Abstract
Neuroblastoma is a sympathetic nervous system tumor, primarily presenting in children under 6 years of age. The long‐term prognosis for patients with high‐risk neuroblastoma (HRNB) remains poor despite aggressive multimodal therapy. This report provides an update to a phase II trial evaluating DFMO as maintenance therapy in HRNB. Event‐free survival (EFS) and overall survival (OS) of 81 subjects with HRNB treated with standard COG induction, consolidation and immunotherapy followed by 2 years of DFMO on the NMTRC003/003b Phase II trial were compared to a historical cohort of 76 HRNB patients treated at Beat Childhood Cancer Research Consortium (BCC) hospitals who were disease‐free after completion of standard upfront therapy and did not receive DFMO. The 2‐ and 5‐year EFS were 86.4% [95% confidence interval (CI) 79.3%‐94.2%] and 85.2% [77.8%‐93.3%] for the NMTRC003/003b subset vs 78.3% [69.5%‐88.3%] and 65.6% [55.5%‐77.5%] for the historical control group. The 2‐ and 5‐year OS were 98.8% [96.4‐100%] and 95.1% [90.5%‐99.9%] vs 94.4% [89.3%‐99.9%] and 81.6% [73.0%‐91.2%], respectively. DFMO maintenance for HRNB after completion of standard of care therapy was associated with improved EFS and OS relative to historical controls treated at the same institutions. These results support additional investigations into the potential role of DFMO in preventing relapse in HRNB.
Keywords: DFMO, high‐risk neuroblastoma, maintenance
Short abstract
What's new?
The long‐term prognosis for patients with high‐risk neuroblastoma (HRNB) remains poor despite aggressive multimodal therapy. This phase II clinical trial update is the first study to demonstrate statistically and clinically significant improvement in event‐free and overall survival in HRNB patients as a result of DFMO administration following standard upfront therapy relative to a closely matched historical control. The findings support DFMO maintenance as a novel strategy to prevent relapse in a high‐risk population for relapse following frontline treatment.
Abbreviations
- BCC
Beat Childhood Cancer (formerly NMTRC)
- CI
confidence interval
- COG
Children's Oncology Group
- Control
BCC001
- CR
complete response
- DFMO
difluoromethylornithine
- EFS
event‐free survival
- HRNB
high‐risk neuroblastoma
- IL‐2
interleukin‐2
- IRB
Institutional Review Board
- NB
neuroblastoma
- NMTRC
Neuroblastoma and Medulloblastoma Translational Research Consortium (now BCC)
- ODC
ornithine decarboxylase
- OS
overall survival
- PR
partial response
- SD
stable disease
- SIOPEN
International Society of Pediatric Oncology European Neuroblastoma
- VGPR
very good partial response
- WIRB
Western Institutional Review Board
1. INTRODUCTION
Neuroblastoma (NB) is a tumor of the autonomic nervous system, typically originating from the adrenal medulla. This cancer occurs in 1/7000 children, with 800 cases annually in the US. Risk stratification of NB is determined by a variety of factors including age, stage, histology and cytology, with MYCN gene amplification serving as the primary prognostic indicator. Patients with low‐ and intermediate‐risk disease often achieve long‐term remission with moderate courses of chemotherapy and/or surgical resection. In contrast, patients with high‐risk NB (HRNB), which represents approximately 50% of cases, demonstrate poorer long‐term survival despite aggressive, multimodal frontline therapy including 5‐8 cycles of multiagent induction chemotherapy followed by surgical resection, consolidation therapy with high‐dose chemotherapy plus autologous stem cell support, radiation therapy and maintenance immunotherapy with dinutuximab plus cis‐retinoic acid. 1 , 2 , 3 , 4 , 5 , 6 Data from the Children's Oncology Group's (COG) pivotal ANBL0032 study show a 2‐year event‐free survival (EFS) from the start of immunotherapy of 66% ± 5%, 5 decreasing to 59% ± 5% at 4 years, 7 thus demonstrating the need for further improvements in post‐immunotherapy maintenance therapy. While more recent data have reported an improved 3‐year EFS and overall survival (OS) for patients receiving tandem courses of high‐dose chemotherapy with stem cell support prior to immunotherapy (73.3% [95% confidence interval (CI), 65.2%‐81.3%] and 84.0% [77.3%‐90.7%], respectively), approximately 25% of patients will still relapse. 8 While patients with relapsed/refractory disease after upfront treatment may respond transiently to salvage therapies, the rate of subsequent relapse remains extremely high at 80%‐90% within 2 years. 9 , 10 , 11 Thus, interventions designed to prevent relapse after upfront therapy may provide an important strategy to improve the long‐term survival of HRNB patients.
