pathway is mediated by three types of proteins: regulatory component of ABA receptor (RCAR)/pyrabactin resistance (PYR)/PYR1‐like proteins (PYLs), protein phosphatase 2Cs (PP2Cs), and sucrose non‐fermenting‐1‐related protein kinase 2s (SnRK2s). In the presence of ABA, SnRK2s are activated, leading to the phosphorylation‐mediated activation or repression of downstream components such as transcription factors and membrane channel proteins (Fujii et al. 2009; Fujita et al. 2013). Most ABA‐induced genes contain a conserved ABA‐responsive cis‐element (ABRE; PyAC GTGG/TC) in their promoter regions. The basic leucine zipper (bZIP) transcription factor ABRE binding protein (AREB) binds to the ABRE motif and activates the expression of ABA‐responsive genes (Choi et al. 2000; Yoshida et al. 2010; Fujita et al. 2013; Wang et al. 2019b). Trehalose‐6‐phosphate phosphatases also participate in the ABA signaling pathway. For example, tppb, tppg, and tppf mutants show altered sensitivity to ABA during seed germination and stomatal opening (Vandesteene et al. 2012). However, the detailed molecular mechanism underlying the roles of TPPs in the ABA signaling pathway remains largely unknown.
In the current study, we examined the effects of exogenous trehalose and ABA treatment on root elongation and stomatal closure in Arabidopsis. TPPE is encoded by the most highly ABA‐responsive TPP family gene in Arabidopsis and we demonstrate that TPPE regulates trehalose accumulation. Furthermore, we show that ABF2 is an upstream transcription factor that mediates ABA‐regulated TPPE expression. Our results reveal the molecular basis of ABA‐regulated trehalose metabolism in Arabidopsis.
RESULTS
Trehalose and ABA act synergistically on root elongation and stomatal closure
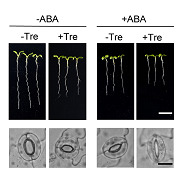
Exogenous trehalose application causes stomatal closure and inhibits root elongation in Arabidopsis (Shi et al. 2019). To determine the role of trehalose in the ABA pathway, we analyzed the effects of trehalose and ABA on root elongation and stomatal aperture. We transferred 4‐d‐old WT seedlings to half‐strength Murashige and Skoog (½ MS) medium with or without 30 μM ABA combined with various concentrations of trehalose. The root length was significantly shorter in seedlings grown on medium supplemented with 10 mM trehalose and 30 μM ABA than in seedlings grown in the presence of 30 μM ABA alone. Root growth was more severely reduced when the seedlings were treated with 20 mM trehalose and 30 μM ABA (Figure 1A,B). To examine the effects of trehalose and ABA on stomatal closure, we added trehalose and ABA to stomatal opening solution. The presence of exogenous trehalose enhanced the effect of ABA‐induced stomatal closure (Figure 1C,D). These results indicate that trehalose and ABA have synergistic effects on root elongation and stomatal closure.
Figure 1.
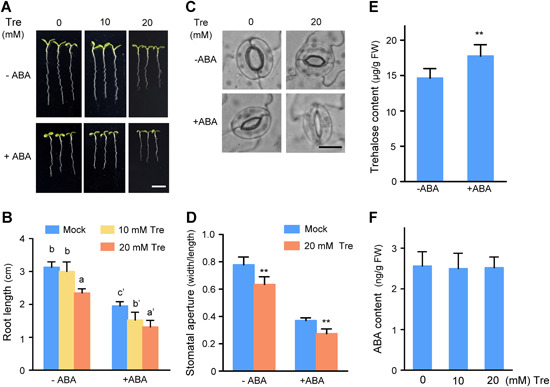
Exogenous application of trehalose enhances the effects of abscisic acid (ABA) on root elongation and stomatal closure in Arabidopsis
(A) Photographs of root elongation in wild type (WT) seedlings grown on ½ MS plates containing 0 μM (−ABA) or 30 µM ABA (+ABA) with different concentrations of trehalose (0, 10, and 20 mM). Scale bar, 1 cm. (B) Statistical analysis of root length of the plants shown in (A). The values are means ± SD (n > 10). Different letters indicate significant differences at P < 0.05 (one‐way analysis of variance (ANOVA)). (C) Stomatal morphology in plants under trehalose and ABA treatment. Scale bar, 10 μm. (D) Statistical analysis of stomatal aperture after trehalose and ABA treatment. Error bars indicate ± SD (n > 100). Significant differences are based on Student's t‐test: *P < 0.05, **P < 0.01. (E) Trehalose content of WT seedlings in the presence of ABA. Values are means ± SD (n = 3). The asterisk indicates a significant difference compared to the control using Student's t‐test (*P < 0.05, **P < 0.01). (F) ABA contents in seedlings under exogenous trehalose treatment. Values are means ± SD (n = 3).
We also examined the trehalose content of plants treated with exogenous ABA and vice versa. ABA treatment induced trehalose accumulation in vivo (Figure 1E), whereas trehalose treatment had no effect on ABA levels (Figure 1F). The expression of ABSCISIC ACID DEFICIENT2 (ABA2) and NINE‐CIS‐EPOXYCAROTENOID DIOXYGENASE3 (NCED3), key genes in the ABA biosynthesis pathway (Iuchi et al. 2001; Endo et al. 2008), did not significantly differ in the presence versus absence of trehalose (Figure S1). These results indicate that trehalose does not affect the ABA biosynthesis pathway, and they suggest that trehalose functions downstream of ABA signaling during root elongation and stomatal closure.
Abscisic acid regulates trehalose content through TPPE
To further explore the molecular network by which trehalose regulates ABA‐mediated root elongation and stomatal closure, we analyzed the expression of TPP gene family members in response to ABA. Based the Arabidopsis eFP browser (https://bar.utoronto.ca/efp/cgi‐bin/efpWeb.cgi), the expression levels of TPPE, TPPF, and TPPI increase significantly after ABA treatment. To verify this, we used quantitative reverse transcription polymerase chain reaction (qRT‐PCR) to measure the expression levels of TPPs in 10‐d‐old WT seedlings before and after ABA treatment. Abscisic acid treatment induced TPPE expression to levels much higher than those of the other TPPs, with TPPE reaching its highest value (7.5‐fold increase) at 3 h after ABA treatment (Figure S2). In addition, we generated reporter lines harboring GUS driven by the TPPE native promoter and performed histochemical staining of seedlings after ABA treatment (Figure S3). GUS activity dramatically increased in the presence of ABA, suggesting that ABA upregulates the expression of TPPE.
TPPE complements the function of the yeast tps2 mutant (Vogel et al. 1998). To identify the function of TPPE in Arabidopsis, we examined the catalytic activity of this enzyme expressed in Escherichia coli. TPPE had higher catalytic activity for T6P than for glucose‐6‐phosphate (G6P) and sorbitol‐6‐phosphate (S6P) in vitro (Figure S4), indicating that T6P is a specific substrate of TPPE.
TPPE participates in ABA‐inhibited root elongation and stomatal movement
To gain a better understanding of the role of TPPE in trehalose production and ABA responses, we measured the trehalose contents in tppe mutants and TPPE overexpression lines. Two independent T‐DNA insertion mutants of TPPE were identified: tppe‐1 (SALK_090223), with a T‐DNA insertion in an intron, and tppe‐2 (GK‐291G05), with a T‐DNA insertion in the coding sequence (Figure S5A, B). TPPE expression was significantly lower in tppe‐1 and tppe‐2 and higher in the 35S:TPPE lines compared to the WT, as determined by qRT‐PCR (Figure S5C, D).
We then analyzed the trehalose content in 10‐d‐old seedlings of these genotypes by LC‐MS. The trehalose content was higher in 35S:TPPE and lower in tppe‐1 and tppe‐2 compared to the WT (Figure 2A). In addition, we measured trehalose contents in the plants in response to the application of 50 μM ABA. The trehalose content increased (1.21‐fold) in the WT after ABA treatment but remained almost unchanged in tppe‐1 and tppe‐2 after this treatment (1.09 and 1.08‐fold, respectively). By contrast, the trehalose content of 35S:TPPE increased 1.26‐fold under ABA treatment. Collectively, these results indicate that ABA induces TPPE expression and positively regulates trehalose accumulation.
Figure 2.
