ABSTRACT
BACKGROUND AND PURPOSE
In 30% of the patients with focal epilepsy, an epileptogenic lesion cannot be visually detected with structural MRI. Ultra‐high field MRI may be able to identify subtle pathology related to the epileptic focus. We set out to assess 7T MRI‐derived volumetric and functional activity lateralization of the hippocampus, hippocampal subfields, temporal and frontal lobe in healthy subjects and MRI‐negative patients with focal epilepsy.
METHODS
Twenty controls and 10 patients with MRI‐negative temporal or frontal lobe epilepsy (TLE and FLE, respectively) underwent a 7T MRI exam. T1‐weigthed imaging and resting‐state fMRI was performed. T1‐weighted images were segmented to yield volumes, while from fMRI data, the fractional amplitude of low frequency fluctuations was calculated. Subsequently, volumetric and functional lateralization was calculated from left‐right asymmetry.
RESULTS
In controls, volumetric lateralization was symmetric, with a slight asymmetry of the hippocampus and subiculum, while functional lateralization consistently showed symmetry. Contrarily, in epilepsy patients, regions were less symmetric. In TLE patients with known focus, volumetric lateralization in the hippocampus and hippocampal subfields was indicative of smaller ipsilateral volumes. These patients also showed clear functional lateralization, though not consistently ipsilateral or contralateral to the epileptic focus. TLE patients with unknown focus showed an obvious volumetric lateralization, facilitating the localization of the epileptic focus. Lateralization results in the FLE patients were less consistent with the epileptic focus.
CONCLUSION
MRI‐derived volume and fluctuation amplitude are highly symmetric in controls, whereas in TLE, volumetric and functional lateralization effects were observed. This highlights the potential of the technique.
Keywords: 7T, fMRI, hippocampus, lateralization, volumes
Introduction
Focal (i.e. localization‐related) epilepsies are the most prevalent form of epilepsy. 1 In 20‐40% of these patients, complete seizure control using antiepileptic drugs cannot be achieved. 2 In this case, surgery is a successful treatment option. 2 , 3 A well‐identified location of the seizure focus has been associated with good surgical and seizure‐free outcome. 4 Unfortunately, in 30% of the focal epilepsy cases, the epileptic focus cannot be visually identified with EEG nor structural MRI (i.e. MRI‐negative). 5 , 6 On one hand, this could be due to the insufficient spatial resolution of structural MRI to detect any morphological abnormality. On the other hand, the delicate, complex morphological nature of the epileptogenic region can evade visual detection. Moreover, the epileptic focus may not be characterized by structural abnormalities, but could be reflected by abnormalities of a more physiological nature. A straightforward and quantitative method to identify local morphological and physiological abnormalities, as a pointer toward the epileptic focus, would be to assess left‐right asymmetry through a lateralization index (LI). 7
The most prevalent form of focal epilepsy is of temporal origin (e.g. temporal lobe epilepsy, TLE). 1 Hippocampal sclerosis is often present or suspected in TLE and patients may suffer from learning and memory problems. 8 Differences in hippocampal volumes between healthy subjects and patients with TLE have been investigated: recent 3T and 7T studies revealed an ipsilateral volume reduction of the hippocampus and hippocampal subfields in epilepsy patients with a known focus location, while in MRI‐negative epilepsy patients this reduction was not observed. 9 , 10 Interestingly, these changes in hippocampal volumes were not only found in TLE, but also in frontal lobe epilepsy (FLE). 11
In previous studies in TLE patients, structural and functional asymmetry metrics have already been used to aid in seizure lateralization. 12 , 13 However, these studies either used FDG‐PET or 3T MRI and focused on the whole hippocampus or the mesial temporal lobe. They did not explore both volumetric and functional asymmetry and, although volumetric hippocampal changes were previously found, these studies did not include patients with FLE. Therefore, whether structural abnormalities are related to functional abnormalities within the hippocampus remains unclear. Moreover, functional lateralization differences of the hippocampal subfields between controls and focal epilepsy patients may provide new information about the mechanisms underlying epilepsy. An underexplored functional imaging measure to assess the functional activity of the hippocampus is the amplitude of spontaneous fluctuations as obtained by resting‐state blood oxygen level dependent (BOLD) time signals. 14 However, the signal‐to‐noise ratio of functional MRI (fMRI) increase with field strength and the BOLD contrast effect increases from 4% at 1.5T, 6% at 3T to 9% at 7T. This allows the use of higher spatial resolutions at an ultra‐high field strength. 15 Furthermore, due to the differences in cellular components and functional specialization of the hippocampal subfields, the identification of functional abnormalities and differentiation between subfields based on physiological MRI signals might be challenging. 16 , 17 Obtaining higher spatial resolution, better signal‐to‐noise ratios and a stronger BOLD effect, through use of 7T MRI, might be a suitable approach to determine morphological and functional abnormalities for the identification of the potential epileptic focus.
The primary aim was to investigate to which extent the volumes of brain (sub)structures, particularly the hippocampus, and their resting‐state spontaneous fluctuations are symmetric in healthy subjects and MRI‐negative patients with focal epilepsy of temporal or frontal origin, TLE or FLE respectively. We hypothesize that in controls the volume and functional amplitude are highly symmetric, but for focal epilepsy patients asymmetric. Secondly, we explored whether the expected asymmetry relates to the lateralization of the seizure focus and how it is expressed in patients with an unknown focus.