The antineoplastic mechanism of difluoromethylornithine (DFMO) is proposed to involve irreversible inhibition of the rate‐limiting enzyme of polyamine biosynthesis, ornithine decarboxylase (ODC). 12 Elevated polyamine levels, due to overexpression of ODC, are thought to contribute to neoplastic transformation and have been identified in NB and other tumors. Furthermore, laboratory studies have demonstrated that a reduction of polyamine levels through DFMOs inhibition of ODC prevents progression through the stages of carcinogenesis both in vitro and in xenograft models. 13 , 14 , 15 , 16 DFMO also possesses downstream activity in the LIN28/Let7 axis and has been shown to reverse cancer stem cell pathways. In vitro and xenograft models have demonstrated decreased neurosphere and tumor formation as a result of decreased LIN28 and increased Let7 after DFMO treatment. 17 , 18
DFMO has been clinically studied through the Beat Childhood Cancer Research Consortium (BCC) since 2010. An initial Phase I trial established the safety of DFMO in multiply relapsed patients at dose levels up to 1500 mg/m2 by mouth twice a day without reaching a maximum tolerated dose; three patients enrolled in our study remain alive >9 years poststudy without further therapy. A subsequent Phase II study evaluated the EFS and OS for patients receiving DFMO as maintenance therapy after completion of upfront (Stratum I) or relapsed/refractory therapy (Stratum II) for HRNB. Those who received DFMO after completion of standard therapy (Stratum I) demonstrated a 2‐year EFS of 84% and OS of 97% while those who received DFMO after therapy for relapsed/refractory disease had a 2‐year EFS of 51% and OS of 84%. 19 The majority of patients enrolled on Stratum I received initial treatment as per the standard of care used by our enrolling hospitals (COG HRNB upfront therapy). BCC conducted a retrospective study (BCC001) to identify a historical control population from the same hospitals as a statistical comparator. The following report updates the results of the subset of patients on NMTRC003/003b who received standard COG upfront therapy and compares them to the outcomes of patients identified by the BCC001 retrospective study.
2. MATERIALS AND METHODS
2.1. NMTRC003/003b study design, subjects and treatment
This Phase II clinical trial evaluated the use of DFMO for patients with HRNB as maintenance therapy after standard upfront therapy (Stratum I) or relapsed/refractory therapy (Stratum II). Subjects were enrolled from June 2012 to February 2016, and results have been previously reported. 19 This trial was approved by the Western Institutional Review Board (WIRB) as well as by all local Institutional Review Boards (IRB) at 22 enrolling hospitals across the United States. Consent for study participation was obtained on all subjects according to institutional guidelines. All methods were performed in accordance with relevant guidelines and regulations. ClinicalTrials.gov identifiers include: NCT01586260, Unique ID: NMTRC003, released April 24, 2012 and NCT02395666, Unique ID: NMTRC003B, released on March 5, 2015.
Patients with HRNB who completed standard upfront therapy without progression were eligible for enrollment. DFMO was initiated within 120 days of the completion of therapy at a dose of 750 ± 250 mg/m2/dose, twice daily. For the purpose of this report, we focus on a subset (n = 81) of the 100 enrolled patients who had been treated with the standard of care according to the COG guidelines for HRNB (induction, surgical resection, consolidation, radiation therapy and immunotherapy maintenance with dinutuximab) followed by maintenance DFMO and compare their outcomes to a retrospective cohort of patients treated at participating BCC hospitals with the same upfront therapies but without DFMO.