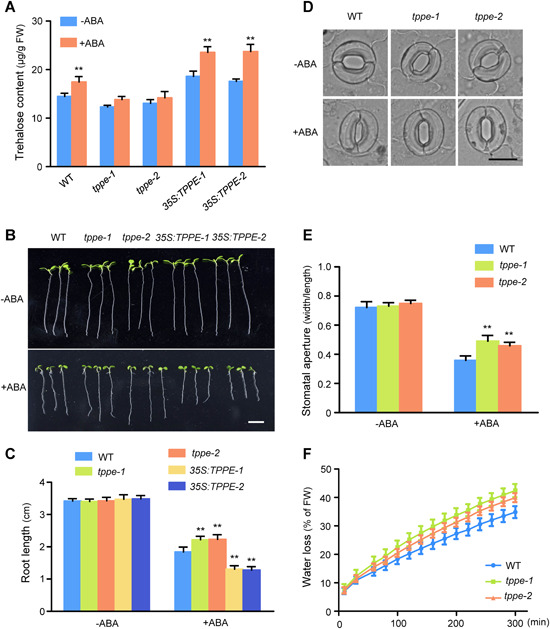
Loss‐of‐function mutants of TPPE are less sensitive to abscisic acid (ABA) than the wild type (WT) in terms of root inhibition and stomatal closure
(A) Trehalose contents in 10‐d‐old WT, tppe‐1, tppe‐2, and TPPE overexpression plants with or without ABA treatment (50 μM). The values are means ± SD (n > 10). *P < 0.05, **P < 0.01 (Student's t‐test). (B) Comparison of root elongation among genotypes on ½ MS with or without 30 μM ABA. Scale bar, 1 cm. (C) Statistical analysis of the differences in root length among the plants shown in (B) The values are means ± SD (n > 10), *P < 0.05, **P < 0.01 (Student's t‐test). (D) Representative images showing stomatal apertures before and after 2 h treatment with 10 μM ABA. Scale bar, 10 μm. (E) Quantification of stomatal apertures. The values are means ± SD (n > 100) from five biological replicates. *P < 0.05, **P < 0.01 (Student's t‐test). (F) The detached leaves of 4‐week‐old WT, tppe‐1, and tppe‐2 plants were used for relative water loss measurements. The experiments were repeated three times with similar results. Each data point represents the mean ± SD (n = 3).
To investigate the role of TPPE in the ABA response, we analyzed the sensitivity of root growth to ABA in the WT, TPPE overexpression lines, and tppe mutants. We transferred 4‐d‐old seedlings grown on ½ MS medium to medium supplemented with 30 μM ABA. After 5 d of growth, root growth was dramatically retarded in the overexpression lines under ABA treatment compared to the WT, whereas root elongation was less sensitive to ABA in tppe seedlings (Figure 2B,C).
We measured stomatal aperture in WT and tppe plants to determine whether TPPE regulates ABA‐dependent stomatal closure. In the light, the stomatal apertures of tppe and WT plants were similar (Figure 2D). However, stomatal closure in the tppe‐1 and tppe‐2 mutants was significantly less sensitive to ABA than the WT (Figure 2E). Water loss measured in detached leaves reflects the stomatal aperture of intact leaves. Consistent with the stomatal closure results, water loss in detached leaves occurred much more rapidly in tppe‐1 and tppe‐2 than in the WT; the water loss rates were approximately 7.6% and 5.2% faster in tppe‐1 and tppe‐2 versus the WT, respectively, at 300 min after detachment (Figure 2F). However, no significant difference was observed in leaf water loss between the overexpression lines and the WT (data not shown). The tppe deletion mutant lines, which were generated using the CRISPR/Cas9 system, showed the same phenotype as the tppe‐1 and tppe‐2 T‐DNA insertion mutants (Figure S6). Taken together, these data demonstrate that TPPE positively regulates ABA‐mediated root‐growth inhibition and stomatal closure.
ABA RESPONSE ELEMENT BINDING FACTOR2 directly binds to the TPPE promoter in vitro and in vivo
To explore the mechanisms by which ABA regulates TPPE expression, we identified transcription factors that directly regulate the transcription of TPPE and predicted the ABREs in the promoter region of TPPE using PlantCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/). Three ABREs in the TPPE promoter were predicted to bind to AREB transcription factors. Using these fragments as bait in yeast one‐hybrid assays (Figure 3A), we identified the bZIP transcription factor ABF2 as binding the TPPE promoter E3 region. We next used full‐length coding region of ABF2 to test if ABF2 binding to the TPPE promoter region could induce gene expression. Indeed, co‐expression with ABF2 induced the expression of the aureobasidin A resistance reporter gene (AbAr) driven by the E3 fragment of the TPPE promoter region (pAbAi‐E3). By contrast, yeast co‐transformed with pAbAi‐E1, pAbAi‐E2, and ABF2 exhibited abnormal growth (Figure 3B).
Figure 3.
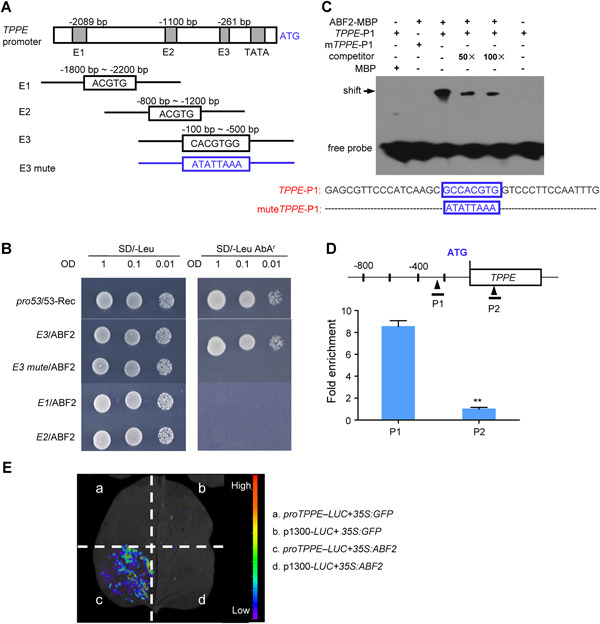
ABA RESPONSE ELEMENT BINDING FACTOR2 (ABF2) binds to TPPE in vivo and in vitro
(A) Sequence analysis of the TPPE promoter. The gray boxes upstream of ATG represent the TATA box and three ABA‐responsive cis‐elements (ABREs). Each ABRE was selected as the core region, and the line indicates the truncated promoter region. (B) Physical interactions of ABF2 with the TPPE promoter in yeast one‐hybrid (Y1H) assays. (C) Electrophoretic mobility shift assay (EMSA) of in vitro binding of ABF2 to the ABRE motif of the TPPE promoter. A probe (mute TPPE‐P1) with the G‐box/ABRE motif mutated was used to test binding specificity. “−” represents the absence and “+” represents the presence of components in the reaction. Sequences of both the wild‐type and mutated probes are shown under the images, with the G‐box/ABRE motif boxed. (D) chromatin immunoprecipitation polymerase chain reaction (ChIP‐PCR) assay of in vivo binding of ABF2‐GFP to the TPPE promoter. Two DNA fragments (P1, P2) spanning these sites were examined by ChIP enrichment tests, as shown in the schematic diagram. Fold enrichment was calculated as the ratio of 35S:ABF2‐GFP to 35S:GFP signal. Data are means ± SD of three repeats, *P < 0.05, **P < 0.01. (E) Transcriptional activity assay in N. benthamiana leaves. ABF2 promotes the expression of the reporter gene LUC driven by the ABRE‐containing promoter.
We then performed an electrophoretic mobility shift assay (EMSA) to determine whether ABF2 directly binds to the TPPE promoter. A 40 bp biotin‐labeled DNA fragment containing the ABRE consensus motif (CTCGTGG), was used as a probe (TPPE‐P1), and a DNA fragment harboring a mutated ABRE was used as a control (mTPPE‐P1). We carried out a competition assay by adding 50‐fold and 100‐fold excess amounts of unlabeled probe. When labeled probes were pre‐incubated with the ABF2‐MBP protein, a shifted band was detected (Figure 3C). By contrast, the binding of the ABF2‐MBP protein to the promoter was abolished by the mutation in mTPPE‐P1, as well as by the addition of excess unlabeled probe. These results suggest that ABF2 physically interacts with the TPPE promoter in vitro.
To determine whether ABF2 directly binds to the promoter of TPPE in vivo, we performed a chromatin immunoprecipitation (ChIP) assay using ABF2:GFP‐overexpressing plants. Chromatin isolated from ABF2:GFP transgenic plants and 35S:GFP control plants was immunoprecipitated with GFP antibody, followed by quantitative polymerase chain reaction (qPCR) to quantify the fold enrichment of the TPPE promoter regions. We observed an 8.4‐fold enrichment of the TPPE promoter region containing the ABRE, but no enrichment in the coding region of TPPE (Figure 3D). These results indicate that ABF2 directly binds to the ABRE‐containing region of the TPPE promoter in vivo.