Methods
Participants
Twenty healthy subjects and 10 focal epilepsy patients with an origin in the temporal lobe (TLE, n = 8) or frontal lobe (FLE, n = 2) were examined with 7T MRI. We excluded subjects with a medical history of (other) neurological diseases and/or contraindications for MR scanning. 18 The study was approved by the ethics committee of Maastricht University Medical Center and registered at the Dutch Trial Register with registration number NTR4879. Written improved consent was obtained from all participants.
The patients (aged 20‐69 years) all had 3T‐MRI‐negative focal epilepsy, as described in Table 1. Potential epileptic focus assessment, based on clinical characteristics and/or scalp EEG, was independently performed by 2 expert neurologists (15 and 10 years of experience), and the independently drawn conclusions were in consensus, see Table 1. A visual assessment of the clinical 7T MRI scans by an expert neuroradiologist (over 20 years of experience) yielded no signs of hippocampal sclerosis and other epileptogenic abnormalities.
Table 1.
Patient Characteristics
| EEG Characteristics | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient Number | Location | Lateralization | Age (Years) | Sex | Age of Onset (Years) | Seizure Frequency | Medication Type | Background Pattern | Focal Abnormalities | Epileptiform Discharges |
| 1 | Temporal | Left | 23 | Male | 2 | Seizure‐free | Valproate | Normal | Left temporal slowing | Left temporal abnormalities |
| 2 | Temporal | Left | 45 | Male | 32 | Seizure‐free | Carbamazepine Valproate | Normal | Left anterior temporal slowing | Left temporal abnormalities |
| 3 | Temporal | Left | 31 | Male | 12 | Seizure‐free | Oxcarbazepine | Normal | Left temporal slowing | None |
| 4 | Temporal | Right | 63 | Female | 38 | <12/year | Lamotrigine | Normal | Slight right temporal slowing | Right temporal abnormalities |
| 5 | Frontal | Left | 20 | Female | 4 | <12/year | Lamotrigine | Normal | Light left temporal focal slowing | Left frontal and temporal abnormalities |
| 6 | Frontal | Right | 25 | Male | 23 | <1/year | Carbamazepine | Normal | Right midtemporal slow activity | Right frontocentral abnormalities |
| 7 | Temporal | Unknown | 44 | Female | 1 | <12/year | Levetiracetam | Normal | None | None |
| 8 | Temporal | Unknown | 69 | Male | 26 | Seizure‐free | Carbamazepine | N/A | N/A | N/A |
| 9 | Temporal | Unknown | 45 | Male | 10 | <12/year | Carbamazepine | N/A | N/A | N/A |
| 10 | Temporal | Unknown | 36 | Male | 18 | <1/year | Carbamazepine | Normal | Left and right midtemporal slow activity | None |
N/A, not available.
We used the values obtained in 20 controls (10 males, aged 22‐65 years) to determine lateralization in a healthy situation. These values were used as reference for comparison with the epilepsy patients.
Image Acquisition
MR images were acquired using a 7T MRI System (Magnetom, Siemens Healthineers, Erlangen, Germany) using a 32‐channel phased‐array coil (Nova Medical, Wilmington, MA, USA). Dielectric pads were placed proximal to the temporal lobe to improve B1 homogeneity. 19
For anatomical reference and volumetric assessment, images were acquired using a sagittal 3D T1‐weigthed Magnetization Prepared 2 Rapid Acquisition Gradient Echo (MP2RAGE) sequence with: repetition time (TR)/echo time (TE): 4,500/2.39 milliseconds; inversion times (TI1/TI2): 900/2,750 milliseconds; flip angles (FA1/FA2): 5o/3o; voxel size: .9 × .9 × .9 mm; generalized autocalibrating partially parallel acquisitions (GRAPPA) factor: 3 in the phase‐encoding (PE) direction with 24 reference lines and an acquisition time (TA): 6 minutes. From this, a quantitative T1‐map was calculated.
Resting‐state T2*‐weighted images sensitive to the BOLD‐contrast were obtained with a transverse, multislice echoplanar‐imaging (EPI) sequence with TR/TE: 1,700/19 milliseconds; FA: 64o; field‐of‐view: 205 × 219 × 152 mm; pixel size: 1.5 × 1.5 mm; slice thickness: 1.5 mm; GRAPPA factor: 3; number of dynamics: 200; TA: 6 minutes; phase encoding in the anterior–posterior direction. For distortion correction, 5 dynamic images were also acquired in reversed phase‐encoding direction. FMRI was only acquired for a subset of the participants: 14 controls and 8 patients (7 TLE and 1 FLE).
Image Processing
The subject‐specific T1‐weighted images were preprocessed by performing bias field correction, skull stripping, and gradient distortion correction. 20 Using Freesurfer version 6.0, 21 the T1‐maps were automatically segmented into the frontal and temporal lobes, the hippocampus and hippocampal subfields, that is, subiculum, cornu ammonis 1 (CA1), CA2/CA3, CA4, granule cell layer of the dentate gyrus (DG) (Fig 1).
Fig 1.