2.2. BCC001 study design, subjects and treatment
The historical control was generated through a retrospective study, BCC001 “Retrospective Chart Review of HRNB Patients”, that was approved by the WIRB as well as all local IRBs of participating BCC sites. Fifteen BCC hospitals performed a retrospective review of all neuroblastoma cases from 2003 to 2018. Clinical case histories were collected including diagnostic and prognostic indicators, treatments, and outcomes. A total of 378 patients were screened. Inclusion criteria for evaluation were defined as completion of COG standard of care therapy for HRNB, meeting the eligibility criteria for enrollment on Stratum I of NMTRC003/003b (including event‐free survival 120 days from completion of antibody), and no treatment with DFMO. Therefore, this control population was matched to the subset of NMTRC003/003b patients in all aspects except DFMO administration. These subjects were utilized for statistical comparison of DFMO treatment effect on EFS and OS.
2.3. Statistical analysis
For each covariate in Table 1, we reported a P‐value of the differences in that covariate between the subset of Stratum 1 NMTRC003/003b group and the control group. For continuous covariates (age), we tested the differences between these groups using a t test. For discrete covariates (sex, stage, MYCN, histology and response to induction), we tested the differences between treatment groups using Chi‐squared tests. The EFS/OS survival curves were compared between the subset of NMTRC003/003b Stratum I and the control group, with additional subset analysis based on MYCN amplification. Time “0” was defined as the time from the end of standard therapy, as this was a consistent time point between the two groups. The R packages survival and survminer were used to create and plot survival curves from the Kaplan‐Meier estimates, including the corresponding 95% pointwise confidence interval bands obtained using Greenwood's method. The P values were provided for the associated log‐rank test. We additionally reported the hazard ratio estimated using a Cox proportional‐hazards regression model, the 95% confidence interval of the hazard ratio and the resulting P‐value. The Cox regression was estimated controlling for the treatment variable only.
TABLE 1.
Subject characteristics
| Subject characteristics | NMTRC003/003b (n = 81) | BCC001 (n = 76) | P value |
|---|---|---|---|
| Mean age at diagnosis, years | 4.5 | 3.4 | .0076 |
| Sex, n (%) | .4862 | ||
| Male | 48 (59.3%) | 48 (63.2%) | |
| Female | 33 (40.7%) | 27 (35.5%) | |
| Unknown | 0 (0%) | 1 (1.3%) | |
| Stage at diagnosis, n (%) | .3610 | ||
| 1 | 0 (0%) | 0 (0%) | |
| 2A | 0 (0%) | 0 (0%) | |
| 2B | 2 (2.5%) | 0 (0%) | |
| 3 | 7 (8.6%) | 5 (6.6%) | |
| 4 | 72 (88.9%) | 70 (92.1%) | |
| 4S | 0 (0%) | 1 (1.3%) | |
| Unknown | 0 (0%) | 0 (0%) | |
| MYCN, n (%) | .3288 | ||
| Amplified | 37 (45.7%%) | 30 (39.5%) | |
| Nonamplified | 41 (50.6%) | 39 (51.3%) | |
| Unknown | 3 (3.7%) | 7 (9.2%) | |
| Histology, n (%) | .0004 | ||
| Unfavorable | 43 (53.1%) | 59 (77.6%) | |
| Favorable | 6 (7.4%) | 8 (10.5%) | |
| Unknown | 32 (39.5%) | 9 (11.8%) | |
| Response to induction therapy, n (%) | .0461 | ||
| CR | 39 (48.1%) | 23 (30.3%) | |
| VGPR | 10 (12.3%) | 11 (14.5%) | |
| PR | 25 (30.9%) | 37 (48.7%) | |
| SD | 5 (6.2%) | 1 (1.3%) | |
| Unknown | 2 (2.5%) | 4 (5.3%) |
Abbreviations: CR, complete response; PR, partial response; SD, stable disease; VGPR, very good partial response.