Next, we performed an in vivo luciferase activation assay to further explore the binding of ABF2 to the TPPE promoter. The TPPE promoter region, that is, a 1.0 kb fragment upstream of the ATG, was fused to the firefly luciferase reporter gene (LUC), transferred into Agrobacterium GV3101, and transiently transformed into Nicotiana benthamiana leaves. Luminescence was detected only in the area co‐transformed with 35S:ABF2 and ProTPPE‐LUC (Figure 3E). These results indicate that ABF2 binds to the TPPE promoter to activate LUC expression in N. benthamiana leaves. Taken together, these results suggest that ABF2 binds to the promoter of TPPE in vitro and in vivo.
ABA RESPONSE ELEMENT BINDING FACTOR2 controls TPPE expression and affects trehalose production
ABA RESPONSE ELEMENT BINDING FACTOR2 is an important transcription factor that bridges ABA signaling and plays key roles in plant responses to environmental stress. To examine whether ABA regulates the expression of TPPE through ABF2, we performed a dual‐luciferase reporter assay using Arabidopsis protoplasts. The ABRE motif between −290 bp and −230 bp upstream of the ATG codon of TPPE was fused to the LUC reporter vector in the form of four repeats and co‐transformed with the effector vector into WT leaf protoplasts with or without ABA treatment. The transient overexpression of ABF2 activated the expression of the reporter gene, and the LUC/REN ratio sharply increased (2.25‐fold) after 10 μM ABA treatment (Figure 4A). These results indicate that ABA induces the expression of TPPE through ABF2.
Figure 4.
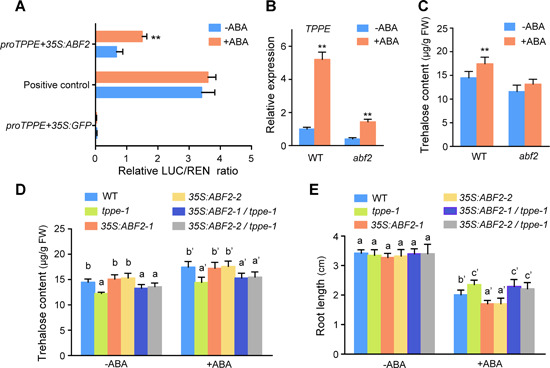
ABA RESPONSE ELEMENT BINDING FACTOR2 (ABF2) mediates abscisic acid (ABA)‐controlled trehalose accumulation and root growth inhibition
(A) Transactivation of the TPPE promoter by ABF2 in protoplasts. Dual‐luciferase assays were carried out to test ABA‐induced binding of ABF2 to the TPPE promoter in Arabidopsis protoplasts. The empty effector construct 35S:GFP was used as a control. Relative luciferase activity was determined 18 h after transfection with or without ABA treatment. Data are means ± SD (Student's t‐test, n > 3, *P < 0.05, **P < 0.01). (B) Expression levels of TPPE in wild type (WT) and abf2 plants after ABA treatment. Induction levels of TPPE in 10‐d‐old plants by ABA (50 μM, 4 h) were determined by qRT‐PCR. Values are mean ± SD (Student's t‐test, n > 3, *P < 0.05, **P < 0.01). (C) Trehalose contents of WT and abf2 plants with or without ABA treatment. (D) Trehalose contents in 10‐d‐old tppe‐1, 35S:ABF2, 35S:ABF2/tppe‐1, and WT plants with or without ABA treatment. The values are means ± SD (n > 3). Different letters indicate significant differences at P < 0.05 (one‐way analysis of variance (ANOVA)). (E) Statistical analysis of root length in plants under ABA treatment. tppe‐1, 35S:ABF2, 35S:ABF2/tppe‐1, and WT seedlings were incubated on ½ MS or ½ MS with ABA for 5 d. Root length was measured, which was repeated at least three times in each experiment. The values are means ± SD (n > 10). Different letters indicate significant differences at P < 0.05 (one‐way ANOVA).
To explore whether ABF2 functions upstream of TPPE in ABA‐induced trehalose accumulation in Arabidopsis, we obtained the T‐DNA insertion mutant abf2 (SALK_002984) (Figure S7A, B). The abf2 and tppe mutants exhibited similar root elongation patterns when grown on ABA‐containing medium (Figure S7D), implying that ABF2 and TPPE act in the same signaling pathway. We examined the expression of TPPE in abf2 via qRT‐PCR. TPPE was expressed at significantly lower levels in abf2 versus the WT (Figure 4B). The expression of TPPE in the mutant increased after 50 μM ABA treatment, but it was still significantly lower than that in the WT. The trehalose content in abf2 did not significantly change in response to ABA treatment (Figure 4C). These results indicate that ABF2 is required for ABA‐induced increases in TPPE expression and trehalose content in vivo.
To investigate whether ABF2 participates in the regulation of trehalose‐modulated root elongation under ABA treatment, we examined whether the tppe‐1 mutation would suppress the phenotypes of ABF2‐overexpressing plants under ABA treatment. We generated 35S:ABF2/tppe‐1 plants by crossing the 35S:ABF2 overexpression line with tppe‐1 (Figure S7E). The trehalose content in 35S:ABF2/tppe‐1 was similar to that in tppe‐1 (Figure 4D). In addition, the seedling growth of the 35S:ABF2/tppe‐1 was less sensitive to ABA compared to the 35S:ABF2 line (Figure 4E). These results indicate that ABF2 is a positive regulator of TPPE that functions in ABA‐mediated root elongation.
TPPE participates in ABA‐induced ROS accumulation
Trehalose promotes ROS production, which can also be elicited by ABA treatment (Yang et al. 2014; Shi et al. 2019). Therefore, we investigated whether TPPE modulates the ABA‐induced accumulation of ROS. Hydrogen peroxide (as revealed by H2DCFDA staining) and superoxide (as revealed by nitroblue tetrazolium chloride (NBT) staining) were detected in the root tips of WT, tppe‐1, tppe‐2, 35S:TPPE‐1, and 35S:TPPE‐2 seedlings. In the absence of ABA, less H2O2 and superoxide accumulated in the root tips of tppe‐1 and tppe‐2 than in the WT, whereas the highest levels of ROS were detected in the root tips of 35S:TPPE‐1 and 35S:TPPE‐2 seedlings. However, ABA‐induced ROS production was partially impaired in tppe‐1 and tppe‐2, whereas 35S:TPPE exhibited enhanced ROS levels compared to WT (Figure 5). Furthermore, we analyzed ROS accumulation and stomatal movement in these plants in response to ABA using the fluorogenic reagent H2DCFDA. The fluorescence intensity of guard cells was lower in tppe‐1 and tppe‐2 versus WT plants after ABA treatment (Figure S8). These results indicate that decreased trehalose levels impair ABA‐induced ROS production and that TPPE participates in ABA‐induced ROS accumulation. Therefore, TPPE is involved in ABA‐induced ROS accumulation in root tips and guard cells.
Figure 5.
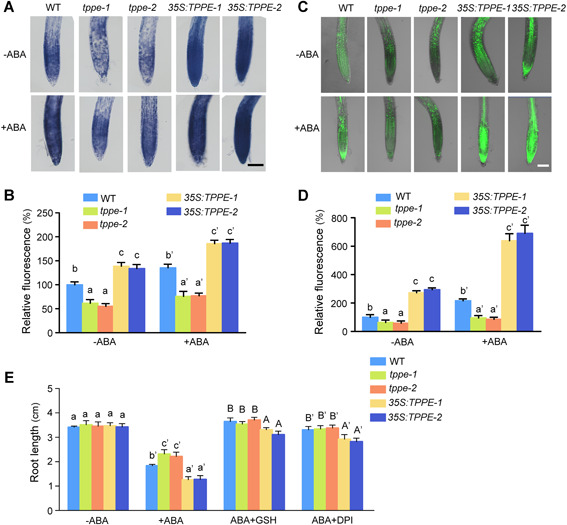
Trehalose enhances abscisic acid (ABA)‐induced reactive oxygen species (ROS) accumulation in roots and stomata
(A) Nitroblue tetrazolium chloride (NBT) staining for superoxide anion in the primary roots of wild type (WT), tppe‐1, tppe‐2, 35S:TPPE‐1, and 35S:TPPE‐2 seedlings after ABA treatment. Scale bar, 100 μm. (B) Relative intensities calculated from (A). The values are means ± SD (n > 20). Different letters indicate significant differences at P < 0.05 (one‐way analysis of variance (ANOVA)). (C) H2DCFDA staining for ROS in primary roots of WT, tppe‐1, tppe‐2, 35S:TPPE‐1, and 35S:TPPE‐2 seedlings after ABA treatment. Scale bar, 100 μm. (D) Relative intensities calculated from (C). The values are means ± SD (n > 10). Different letters indicate significant differences at P < 0.05 (one‐way ANOVA). (E) Statistical analysis of the root lengths of seedlings grown on ½ MS medium supplemented with ABA and 100 µM GSH or 2 µM DPI. The values are means ± SD (n > 10). Different letters indicate significant differences at P < 0.05 (one‐way ANOVA).