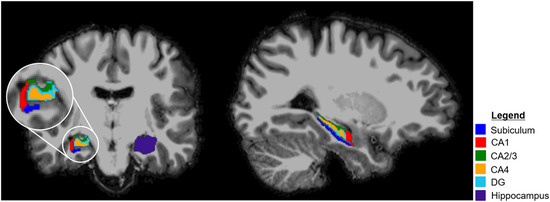
Example of hippocampal segmentation in a healthy subject (male, 25 years). CA, cornu ammonis; DG, dentate gyrus.
Preprocessing of the functional images included discarding the first 6 timepoints to allow for magnetic stabilization, brain extraction, motion correction, and distortion correction (FSL, Analysis Group, FMRIB, Oxford, UK). Further preprocessing included correction for slice‐timing effects (SPM12 with MATLAB R2018b), and correction for general physiological fluctuations by regressing out the mean time signals from the white matter and CSF. Finally, spatial coregistration of the functional images to the high‐resolution (segmented) T1‐maps was performed.
Image Analysis
Fractional Amplitude of Low‐Frequency Fluctuations (fALFF)
For each voxel, the signal intensity time‐series was Fourier transformed to the frequency domain. The power spectrum was calculated for the conventional frequency range (10‐100 mHz). 22 The fALFF was computed for all regions of interest (ROI), in both hemispheres separately, as the ratio of the amplitude in the conventional frequency range (10‐100 mHz) to that of the full frequency range (0‐294 mHz). 14
Morphological and Functional Lateralization
For both the controls and patients, the segmented ROIs of the hippocampus, hippocampal subfields, and temporal and frontal lobes were selected to calculate the LI, 23 for the left‐right differences in volume and fluctuation amplitude by:
Theoretically, LI ranges between −1 and +1. Positive indices indicate larger left‐sided volume or fluctuation amplitude, while negative scores imply a larger right‐sided volume or fluctuation amplitude.
Statistical Analysis
Normative values of the controls were expressed as the median value and the 95% confidence intervals (CI). Shapiro–Wilk tests were performed to check normality of the LI values in the controls. All volume and fluctuation amplitude LI values were normally distributed (P > .15 and P > .12, respectively). Pearson's correlation coefficients between age and volume, volume LI and fluctuation amplitude LI were calculated to assess whether correction for age was necessary.
To determine whether significant volumetric and functional lateralization was present in controls, a Student's t‐test was performed, and LI values were compared to zero. When P < .05, statistically significant lateralization was inferred. Significant lateralization for each region of every individual patient was inferred, in case the LI value was outside this 95% CI of the control group.
Results
Volume Asymmetry
Neuroradiological assessment of T1‐weighted, T2‐weighted, and FLAIR images revealed a degree of atrophy in several participants, which could not be linked to origin of the epilepsy. However, this did not result in an obvious visual asymmetry. Also, neither hippocampal sclerosis nor hippocampal left‐right differences were observed. Representative images are shown in Figure 2.
Fig 2.
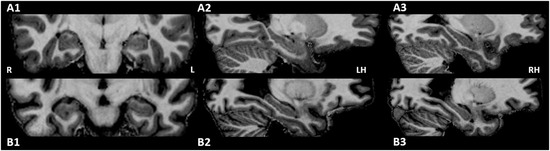
Example illustrating the symmetric hippocampal region in a healthy subject (male, 25 years, A1, A2, A3) and the left‐sided TLE patient with the largest volumetric lateralization (#3, male, 45 years, B1, B2, B3). Note the atrophy in the patient. R, right; L, Left; LH, left hemisphere; RH, right hemisphere.
Volumes in the left and right hemispheres in controls were not different (Table 2). Age‐related differences between controls were found in the CA1, DG, temporal, and frontal lobes, with a significant decrease in volume with age (Pearson correlation r < .40, P < .03). However, no significant correlation between the LI and age was found (P > .29). Therefore, the comparisons between individual patients and the control group were not corrected for age.
Table 2.
Regional Volumes, fALFF, and LI of the Controls
| Hippocampus | Subiculum | CA1 | CA2/3 | CA4 | DG | Temporal Lobe | Frontal Lobe | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Volume (cm3) | Left | 3.00 ± .27 | .41 ± .05 | .59 ± .07 | .18 ± .02 | .24 ± .03 | .29 ± .03 | 15.02 ± 1.50 | 30.70 ± 30.20 | |
| Right | 2.93 ± .29 | .39 ± .04 | .60 ± .08 | .18 ± .02 | .24 ± .03 | .28 ± .03 | 15.07 ± 1.47 | 31.07 ± 2.92 | ||
| Volume LI (×10−2) | Median | 1.4 * | 2.8 * | −1.0 | −.6 | .3 | .6 | −.3 | −.1 | |
| 95% CI | Lower | .3 | .5 | −1.9 | −3.1 | −.3 | −.3 | −.9 | −1.6 | |
| Upper | 2.0 | 4.3 | .4 | 1.3 | 2.2 | 2.0 | .7 | .3 | ||
| fALFF | Left | 1.18 ± .11 | 1.10 ± .08 | 1.12 ± .10 | 1.04 ± .08 | 1.06 ± .09 | 1.12 ± .08 | 1.27 ± .10 | 1.37 ± .11 | |
| Right | 1.14 ± .09 | 1.13 ± .10 | 1.14 ± .08 | 1.08 ± .09 | 1.08 ± .11 | 1.10 ± .09 | 1.27 ± .09 | 1.36 ± .10 | ||
| Fluctuation amplitude LI (×10−2) | Median | 2.4 | −2.1 | −.2 | −1.5 | −2.2 | 1.4 | −.08 | .2 | |
| 95% CI | Lower | −.01 | −3.0 | −2.3 | −4.2 | −2.8 | −.7 | −.4 | −.4 | |
| Upper | 3.7 | .6 | .9 | .4 | 1.7 | 2.8 | 1.2 | .7 |
Significantly different from zero. Data represent mean ± standard deviation unless otherwise indicated.