3. RESULTS
3.1. Subject characteristics
Of the 100 subjects eligible for enrollment on Stratum I of NMTRC003/003b, 81 subjects had been treated per COG standard of care (including completion of dinutuximab after enrollment on the postrandomization extension of COG ANBL0032) and started DFMO within 120 days of completion of immunotherapy. The BCC001 retrospective chart review identified 76 subjects treated at the same centers as NMTRC003/003b who met inclusion criteria for Stratum I of NMTRC003/003b (ie, treated per standard COG therapy with dinutuximab on ANBL0032 and remaining event‐free at least 120 days from completion of immunotherapy) and did not receive DFMO as maintenance therapy. See consort diagram for an outline of the flow of inclusion (Figure 1A). Subject characteristics for both groups have been outlined in Table 1. High‐risk features of the subject populations for both NMTRC003/003b and BCC001 groups are similar to those previously reported for HRNB population studies regarding age, stage, MYCN, histology, ploidy and induction response. 5 , 20 The groups are statistically similar with the exception of histology, age, and induction response. The large number of unknown histology found in the NMTRC003/003b group can account for this difference; when “unknown” is removed as a variable, the percentage of patients with unfavorable histology in each group is virtually identical with NMTRC003/003b at 87.8% and BCC001 at 88.1%. The BCC001 group showed a statistically significant difference in median age (3.4‐4.5 years). However, these patients are within the same risk group for children >18 months. The response to induction therapy was on the cusp of statistical significance, with more CR reported in the NMTRC003/003b group and more PR in the BCC001 group.
FIGURE 1.
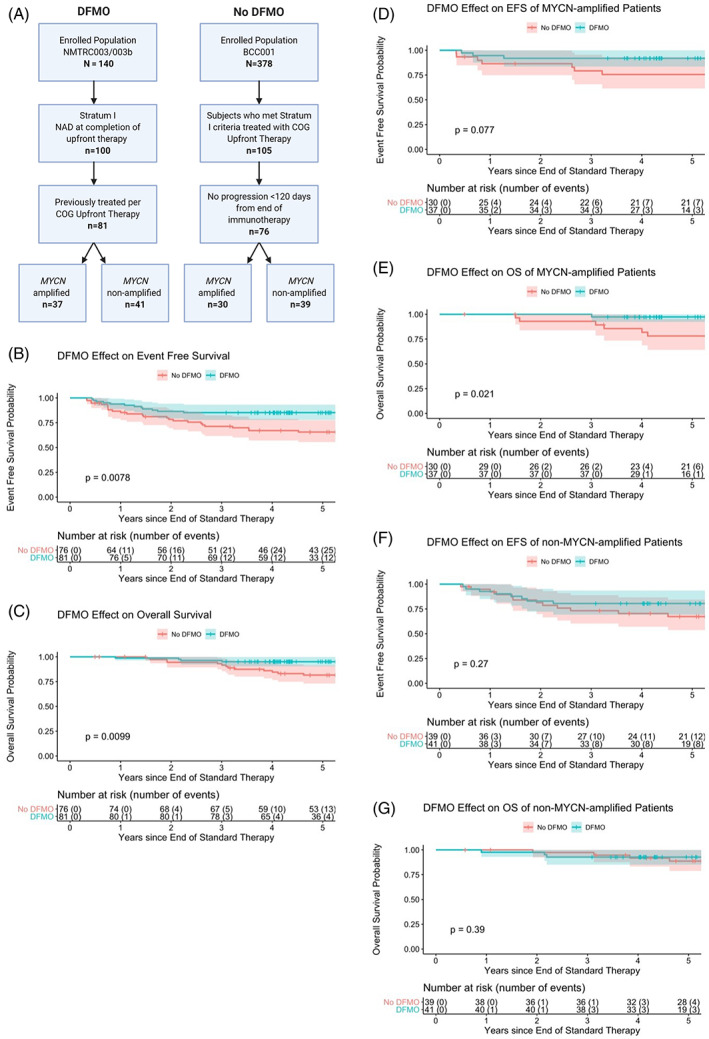
A, Consort diagram of comparison groups between NMTRC003/003b (DFMO) and BCC001 (No DFMO). B, EFS of NMTRC003/003b vs BCC001 subjects with comparison at 2 and 5 years of DFMO (86.4%, 85.2%) vs no DFMO (78.3%, 65.6%), respectively. C, OS of NMTRC003/003b vs BCC001 subjects with comparison at 2 and 5 years of DFMO (98.8%, 95.1%) and vs no DFMO (94.4%, 81.6%), respectively. D, EFS of MYCN amplified subjects, with comparison at 2 and 5 years DFMO (91.9%, 91.9%) vs no DFMO (86.4%, 75.6%). E, OS of MYCN amplified subjects, with comparison at 2 and 5 years DFMO (100%, 97.3%) vs no DFMO (92.9%, 78.1%). F, EFS of MYCN non‐amplified subjects, with comparison at 2 and 5 years DMFO (82.9%, 80.5%) vs no DFMO (81.3%, 67.3%). G, OS of MYCN nonamplified subjects, with comparison at 2 and 5 years DFMO (97.6%, 92.7%) vs no DFMO (97.3%, 88.7%)
3.2. Outcomes