Finally, to verify the notion that TPPE participates in ABA‐induced ROS accumulation to inhibit root elongation, we transferred 4‐d‐old WT, tppe‐1, tppe‐2, 35:TPPE‐1, and 35:TPPE‐2 seedlings to medium supplemented with ABA and the antioxidant reduced glutathione (GSH) or the NADPH oxidase inhibitor diphenyleneiodonium chloride (DPI). Compared to the WT, tppe‐1, and tppe‐2 seedlings were less sensitive to ABA, whereas 35:TPPE‐1 and 35:TPPE‐2 seedling showed increased sensitivity to 30 μM ABA in the medium. Moreover, the roots length were significantly greater in 35:TPPE‐1 and 35:TPPE‐2 plants grown in ABA medium containing GSH or DPI compared to these lines in ABA medium alone, indicating that the inhibition of root elongation by ABA was partially restored by treatment with a reducing reagent (Figure 5E). Together, these results suggest that TPPE is involved in ABA‐inhibited root elongation by modulating ROS accumulation.
DISCUSSION
Trehalose is an essential molecule required for plant growth and development. This carbohydrate can also act as a stress‐protective agent to reduce damage to plant tissues and improve stress resistance (O'Hara et al. 2013; Delorge et al. 2014; Zhang et al. 2017). Although recent work showed that ABA and trehalose contents in plants increase under drought conditions (Lin et al. 2019), the relationship between trehalose and the ABA signaling pathway had been unclear. In the current study, we demonstrated that trehalose and ABA have synergistic effects on root growth and stomatal closure in Arabidopsis, implying that trehalose participates in the ABA signal transduction process. TPPE, the most ABA‐responsive gene in the TPP gene family, plays a key role in trehalose production. However, the mechanisms underlying the role of TPPE in ABA signaling in response to stress remain elusive. Here, we uncovered a possible regulatory network governing trehalose metabolism and ABA signaling to control root growth and stomatal movement.
We determined that TPPE plays an important role in ABA‐controlled root elongation and stomatal closure based on the following evidence. First, ABA induced trehalose accumulation in WT plants but not tppe mutants. The tppe mutants showed reduced sensitivity to ABA‐induced inhibition of root elongation and stomatal closure (Figure 2). Second, ABF2 directly binds to the promoter of TPPE and controls its expression (Figure 3). Third, overexpressing ABF2 did not restore the phenotype of tppe, that is, its reduced sensitivity to ABA‐inhibited root elongation, implying that TPPE acts downstream of ABF2 (Figure 4). Taken together, these results indicate that ABA signaling modulates trehalose biosynthesis and that trehalose functions synergistically with ABA. Such a regulatory mechanism might help the plant respond rapidly to adverse conditions by balancing plant growth and defense responses.
Abscisic acid induces a rapid stomatal response to environmental stimuli by activating or repressing ion channels and other signaling components (Munemasa et al. 2015; Wang et al. 2019a). Our results indicate that ABA induces the expression of members of the TPP family to some extent. Interestingly, the expression levels of TPPs remained above basal levels 6 h after rapid upregulation, suggesting that relatively high levels of trehalose are associated with the long‐term effect of ABA on plant growth. Abscisic acid contents fluctuate in a rhythmic fashion, reaching their highest levels at 12 h after dawn (Adams et al. 2018; Belbin and Dodd 2018). The changes in trehalose contents follow the same rhythmical pattern, reaching maximum levels after 12 h of daylight (Carillo et al. 2013). Therefore, our results support the correlation between ABA levels and trehalose accumulation.
ABA RESPONSE ELEMENT BINDING FACTOR2, ABF3, and ABF4 are key transcription factors that function in various plant responses to ABA signals induced by cold and osmotic stress (Choi et al. 2000; Yoshida et al. 2010; Fujita et al. 2013; Yoshida et al. 2014). However, our study provided no evidence that ABF3 and ABF4 directly bind to the TPPE promoter. ABF3 and ABF4 play important roles in regulating seed germination and plant responses to ABA and various stresses, whereas ABF2 primarily functions in ABA responses during vegetative growth (Kim et al. 2004; Yoshida et al. 2015; Zandkarimi et al. 2015). TPPE expression and trehalose content were significantly lower in the abf2 mutants compared to the WT (Figure 4B, C). In addition, our results demonstrate that trehalose content is controlled by ABF2 in an ABA‐dependent manner (Figure 4A), suggesting that core ABA signaling modulates trehalose metabolism. This idea is further supported by the finding that the trehalose content in open stomata1 (OST1) mutants (which lack a kinase downstream of ABA perception) remained unchanged after ABA treatment (data not shown). However, some stress‐response genes function through an ABA‐independent pathway. Analysis of the promoters of these genes revealed that the dehydration response element (DRE; TACCGACAT) and C‐repeat (CRT) element (G/ACCGAC) are primarily activated independently of ABA (Baker et al. 1994; Yamaguchi‐Shinozaki and Shinozaki 1994). DREB1A combines with DRE/CRT at the TPPF promoter and activates its expression to increase soluble sugar contents, resulting in improved drought resistance (Lin et al. 2019). Although it remains unclear whether TPPF activity is independent of ABA, Lin demonstrated that trehalose content is regulated in many different manners in plants coping with different stresses. It remains to be elucidated whether these pathways are interrelated or integrated into a regulatory network.
Compared to other disaccharides, trehalose levels are very low in plants (Lunn et al. 2014). Transgenic plants expressing bacterial or plant TPS or TPP were previously generated in studies aiming to improve plant stress tolerance; however, although these plants showed drought tolerance, changes in trehalose contents were not obvious in the transgenic plants (Romero et al. 1997; Pramanik and Imai 2005; Li et al. 2011). Another study used constitutive or stress‐inducible promoters to express TPS genes and also induced drought tolerance (Karim et al. 2007). Trehalose is thought to function as a signaling molecule affecting sugar metabolism, cell wall modification, and ROS production (Chary et al. 2008; Van Houtte et al. 2013; Yang et al. 2014). Exogenous treatment with trehalose increases the cellular levels of superoxide anion (O2 −) and hydrogen peroxide (H2O2) by upregulating the expression of NADPH oxidase genes RBOHD/F (Shi et al. 2019). In the current study, increasing endogenous trehalose contents induced ROS production. Glutathione and DPI treatment partially reduced the synergistic effects of ABA and trehalose on inhibiting root elongation (Figure 5E). Therefore, the effects of ABA and trehalose on the suppression of root elongation could be attributed, at least in part, to increased ROS production.
Trehalose induces stomatal closure via ROS accumulation (Shi et al. 2019), whereas ABA has little effect on stomatal movement in the tppg and tps1 mutants (Gomez et al. 2010; Vandesteene et al. 2012). Here, we show that stomatal closure was less sensitive to ABA in the tppe mutants than in the WT (Figure 2C), suggesting that TPPE‐controlled trehalose plays an important role in stomatal closure. However, we also detected increased ROS accumulation in guard cells of TPPE‐overexpressing plants, but their stomatal response to ABA and the rate of water loss in detached leaves were similar to those of WT plants. These results suggest that overexpressing TPPE has antagonistic effects on ROS‐promoted stomatal closure and that stomatal movement is controlled by a complex regulatory network.
Based on our results, we propose a model in which a trehalose‐mediated branch of ABA signaling controls root growth (Figure 6). In the presence of ABA or under stress conditions, ABA signaling is activated. Consequently, ABF2, a key transcription factor that functions downstream of ABA signaling, activates the expression of TPPE, which leads to trehalose accumulation. Trehalose‐induced ROS accumulation enhances the effects of ABA. In the absence of ABA or when the stress conditions end, the plant returns to normal growth conditions, ABF2 loses its regulatory effect on TPPE, and TPPE expression and trehalose production return to basal levels to maintain normal plant growth and development. Our study provides a molecular basis for the role of trehalose in plants under abiotic stress and uncovers a new downstream regulatory branch of the ABA signaling pathway.
Figure 6.
