Abstract
Objective
Sex steroid hormones potently shape brain functions, including those critical to maintain mental health such as serotonin signaling. Use of oral contraceptives (OCs) profoundly changes endogenous sex steroid hormone levels and dynamics. Recent register‐based studies show that starting an OC is associated with increased risk of developing depression. Here, we investigate whether use of OCs in healthy women is associated with a marker of the serotonin system in terms of serotonin 4 receptor (5‐HT4R) brain imaging.
Methods
[11C]SB207145‐PET imaging data on 53 healthy women, of whom 16 used OCs, were available from the Cimbi database. We evaluated global effects of OC use on 5‐HT4R binding in a latent variable model based on 5‐HT4R binding across cortical and subcortical regions.
Results
We demonstrate that OC users have 9–12% lower global brain 5‐HT4R binding potential compared to non‐users. Univariate region‐based analyses (pallidostriatum, caudate, hippocampus, amygdala, anterior cingulate cortex, and neocortex) supported the global effect of OC use with the largest difference present in the hippocampus (−12.8% (95% CI [−21.0; −3.9], P corrected = 0.03).
Conclusion
We show that women who use OCs have markedly lower brain 5‐HT4R binding relative to non‐users, which constitutes a plausible molecular link between OC use and increased risk of depressive episodes. We propose that this reflects a reduced 5‐HT4R gene expression, possibly related to a blunted ovarian hormone state among OC users.
Keywords: oral contraceptives, (11C)SB207145, serotonin 4 receptor, sex steroid hormones, major depressive disorder
Significant outcomes.
Healthy women who use oral contraceptives have 9–12% lower global brain serotonin 4 receptor binding potential compared to non‐users.
The lower serotonin 4 receptor binding potential provides a plausible molecular mechanism by which oral contraceptive use can be associated with increased risk of developing depression.
Compared to previous studies, the effect size of oral contraceptive use is almost twice as big as the effect size of selective serotonin reuptake inhibitors (SSRIs) on serotonin 4 receptor binding potential. This raises the question if concomitant oral contraceptive use affects SSRI antidepressant efficacy.
Limitations.
The study is cross‐sectional, and thus, causality cannot be inferred.
We only included healthy women, which increases the risk of 'survival effect' in the oral contraceptive user group, and hence potentially reduces the effect size of oral contraceptive use on serotonin 4 receptor binding potential.
Introduction
More than 100 million women worldwide use oral contraceptives (OCs) (1, 2), and indeed, they have profound implications for women's health including the avoidance of unwanted pregnancies and abortions as well as menstrual cycle disturbances (3). However, not all women tolerate OCs; some are at excessive risk of thromboembolic events (4), and others are forced to discontinue OC use because of experienced side effects including mood deterioration (5). Recently, register‐based epidemiological work has shown that starting on hormonal contraceptives is associated with an increased risk of developing a depressive episode, based on either subsequent first‐time use of an antidepressant or receiving a first diagnosis of major depressive disorder (MDD) (6, 7), including the severe cases that involve suicide (8). Additionally, these studies point out that adolescents were at higher risk relative to women older than 20 years, which has since been replicated in an independent prospective cohort study (9). Further, another study suggests that first time use of OCs in adolescence compared to in adulthood is associated with longer lasting vulnerability to MDD through adulthood (10). The literature, however, shows conflicting results as smaller studies have reported positive or no mood effect of OC use (11). Nevertheless, as adolescent females start on OCs at an increasingly young age (12) their brains are more susceptible to the potential impact of exogenous hormones as they go through critical stages of brain maturation (13). This emphasizes the importance of investigating how OCs may affect brain biology, which is putatively critical to mental health.
It remains unknown why use of OCs may increase the risk of a depressive episode, at least in a subgroup of women and how these women may be identified. Previous findings indicate that OC use can affect brain structure (14) and function (15), which is important for affective control such as fear processing (16), and further OC use distorts the hypothalamic–pituitary–adrenal axis into a state comparable to chronic stress (16, 17). However, to our knowledge, the effects of OC use on neurotransmission, including the serotonin brain system, are very scarcely studied.
OCs suppress the hypothalamic–pituitary–gonadal hormonal axis, which drives the reproductive system, and consequently downregulates endogenous ovarian sex steroid hormone production and disrupts follicular maturation and ovulation (18). This suppression is profound and clearly visible by ultrasound where OC use may halve the size of the ovaries (18). The synthetic OC steroids, that is, ethinyl estradiol and a generation‐specific progestin, substitute the endogenous hormones only to some extent; in OC users, the blood concentration of total estrogen (exogenous + endogenous) is considerably low compared to in non‐users, but when considering the relative binding affinity for the estradiol receptor (ER), the average daily estrogen exposure compares to the natural exposure. In contrast, the progesterone exposure can be eight‐fold increased (19). However, the synthetic steroids upregulate the level of sex hormone‐binding globulin, which reduces the freely available sex steroid hormones (20). Such changes in sex steroid milieu can shape human brain biology, including the serotonergic system (21, 22), which is key in maintaining mental health and is critically involved in the risk for and treatment of MDD (23). Estradiol, in particular, directly targets serotonergic neurons and affects key features of the serotonin signaling system (21) including gene expression of the serotonin transporter, the main regulator of synaptic serotonin (24).
One of the most recent advances in serotonin brain positron emission tomography (PET) imaging is the quantification of the postsynaptic serotonin 4 receptor (5‐HT4R) by the [11C]SB207145‐PET radioligand (25). The 5‐HT4R is itself a potential target in MDD as 5‐HT4R stimulation has fast acting antidepressant‐like properties (26), and further, it is distributed abundantly throughout subcortical regions (27) along with sex hormone receptors (22). Animal and human studies suggest that 5‐HT4R binding is inversely correlated to serotonergic brain tonus (28, 29, 30). Thus, 5‐HT4R brain binding constitutes an informative marker of serotonergic neurotransmission and provides an opportunity to elucidate a potential molecular link between OC use and risk of developing MDD.
Aims of the study
In this cross‐sectional study, we investigate whether healthy women who use oral contraceptives differ relative to non‐users in terms of global serotonin 4 receptor binding in the brain.
Material and Methods
Participants
[11C]SB207145‐PET neuroimaging binding potential data and associated data from a total of 74 healthy women were extracted from the Center for Integrated Molecular Brain Imaging (Cimbi) database (1). Participants had been recruited for different neuroimaging projects between 2006 and 2018. All projects had been approved by the Ethics Committee of Copenhagen and Frederiksberg or of RegionH, Denmark ((KF)01‐274821, (KF)01‐2006‐20, H‐15004506, H‐1‐2010‐085, H‐4‐2012‐105, H‐6‐2014‐057, H‐15017713). Participants had normal physical and neurological examinations as well as unremarkable brain magnetic resonance imaging (MRI) scans. Women were not included if they were pregnant or breastfeeding and had a significant medical history including psychiatric or neurological diseases or dependence of drugs or alcohol. Of the 74 women from the database, we included 61 women with available information about hormonal contraceptive use, younger than 50 years (i.e., presumably premenopausal), and body mass index (BMI) <30 kg/m2, as large deposits of fat tissue produce estrogen (31). Further, one participant was excluded because of polycystic ovary syndrome, two because of PET‐tracer doses that exceeded our upper limit of cold mass injected (32), and four because of recent (≤2 months) discontinuation of OCs or vaginal ring (33). Finally, one OC user was excluded because her progesterone levels were 24.5 nmol/l, indicating non‐compliance (18). Thus, 53 healthy women were available for analyses, of which 16 used OCs.