The comparison between the NMTRC003/003b subset and the BCC001 historical control, outlined in Table 2 and Figure 1B,C, demonstrates statistically significant differences for both EFS and OS. The 2‐ and 5‐year EFS probabilities for NMTRC003/003b were 86.4% [95% CI, 79.3%‐94.2%] and 85.2% [95% CI, 77.8%‐93.3%], respectively. The 2‐ and 5‐year EFS for the control group were 78.3% [95% CI, 69.5%‐88.3%] and 65.6% [95% CI, 55.5%‐77.5%]. The 2‐ and 5‐year OS probabilities for NMTRC003/003b were 98.8% [95% CI, 96.4%‐100%] and 95.1% [95% CI, 90.5%‐99.9%]. The 2‐ and 5‐year OS for the control group were 94.4% [95% CI, 89.3%‐99.9%] and 81.6% [95% CI, 73.0%‐91.2%]. The hazard ratio from a Cox proportional hazards model conditioning on treatment only for EFS is 0.404 with a 95% confidence interval of (0.203, 0.805). For OS, the hazard ratio from the Cox model conditioning on treatment only is 0.257, with a confidence interval (0.085, 0.779).
TABLE 2.
EFS and OS Outcomes for NMTRC003/003b vs BCC001 groups
| Study | |||||
|---|---|---|---|---|---|
| EFS | 2‐year | 5‐year | P value | HR | P value |
| NMTRC003/003b (DFMO) | 0.864 (0.793, 0.942) | 0.852 (0.778, 0.933) | .0078* | 0.404 (0.203, 0.805) | .0100* |
| BCC001 (No DFMO) | 0.783 (0.695, 0.883) | 0.656 (0.555, 0.775) | |||
| OS | |||||
| NMTRC003/003b (DMFO) | 0.988 (0.964, 1.000) | 0.951 (0.905, 0.999) | .0099* | 0.257 (0.085, 0.779) | .0163* |
| BCC001 (No DFMO) | 0.944 (0.893, 0.999) | 0.816 (0.730, 0.912) | |||
| EFS | |||||
| MYCN‐A | |||||
| DFMO | 0.919 (0.835, 1.000) | 0.919 (0.835, 1.000) | .0766 | 0.315 (0.081, 1.217) | .0938 |
| No DFMO | 0.864 (0.749, 0.997) | 0.756 (0.614, 0.931) | |||
| OS | |||||
| MYCN‐A | |||||
| DFMO | 1.000 (1.000, 1.000) | 0.973 (0.922, 1.000) | .0207* | 0.123 (0.015, 1.023) | .0525 |
| No DFMO | 0.929 (0.838, 1.000) | 0.781 (0.641, 0.953) | |||
| EFS | |||||
| Non‐MYCN‐A | |||||
| DFMO | 0.829 (0.722, 0.953) | 0.805 (0.692, 0.936) | .2697 | 0.607 (0.248, 1.486) | .2750 |
| No DFMO | 0.813 (0.697, 0.948) | 0.673 (0.537, 0.844) | |||
| OS | |||||
| Non‐MYCN‐A | |||||
| DFMO | 0.976 (0.930, 1.000) | 0.927 (0.850, 1.000) | .3948 | 0.553 (0.139, 2.201) | .4010 |
| No DFMO | 0.973 (0.922, 1.000) | 0.887 (0.788, 0.998) |
Analysis of the effect in MYCN amplified and nonamplified tumor subgroups (Figure 1D‐G) shows a trend toward improved EFS and OS in both groups at 5 years, although most comparisons did not reach statistical significance. The OS difference in MYCN amplified tumors who received DFMO did reach statistical significance (P = .0207) with an increase in OS from 78.1% to 97.3% at 5 years. This analysis was limited by the small sample size of these subgroups and needs further investigation.