CA, cornu ammonis; CI, confidence interval; DG, dentate gyrus; fALFF, fractional amplitude of low frequency fluctuations; LI, lateralization index.
In healthy participants, LI values were not significantly different from zero, except for a subtle left lateralization of the hippocampus (median LI = .014, P < .05) and subiculum (median LI = .043, P < .02), see Table 2.
All TLE patients with known focus showed significant hippocampal lateralization in comparison to the controls (Table 3). The strongest LI value of the left‐sided TLE patients was negative and was observed in the CA2/3. Furthermore, hippocampal subfields with significant (negative) lateralization were spatially adjacent. The observed LI values of the right‐sided TLE patient were mainly positive. In all TLE patients with known focus, the hippocampus, subiculum and CA2/3 revealed asymmetric volumes, with consistently smaller ipsilateral than contralateral volumes.
Table 3.
The Volumetric LI in the Epilepsy Group
| LI (×10−2) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Focal Epilepsy | Location | Patient Number | Hippocampus | Subiculum | CA1 | CA2/3 | CA4 | DG | Temporal Lobe | Frontal Lobe |
| Temporal | Left | 1 | −1.9 * | −2.2 * | −3.3 * | −6.1 * | −5.3 * | −4.8 * | −1.1 * | −.7 |
| 2 | −2.4 * | −1.2 * | −1.8 | −5.4 * | −4.3 * | −3.2 * | .4 | −.4 | ||
| 3 | −2.2 * | −2.2 * | −7.6 * | −11.1 * | −4.2 * | −6.1 * | .08 | −2.5 * | ||
| Right | 4 | 2.8 * | .4 * | 1.7 = | 4.0 * | .3 | .9 | −.4 | −.9 | |
| Frontal | Left | 5 | −.5 * | 2.9 | −.9 | −1.9 | −1.0 * | −.5* | −1.3 * | .8 * |
| Right | 6 | 1.9 | 3.5 | 3.6 * | .5 | 1.7 | 2.3 * | 1.0 * | .9 * | |
| Temporal | Unknown | 7 | −.9 * | .3 * | −3.3 * | −.06 | 1.1 | .5 | −.8 | −1.5 |
| 8 | −2.1 * | .8 | −1.9 | −2.4 | −3.4 * | −3.4 * | −.03 | −1.1 | ||
| 9 | 2.2 * | 5.5 * | 2.6 * | 2.4 * | −1.2 * | .003 | 2.6 * | −.02 | ||
| 10 | 2.1 * | 1.4 | −2.4 * | .8 | 1.6 | 3.0 * | −2.1 * | .4 * | ||
LI metrics located outside the 95% CI of those of controls.
CA, cornu ammonis; CI, confidence interval; DG, dentate gyrus; LI, lateralization index.
Two of the TLE patients with unknown focus (#7 and #8) had a dominant and consistent LI on the left side. All significant LI values were lower compared to those of the control subjects and the strongest value was negative, which was consistent with the left‐sided TLE patients. One TLE patient with unknown focus (#9) had the highest (positive) LI value for the subiculum, consistent with a dominance of the right side. Finally, the last TLE patient with unknown focus (#10) showed rather variable lateralization, with a significant positive hippocampus lateralization, which could indicate a dominance for the right side.
For the left FLE patient, we observed generally negative LI values, while for the right FLE patient the values were mostly positive. However, for most regions the LI values were not different from the values of the controls.
Fluctuation Amplitude Asymmetry
FALFF maps showed overall higher amplitudes for patients compared to controls (Fig 3). Fluctuation amplitude LI and age did not significantly correlate in controls (r < .40, P > .16). Hence, no age correction was applied for comparisons between individual patients and the control group. In the controls, all regions were symmetric (P > .1, Table 2).
Fig 3.
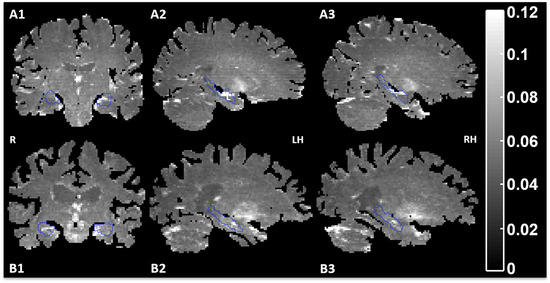
Example of the fALFF maps in a healthy subject (male, 25 years, A1, A2, A3) and a left‐sided TLE patient (#3) (male, 45 years, B1, B2, B3). The hippocampi are delineated with a blue contour. Brighter spots indicate higher activity. R, right; LH, left hemisphere; RH, right hemisphere.