Women using a hormonal intrauterine device (IUD) were included as non‐users because the majority are expected to have a normal ovulatory cycle (34). In a supplementary analysis, we tested if they differed from other non‐users in terms of 5‐HT4R binding potentials, which was not the case (Table S1). Screening for MDD and mood disturbances as well as other psychopathology was done using the 10 item Major Depression Inventory (MDI) questionnaire (35), the Cohen's Perceived Stress Scale, the Profile of Mood States‐Total Mood Disturbance (POMS‐TMD), and the Symptom Checklist‐92 Global Severity Index (SCL‐92 GSI). Familial risk for MDD was acquired through the Family History Assessment Module (FHAM) questionnaire (36). Neuroticism, a personality trait associated with MDD (37), was assessed via the Danish version of the five‐factor NEO Personality Inventory‐Revised (NEO PI‐R) (38). Educational level was scored via a 5‐point Likert scale, scoring one if having no vocational degree and five if having more than four years of higher academic education. Information about relationship status was acquired on the scan date via interview.
Oral contraceptives
Information about use of hormonal contraceptives was achieved by face‐to‐face interview at the day of PET scan and by written questionnaires including a question about the type of OC used. Those with missing information were contacted at a later time point by e‐mail or phone to specify type of OC used and in two cases if OC was used at time of the scan. The type of OC is known for 12 out of the 16 users, all of which were 'combined OCs'; 2nd and 3rd generation combined OCs containing ethinylestradiol in combination with levonorgestrel or gestodene (Table S2).
Plasma levels of sex steroid hormones
Measurements of sex steroid hormones were included in the standard program for healthy volunteers as of March 2011 (1), and thus, data were available for 42 (11 OC users and 31 non‐users) of the 53 women. However, because of changes in hospital analysis method of plasma sex steroids (change of antibody reagents from Estradiol II/Progesterone II to Elecsys® Estradiol III/Progesterone III on Cobas 8000 e602 module, Roche), 11 progesterone measurements were excluded because the analysis methods were non‐compatible resulting in 31 included samples (9 OC users and 22 non‐users). Estradiol measurements were compatible between the analysis methods by use of a conversion factor (validation reports can be obtained upon request). The new method resulted in a higher lower detection limit for the plasma estradiol level (0.09 nmol/l compared to 0.04 nmol/l). For the descriptive statistics, the estradiol samples below the detection limit (10 out of the 42 measurements) were imputed to the respective lower limit value and an extended version of Wilcoxon test, the Gehan test (39), was performed to compare the plasma estradiol concentrations between the groups.
Imaging
The acquisition of scans and image analysis is detailed elsewhere (25). A brief overview is presented here. High‐resolution T1‐weighted structural MR images were acquired on three different scanners: Siemens MAGNETOM 3T MRI scanners (Prisma (n = 41), Verio (n = 2), and Trio (n = 10)). MR images were segmented into cerebrospinal fluid, gray matter, and white matter by use of Statistical Parametric Mapping (SPM5 or SPM8, The Wellcome Centre for Human Neuroimaging, UCL, London, UK). Individual PET and MR images for each subject were co‐registered using the automatic methods from SPM5 or SPM8. Regions were automatically delineated using each subject's MR image via the user‐independent algorithm in the Pvelab software package (40). Regions of interest included hippocampus, amygdala, anterior cingulate cortex (ACC), neocortex, pallidostriatum, and caudate. The former four regions were chosen because of their high expression level of sex hormone receptors and sensitivity to hormonal changes (22), and the latter two because they have a high density of 5‐HT4Rs (27).
[11C]SB207145‐PET scans were acquired either on an 18‐ring GE‐Advance scanner (General Electric, Milwaukee, WI, USA) (n = 6) with an approximate in‐plane resolution of 6 mm, or a High‐Resolution Research Tomograph (HRRT) PET scanner (CTI/Siemens, Knoxville, TN, USA) (n = 47) with a 1.4‐mm resolution (centre of field of view). All scans were obtained from a 120‐minute dynamic acquisition starting immediately after bolus injection of [11C]SB207145. Framing of the dynamic scans and procedures for head motion correction are described elsewhere (28). Non‐displaceable binding potentials (BPND) were estimated using the simplified reference tissue model with cerebellum as reference tissue (25) using an in‐house batch‐mode algorithm validated against kinetic modeling with PMOD software version 2.95 (PMOD Inc., Zurich, Switzerland, http://www.pmod.com/web).
For illustrative purposes, PXMOD was used to generate single‐subject voxel‐based parametric BPND images. For this, we only used the HRRT PET images (n = 47), which were smoothened across adjacent voxels by applying a Gaussian filter within each time frame of the dynamic PET image and were normalized into standard space with SPM12 and visualized in FreeView (FreeSurfer software, http://surfer.nmr.mgh.harvard.edu/, v6.0.0). A percent difference map was generated by fitting our multiple linear regression model (described below) at each voxel, with mean‐centered continuous covariates, and dividing the voxel‐specific marginal OC use effect by its model intercept, multiplied by 100.
Statistics
We evaluated whether OC use is associated with a global pattern of 5‐HT4R brain binding in a linear latent variable model (LVM) framework where the effect of OC use is mediated through a single latent variable across brain regions of low (neocortex), intermediate (ACC, hippocampus, and amygdala), and high (pallidostriatum and caudate) 5‐HT4R binding (27). The latent variable enables to model the large correlation in 5‐HT4R binding between brain regions (41). Covariates included age, scanner type (GE‐Advance PET scanner vs. HRRT Siemens PET scanner), injected [11C]SB207145 mass per kg bodyweight, and familial risk for MDD (as binary factor, i.e., having none vs. one or more first‐degree relatives with MDD), which are known to influence 5‐HT4R PET measurements (42, 43, 44). This adjustment was done independently in each region, that is, not mediated through the latent variable.
To support the LVM and provide a more conceptually accessible description of the effect of OC use on 5‐HT4R brain binding, we also present a univariate multiple linear regression analysis of the association between OC use and 5‐HT4R BPND in the six regions of interest. These models included the same covariates as the LVM. In both models, 5‐HT4R BPND values were log‐transformed prior to modeling and the estimated OC effect on the regional binding is expressed as a percent difference in 5‐HT4R binding between OC users and non‐users.
Diagnostic tools were used to assess the adequacy of the model's assumptions. In case of unequal residual variance, the linear model was re‐estimated while allowing for different residual variances between the groups. For the LVM, the specification of the covariance structure was assessed using a chi‐squared test. When suboptimal specification was indicated (chi‐square P < 0.05), score tests (see section 6.2.4 in (45)) were used to identify covariance parameters to include in the model. This procedure was iteratively performed until the chi‐squared test was no longer significant (chi‐square P > 0.05). Two‐sided statistical tests were used, and P < 0.05 was considered statistically significant. When testing the OC effect on each region separately, P‐values and confidence intervals were adjusted for multiple comparisons using the single‐step Dunnett's procedure (46). Statistical analyses were performed in R (47), and the lava package (45) was used to create the LVM.
Results
Study population profile
Demographic, psychometric, endogenous hormone profiles, and PET parameters of the study population are shown in Table 1 . The groups (OC user vs. non‐user) were similar in age, BMI, educational level, relationship status, and neuroticism score. However, the OC user group tended to have more first‐degree relatives with MDD (P = 0.05). Since this may be a relevant confounder (43), accordingly, it was taken into consideration in the regression model evaluating differences in 5‐HT4R between groups. The study population scored within the range of a mentally healthy population in terms of no signs of depression; that is, MDI averages 4.8 (range 0–12) (48), and no signs of other psychopathology evaluated by the global severity index (SLC‐92 GSI) (49). Plasma estradiol levels in OC users (mean ± standard deviation: 0.09 ± 0.06 nmol/l) were lower relative to non‐users (mean ± standard deviation: 0.46 ± 0.40 nmol/l), P < 0.001. Progesterone levels were also reduced in OC users (P = 0.04).
Table 1.