4. DISCUSSION
In this report, we provide a comparison of the EFS and OS of a subset of subjects from Stratum I of the NMTRC003/003b study who received DFMO after treatment as per the contemporaneous COG standard of care with a historical control group of similar patients treated at BCC centers who did not receive DFMO. Our results show an improvement in 2‐ and 5‐year EFS and OS in subjects who received DFMO for 2 years vs the historical control. Also, in contrast to the historical control, survival after DFMO treatment is maintained and demonstrates little decrease in EFS and OS over time through 5 years. These results suggest that there is a clinically significant benefit to maintenance DFMO, justifying further study of this drug as maintenance therapy.
To evaluate comparable subjects, we used a subset of enrolled subjects (n = 81 of those on Stratum I) who were treated per COG standard of care (used at all BCC hospitals) and received dinutuximab immediately prior to enrollment on NMTRC003/003b. For comparison, a cohort of subjects treated at the same institutions using similar COG treatment regimens for HRNB, including dinutuximab but without DFMO, was obtained via a retrospective chart review. The investigators recognize that historical control group comparisons have inherent limitations including sampling variability, drift and selection bias. 21 However, all patients evaluated in our study received similar upfront treatment at the same cohort of hospitals, regardless of whether they received DFMO, thereby mitigating the effect of drift. To decrease biases in the historical control group, only patients who met all NMTRC003/003b Stratum I inclusion criteria, including being event‐free 120 days after completion of immunotherapy (latest possible date to start therapy on NMTRC003/003b), were included in this evaluation. This excluded any subjects from the control group who would have been screen failures for entry onto NMTRC003/003b secondary to disease progression after immunotherapy. This approach has the potential to favor the control group in terms of EFS as all patients were required to be event‐free 120 days after completion of immunotherapy, whereas enrollment on NMTRC003/003b typically occurred less than 120 days from completion of immunotherapy. The patient characteristics in each group were well matched, although the minor difference in induction response (0.046) should be acknowledged as a potential limitation influencing outcome. The differences in histology were due to the unknown group as known categories were similar. The age difference is within the same risk group and unlikely to influence outcome.
In the COG ANBL0032 clinical trial, the 2‐year EFS, from start of immunotherapy, was 66 ± 5% for those in the experimental arm who received immunotherapy with dinutuximab. 5 The European experience with dinutuxmab antibody in HRNB patients who were treated with a similar overall therapy strategy had a 3‐year EFS of 46.5% ± 4.1% and OS of 86.5% ± 3.9%. 3 More recent results of the SIOPEN HR‐NBL1 trial 22 in which 406 patients were randomized to receive dinutuximab beta with or without subcutaneous interleukin‐2 (IL‐2) reported a 3‐year EFS, from start of immunotherapy, of 56% (95% CI, 49%‐63%) with dinutuximab beta and 60% (95% CI, 53%‐66%) with dinutuximab beta plus IL‐2. Three‐ and five‐year OS were 68% (95% CI, 62%‐75%) and 63% (95% CI, 55%‐69%) with dinutuximab beta vs 70% (95% CI, 63%‐75%) and 62% (95% CI, 55%‐69%) with dinutuximab beta plus IL‐2. These studies, along with their predecessor therapies for HRNB, have all shown progressive, refractory and relapsed disease occurring during upfront therapy. Therefore, initiation of DFMO at an earlier timepoint, such as during immunotherapy, may play a role in prevention of early relapse.