For the left‐sided TLE patients, the lateralization pointed at significantly higher fluctuation amplitude (highest LI and adjacent subfields with positive values) in the left hippocampus (Table 4). Contrary, the right‐sided TLE patient generally showed higher fluctuation amplitude contralateral to the seizure focus. LI values in the left‐sided FLE patient revealed a dominant fluctuation amplitude lateralized to the left side in all significant regions, except for a right‐sided lateralization in the temporal lobe, while no clear lateralization was observed in the frontal lobe. The LI in the TLE patients with unknown focus varied strongly over the regions.
Table 4.
The Fluctuation Amplitude LI in the Epilepsy Group
| LI (×10−2) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Focal Epilepsy | Location | Patient Number | Hippocampus | Subiculum | CA1 | CA2/3 | CA4 | DG | Temporal Lobe | Frontal Lobe |
| Temporal | Left | 1 | 5.3 * | 6.9 * | 5.4 * | .9 * | 3.6 * | 6.9 * | −1.6 * | .2 |
| 3 | 7.5 * | 3.7 * | 1.4 * | 4.6 * | −1.3 | 4.2 * | 2.8 * | −.2 | ||
| Right | 4 | 6.6 * | −1.3 | 3.8 * | 1.9 * | −1.9 | −.9 * | −.03 | −1.9 * | |
| Frontal | Left | 5 | 4.7 * | .6 * | 6.3 * | 3.3 * | −.8 | 2.7 | −1.6 * | .04 |
| Temporal | Unknown | 7 | −1.6 * | .8 * | −3.2 * | 3.9 * | −1.9 | 2.0 | .9 | −.1 |
| 8 | .2 | −9.5 * | −1.0 | 5.3 * | 4.0 * | 1.7 | −2.2 * | −1.1 * | ||
| 9 | 4.8 * | 10.6 * | −4.3 * | .3 | −.8 | −1.6 * | −3.0 * | −.5 * | ||
| 10 | 2.3 * | −6.0 * | −2.0 | −1.4 | 8.8 * | 8.2 * | −1.9 * | −.05 | ||
LI metrics located outside the 95% CI of those of controls.
CA, cornu ammonis; CI, confidence interval; DG, dentate gyrus; LI, lateralization index.
Relationship Between Volume and Fluctuation Amplitude Lateralization in Epilepsy
For the left‐sided TLE patients, the ipsilateral hippocampus was smaller and exhibited higher fluctuation amplitude (i.e. LI had opposite signs for volume and fluctuation amplitude). Interestingly, the right‐sided TLE patient also showed a smaller ipsilateral hippocampal volume, but a larger contralateral fluctuation amplitude was observed.
Discussion
We explored the volume and fluctuation amplitude lateralization of controls and TLE and FLE patients in a small pilot study. The results generally confirmed that the hippocampal formation and frontal and temporal lobes are symmetric in healthy subjects. In TLE patients with known focus, we observed slightly larger contralateral volumes compared to ipsilateral volumes and the largest volumetric LI was found in a hippocampal subfield: CA2/3. In all TLE patients with unknown seizure focus, volumetric LI's suggest a lateralization of the epileptic focus. Even though differences in fluctuation amplitude lateralization indices of epilepsy patients in comparison with controls were found, no general consistency was observed with their location of the epileptic focus.
Volumetric Lateralization
Volumes were generally symmetric in healthy subjects, but a subtle but significant left‐sided volumetric asymmetry of the hippocampus and subiculum was found (median LI 1.4·10−2 and 2.8·10−2, respectively). Previous studies found either no asymmetry of the hippocampus (at 1.0T), 24 or larger right hippocampal volumes at 1.5T. 25 Laakso and others revealed an effect of slice thickness on hippocampal volume and asymmetry, with slice thicknesses <2 mm showing larger left‐right differences compared to volumes obtained with slice thickness >2 mm. 26 As both field strength and slice thickness are likely to influence volume and asymmetry, comparison is challenging.
We found small LI values, such as a hippocampal volumetric LI of 1.4 in controls, corresponding to a difference of .084 mL. This might be too subtle to visually detect, which might explain why the neuroradiological assessment did not reveal epileptogenic abnormalities.
The volume estimates of the hippocampal formation and its subfields in controls were comparable with those in literature. 24 Here, the volume of the left hippocampus was generally slightly smaller in left TLE patients compared to the control group, while the right hippocampal volume was similar or slightly larger. Previously at lower field strengths, a smaller ipsilateral hippocampal volume was found in patients with TLE, 12 , 27 which concurs with the findings of the current study. Interestingly, some of the volumetric asymmetry ratios were similar to those of the controls, which is in agreement with evidence from pathology studies, as hippocampal sclerosis can also express bilaterally, 28 resulting in symmetric volume ratios. Volumetric asymmetry of the hippocampus was more prominent in TLE than FLE patients, which is consistent with literature in which the epileptic focus of FLE patients is often reported outside the mesial temporal lobe. 29 , 30 However, a significantly smaller ipsilateral hippocampal volume was found in the left FLE patient, but not in the right FLE patient. This is consistent with previous literature. 11
Previous studies showed that routine EEG was correlated with the seizure origin and MRI volumetry findings. 31 Hence, improving volumetric assessments can further aid the identification of the epileptic focus.