Clinical profiles and PET parameters
| Clinical parameters | Non‐users (n = 37) | OC users (n = 16) | P‐value (OC users vs. non‐users)† | ||
|---|---|---|---|---|---|
| HC non‐users (n = 29) (SD) | Hormonal IUD users (n = 8) (SD) | (SD) | n | ||
| Age | 26.9 (6.4) | 24.5 (2.0) | 25.8 (4.2) | 0.71 | 53 |
| BMI | 23.3 (2.8) | 23.9 (3.3) | 22.2 (2.7) | 0.13 | 53 |
| Educational level | 4.1 (1.4) | 4.1 (1.5) | 4.2 (1.1) | 0.75 | 53 |
| Individuals in relationship | 7 (26.9%) | 3 (37.5%) | 3 (25.0%) | 1.0 | 46 |
| Neuroticism score | 89.0 (19.9) | 82.9 (19.3) | 87.4 (21.6) | 0.97 | 53 |
| Individuals with familial risk for MDD | 4 (13.8%) | 0 (0.0%) | 6 (37.5%) | 0.05 | 53 |
| Cohen's PSS | 9.4 (5.5) | 8.6 (5.3) | 10.1 (6.5) | 0.66 | 51 |
| MDI | 4.9 (3.2) | 4.8 (4.7) | 4.7 (3.1) | 0.85 | 53 |
| SCL‐92 GSI | 0.2 (0.2) | 0.4 (0.2) | 0.2 (0.2) | 0.83 | 46 |
| POMS‐TMD | 2.5 (18.6) | 2.0 (18.3) | 0.3 (12.3) | 0.66 | 47 |
| P‐estradiol (nmol/l) | 0.5 (0.4) | 0.4 (0.5) | 0.09 (0.06) | <0.001 | 42 |
| P‐progesterone (nmol/l) | 3.4 (4.3) | 1.5 (0.5) | 1.2 (0.6) | 0.04 | 31 |
| PET parameters | |||||
| Individuals with HRRT scans | 26 (89.7%) | 8 (100.0%) | 13 (81.2%) | 0.35 | 53 |
| Injected dose (MBq) | 556.9 (71.1) | 592.8 (13.7) | 561.5 (105.8) | 0.91 | 53 |
| Injected SB mass/kg (μg/kg) | 0.03 (0.02) | 0.01 (0.002) | 0.02 (0.02) | 0.74 | 53 |
| Specific SB activity (GBq/μmol) | 308.4 (277.0) | 310.4 (59.9) | 265.2 (176.4) | 0.47 | 53 |
OC: oral contraceptive, HC: hormonal contraceptive, hormonal IUD: hormonal intrauterine device, BMI: body mass index, MDD: major depressive disorder, Cohen's PSS: Cohen's Perceived Stress Scale, MDI: Major Depression Inventory, SCL‐92 GSI: Symptom Checklist‐92 Global Severity Index, POMS‐TMD: Profile of Mood States‐Total Mood Disturbance, HRRT: High‐Resolution Research Tomograph, SB: [11C]SB207145.
The statistical tests performed were two‐sided t‐test for the continuous variables, Fischer's exact test for the categorical variables, and Gehan test for P‐estradiol because of censored values.
Effects of OC use on serotonin 4 receptor binding
Support for the LVM structure was evidenced by all region‐specific bindings loading onto the latent variable (P < 0.001). To satisfy the chi‐squared test, two covariance parameters (between neocortex and ACC and between hippocampus and amygdala) were added to the LVM, which only had a minor impact on the estimate of the effect of OC use. No additional parameter modeling region‐specific effect of OC use was found necessary. The LVM identified a global pattern of association between OC use and 5‐HT4R binding; the effect of OC use was mediated by the global latent variable with a negative association between OC use and the individual 5‐HT4R latent levels (γ = −0.099, P = 0.0039, Fig. 1). Regional loadings varied, corresponding to an effect of OC use of −9.4% to −11.7% in BPND. The global effect is illustrated in parametric maps in Fig. 2. No gross deviation from the normality assumption was observed.
Fig. 1.
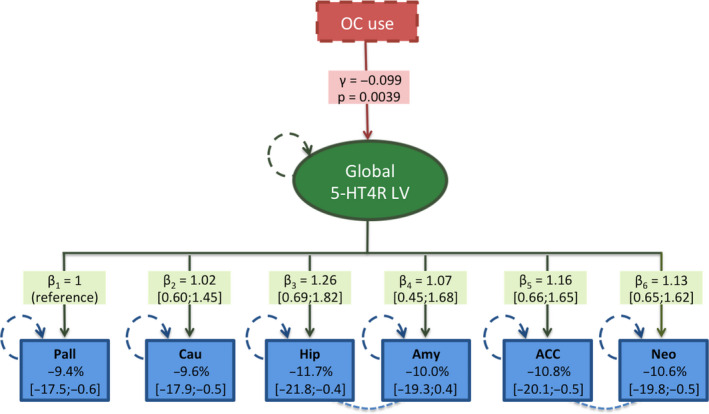
Illustration of the latent variable model showing the effect of OC use (represented by the γ‐value (on the log‐scale) and the corresponding P‐value) mediated by the global 5‐HT4R latent variable (LV) (green oval) on the six brain regions (blue boxes). The loading effects on each region are shown as β‐values (on the log‐scale) with 95% confidence intervals, and the corresponding OC effect on the regional 5‐HT4R binding potential (BPND) is shown as percent difference in each blue box. The hatched lines between hippocampus and amygdala and between ACC and neocortex illustrate partial correlation, which were included as covariance parameters. Circular green and blue hatched lines reflect variables estimated with error. Each regional BPND is independently corrected for age, scanner type, injected [11C]SB207145 mass per kg bodyweight, and familial risk for MDD (not illustrated). The percentage difference in BPND reported in the blue boxes was obtained after back transformation using (exp(γ × β)−1) × 100. Pall, pallidostriatum; Cau, caudate; ACC, anterior cingulate cortex; Neo, neocortex; Hip, hippocampus; Amy, amygdala. [Colour figure can be viewed at wileyonlinelibrary.com]
Fig. 2.
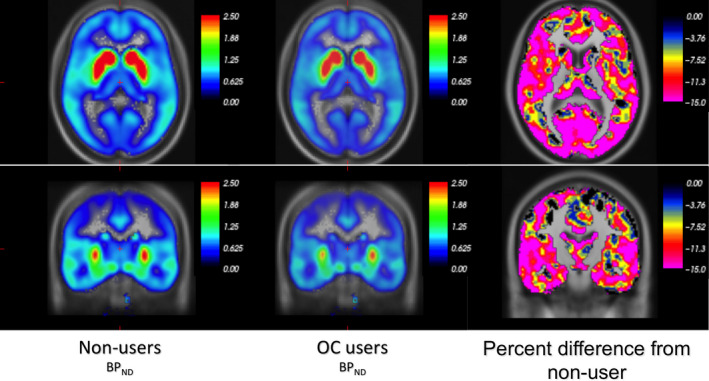
Illustration of the global effect of OC use on 5‐HT4R BPND via voxel‐based parametric images. The first two panels show the group's average non‐displaceable binding potentials (BPND) (color bars represent BPND units). OC users appear paler both in subcortical and cortical areas. The third panel shows the percentage difference in BPND in OC users from non‐users where the color bar scale illustrates percentage from 0 to −15%. [Colour figure can be viewed at wileyonlinelibrary.com]
The univariate region‐based analyses supported the global effect of OC use as depicted in Table 2. However, amygdala, which was significantly associated with the shared latent variable in the LVM, did not display statistical significance in the univariate linear model, and this may reflect low signal to noise problems well‐known for PET imaging of smaller regions. The largest difference was present in the hippocampus (−12.8% (95% CI [−21.0; −3.9], P corrected = 0.03, Fig. 3).