The proposed mechanism of action of DFMO, studied in HRNB, is inhibition of ODC resulting in a decrease in MYCN. 16 , 17 , 23 MYCN amplification is present in 30% to 40% of HRNB patients and is associated with a poorer prognosis. Therefore, treatment with DFMO, with subsequent inhibition of MYCN, would be expected to play a more important role in this subset of patients. 16 , 17 , 23 However, subjects on NMTRC003/003b study showed improvement in EFS and OS relative to historical controls regardless of MYCN status, although this subset analysis was not powered for significance due to small sample size. The observation that the baseline EFS for MYCN amplified (75.6%) is greater than that for MYCN nonamplified (67.3%) patients, though not statistically significant, is unexpected and may be due to either the effect of previous antibody therapy or to small sample size. The 5‐year EFS/OS improved from 75.6%/78.1% to 91.9%/97.3% for MYCN amplified tumors and from 67.3%/88.7% to 80.5%/92.7% for MYCN nonamplified tumors at 5 years. These results suggest that DFMOs targeting of ODC and MYCN may provide a greater benefit in terms of improved survival for the MYCN amplified group, which has historically had a worse outcome. However, because DFMO also appeared to improve survival in the MYCN nonamplified group, it may be of interest to study c‐MYC as a potential target as it has been noted that c‐MYC is found in up to 20% of neuroblastoma. 24 In colon cancer, DFMO inhibition of c‐MYC has been shown to be important in tumor response. 25 The outcome differences between MYCN amplified and nonamplified patients should be further studied prospectively to elucidate the mechanisms and differences in these subgroups.
In conclusion, comparison of children with HRNB from BCC hospitals who received DFMO after standard therapy showed a significant increase in EFS and OS relative to those that did not receive DFMO. It is possible that earlier addition of DFMO within maintenance therapy in combination with immunotherapy and cis‐retinoic acid may further improve these outcomes. This concept is being studied in a randomized clinical trial. In addition, further study of DFMO involving dose escalation as maintenance therapy is underway.
CONFLICT OF INTEREST
Dr Donald Berry is a co‐owner and statistical consultant with Berry Consultants, LLC. The remaining authors declare no competing interests.
ACKNOWLEDGEMENTS
The funding sources include the Beat NB Foundation, Meryl and Charles Witmer Foundation, Lillie's Friends Foundation, Dick and Betsy DeVos Family Foundation, Owen Moscone Foundation, Brooke's Blossoming Hope for Childhood Cancer Foundation, Ethan's Rodeo and the Jesse Heikkila Foundation. The funding organizations did not have a role in the research or writing of the manuscript.
Lewis EC, Kraveka JM, Ferguson W, et al. A subset analysis of a phase II trial evaluating the use of DFMO as maintenance therapy for high‐risk neuroblastoma. Int. J. Cancer. 2020;147:3152–3159. 10.1002/ijc.33044
DATA ACCESSIBILITY
The datasets generated during the NMTRC003/003b study are available in the clinicaltrials.gov repository: https://clinicaltrials.gov/ct2/show/NCT02395666. The data obtained via the BCC001 retrospective review that support the findings of our study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Irwin MS, Park JR. Neuroblastoma: paradigm for precision medicine. Pediatr Clin North Am. 2015;62:225‐256. 10.1016/j.pcl.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 2. Pinto NR, Applebaum MA, Volchenboum SL, et al. Advances in risk classification and treatment strategies for Neuroblastoma. J Clin Oncol. 2015;33:3008‐3017. 10.1200/JCO.2014.59.4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Simon T, Hero B, Faldum A, Handgretinger R, Schrappe M. Niethammer, D. & Berthold, F. consolidation treatment with chimeric anti‐GD2‐antibody ch14.18 in children older than 1 year with metastatic neuroblastoma. J Clin Oncol. 2004;22:3549‐3557. 10.1200/JCO.2004.08.143. [DOI] [PubMed] [Google Scholar]
- 4. Berthold F, Boos J, Burdach S, et al. Myeloablative megatherapy with autologous stem‐cell rescue versus oral maintenance chemotherapy as consolidation treatment in patients with high‐risk neuroblastoma: a randomised controlled trial. Lancet Oncol. 2005;6:649‐658. 10.1016/S1470-2045(05)70291-6. [DOI] [PubMed] [Google Scholar]
- 5. Yu AL, Gilman AL, Ozkaynak MF, et al. Anti‐GD2 antibody with GM‐CSF, interleukin‐2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363:1324‐1334. 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cheung NK, Cheung IY, Kushner BH, et al. Murine anti‐GD2 monoclonal antibody 3F8 combined with granulocyte‐macrophage colony‐stimulating factor and 13‐cis‐retinoic acid in high‐risk patients with stage 4 neuroblastoma in first remission. J Clin Oncol. 2012;30:3264‐3270. 10.1200/JCO.2011.41.3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yu A, Ozkaynak FM, Sondel PM, et al. Update of outcome for high‐risk Neuroblastoma treated on a randomized trial of chimeric anti‐GD2 antibody (ch14.18) +GM‐CSF/IL2 immunotherapy in 1st response: a Children's Oncology group study. Paper preseted at: Advances in Neuroblastoma Research Association Meetings; Cologne, Germany; 2014.