Fluctuation Amplitude Lateralization
The fALFF values in controls were in the same range as previously reported by studies performed at field strengths of 1.5, 3, and 7T. 32 , 33 An earlier study by Chen and others already showed the potential of resting‐state fMRI at 3T in 42 focal epilepsy patients. 34 They showed that the sensitivity of fMRI based on regional homogeneity analysis, amplitude of low frequency fluctuations, and fALFF was comparable with FDG‐PET and specificity was similar with video‐EEG in the mesial temporal lobe. 34
Previous functional connectivity (FC) studies using resting‐state fMRI in left‐sided TLE demonstrated a decreased ipsilateral FC of the hippocampus compared to controls. In right‐sided TLE patients, this decrease was also, less consistently, observed. 35 Contrarily, in MRI‐negative TLE patients, a greater ipsilateral connectivity was previously observed in the hippocampus and CA1, and a trend in all the other subfields. 10 In the current study, a higher left‐sided fluctuation amplitude was found in left‐ and right‐sided TLE and FLE patients. This was most consistently observed in the hippocampus, CA1 and CA2/3, with a trend for all other subfields. The left hippocampus is likely more dominant and responsive compared to the right hippocampus, due to the dominance for language in the left hemisphere. 36
Link Between Volumetric and Fluctuation Amplitude Lateralization
We demonstrated that left‐sided TLE patients had smaller ipsilateral hippocampal volumes and generally higher fluctuation amplitude than the contralateral hippocampus (ie LI had opposite signs for volume and fluctuation amplitude). Contrarily, the right‐sided TLE patient, showed a smaller ipsilateral hippocampal volume and a lower ipsilateral fluctuation amplitude compared to the contralateral side.
Importantly, in all TLE patients with known epileptic focus, we observed that the volumetric and fluctuation amplitude lateralization of the hippocampus exceeded the 95% CI of the control group. Most of the hippocampal subfields also showed these differences in lateralization, but results were less consistent, which might imply that it is more robust to consider the hippocampus rather than its subfields. However, including the subfields in the analysis seems fruitful, as the largest LI values were detected in the hippocampal subfields, thus, considering subfields might increase sensitivity.
Clinical Significance
When the epileptic focus cannot be identified on MR images, the success rate of the surgery for complete seizure control drops to about 36% compared to 62‐80% when MRI can identify the epileptic focus. 4 In this pilot study, we demonstrate that particularly volumetric lateralization assessment can help in localizing the epileptic focus in TLE patients with unknown seizure focus and possibly aiding us with the prediction of the location of the epileptic focus. As the clinical workup prior to epilepsy surgery treatment often includes a specialized MR protocol, including both structural and functional sequences, it will be relatively straightforward to add the lateralization analyses. The promising results in this pilot study should ideally be confirmed with detailed intracranial electrophysiology, postsurgical histology, or assessment of seizure freedom after surgery.
Study Considerations
One of the limitations of this study is the relatively small size and heterogeneity of the epilepsy group and clinical parameters; thus, the findings of the present study should be considered as preliminary. Small sample sizes, between 6 and 15 participants, are characteristic for 7T epilepsy studies and might reflect both the cost and challenges of imaging at ultra‐high field strengths, such as contraindications for 7T MRI such as metallic implants. 37 Moreover, in 4 of the 10 patients participating in this study, the location of the epileptic focus was unknown, which, even though a small number of patients is included, is consistent with the percentage found in literature. 5 , 6
The current study demonstrates the potential of MRI lateralization assessment in aiding the side of the localization of the epileptic focus in focal epilepsy patients. Unfortunately, in our study, focal epilepsy patients were not confirmed to be of temporal or frontal lobe onset with intracranial EEGs. Therefore, patients could have multifocal epilepsy or an occult onset in one of the other lobes or in deeper structures. However, the epileptic focus was independently assessed by 2 neurologists based on previous medical examinations, which reflects the clinical practice in relatively stable epilepsy patients. The current study considered a relatively stable group of epilepsy patients, in which relatively small LIs were observed. In large epilepsy centers, patients with higher seizure frequencies or without complete seizure control are treated. In these patients, different values of LIs might be found. Therefore, generalization of the current results is limited and confirmation in larger studies is needed.
Another limitation of the current study is that only the lateralization of the hippocampal formation and entire temporal and frontal lobes were investigated. Although in TLE, hippocampal sclerosis is considered as the most common underlying etiology, 38 other subregions of the temporal and frontal lobes could also be lateralized. In a larger study, investigating these regions could be interesting.
Due to its composite nature, alterations in LI do not have an unambiguous meaning, as changes in both left and/or right can result in the same LI. However, the LI is a rather straightforward and robust measure, which is easy to calculate and to apply. 23 Additionally, the volumetric LI is insensitive to atrophy due to ageing.