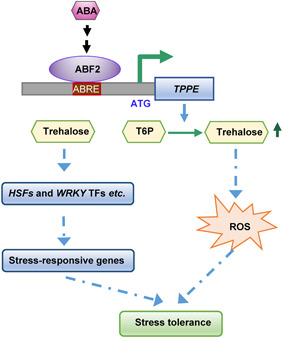
Proposed model of the role of trehalose in abscisic acid (ABA)‐controlled root growth and stomatal responses
In the presence of ABA, ABA RESPONSE ELEMENT BINDING FACTOR2 (ABF2) enhances TPPE expression by directly binding to its promoter. The trehalose content increases due to enhanced TPPE expression. Trehalose triggers reactive oxygen species (ROS) accumulation in roots and stomata, which enhances the effect of ABA on inhibiting root growth and promoting stomatal closure, allowing the plant to respond rapidly to stress conditions. In addition, trehalose acts upstream of transcription factors to regulate stress‐responsive gene expression (Shi et al. 2019).
MATERIALS AND METHODS
Plant materials and growth conditions
The A. thaliana ecotype Columbia‐0 (Col‐0) was used as the WT control. T‐DNA insertion mutants tppe‐1 (Salk_090223), tppe‐2 (GK‐291G05), and abf2 (Salk_002984) were obtained from ABRC (Arabidopsis Biological Resource Center). The seeds were surface sterilized for 15 min in 10% bleach, washed four times with sterile water, and plated on half‐strength Murashige and Skoog (½ MS) medium. The seeds were stratified at 4°C for 2 d in the dark and transferred to a phytotron at 22°C with a 16‐h light: 8‐h dark photoperiod (light intensity 100 μmol /(m2·s)). N. benthamiana was grown under a 16‐h light: 8‐h dark photoperiod. All experiments were repeated at least three times.
Construction of plasmids and generation of transgenic plants
TPPE‐overexpressing lines 35S:TPPE‐1 and 35S:TPPE‐2 were generated by cloning the coding sequence of TPPE into the pCAMBIA1300 vector under the control of the CaMV 35S promoter. The construct was used for transformation (via Agrobacterium strain GV3101) by the floral‐dip method. Transgenic seedlings were selected on ½ MS medium containing 25 mg/L hygromycin (Sigma‐Aldrich). We obtained single‐copy lines and used T3 seeds for subsequent experiments.
Stomatal movement assay
Stomatal movement assays were performed as described previously (Dong et al. 2018). Four‐week‐old rosette leaves were harvested and epidermal strips were incubated in stomatal opening solution containing 50 mM KCl, 10 μM CaCl2, and 10 mM MES‐KOH (pH 6.1) for 2.5 h in the light, followed by the addition of 10 μM ABA and 2 h of incubation. Subsequently, the epidermal strips were mounted on glass slides, imaged under a Nikon 80i upright microscope, and measured using ImageJ software. The stomatal aperture values are means from at least 100 stomata, measured in five plants per treatment.
RNA isolation and quantitative RT‐PCR
The Arabidopsis seedlings (100 mg) were harvested in liquid ½ MS medium (with or without 50 μM ABA). Total RNA was extracted from the samples using Trizol reagent (Invitrogen), and DNA contamination was removed by DNaseI (Invitrogen) treatment. First‐strand complementary DNA (cDNA) was synthesized with the Reverse Transcription System (Promega) and used as a template for qRT‐PCR with LightCycler 480 SYBR Green I Master (Roche). qRT‐PCR was performed on a Roche 480 Real‐Time PCR System following the manufacturer's instructions. Results were normalized to ACTIN2/8.
Measuring trehalose content
To measure trehalose contents, 10‐d‐old Arabidopsis seedlings (100 mg) were harvested and rapidly frozen in liquid nitrogen. After being ground to powder, each sample was evenly mixed with 500 μL chloroform: acetonitrile (3:7, v‐v) and incubated in a ‐10°C freezer with occasional shaking. The organic matter was extracted with 400 μL water at 4°C and centrifuged for 4 min at 10,000 g; this step was repeated twice. The supernatants were combined and dried in a centrifugal vacuum dryer. The dry samples were dissolved in 500 μL methanol: water (1:1, v‐v) and filtered through a 0.22 μm membrane at room temperature.
ACQUITY UPLC I‐Class (Waters) coupled with a Xevo G2 Q‐TOF high‐resolution mass spectrometer (Waters) was used to measure trehalose content. Chromatographic separation was conducted using a UPLC BEH Amide column (1.7 μm, 2.1 mm × 100 mm, Waters). Elution was performed with mobile 90% phase A (water, 0.1% ammonia) and 10% phase B (acetonitrile: water = 95:5, 0.1% ammonia, 2 mM ammonium acetate) at a flow rate of 0.3 mL/min.
Measuring ABA content
Ten‐d‐old seedlings (100 mg) were subjected to endogenous ABA measurements. After treatment with trehalose in ½ MS liquid medium for 4 h, the seedlings were harvested and immediately frozen in liquid nitrogen, then ground to powder. Extraction solvent (acetone, water, acetic acid, 80:19:1, v‐v:v) was added to the sample and carefully mixed. The supernatant was recovered by centrifugation; this step was repeated twice. The extraction solvent was evaporated and the residue was resuspended in 0.5 mL dissolution solvent (acetonitrile, water, 30:70, v‐v). Abscisic acid was quantified using an LC‐ESI‐MS‐MS system (Quattro LC, Waters) in negative ionization and multiple reaction‐monitoring mode.
Yeast one‐hybrid assay
The yeast one‐hybrid (Y1H) assay was carried out using the Matchmaker Gold Yeast One‐Hybrid System (Clontech). The three bait fragments (E1, E2, and E3 fragments) of the TPPE promoter were amplified by PCR and inserted into the pAbAi vector independently; this vector harbors the AbA r reporter gene. The constructs were linearized by BstBI digestion and transformed into Y1H Gold cells. The full‐length coding sequences of the ABF2 were cloned into the pGADT7 vector. The constructs were transformed into the Y1H bait strain and cultured on plates containing SD/‐Leu medium supplemented with 100 ng/mL Aureobasidin A (Clontech). The mE3 fragment was synthesized and used as a negative control.
Electrophoretic mobility shift assay
For EMSA, the full‐length cDNA of ABF2 was amplified and cloned into the pMAL‐c5E vector (New England Biolabs). The recombinant MBP‐ABF2 protein was purified from E. coli. Oligonucleotides based on the TPPE promoter were synthesized and labeled with biotin at the 3′‐end. Unlabeled versions of the same oligonucleotides were used as competitors. The probes were obtained by annealing using biotin‐labeled or unlabeled primers. Electrophoretic mobility shift assay was performed using a LightShift Chemiluminescent EMSA kit (Thermo Fisher Scientific). Briefly, a 20 μL reaction mixture containing 2 μL of binding buffer, 0.5 μL of poly (dI‐dC), 1 μg of purified fusion protein, and 1 μL of biotin‐labeled probe was incubated at room temperature for 20 min. For competition with unlabeled probes, unlabeled probes were added to the reactions, which were incubated for 20 min. The reactions mixture were resolved in 5% native polyacrylamide gels with 1 × TBE buffer. The assays were repeated three times.
Chromatin immunoprecipitation assay
Chromatin immunoprecipitation assay was performed as described previously (Wang et al. 2019a). Two‐week‐old 35S:ABF2‐GFP transgenic plants and 35S:GFP plants grown on ½ MS medium (5 g) were collected for chromatin extraction using a ChIP‐A kit (Millipore). The isolated chromatin was sonicated to produce 0.5–1.0 kb DNA fragments. The DNA fragments were purified and resuspended in double‐distilled water, and enriched DNA fragments were quantified by qPCR. Fold enrichment of each region in the 35S:ABF2‐GFP line was calculated compared to that in the 35S:GFP line.
Transient expression assays in Nicotiana benthamiana
The full‐length cDNA of ABF2 was inserted into the pCAMBIA1300 vector under the control of the 35S promoter, and the TPPE promoter fragment was cloned into the pCAMBIA1300‐LUC vector. For transient expression, plasmids were transferred into Agrobacterium strain GV3101. Nicotiana benthamiana plants were grown for 5‐6 weeks in a growth chamber. For infiltration, Agrobacterium cultures were grown overnight, collected by centrifugation (4,000 g), and resuspended in infiltration medium (10 mM MES, pH 5.6, 10 mM MgCl2 and 200 μM acetosyringone) to a final optical density at 600 nm (OD600) of 0.3. The suspensions were mixed and incubated for 2 h at room temperature on a horizontal rolling mixer. Co‐infiltration into the abaxial surfaces of N. benthamiana leaves was performed using a 1 mL needle‐free syringe. The infiltrated plants were incubated under a 10‐h light: 14‐h dark photoperiod at 22°C for 48 h to express LUC proteins. The LUC luminescence intensity of the infiltrated leaves was observed by bioluminescence imaging. In each experiment, 10 independent N. benthamiana leaves were infiltrated and analyzed.