Table 2.
Effects of oral contraceptive use on serotonin 4 receptor BPND via univariate multiple linear regression analyses
| (A) All PET scans (n = 53, 16 users) | (B) HRRT PET scans only (n = 47, 13 users) | (C) No familial risk for MDD (n = 39, 9 users) | ||||
|---|---|---|---|---|---|---|
| ROIs | Pct. diff. from non‐users [95% CI] | P‐value (†) | Pct. diff. from non‐users [95% CI] | P‐value (†) | Pct. diff. from non‐users [95% CI] | P‐value (†) |
| Pallidostriatum | −8.4 [−14.8; −1.5] | 0.02 (0.07) | −7.0 [−13.8;0.3] | 0.06 (0.21) | −9.0 [−16.5;−0.9] | 0.03 (0.11) |
| Caudate | −10.7 [−17.1;−3.9] | 0.003 (0.01) | −9.0 [−15.8;−1.6] | 0.02 (0.08) | −9.8 [−17.8;−0.9] | 0.03 (0.12) |
| Hippocampus | −12.8 [−21.0;−3.9] | 0.007 (0.03) | −14.7 [−21.6;−7.1] | 0.0007 (0.01) | −16.5 [−24.4;−7.7] | 0.001 (0.02) |
| Amygdala | −6.9 [−16.1;3.3] | 0.17 (0.49) | −6.0 [−15.9;5.1] | 0.27 (0.69) | −6.4 [−17.8;6.7] | 0.31 (0.74) |
| ACC | −10.5 [−18.0;−2.4] | 0.01 (0.05) | −9.6 [−16.6;−1.9] | 0.02 (0.07) | −9.8 [−17.6;−1.4] | 0.02 (0.09) |
| Neocortex | −10.9 [−18.2;−3.0] | 0.009 (0.04) | −9.7 [−17.2;−1.6] | 0.02 (0.09) | −10.3 [−18.8;−0.9] | 0.03 (0.12) |
(A) Main analysis with all scans; (B) only High‐Resolution Research Tomograph (HRRT) PET scans; (C) only HRRT PET scans of the women without any familial risk for major depressive disorder.
P‐values after adjustment for six comparisons using a single‐step Dunnett's procedure. BPND; non‐displaceable binding potentials, ROIs; regions of interest, ACC; anterior cingulate cortex.
Fig. 3.
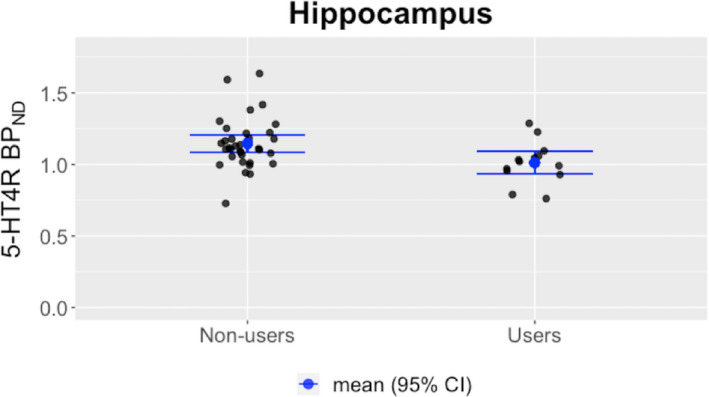
Mean value and bars indicating the 95% confidence interval are shown. Binding potentials (BPND's) are shown as unadjusted values, and for illustrative purposes, only data from High‐Resolution Research Tomograph (HRRT) PET scans are shown. [Colour figure can be viewed at wileyonlinelibrary.com]
Follow‐up analyses on robustness, hormonal IUD use, and ovarian hormone associations with 5‐HT4R
To overcome the issue of pooling scans from different PET scanners and having an overrepresentation of familial risk for MDD in the OC user group, other than incorporating them as covariates, we performed two separate sensitivity analyses in two restricted study groups (Table 2), (B) only HRRT scans, and (C) only HRRT scans of women with no familial risk for MDD. Both analyses supported that neither scanner type nor the distribution of familial risk for MDD was driving the observed OC effect. A supplementary analysis did not demonstrate a statistically significant association between use of hormonal IUD (n = 8) and 5‐HT4R binding relative to no use of hormonal contraception in any of the six regions; however, the analysis had clearly suboptimal power (Table S1). Further, in a supplementary analysis (Tables S3 and S4), we evaluated if the ovarian hormone status, that is, estradiol and progesterone levels, was associated with 5‐HT4R binding and if any such associations depended on hormonal cycle dynamics, that is, a group interaction effect. The interaction between OC user status and the plasma estradiol level on 5‐HT4R BPND was borderline significant in neocortex (P = 0.05, not corrected for multiple comparisons), pointing to a positive association between BPND and suppressed levels of plasma estradiol among OC users only. Also, the interaction was borderline significant for progesterone in ACC (P = 0.08, not corrected for multiple comparisons), such that for OC users, the BPND appeared to be negatively associated with the progesterone level.
Discussion
Here, we show that OC users have a markedly 9–12% reduction in global 5‐HT4R brain binding relative to non‐users. These findings are to our knowledge the first in vivo evidence of an effect of OC use on the brain serotonin system in healthy women.
Sex steroids and serotonin signaling
Existing literature strongly supports that sex hormones, especially estradiol, affect serotonergic neuromodulation (21, 22). More specifically, both estradiol and progesterone, which directly target serotonergic neurons, may affect key features of serotonergic signaling in terms of serotonin synthesis (50, 51), degradation (51, 52), reuptake (51, 53), neuronal firing (54), and postsynaptic serotonin receptor availability on non‐serotonergic neurons (55, 56). We speculate that the observed OC‐associated reduction in 5‐HT4R binding is a result of either (1) a direct effect of the OC's synthetic steroids or (2) an OC‐suppressed endogenous hormone state. Knowledge on how the 5‐HT4R is regulated in brain tissue is limited (57). Animal and human studies show that increased synaptic serotonergic tonus leads to a reduction in 5‐HT4R (28, 29, 30, 44). Accordingly, in the context of our findings, this could imply that OC users have higher synaptic 5‐HT levels. Estradiol is generally believed to support serotonergic neurotransmission (58). Therefore, if an increased 5‐HT tonus should explain the lower levels of 5‐HT4R in OC users, it may be related to synthetic steroid exposure from OCs. However, since estrogen mainly target serotonergic neurons via ERβ activation (59, 60, 61, 62), ethinyl estradiol, most likely, does not directly stimulate serotonergic neurotransmission since it primarily stimulates ERα (63). This may even compromise serotonergic neurotransmission as ERα activation increases the 5‐HT1A auto‐receptor expression, which can inhibit 5‐HT‐firing from dorsal raphe nucleus (64, 65). Taken together, this tends to argue against increased 5HT‐levels induced by ethinyl estradiol from OCs. On the other hand, the progestin from OCs could interfere with the serotonergic neurotransmission, but this is less well understood. Alternative to an increased serotonergic tonus, the lower 5‐HT4R binding may be caused by a hormone‐dependent reduction of gene expression of the 5‐HT4 receptor (66) because of OC‐induced suppression of endogenous estradiol (Table 1). In support of this explanation, estradiol increases the expression levels of the 5‐HT4R in the rat anterior pituitary cells (67). Also, our group has previously shown that plasma estradiol is positively associated with 5‐HT4R binding in the amygdala of healthy men (68).