Finally, a limitation is the spatial resolution of fMRI (cubic voxel size 1.5 mm). However, the ROIs used have a substantial number of voxels, with the smallest ROI containing 54 voxels. In an effort to limit partial volume effects, no spatial smoothing was applied to maintain the spatial specificity of the measured fluctuation amplitude as much as possible. 39 The feasibility of assessing the FC of the hippocampal subfields at 7T has already been demonstrated previously. 10 , 39 , 40 Moreover, this study was limited to one fMRI measure, the fALFF, while other measures also could be evaluated, for instance regional homogeneity (ReHo) or FC. 41 The fALFF was chosen as it reflects the amplitude of local spontaneous fluctuations per pixel. While ReHo is also a regional measure, reflecting the concordance (Kendall's correlation coefficient) of the time‐series of neighboring voxels, and was therefore possibly less suitable in the small hippocampal subfields. FC has more global properties as it measures the correlation of a region with all other regions, and therefore is not a regional measure.
Conclusions
Volumes and fluctuation amplitude of the hippocampus, hippocampal subfields, and frontal and temporal lobes are, in general, highly symmetric in healthy subjects. Interestingly, in TLE, though not FLE, hippocampal volumetric and fluctuation amplitude lateralization effects were observed, highlighting the potential for the application of detecting local abnormalities. As the strongest lateralization was often observed in the hippocampal subfields, investigating hippocampal subfield lateralization might be a sensitive approach. Future work in larger cohorts is warranted to assess the clinical merits.
Acknowledgement: The authors thank Esther Steijvers, Lotty Huijboom, and Chris Wiggins for help with data acquisition. This study was funded by the University Fund Limburg/SWOL (S.2014.14).
References
- 1. Téllez‐Zenteno JF, Hernández‐Ronquillo L. A review of the epidemiology of temporal lobe epilepsy. Epilepsy Res Treat 2011;2012:1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wiebe S, Jette N. Pharmacoresistance and the role of surgery in difficult to treat epilepsy. Nat Rev Neurol 2012;8:669‐77. [DOI] [PubMed] [Google Scholar]
- 3. Wiebe S, Blume WT, Girvin JP, et al. A randomized, controlled trial of surgery for temporal‐lobe epilepsy. N Engl J Med 2001;345:311‐8. [DOI] [PubMed] [Google Scholar]
- 4. Téllez‐Zenteno JF, Ronquillo LH, Moien‐Afshari F, et al. Surgical outcomes in lesional and non‐lesional epilepsy: a systematic review and meta‐analysis. Epilepsy Res 2010;89:310‐8. [DOI] [PubMed] [Google Scholar]
- 5. Cascino GD, Jack CR Jr, Parisi JE, et al. Magnetic resonance imaging‐based volume studies in temporal lobe epilepsy: pathological correlations. Ann Neurol 1991;30:31‐6. [DOI] [PubMed] [Google Scholar]
- 6. Muhlhofer W, Tan YL, Mueller SG, et al. MRI‐negative temporal lobe epilepsy—what do we know? Epilepsia 2017;58:727‐42. [DOI] [PubMed] [Google Scholar]
- 7. Toga AW, Thompson PM. Mapping brain asymmetry. Nat Rev Neurosci 2003;4:37‐48. [DOI] [PubMed] [Google Scholar]
- 8. Bell B, Lin JJ, Seidenberg M, et al. The neurobiology of cognitive disorders in temporal lobe epilepsy. Nat Rev Neurol 2011;7:s154‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sone D, Sato N, Maikusa N, et al. Automated subfield volumetric analysis of hippocampus in temporal lobe epilepsy using high‐resolution T2‐weighed MR imaging. NeuroImage Clin 2016;12:57‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shah P, Bassett DS, Wisse LEM, et al. Structural and functional asymmetry of medial temporal subregions in unilateral temporal lobe epilepsy: a 7T MRI study. Hum Brain Mapp 2019;40:2390‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Widjaja E, Zamyadi M, Raybaud C, et al. Volumetric changes in hippocampal subregions and their relation to memory in pediatric nonlesional localization‐related epilepsy. Epilepsia 2014;55:519‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Farid N, Girard HM, Kemmotsu N, et al. Temporal lobe epilepsy: quantitative MR volumetry in detection of hippocampal atrophy. Radiology 2012;264:542‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goffin K, Van Paesschen W, Dupont P, et al. Anatomy‐based reconstruction of FDG‐PET images with implicit partial volume correction improves detection of hypometabolic regions in patients with epilepsy due to focal cortical dysplasia diagnosed on MRI. Eur J Nucl Med Mol Imag 2010;37:1148‐55. [DOI] [PubMed] [Google Scholar]
- 14. Zou Q‐H, Zhu C‐Z, Yang Y, et al. An improved approach to detection of amplitude of low‐frequency fluctuation (ALFF) for resting‐state fMRI: fractional ALFF. J Neurosci Method 2008;172:137‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van der Zwaag W, Francis S, Head K, et al. fMRI at 1.5, 3 and 7 T: characterising BOLD signal changes. Neuroimage 2009;47:1425‐34. [DOI] [PubMed] [Google Scholar]