Protoplast transformation and dual‐luciferase reporter assay
Protoplasts were isolated from the rosette leaves of 4‐week‐old WT plants as described (Yoo et al. 2007). pCAMBIA1300‐LUC vector containing four tandem copies of the ABRE‐containing fragment was used as reporter, and the 35S:ABF2‐GFP construct was used as an effector. The constructs were co‐transfected into protoplasts by polyethylene glycol‐mediated transfection. For ABA treatment, protoplasts were incubated overnight in 10 μM ABA after transfection. Firefly and Renilla luciferase activities were quantified using a dual‐luciferase assay kit (Promega) and detected using a Synergy 2 multi‐mode microplate reader (Bio‐Tek).
Measuring ROS in plants
Four‐d‐old WT, tppe‐1, tppe‐2, 35S:TPPE‐1, and 35S:TPPE‐2 seedlings were incubated in liquid ½ MS with or without 50 μM ABA for 4 h before staining. For NBT staining to detect superoxides, the seedlings were incubated in buffer containing 1 mM NBT (Sigma‐Aldrich), 20 mM K‐phosphate, and 0.1 M NaCl at pH 6.2 for 15 min. The seedlings were cleared by washing three times with water, transferred to a solution containing 7% NaOH and 60% ethanol, and incubated for 15 min at room temperature. The seedlings were further incubated for 10 min at 90°C in the following ethanol series: 50% ethanol, 70% ethanol, and 90% ethanol. The seedlings were examined under an Olympus BX53 microscope. For 2′, 7′‐dichlorodihydrofluorescein diacetate (H2DCFDA) staining to detect H2O2, the seedlings were incubated in buffer containing 5 μM H2DCFDA (Sigma‐Aldrich) and 20 mM PBS in the dark for 10 min. The roots were photographed under a Zeiss LSM510 META confocal microscope with excitation at 488 nm. The intensities of the fluorescent signals were statistically compared using Student's t‐test.
AUTHOR CONTRIBUTIONS
C.‐P.S. and X.Z. conceived and designed research; W.W., Q.C., and S.X. performed the experiments; W.‐C.L. interpreted and analyzed the results; C.‐P. S., W.W., and X.Z. analyzed the data and wrote the manuscript.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Supporting information
Additional Supporting Information may be found online in the supporting information tab for this article: http://onlinelibrary.wiley.com/doi/10.1111/jipb.12925/suppinfo
Supporting information.
Figure S1.
Expression levels of the genes in ABA biosynthetic pathway in the presence of exogenous trehalose
Ten‐d‐old WT seedlings grown on ½ MS medium were transferred to ½ MS liquid medium with or without trehalose (10 mM, 20 mM) for 12 h, and gene transcripts were analyzed by qRT‐PCR. Values show average ± SD (n = 3).
Figure S2.
Relative expression of TPPs induced by ABA
qRT‐PCR analysis reveals that TPPs expression is induced by ABA. Ten‐d‐old seedlings were treated with 50 μM ABA and collected for RNA extraction. Actin2/8 was used as an internal standard. Values are the mean ± SD of three independent biological replicates.
Figure S3.
Histochemical analysis of TPPE promoter activity under ABA treatments
(A) Ten‐d‐old proTPPE:GUS transgenic seedlings were treated by ABA (50 μM) for 4 h and then harvested for GUS staining. Scale bar, 1 mm. (B) Quantitative analysis of GUS activity in proTPPE:GUS transgenic seedlings under ABA treatments. Values are mean ± SD of three replicate experiments (Student's t‐test, **P < 0.01).
Figure S4.
Analysis of TPPE enzymatic catalytic activity
(A) Standard curve of phosphate derived from the reaction of catalytic activity of TPPE. (B) Comparison of TPPE catalytic activities with different substrates.
Figure S5.
Identification of tppe mutant and 35S:TPPE transgenic lines
(A) Schematic diagram of TPPE T‐DNA insertion lines. Black boxes are exons and lines between the boxes are introns. ATG and TGA are the start codon and termination codon, respectively. The position of the T‐DNA insertion is indicated by a triangle. (B) PCR analysis of the tppe insertion mutants. The genomic DNA products were PCR‐amplified using primer pairs LP + RP, LP + LBa1. (C) qRT‐PCR analysis of TPPE transcript levels in tppe mutants. (D) qRT‐PCR analysis of TPPE transcript levels in 35S:TPPE lines. Ten‐d‐old seedlings were used for qRT‐PCR analysis.
Figure S6.
The generation and phenotype analysis of the TPPE CRISPR/Cas9 mutants
(A) The schematic map of the gRNA targeted sites of TPPE. (B) The sequencing chromatograms show the positions of the deletion in tppe‐cas9‐1 and tppe‐cas9‐2 mutants. (C) Phenotypic analysis of WT and tppe‐cas9 lines under ABA treatment. Scale bar, 1 cm. (D) Statistical analysis of the root length corresponding to (C). Error bars indicate ± SD (n = 9), *P < 0.05, **P < 0.01. (E) Water loss from the detached leaves of WT, tppe‐cas9‐1 and tppe‐cas9‐2. The experiments were repeated three times with similar results. Each data point represents the means ± SD (n = 3).
Figure S7.
Phenotype analysis of abf2 mutant and ABF2 overexpression lines
(A) Schematic diagram of T‐DNA insertion lines of abf2. Black boxes are exons, and lines between the boxes are introns. ATG and TGA are the start codon and termination codon. (B) Identification of abf2 mutants by PCR. The genomic DNA products were PCR‐amplified using primer pairs LP + RP, LP + LBa1. (C) qRT‐PCR analysis of the expression of ABF2 in mutant and overexpression lines. (D) The root length of mutants and overexpression lines under ABA treatment. The values are means ± SD (n > 10). Different letters indicate statistical differences at P < 0.05 (one‐way ANOVA). (E) The expression of TPPE and ABF2 in double mutants. The gene expression was detected by qRT‐PCR.
Figure S8.
ABA induces ROS production in the guard cells of WT, tppe‐1, tppe‐2, 35S:TPPE‐1 and 35S:TPPE‐2 plants
(A) H2DCFDA staining for ROS in guard cells, Scale bar, 10 μm. (B) The intensity of the fluorescence signal was measured by Image J. The values are means ± SD (n > 10). Different letters indicate statistical differences at P < 0.05 (one‐way ANOVA).
ACKNOWLEDGEMENTS
We thank Professor Qijun Chen (China Agricultural University) and Dr. Huili Xing (China Agricultural University) for providing the CRISPR/Cas9 binary vector pHSN401. We are grateful to Dr. Yuan Zheng for providing the pCAMBIA1300‐LUC vector. This work was supported by the Ministry of Agriculture of China (2016ZX08009003‐002) and Key Project of Natural Science Foundation of China (U1604233).