As expected, the OC users had suppressed estradiol and progesterone levels compared to non‐users (69). It is well established that OC users have a non‐dynamic hormone status compared to non‐users (18). We speculate that a dynamic vs. non‐dynamic hormone status may be critical for the regulation and adaptive capacity of cerebral 5‐HT4R. Similarly, this might be why hormonal IUD use shows no effect on 5‐HT4R binding. However, because of low statistical power on the supplementary hormonal IUD analysis, we cannot conclude on this. Future work on 5‐HT4R BPND and hormonal IUD use is wanted since IUD also has been associated with the development of depression (6). Nevertheless, confounding by indication can also play a role in the earlier observed association between hormonal IUD use and depression‐related phenomena.
We observed a trend toward an association of estradiol and progesterone with the binding in neocortex and ACC in the OC user group but not in the non‐user group (Tables S3 and S4), that is, a positive association for estradiol and negative association for progesterone on the 5‐HT4R binding. Larger independent datasets are needed to corroborate these findings and determine if the effect can be attributed to blunted levels of specific hormones or related metabolites. For example, the neuroactive progesterone metabolite, allopregnanolone, which is reduced in OC users (69), is of particular interest in that context. So far, no human data exist to clarify whether allopregnanolone is associated with 5‐HT4R binding; however, allopregnanolone appears to be negatively associated with cerebral serotonin transporter binding in naturally cycling women (70).
OC use, serotonin, depression, and cognitive health
Given the important role of the serotonergic system in MDD (23), our finding of lower 5‐HT4R BPND among OC users represents a potential mechanism by which OC use can trigger depressive episodes (6, 7). However, obviously from our data alone we are not able to conclude if the lower 5‐HT4R binding level has protective or harmful effects in the context of depression. Previous research on the association between OC use and depression is inconsistent (11). One of the reasons might be that the mood‐ and affect‐related effects of OC use are subclinical for most women, which is supported by studies showing that OC users appear less responsive to emotional stimuli including positive stimuli (71, 72).
Other hormonal transitions may also be linked to depressive states: For example, earlier data from our group link the emergence of depressive symptoms with sex hormone manipulations and changes in serotonin transporter binding, which tend to compromise serotonin signaling (73). Further, evidence converges to support the notion that hormonal transitions may cause depressive symptoms in hormone‐sensitive individuals: (i) Estradiol fluctuations around menopausal transition are associated with first‐time onset of MDD (74) and appears to be preventable by hormonal replacement (75), (ii) hormone manipulations trigger depressive symptoms in women with a history of postpartum depression (76), and (iii) 'estrogen sensitivity' at the level of gene transcription and DNA methylation also appears to increase risk for postpartum depression (77, 78). Intriguingly, we do not yet know if depressive responses to OC use may be a marker of such hormone sensitivity.
Another line of evidence directly links 5‐HT4Rs to MDD (57); two polymorphisms in the splice variant region of the 5‐HT4R gene have been associated to MDD (79), and hippocampal 5‐HT4R binding is reduced in the Flinders Sensitive Line rat model of depression (29). 5‐HT4Rs appear to play a role in familial risk for MDD (43). As 5‐HT4R activation has widespread effects on serotonergic neurotransmission, that is, stimulates the serotonergic neuronal firing from the dorsal raphe nuclei to the rest of the brain (80), OC‐induced changes in 5‐HT4R agonism capacity may distort the serotonin system and increase risk of depression and/or play a role in antidepressant treatment. Notably, rodent data support fast acting antidepressant‐like effect of 5‐HT4R partial agonists (26), and that antidepressant‐like effects of selective serotonin reuptake inhibitor (SSRI) is dependent on 5‐HT4R activation (81).
SSRIs are known to reduce 5‐HT4R binding as shown in animal (29, 30) and human (28) studies. In healthy men, three weeks of SSRI treatment reduces the 5‐HT4 BPND by 5% (28), and this most likely represents as an adaptive response to the heightened serotonergic tone. The observed effect size of OC use on the 5‐HT4R is comparatively large, that is, approximately twice as big. Also, it is considerably larger than the test–retest difference for [11C]SB207145 ligand, which is 4–7% depending on the region (25). This questions whether concomitant use of OCs in depressed individuals could affect SSRI treatment response. As far as we know surprisingly, only the STAR*D study has addressed this question, which followed 896 depressed premenopausal women, 226 of whom used hormonal contraceptives (82). The authors found a trend toward better remission rate among the hormonal contraceptive users. This may argue for a protective effect of the reduced 5‐HT4R level in OC users, but alternatively it also reconciles with the notion that if OC use contributes to the depressed state by suppressing 5‐HT4R capacity, then SSRIs may work particularly well in OC users by increasing serotonergic tone and thus support 5‐HT4R agonism.
Interestingly and yet unstudied, withdrawal of OCs may itself have an antidepressant effect. Further studies are warranted to clarify whether (i) concomitant use of OCs affect SSRI treatment response and (ii) whether women who develop a depressive episode while taking an OC (even in cases where OCs were not the trigger of MDD) may benefit from withdrawal of OCs either alone or in combination with SSRI treatment.
It is important to emphasize that not all women using OCs develop depressive episodes, and correspondingly, none of the OC using women in this study were depressed. Nevertheless, an OC‐induced decrease in 5‐HT4R agonism capacity may trigger depressive episodes in some women, perhaps a particular subgroup of hormone‐sensitive women. How lower 5‐HT4R BPND otherwise may affect healthy women using OCs remains elusive but can include effects on cognition. Intriguingly, we do see the largest effect size seen in the hippocampus, which is key in memory functions. OC use may affect memory performance (83), where 5‐HT4Rs also appear related (84), as shown repeatedly in humans, in particular for hippocampal 5‐HT4R binding (85, 86). This might be related to the role of 5‐HT4R activation in hippocampal neurogenesis (87). Several studies have investigated the effect of OC use on hippocampal volume, but results are inconsistent (17, 88, 89). Future studies are needed to address neurodegenerative long‐term effects of OC use, for example, Alzheimer's disease, where 5‐HT4Rs may play a protective role (90, 91), as well as to what extent withdrawal from OCs rescues 5‐HT4R brain binding.
Taken together, OC users have 9–12% lower 5‐HT4R BPND. This adds new insight into a plausible mechanism by which OC use may increase the risk of developing depressive episodes. If confirmed in future longitudinal studies and if translated to other hormonal transitions across reproductive age, a history of OC‐induced depressive symptoms may serve as a key marker for healthcare professionals to guide psychoeducation or personalize prevention and treatment strategies.
Methodological considerations
Some limitations should be considered when interpreting the results of this study. First, as no information was collected about pill‐free days or experienced OC‐related mood deterioration, we cannot relate these to potential patterns of 5‐HT4R binding. Also because of limited power, no firm conclusion could be drawn from the current dataset on a potential moderate to small effect of menstrual phase on 5‐HT4R binding; however, we saw no effect of menstrual cycle phase per se in our data (8 in luteal phase vs. 14 in follicular phase, P > 0.2). Likewise, existing data on menstrual phase effects on other serotonergic markers; that is, the serotonin 1A receptor and the serotonin transporter do not support menstrual phase to affect serotonergic signaling in a detectable manner in the absence of psychopathology (92). Further, we were underpowered to take potential effects of smoking on 5‐HT4R binding into account in our analyses, since we only had 3 smokers. When ignoring these three in the analyses, it did not affect the results. Second, we cannot rule out a possible 'survival effect' among the OC users, such that those sensitive to OC‐depressogenic effects converted to non‐users before inclusion or those who developed depression on OCs were not included as healthy controls. Therefore, our sample might be biased, so if more 'vulnerable' women had been included, a larger effect may have appeared. Third, three different Siemens MAGNETOM MRI scanners were used. The effect of MR scanner on 5‐HT4R BPND is expected to be low and thus not expected to drive our results, which was confirmed by sustained effects seen in analyses restricted to Prisma scans only (n = 41, results not presented). Fourth, because of a limited sample size, the derived effect estimates of the associations between ovarian hormone steroids and 5‐HT4R binding are estimated with large uncertainty.