- 16. Bienkowski MS, Bowman I, Song MY, et al. Integration of gene expression and brain‐wide connectivity reveals the multiscale organization of mouse hippocampal networks. Nat Neurosci 2018;21:1628‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Duvernoy HM, Cattin F, Risold PY, et al. The Human Hippocampus: Functional Anatomy, Vascularization and Serial Sections with MRI. 4th ed. Berlin Heidelberg: Springer‐Verlag, 2013. [Google Scholar]
- 18. Veenendaal TM Van, Backes WH, Tse DHY, et al. High field imaging of large‐scale neurotransmitter networks: proof of concept and initial application to epilepsy. NeuroImage Clin 2018;19:47‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Teeuwisse WM, Brink WM, Webb AG. Quantitative assessment of the effects of high‐permittivity pads in 7 Tesla MRI of the brain. Magn Reson Med 2012;67:1285‐93. [DOI] [PubMed] [Google Scholar]
- 20. Haast RAM, Ivanov D, Uludağ K. The impact of B1+ correction on MP2RAGE cortical T 1 and apparent cortical thickness at 7T. Hum Brain Mapp 2018;39:2412‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 2002;33:341‐55. [DOI] [PubMed] [Google Scholar]
- 22. Yu‐Feng Z, Yong H, Chao‐Zhe Z, et al. Altered baseline brain activity in children with ADHD revealed by resting‐state functional MRI. Brain Dev 2007;29:83‐91. [DOI] [PubMed] [Google Scholar]
- 23. Binder JR, Swanson SJ, Hammeke TA, et al. Determination of language dominance using functional MRI: a comparison with the Wada test. Neurology 1996;46:978‐84. [DOI] [PubMed] [Google Scholar]
- 24. Pedraza O, Bowers D, Gilmore R. Asymmetry of the hippocampus and amygdala in MRI volumetric measurements of normal adults. J Int Neuropsychol Soc 2004;10:664‐78. [DOI] [PubMed] [Google Scholar]
- 25. Jack CR, Theodore WH, Cook M, et al. MRI‐based hippocampal volumetrics: data acquisition, normal ranges, and optimal protocol. Magn Reson Imaging 1995;13:1057‐64. [DOI] [PubMed] [Google Scholar]
- 26. Laakso MP, Juottonen K, Partanen K, et al. MRI volumetry of the hippocampus: the effect of slice thickness on volume formation. Magn Reson Imaging 1997;15:263‐5. [DOI] [PubMed] [Google Scholar]
- 27. Voets NL, Hodgetts CJ, Sen A, et al. Hippocampal MRS and subfield volumetry at 7T detects dysfunction not specific to seizure focus. Sci Rep 2017;7:1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. García‐Fiñana M, Denby CE, Keller SS, et al. Degree of hippocampal atrophy is related to side of seizure onset in temporal lobe epilepsy. Am J Neuroradiol 2006;27:1046‐52. [PMC free article] [PubMed] [Google Scholar]
- 29. Cook MJ, Fish DR, Shorvon SD, et al. Hippocampal volumetric and morphometric studies in frontal and temporal lobe epilepsy. Brain 1992;115:1001‐15. [DOI] [PubMed] [Google Scholar]
- 30. Santyr BG, Goubran M, Lau JC, et al. Investigation of hippocampal substructures in focal temporal lobe epilepsy with and without hippocampal sclerosis at 7T. J Magn Reson Imaging 2017;45:1359‐70. [DOI] [PubMed] [Google Scholar]
- 31. Cascino GD, Trenerry MR, So EL, et al. Routine EEG and temporal lobe epilepsy: relation to long‐term EEG monitoring, quantitative MRI, and operative outcome. Epilepsia 1996;37:651‐6. [DOI] [PubMed] [Google Scholar]
- 32. McHugo M, Rogers BP, Talati P, et al. Increased amplitude of low frequency fluctuations but normal hippocampal‐default mode network connectivity in schizophrenia. Front Psychiatr 2015;6:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Duan G, Liu H, Pang Y, et al. Hippocampal fractional amplitude of low‐frequency fluctuation and functional connectivity changes in premenstrual syndrome. J Magn Reson Imaging 2018;47:545‐53. [DOI] [PubMed] [Google Scholar]
- 34. Chen Z, An Y, Zhao B, et al. The value of resting‐state functional magnetic resonance imaging for detecting epileptogenic zones in patients with focal epilepsy. PLoS One 2017;12:1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pereira FRS, Alessio A, Sercheli MS, et al. Asymmetrical hippocampal connectivity in mesial temporal lobe epilepsy: evidence from resting state fMRI. BMC Neurosci 2010;11:66‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hickok G, Bellugi U, Klima ES. The neurobiology of sign language and its implications for the neural basis of language. Nature 1996;381:699‐702. [DOI] [PubMed] [Google Scholar]
- 37. Van Der Kolk AG, Hendrikse J, Zwanenburg JJM, et al. Clinical applications of 7 T MRI in the brain. Eur J Radiol 2013;82:708‐18. [DOI] [PubMed] [Google Scholar]
- 38. Thom M, Eriksson S, Martinian L, et al. Temporal lobe sclerosis associated with hippocampal sclerosis in temporal lobe epilepsy: neuropathological features. J Neuropathol Exp Neurol 2009;68:928‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shah P, Bassett DS, Wisse LEM, et al. Mapping the structural and functional network architecture of the medial temporal lobe using 7T MRI. Hum Brain Mapp 2018;39:851‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Suthana NA, Donix M, Wozny DR, et al. High‐resolution 7T fMRI of human hippocampal subfields during associative learning. J Cogn Neurosci 2015;27:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Soares JM, Magalhães R, Moreira PS, et al. A Hitchhiker's guide to functional magnetic resonance imaging. Front Neurosci 2016;10:1‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]