Edited by: Jin‐Song Zhang, Institute of Genetics and Developmental Biology, CAS, China
Online on Mar. 13, 2020
REFERENCES
- Adams S, Grundy J, Veflingstad SR, Dyer NP (2018) Circadian control of abscisic acid biosynthesis and signalling pathways revealed by genome‐wide analysis of LHY binding targets. New Phytol 220: 893–907 [DOI] [PubMed] [Google Scholar]
- Avonce N, Mendoza‐Vargas A, Morett E, Iturriaga G (2006) Insights on the evolution of trehalose biosynthesis. BMC Evol Biol 6: 109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SS, Wilhelm KS, Thomashow MF (1994) The 5'‐region of Arabidopsis thaliana cor15a has cis‐acting elements that confer cold‐, drought‐ and ABA‐regulated gene expression. Plant Mol Biol 24: 701–713 [DOI] [PubMed] [Google Scholar]
- Belbin FE, Dodd AN (2018) ABA signalling is regulated by the circadian clock component LHY. New Phytol 220: 661–663 [DOI] [PubMed] [Google Scholar]
- Carillo P, Feil R, Gibon Y, Satoh‐Nagasawa N, Jackson D, Blasing OE, Stitt M, Lunn JE (2013) A fluorometric assay for trehalose in the picomole range. Plant Methods 9: 21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chary SN, Hicks GR, Choi YG, Carter D, Raikhel NV (2008) Trehalose‐6‐phosphate synthase/phosphatase regulates cell shape and plant architecture in Arabidopsis. Plant Physiol 146: 97–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H, Hong J, Ha J, Kang J, Kim SY (2000) ABFs, a family of ABA‐responsive element binding factors. J Biol Chem 275: 1723–1730 [DOI] [PubMed] [Google Scholar]
- Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR (2010) Abscisic acid: Emergence of a core signaling network. Annu Rev Plant Biol 61: 651–679 [DOI] [PubMed] [Google Scholar]
- Delorge I, Janiak M, Carpentier S, Van Dijck P (2014) Fine tuning of trehalose biosynthesis and hydrolysis as novel tools for the generation of abiotic stress tolerant plants. Front Plant Sci 5: 147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Bai L, Zhang Y, Zhang G, Mao Y, Min L, Xiang F, Qian D, Zhu X, Song CP (2018) Modulation of guard cell turgor and drought tolerance by a peroxisomal acetate‐malate shunt. Mol Plant 11: 1278–1291 [DOI] [PubMed] [Google Scholar]
- Elbein AD, Pan YT, Pastuszak I, Carroll D (2003) New insights on trehalose: A multifunctional molecule. Glycobiol 13: 17R–27R [DOI] [PubMed] [Google Scholar]
- Endo A, Sawada Y, Takahashi H, Okamoto M, Ikegami K, Koiwai H, Seo M, Toyomasu T, Mitsuhashi W, Shinozaki K, Nakazono M, Kamiya Y, Koshiba T, Nambara E (2008) Drought induction of Arabidopsis 9‐cis‐epoxycarotenoid dioxygenase occurs in vascular parenchyma cells. Plant Physiol 147: 1984–1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa CM (2016) A tale of two sugars: Trehalose 6‐phosphate and sucrose. Plant Physiol 172: 7–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii H, Chinnusamy V, Rodrigues A, Rubio S, Antoni R, Park SY, Cutler SR, Sheen J, Rodriguez PL, Zhu JK (2009) In vitro reconstitution of an abscisic acid signalling pathway. Nature 462: 660–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y, Yoshida T, Yamaguchi‐Shinozaki K (2013) Pivotal role of the AREB/ABF‐SnRK2 pathway in ABRE‐mediated transcription in response to osmotic stress in plants. Physiol Plant 147: 15–27 [DOI] [PubMed] [Google Scholar]
- Ge LF, Chao DY, Shi M, Zhu MZ, Gao JP, Lin HX (2008) Overexpression of the trehalose‐6‐phosphate phosphatase gene OsTPP1 confers stress tolerance in rice and results in the activation of stress responsive genes. Planta 228: 191–201 [DOI] [PubMed] [Google Scholar]
- Gomez LD, Gilday A, Feil R, Lunn JE, Graham IA (2010) AtTPS1‐mediated trehalose 6‐phosphate synthesis is essential for embryogenic and vegetative growth and responsiveness to ABA in germinating seeds and stomatal guard cells. Plant J 64: 1–13 [DOI] [PubMed] [Google Scholar]
- Han B, Fu L, Zhang D, He X, Chen Q, Peng M, Zhang J (2016) Interspecies and intraspecies analysis of trehalose contents and the biosynthesis pathway gene family reveals crucial roles of trehalose in osmotic‐stress tolerance in cassava. Int J Mol Sci 17: 1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsa CG, Brandt EV, Thevelein J, Hohmann S, Prior BA (1998) Role of trehalose in survival of Saccharomyces cerevisiae under osmotic stress. Microbiology 144: 671–680 [DOI] [PubMed] [Google Scholar]
- Iuchi S, Kobayashi M, Taji T, Naramoto M, Seki M, Kato T, Tabata S, Kakubari Y, Yamaguchi‐Shinozaki K, Shinozaki K (2001) Regulation of drought tolerance by gene manipulation of 9‐cis‐epoxycarotenoid dioxygenase, a key enzyme in abscisic acid biosynthesis in Arabidopsis. Plant J 27: 325–333 [DOI] [PubMed] [Google Scholar]
- Karim S, Aronsson H, Ericson H, Pirhonen M, Leyman B, Welin B, Mantyla E, Palva ET, Van Dijck P, Holmstrom KO (2007) Improved drought tolerance without undesired side effects in transgenic plants producing trehalose. Plant Mol Biol 64: 371–386 [DOI] [PubMed] [Google Scholar]
- Kim S, Kang JY, Cho DI, Park JH, Kim SY (2004) ABF2, an ABRE‐binding bZIP factor, is an essential component of glucose signaling and its overexpression affects multiple stress tolerance. Plant J 40: 75–87 [DOI] [PubMed] [Google Scholar]
- Kondrak M, Marincs F, Antal F, Juhasz Z, Banfalvi Z (2012) Effects of yeast trehalose‐6‐phosphate synthase 1 on gene expression and carbohydrate contents of potato leaves under drought stress conditions. BMC Plant Biol 12: 74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondrak M, Marincs F, Kalapos B, Juhasz Z, Banfalvi Z (2011) Transcriptome analysis of potato leaves expressing the trehalose‐6‐phosphate synthase 1 gene of yeast. PLoS One 6: e23466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasensky J, Broyart C, Rabanal FA, Jonak C (2014) The redox‐sensitive chloroplast trehalose‐6‐phosphate phosphatase AtTPPD regulates salt stress tolerance. Antioxid Redox Signal 21: 1289–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HW, Zang BS, Deng XW, Wang XP (2011) Overexpression of the trehalose‐6‐phosphate synthase gene OsTPS1 enhances abiotic stress tolerance in rice. Planta 234: 1007–1018 [DOI] [PubMed] [Google Scholar]
- Li P, Ma S, Bohnert HJ (2008) Coexpression characteristics of trehalose‐6‐phosphate phosphatase subfamily genes reveal different functions in a network context. Physiol Plant 133: 544–556 [DOI] [PubMed] [Google Scholar]
- Lin Q, Yang J, Wang Q, Zhu H, Chen Z, Dao Y, Wang K (2019) Overexpression of the trehalose‐6‐phosphate phosphatase family gene AtTPPF improves the drought tolerance of Arabidopsis thaliana . BMC Plant Biol 19: 381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunn JE, Delorge I, Figueroa CM, Van Dijck P, Stitt M (2014) Trehalose metabolism in plants. Plant J 79: 544–567 [DOI] [PubMed] [Google Scholar]
- Ma S, Gong Q, Bohnert HJ (2007) An Arabidopsis gene network based on the graphical Gaussian model. Genome Res 17: 1614–1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda JA, Avonce N, Suarez R, Thevelein JM, Van Dijck P, Iturriaga G (2007) A bifunctional TPS‐TPP enzyme from yeast confers tolerance to multiple and extreme abiotic‐stress conditions in transgenic Arabidopsis. Planta 226: 1411–1421 [DOI] [PubMed] [Google Scholar]
- Munemasa S, Hauser F, Park J, Waadt R, Brandt B, Schroeder JI (2015) Mechanisms of abscisic acid‐mediated control of stomatal aperture. Curr Opin Plant Biol 28: 154–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hara LE, Paul MJ, Wingler A (2013) How do sugars regulate plant growth and development? New insight into the role of trehalose‐6‐phosphate. Mol Plant 6: 261–274 [DOI] [PubMed] [Google Scholar]
- Paul MJ, Gonzalez‐Uriarte A (2018) The role of trehalose 6‐phosphate in crop yield and resilience. Plant Physiol 177: 12–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul MJ, Primavesi LF, Jhurreea D, Zhang Y (2008) Trehalose metabolism and signaling. Annu Rev Plant Biol 59: 417–441 [DOI] [PubMed] [Google Scholar]
- Pramanik MHR, Imai R (2005) Functional identification of a trehalose 6‐phosphate phosphatase gene that is involved in transient induction of trehalose biosynthesis during chilling stress in rice. Plant Mol Biol 58: 751–762 [DOI] [PubMed] [Google Scholar]
- Ramon M, De Smet I, Vandesteene L, Naudts M, Leyman B, Van Dijck P, Rolland F, Beeckman T, Thevelein JM (2009) Extensive expression regulation and lack of heterologous enzymatic activity of the Class II trehalose metabolism proteins from Arabidopsis thaliana . Plant Cell Environ 32: 1015–1032 [DOI] [PubMed] [Google Scholar]