Declaration of interest
VGF and GMK declare to have received honorarium for advisory meetings at Sage Therapeutics and VGF for a symposium lecture at Lundbeck Pharma A/S. All others authors declare no potential conflict of interest.
Peer Review
The peer review history for this article is available at https://publons.com/publon/10.1111/acps.13211. [Correction added on 14 October 2020, after first online publication: Peer review history statement has been added.]
Supporting information
Table S1 No significant association between hormonal IUD use and serotonin 4 receptor BPND.
Table S2 Overview of the specific type of combined oral contraceptives used.
Table S3 Group BY plasma estradiol interaction effects on serotonin 4 receptor BPND at different plasma estradiol imputations.
Table S4 Group BY plasma progesterone interaction effects on serotonin 4 receptor BPND.
Acknowledgements
We wish to thank Lone Freyr, Dorthe Givard, Birgit Tang, Arafat Nasser, Jacob Madsen, Sune Keller, Svitlana Olsen, and Gerda Thomsen and technical staff at the Dept. of Nuclear Medicine, Rigshospitalet for superb technical assistance. The study was funded by Independent Research Fund Denmark, Lundbeckfonden, and Desirée and Yde Foundation. Desirée og Niels Ydes Fond (481‐17), Det Frie Forskningsråd (7016‐00265B, 7090‐00022B), Lundbeckfonden.
Larsen SV, Köhler-Forsberg K, Dam VH, Poulsen AS, Svarer C, Jensen PS, Knudsen GM, Fisher PM, Ozenne B, Frokjaer VG. Oral contraceptives and the serotonin 4 receptor: a molecular brain imaging study in healthy women.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request and from the Cimbi database of molecular brain PET in healthy humans (1). Access can be requested through the procedures outlined here: https://cimbi.dk/index.php/documents/category/3‐cimbi‐database.
References
- 1. Knudsen GM, Jensen PS, Erritzoe D et al. The Center for Integrated Molecular Brain Imaging (Cimbi) database. NeuroImage 2020;124:1213–1219. [DOI] [PubMed] [Google Scholar]
- 2. Christin‐Maitre S. History of oral contraceptive drugs and their use worldwide. Best Pract Res Clin Endocrinol Metab 2013;27:3–12. [DOI] [PubMed] [Google Scholar]
- 3. Liao PV, Dollin J. Half a century of the oral contraceptive pill: historical review and view to the future. Can Fam Physician 2012;58:e757–e760. [PMC free article] [PubMed] [Google Scholar]
- 4. Lidegaard O, Lokkegaard E, Svendsen AL et al. Hormonal contraception and risk of venous thromboembolism: national follow‐up study. BMJ 2009;339:b2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rosenberg MJ, Waugh MS. Oral contraceptive discontinuation: a prospective evaluation of frequency and reasons. Am J Obstet Gynecol 1998;179:577–582. [DOI] [PubMed] [Google Scholar]
- 6. Skovlund CW, Morch LS, Kessing LV et al. Association of hormonal contraception with depression. JAMA Psychiatry 2016;73:1154–1162. [DOI] [PubMed] [Google Scholar]
- 7. Zettermark S, Perez Vicente R, Merlo J. Hormonal contraception increases the risk of psychotropic drug use in adolescent girls but not in adults: A pharmacoepidemiological study on 800 000 Swedish women. PLoS One 2018;13:e0194773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Skovlund CW, Morch LS, Kessing LV et al. Association of hormonal contraception with suicide attempts and suicides. Am J Psychiatry 2018;175:336–342. [DOI] [PubMed] [Google Scholar]
- 9. de Wit AE, Booij SH, Giltay EJ et al. Association of use of oral contraceptives with depressive symptoms among adolescents and young women. JAMA Psychiatry 2020;77:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Anderl C, Li G, Chen FS. Oral contraceptive use in adolescence predicts lasting vulnerability to depression in adulthood. J Child Psychol Psychiatry 2020;61:148–156. [DOI] [PubMed] [Google Scholar]
- 11. Schaffir J, Worly BL, Gur TL. Combined hormonal contraception and its effects on mood: a critical review. Eur J Contracept Reprod Health Care 2016;21:347–355. [DOI] [PubMed] [Google Scholar]
- 12. Lokkegaard E, Nielsen AK. Adolescent girls in Denmark use oral contraceptives at an increasingly young age, and with more pauses and shifts. Dan Med J 2014;61:A4936. [PubMed] [Google Scholar]
- 13. Cahill L. How does hormonal contraception affect the developing human adolescent brain? Curr Opin Behav Sci 2018;23:131–135. [Google Scholar]
- 14. Pletzer BA, Kerschbaum HH. 50 years of hormonal contraception‐time to find out, what it does to our brain. Front Neurosci 2014;8:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Toffoletto S, Lanzenberger R, Gingnell M et al. Emotional and cognitive functional imaging of estrogen and progesterone effects in the female human brain: a systematic review. Psychoneuroendocrinology 2014;50:28–52. [DOI] [PubMed] [Google Scholar]
- 16. Montoya ER, Bos PA. How oral contraceptives impact social‐emotional behavior and brain function. Trends Cogn Sci 2017;21:125–136. [DOI] [PubMed] [Google Scholar]
- 17. Hertel J, Konig J, Homuth G et al. Evidence for stress‐like alterations in the HPA‐axis in women taking oral contraceptives. Sci Rep 2017;7:14111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. D'Arpe S, Di Feliciantonio M, Candelieri M et al. Ovarian function during hormonal contraception assessed by endocrine and sonographic markers: a systematic review. Reprod Biomed Online 2016;33:436–448. [DOI] [PubMed] [Google Scholar]
- 19. Lovett JL, Chima MA, Wexler JK et al. Oral contraceptives cause evolutionarily novel increases in hormone exposure: A risk factor for breast cancer. Evol Med Public Health 2017;2017:97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Panzer C, Wise S, Fantini G et al. Impact of oral contraceptives on sex hormone‐binding globulin and androgen levels: a retrospective study in women with sexual dysfunction. J Sex Med 2006;3:104–113. [DOI] [PubMed] [Google Scholar]
- 21. Bethea CL, Lu NZ, Gundlah C et al. Diverse actions of ovarian steroids in the serotonin neural system. Front Neuroendocrinol 2002;23:41–100. [DOI] [PubMed] [Google Scholar]
- 22. Barth C, Villringer A, Sacher J. Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods. Front Neurosci 2015;9:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Martinowich K, Lu B. Interaction between BDNF and serotonin: role in mood disorders. Neuropsychopharmacology 2008;33:73–83. [DOI] [PubMed] [Google Scholar]
- 24. Lu NZ, Eshleman AJ, Janowsky A et al. Ovarian steroid regulation of serotonin reuptake transporter (SERT) binding, distribution, and function in female macaques*. Mol Psychiatry 2003;8:353. [DOI] [PubMed] [Google Scholar]
- 25. Marner L, Gillings N, Comley RA et al. Kinetic modeling of 11C‐SB207145 binding to 5‐HT4 receptors in the human brain in vivo. J Nucl Med 2009;50:900–908. [DOI] [PubMed] [Google Scholar]
- 26. Lucas G, Rymar VV, Du J et al. Serotonin(4) (5‐HT(4)) receptor agonists are putative antidepressants with a rapid onset of action. Neuron 2007;55:712–725. [DOI] [PubMed] [Google Scholar]
- 27. Beliveau V, Ganz M, Feng L et al. A high‐resolution in vivo atlas of the human brain's serotonin system. J Neurosci 2017;37:120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Haahr ME, Fisher PM, Jensen CG et al. Central 5‐HT4 receptor binding as biomarker of serotonergic tonus in humans: a [11C]SB207145 PET study. Mol Psychiatry 2014;19:427–432. [DOI] [PubMed] [Google Scholar]