- Romero C, Belles JM, Vaya JL, Serrano R, Culianez‐Macia FA (1997) Expression of the yeast trehalose‐6‐phosphate synthase gene in transgenic tobacco plants: Pleiotropic phenotypes include drought tolerance. Planta 201: 293–297 [DOI] [PubMed] [Google Scholar]
- Shi Y, Sun H, Wang X, Jin W, Chen Q, Yuan Z, Yu H (2019) Physiological and transcriptomic analyses reveal the molecular networks of responses induced by exogenous trehalose in plant. PLoS One 14: e0217204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Houtte H, Lopez‐Galvis L, Vandesteene L, Beeckman T, Van Dijck P (2013) Redundant and non‐redundant roles of the trehalose‐6‐phosphate phosphatases in leaf growth, root hair specification and energy‐responses in Arabidopsis. Plant Signal Behav 8: e23209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandesteene L, Lopez‐Galvis L, Vanneste K, Feil R, Maere S, Lammens W, Rolland F, Lunn JE, Avonce N, Beeckman T, Van Dijck P (2012) Expansive evolution of the trehalose‐6‐phosphate phosphatase gene family in Arabidopsis. Plant Physiol 160: 884–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandesteene L, Ramon M, Le Roy K, Van Dijck P, Rolland F (2010) A single active trehalose‐6‐P synthase (TPS) and a family of putative regulatory TPS‐like proteins in Arabidopsis. Mol Plant 3: 406–419 [DOI] [PubMed] [Google Scholar]
- Vogel G, Aeschbacher RA, Muller J, Boller T, Wiemken A (1998) Trehalose‐6‐phosphate phosphatases from Arabidopsis thaliana: Identification by functional complementation of the yeast tps2 mutant. Plant J 13: 673–683 [DOI] [PubMed] [Google Scholar]
- Wang W, Chen Q, Botella JR, Guo S (2019a) Beyond light: Insights into the role of constitutively photomorphogenic1 in plant hormonal signaling. Front Plant Sci 10: 557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Guo C, Peng J, Li C, Wan F, Zhang S, Zhou Y, Yan Y, Qi L, Sun K, Yang S, Gong Z, Li J (2019b) ABRE‐BINDING FACTORS play a role in the feedback regulation of ABA signaling by mediating rapid ABA induction of ABA co‐receptor genes. New Phytol 221: 341–355 [DOI] [PubMed] [Google Scholar]
- Yamaguchi‐Shinozaki K, Shinozaki K (1994) A novel cis‐acting element in an Arabidopsis gene is involved in responsiveness to drought, low‐temperature, or high‐salt stress. Plant Cell 6: 251–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HL, Liu YJ, Wang CL, Zeng QY (2012) Molecular evolution of trehalose‐6‐phosphate synthase (TPS) gene family in Populus, Arabidopsis and rice. PLoS One 7: e42438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Zhao X, Zhu H, Paul M, Zu Y, Tang Z (2014) Exogenous trehalose largely alleviates ionic unbalance, ROS burst, and PCD occurrence induced by high salinity in Arabidopsis seedlings. Front Plant Sci 5: 570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SD, Cho YH, Sheen J (2007) Arabidopsis mesophyll protoplasts: A versatile cell system for transient gene expression analysis. Nat Protoc 2: 1565–1572 [DOI] [PubMed] [Google Scholar]
- Yoshida T, Fujita Y, Maruyama K, Mogami J, Todaka D, Shinozaki K, Yamaguchi‐Shinozaki K (2015) Four Arabidopsis AREB/ABF transcription factors function predominantly in gene expression downstream of SnRK2 kinases in abscisic acid signalling in response to osmotic stress. Plant Cell Environ 38: 35–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Fujita Y, Sayama H, Kidokoro S, Maruyama K, Mizoi J, Shinozaki K, Yamaguchi‐Shinozaki K (2010) AREB1, AREB2, and ABF3 are master transcription factors that cooperatively regulate ABRE‐dependent ABA signaling involved in drought stress tolerance and require ABA for full activation. Plant J 61: 672–685 [DOI] [PubMed] [Google Scholar]
- Yoshida T, Mogami J, Yamaguchi‐Shinozaki K (2014) ABA‐dependent and ABA‐independent signaling in response to osmotic stress in plants. Curr Opin Plant Biol 21: 133–139 [DOI] [PubMed] [Google Scholar]
- Zandkarimi H, Ebadi A, Salami SA, Alizade H, Baisakh N (2015) Analyzing the expression profile of AREB/ABF and DREB/CBF genes under drought and salinity stresses in grape (Vitis vinifera L.). PLoS One 10: e0134288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Li J, Li F, Liu H, Yang W, Chong K, Xu Y (2017) OsMAPK3 phosphorylates OsbHLH002/OsICE1 and inhibits its ubiquitination to activate OsTPP1 and enhances rice chilling tolerance. Dev Cell 43: 731–743 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found online in the supporting information tab for this article: http://onlinelibrary.wiley.com/doi/10.1111/jipb.12925/suppinfo
Supporting information.
Figure S1.
Expression levels of the genes in ABA biosynthetic pathway in the presence of exogenous trehalose
Ten‐d‐old WT seedlings grown on ½ MS medium were transferred to ½ MS liquid medium with or without trehalose (10 mM, 20 mM) for 12 h, and gene transcripts were analyzed by qRT‐PCR. Values show average ± SD (n = 3).
Figure S2.
Relative expression of TPPs induced by ABA
qRT‐PCR analysis reveals that TPPs expression is induced by ABA. Ten‐d‐old seedlings were treated with 50 μM ABA and collected for RNA extraction. Actin2/8 was used as an internal standard. Values are the mean ± SD of three independent biological replicates.
Figure S3.
Histochemical analysis of TPPE promoter activity under ABA treatments
(A) Ten‐d‐old proTPPE:GUS transgenic seedlings were treated by ABA (50 μM) for 4 h and then harvested for GUS staining. Scale bar, 1 mm. (B) Quantitative analysis of GUS activity in proTPPE:GUS transgenic seedlings under ABA treatments. Values are mean ± SD of three replicate experiments (Student's t‐test, **P < 0.01).
Figure S4.
Analysis of TPPE enzymatic catalytic activity
(A) Standard curve of phosphate derived from the reaction of catalytic activity of TPPE. (B) Comparison of TPPE catalytic activities with different substrates.
Figure S5.
Identification of tppe mutant and 35S:TPPE transgenic lines
(A) Schematic diagram of TPPE T‐DNA insertion lines. Black boxes are exons and lines between the boxes are introns. ATG and TGA are the start codon and termination codon, respectively. The position of the T‐DNA insertion is indicated by a triangle. (B) PCR analysis of the tppe insertion mutants. The genomic DNA products were PCR‐amplified using primer pairs LP + RP, LP + LBa1. (C) qRT‐PCR analysis of TPPE transcript levels in tppe mutants. (D) qRT‐PCR analysis of TPPE transcript levels in 35S:TPPE lines. Ten‐d‐old seedlings were used for qRT‐PCR analysis.
Figure S6.
The generation and phenotype analysis of the TPPE CRISPR/Cas9 mutants
(A) The schematic map of the gRNA targeted sites of TPPE. (B) The sequencing chromatograms show the positions of the deletion in tppe‐cas9‐1 and tppe‐cas9‐2 mutants. (C) Phenotypic analysis of WT and tppe‐cas9 lines under ABA treatment. Scale bar, 1 cm. (D) Statistical analysis of the root length corresponding to (C). Error bars indicate ± SD (n = 9), *P < 0.05, **P < 0.01. (E) Water loss from the detached leaves of WT, tppe‐cas9‐1 and tppe‐cas9‐2. The experiments were repeated three times with similar results. Each data point represents the means ± SD (n = 3).
Figure S7.
Phenotype analysis of abf2 mutant and ABF2 overexpression lines
(A) Schematic diagram of T‐DNA insertion lines of abf2. Black boxes are exons, and lines between the boxes are introns. ATG and TGA are the start codon and termination codon. (B) Identification of abf2 mutants by PCR. The genomic DNA products were PCR‐amplified using primer pairs LP + RP, LP + LBa1. (C) qRT‐PCR analysis of the expression of ABF2 in mutant and overexpression lines. (D) The root length of mutants and overexpression lines under ABA treatment. The values are means ± SD (n > 10). Different letters indicate statistical differences at P < 0.05 (one‐way ANOVA). (E) The expression of TPPE and ABF2 in double mutants. The gene expression was detected by qRT‐PCR.
Figure S8.
ABA induces ROS production in the guard cells of WT, tppe‐1, tppe‐2, 35S:TPPE‐1 and 35S:TPPE‐2 plants
(A) H2DCFDA staining for ROS in guard cells, Scale bar, 10 μm. (B) The intensity of the fluorescence signal was measured by Image J. The values are means ± SD (n > 10). Different letters indicate statistical differences at P < 0.05 (one‐way ANOVA).