- 29. Licht CL, Marcussen AB, Wegener G et al. The brain 5‐HT4 receptor binding is down‐regulated in the Flinders Sensitive Line depression model and in response to paroxetine administration. J Neurochem 2009;109:1363–1374. [DOI] [PubMed] [Google Scholar]
- 30. Vidal R, Valdizan EM, Mostany R et al. Long‐term treatment with fluoxetine induces desensitization of 5‐HT4 receptor‐dependent signalling and functionality in rat brain. J Neurochem 2009;110:1120–1127. [DOI] [PubMed] [Google Scholar]
- 31. Siiteri PK. Adipose tissue as a source of hormones. Am J Clin Nutr 1987;45:277–282. [DOI] [PubMed] [Google Scholar]
- 32. Madsen K, Marner L, Haahr M et al. Mass dose effects and in vivo affinity in brain PET receptor studies–a study of cerebral 5‐HT4 receptor binding with [11C]SB207145. Nucl Med Biol 2011;38:1085–1091. [DOI] [PubMed] [Google Scholar]
- 33. Mulders TM, Dieben TO. Use of the novel combined contraceptive vaginal ring NuvaRing for ovulation inhibition. Fertil Steril 2001;75:865–870. [DOI] [PubMed] [Google Scholar]
- 34. Xiao B, Zeng T, Wu S et al. Effect of levonorgestrel‐releasing intrauterine device on hormonal profile and menstrual pattern after long‐term use. Contraception 1995;51:359–365. [DOI] [PubMed] [Google Scholar]
- 35. Bech P, Rasmussen NA, Olsen LR et al. The sensitivity and specificity of the Major Depression Inventory, using the Present State Examination as the index of diagnostic validity. J Affect Disord 2001;66:159–164. [DOI] [PubMed] [Google Scholar]
- 36. Rice JP, Reich T, Bucholz KK et al. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcohol Clin Exp Res 1995;19:1018–1023. [DOI] [PubMed] [Google Scholar]
- 37. Kendler KS, Myers J. The genetic and environmental relationship between major depression and the five‐factor model of personality. Psychol Med 2010;40:801–806. [DOI] [PubMed] [Google Scholar]
- 38. Hansen SH, Mortensen LM, Schiøtz HK. NEO PI‐R, manual—klinisk. 1. udgave, 5. oplag ed. Copenhagen: Hogrefe Psykologisk Forlag; 2011. [Google Scholar]
- 39. Gehan EA. A generalized two‐sample Wilcoxon test for doubly censored data. Biometrika 1965;52:650–653. [PubMed] [Google Scholar]
- 40. Svarer C, Madsen K, Hasselbalch SG et al. MR‐based automatic delineation of volumes of interest in human brain PET images using probability maps. NeuroImage 2005;24:969–979. [DOI] [PubMed] [Google Scholar]
- 41. Fisher PM, Ozenne B, Svarer C et al. BDNF val66met association with serotonin transporter binding in healthy humans. Transl Psychiatry 2017;7:e1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Madsen K, Haahr MT, Marner L et al. Age and sex effects on 5‐HT(4) receptors in the human brain: a [(11)C]SB207145 PET study. J Cereb Blood Flow Metab 2011;31:1475–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Madsen K, Torstensen E, Holst KK et al. Familial risk for major depression is associated with lower striatal 5‐HT4 receptor binding. Int J Neuropsychopharmacol 2014;18:pyu034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fisher PM, Holst KK, Adamsen D et al. BDNF Val66met and 5‐HTTLPR polymorphisms predict a human in vivo marker for brain serotonin levels. Hum Brain Mapp 2015;36:313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Holst K, Budtz‐Jørgensen E. Linear latent variable models: the lava‐package. Computat Stat 2013;28:1385–1452. [Google Scholar]
- 46. Dmitrienko A, D'Agostino R Sr. Traditional multiplicity adjustment methods in clinical trials. Stat Med 2013;32:5172–5218. [DOI] [PubMed] [Google Scholar]
- 47. R Core Team . R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2017. https://www.R‐project.org/ [Google Scholar]
- 48. Bech P, Timmerby N, Martiny K et al. Psychometric evaluation of the Major Depression Inventory (MDI) as depression severity scale using the LEAD (Longitudinal Expert Assessment of All Data) as index of validity. BMC Psychiatry 2015;15:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Olsen LR, Mortensen EL, Bech P. The SCL‐90 and SCL‐90R versions validated by item response models in a Danish community sample. Acta Psychiatr Scand 2004;110:225–229. [DOI] [PubMed] [Google Scholar]
- 50. Bethea CL, Mirkes SJ, Shively CA et al. Steroid regulation of tryptophan hydroxylase protein in the dorsal raphe of macaques. Biol Psychiatry 2000;47:562–576. [DOI] [PubMed] [Google Scholar]
- 51. Smith LJ, Henderson JA, Abell CW et al. Effects of ovarian steroids and raloxifene on proteins that synthesize, transport, and degrade serotonin in the raphe region of macaques. Neuropsychopharmacology 2004;29:2035–2045. [DOI] [PubMed] [Google Scholar]
- 52. Gundlah C, Lu NZ, Bethea CL. Ovarian steroid regulation of monoamine oxidase‐A and ‐B mRNAs in the macaque dorsal raphe and hypothalamic nuclei. Psychopharmacology 2002;160:271–282. [DOI] [PubMed] [Google Scholar]
- 53. Sánchez MG, Morissette M, Di Paolo T. Oestradiol modulation of serotonin reuptake transporter and serotonin metabolism in the brain of monkeys. J Neuroendocrinol 2013;25:560–569. [DOI] [PubMed] [Google Scholar]
- 54. Robichaud M, Debonnel G. Oestrogen and testosterone modulate the firing activity of dorsal raphe nucleus serotonergic neurones in both male and female rats. J Neuroendocrinol 2005;17:179–185. [DOI] [PubMed] [Google Scholar]
- 55. Kugaya A, Epperson CN, Zoghbi S et al. Increase in prefrontal cortex serotonin 2A receptors following estrogen treatment in postmenopausal women. Am J Psychiatry 2003;160:1522–1524. [DOI] [PubMed] [Google Scholar]
- 56. Moses‐Kolko EL, Berga SL, Greer PJ et al. Widespread increases of cortical serotonin type 2A receptor availability after hormone therapy in euthymic postmenopausal women. Fertil Steril 2003;80:554–559. [DOI] [PubMed] [Google Scholar]
- 57. Rebholz H, Friedman E, Castello J. Alterations of expression of the serotonin 5‐HT4 receptor in brain disorders. Int J Mol Sci 2018;19:3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hernández‐Hernández OT, Martínez‐Mota L, Herrera‐Pérez JJ et al. Role of estradiol in the expression of genes involved in serotonin neurotransmission: implications for female depression. Curr Neuropharmacol 2019;17:459–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Donner N, Handa RJ. Estrogen receptor beta regulates the expression of tryptophan‐hydroxylase 2 mRNA within serotonergic neurons of the rat dorsal raphe nuclei. Neuroscience 2009;163:705–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hiroi R, Handa RJ. Estrogen receptor‐beta regulates human tryptophan hydroxylase‐2 through an estrogen response element in the 5' untranslated region. J Neurochem 2013;127:487–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hou X, Adeosun SO, Zhao X et al. ERbeta agonist alters RNA splicing factor expression and has a longer window of antidepressant effectiveness than estradiol after long‐term ovariectomy. J Psychiatry Neurosci 2018;43:170199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Benmansour S, Weaver RS, Barton AK et al. Comparison of the effects of estradiol and progesterone on serotonergic function. Biol Psychiatry 2012;71:633–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Escande A, Pillon A, Servant N et al. Evaluation of ligand selectivity using reporter cell lines stably expressing estrogen receptor alpha or beta. Biochem Pharmacol 2006;71:1459–1469. [DOI] [PubMed] [Google Scholar]
- 64. Wissink S, van der Burg B, Katzenellenbogen BS et al. Synergistic activation of the serotonin‐1A receptor by nuclear factor‐kappa B and estrogen. Mol Endocrinol 2001;15:543–542. [DOI] [PubMed] [Google Scholar]
- 65. Courtney NA, Ford CP. Mechanisms of 5‐HT1A receptor‐mediated transmission in dorsal raphe serotonin neurons. J Physiol 2016;594:953–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Marino M, Galluzzo P, Ascenzi P. Estrogen signaling multiple pathways to impact gene transcription. Curr Genomics 2006;7:497–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Papageorgiou A, Denef C. Estradiol induces expression of 5‐hydroxytryptamine (5‐HT) 4, 5‐HT5, and 5‐HT6 receptor messenger ribonucleic acid in rat anterior pituitary cell aggregates and allows prolactin release via the 5‐HT4 receptor. Endocrinology 2007;148:1384–1395. [DOI] [PubMed] [Google Scholar]
- 68. Perfalk E, Cunha‐Bang SD, Holst KK et al. Testosterone levels in healthy men correlate negatively with serotonin 4 receptor binding. Psychoneuroendocrinology 2017;81:22–28. [DOI] [PubMed] [Google Scholar]
- 69. Rapkin AJ, Morgan M, Sogliano C et al. Decreased neuroactive steroids induced by combined oral contraceptive pills are not associated with mood changes. Fertil Steril 2006;85:1371–1378. [DOI] [PubMed] [Google Scholar]
- 70. Sundström Poromaa I, Comasco E, Bäckström T et al. Negative association between allopregnanolone and cerebral serotonin transporter binding in healthy women of fertile age. Front Psychol 2019;9:2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Jarva JA, Oinonen K. Do oral contraceptives act as mood stabilizers? Evidence of positive affect stabilization. Archives Women's Mental Health 2007;10:225–234. [DOI] [PubMed] [Google Scholar]
- 72. Gingnell M, Engman J, Frick A et al. Oral contraceptive use changes brain activity and mood in women with previous negative affect on the pill–a double‐blinded, placebo‐controlled randomized trial of a levonorgestrel‐containing combined oral contraceptive. Psychoneuroendocrinology 2013;38:1133–1144. [DOI] [PubMed] [Google Scholar]
- 73. Frokjaer VG, Pinborg A, Holst KK et al. Role of serotonin transporter changes in depressive responses to sex‐steroid hormone manipulation: a positron emission tomography study. Biol Psychiatry 2015;78:534–543. [DOI] [PubMed] [Google Scholar]
- 74. Freeman EW, Sammel MD, Lin H et al. Associations of hormones and menopausal status with depressed mood in women with no history of depression. Arch Gen Psychiatry 2006;63:375–382. [DOI] [PubMed] [Google Scholar]
- 75. Gordon JL, Rubinow DR, Eisenlohr‐Moul TA et al. Efficacy of transdermal estradiol and micronized progesterone in the prevention of depressive symptoms in the menopause transition: a randomized clinical trial. JAMA Psychiatry 2018;75:149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bloch M, Schmidt P, Ma D et al. Effects of gonadal steroids in women with a history of postpartum depression. Am J Psychiatry 2000;157:924–930. [DOI] [PubMed] [Google Scholar]
- 77. Mehta D, Newport DJ, Frishman G et al. Early predictive biomarkers for postpartum depression point to a role for estrogen receptor signaling. Psychol Med 2014;44:2309–2322. [DOI] [PubMed] [Google Scholar]
- 78. Mehta D, Rex‐Haffner M, Sondergaard HB et al. Evidence for oestrogen sensitivity in perinatal depression: pharmacological sex hormone manipulation study. Br J Psychiatry 2019;215:519–527. [DOI] [PubMed] [Google Scholar]
- 79. Ohtsuki T, Ishiguro H, Detera‐Wadleigh SD et al. Association between serotonin 4 receptor gene polymorphisms and bipolar disorder in Japanese case‐control samples and the NIMH Genetics Initiative Bipolar Pedigrees. Mol Psychiatry 2002;7:954–961. [DOI] [PubMed] [Google Scholar]
- 80. Lucas G, Compan V, Charnay Y et al. Frontocortical 5‐HT4 receptors exert positive feedback on serotonergic activity: viral transfections, subacute and chronic treatments with 5‐HT4 agonists. Biol Psychiatry 2005;57:918–925. [DOI] [PubMed] [Google Scholar]
- 81. Mendez‐David I, David DJ, Darcet F et al. Rapid anxiolytic effects of a 5‐HT4 receptor agonist are mediated by a neurogenesis‐independent mechanism. Neuropsychopharmacology 2014;39:1366–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kornstein SG, Toups M, Rush AJ et al. Do menopausal status and use of hormone therapy affect antidepressant treatment response? Findings from the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study. J Womens Health 2013;22:121–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Warren A, Gurvich C, Worsley R et al. A systematic review of the impact of oral contraceptives on cognition. Contraception 2014;90:111–116. [DOI] [PubMed] [Google Scholar]
- 84. King MV, Marsden CA, Fone KC. A role for the 5‐HT(1A), 5‐HT4 and 5‐HT6 receptors in learning and memory. Trends Pharmacol Sci 2008;29:482–492. [DOI] [PubMed] [Google Scholar]
- 85. Haahr ME, Fisher P, Holst K et al. The 5‐HT4 receptor levels in hippocampus correlates inversely with memory test performance in humans. Hum Brain Mapp 2013;34:3066–3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Stenbaek DS, Fisher PM, Ozenne B et al. Brain serotonin 4 receptor binding is inversely associated with verbal memory recall. Brain Behav 2017;7:e00674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Samuels BA, Mendez‐David I, Faye C et al. Serotonin 1A and serotonin 4 receptors: essential mediators of the neurogenic and behavioral actions of antidepressants. Neuroscientist 2016;22:26–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Pletzer B. Sex hormones and gender role relate to gray matter volumes in sexually dimorphic brain areas. Front Neurosci 2019;13:592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Petersen N, Touroutoglou A, Andreano JM et al. Oral contraceptive pill use is associated with localized decreases in cortical thickness. Hum Brain Mapp 2015;36:2644–2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Baranger K, Giannoni P, Girard SD et al. Chronic treatments with a 5‐HT4 receptor agonist decrease amyloid pathology in the entorhinal cortex and learning and memory deficits in the 5xFAD mouse model of Alzheimer's disease. Neuropharmacology 2017;126:128–141. [DOI] [PubMed] [Google Scholar]
- 91. Madsen K, Neumann WJ, Holst K et al. Cerebral serotonin 4 receptors and amyloid‐beta in early Alzheimer's disease. J Alzheimers Dis 2011;26:457–466. [DOI] [PubMed] [Google Scholar]
- 92. Jovanovic H, Karlsson P, Cerin Å et al. 5‐HT1A receptor and 5‐HTT binding during the menstrual cycle in healthy women examined with [11C] WAY100635 and [11C] MADAM PET. Psychiatry Res Neuroimag 2009;172:31–37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 No significant association between hormonal IUD use and serotonin 4 receptor BPND.
Table S2 Overview of the specific type of combined oral contraceptives used.
Table S3 Group BY plasma estradiol interaction effects on serotonin 4 receptor BPND at different plasma estradiol imputations.
Table S4 Group BY plasma progesterone interaction effects on serotonin 4 receptor BPND.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request and from the Cimbi database of molecular brain PET in healthy humans (1). Access can be requested through the procedures outlined here: https://cimbi.dk/index.php/documents/category/3‐cimbi‐database.