- 8. Park JR, Kreissman SG, London WB, et al. Effect of tandem autologous stem cell transplant vs single transplant on event‐free survival in patients with high‐risk Neuroblastoma: a randomized clinical trial. JAMA. 2019;322:746‐755. 10.1001/jama.2019.11642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wagner LM, Danks MK. New therapeutic targets for the treatment of high‐risk neuroblastoma. J Cell Biochem. 2009;107:46‐57. 10.1002/jcb.22094. [DOI] [PubMed] [Google Scholar]
- 10. Santana VM, Furman WL, McGregor LM, Billups CA. Disease control intervals in high‐risk neuroblastoma. Cancer. 2008;112:2796‐2801. 10.1002/cncr.23507. [DOI] [PubMed] [Google Scholar]
- 11. London WB, Bagatell R, Weigel BJ, et al. Historical time to disease progression and progression‐free survival in patients with recurrent/refractory neuroblastoma treated in the modern era on Children's Oncology group early‐phase trials. Cancer. 2017;123:4914‐4923. 10.1002/cncr.30934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Meyskens FL Jr, Gerner EW. Development of difluoromethylornithine (DFMO) as a chemoprevention agent. Clin Cancer Res. 1999;5:945‐951. [PubMed] [Google Scholar]
- 13. Samal K, Zhao P, Kendzicky A, et al. AMXT‐1501, a novel polyamine transport inhibitor, synergizes with DFMO in inhibiting neuroblastoma cell proliferation by targeting both ornithine decarboxylase and polyamine transport. Int J Cancer. 2013;133:1323‐1333. 10.1002/ijc.28139. [DOI] [PubMed] [Google Scholar]
- 14. Hixson LJ, Garewal HS, McGee DL, et al. Ornithine decarboxylase and polyamines in colorectal neoplasia and mucosa. Cancer Epidemiol Biomarkers Prev. 1993;2:369‐374. [PubMed] [Google Scholar]
- 15. Hogarty MD, Norris MD, Davis K, et al. ODC1 is a critical determinant of MYCN oncogenesis and a therapeutic target in neuroblastoma. Cancer Res. 2008;68:9735‐9745. 10.1158/0008-5472.CAN-07-6866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Koomoa DL, Yco LP, Borsics T, Wallick CJ, Bachmann AS. Ornithine decarboxylase inhibition by alpha‐difluoromethylornithine activates opposing signaling pathways via phosphorylation of both Akt/protein kinase B and p27Kip1 in neuroblastoma. Cancer Res. 2008;68:9825‐9831. 10.1158/0008-5472.CAN-08-1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lozier AM, Rich ME, Grawe AP, et al. Targeting ornithine decarboxylase reverses the LIN28/Let‐7 axis and inhibits glycolytic metabolism in neuroblastoma. Oncotarget. 2015;6:196‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tracey Avequin, AG , Zagorski J, Maser T, Saulnier Sholler GL. DFMO preferentially drives cancer stem cells in neuroblastoma towards senescence. Paper presented at: AACR Annual Meeting 2018; April 14‐18, 2018; American Association for Cancer Research, Chicago, IL.
- 19. Sholler GLS, Ferguson W, Bergendahl G, et al. Maintenance DFMO increases survival in high risk Neuroblastoma. Sci Rep. 2018;8:14445 10.1038/s41598-018-32659-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet. 2007;369:2106‐2120. 10.1016/S0140-6736(07)60983-0. [DOI] [PubMed] [Google Scholar]
- 21. Viele K, Berry S, Neuenschwander B, et al. Use of historical control data for assessing treatment effects in clinical trials. Pharm Stat. 2014;13:41‐54. 10.1002/pst.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ladenstein R, Potschger U, Valteau‐Couanet D, et al. Interleukin 2 with anti‐GD2 antibody ch14.18/CHO (dinutuximab beta) in patients with high‐risk neuroblastoma (HR‐NBL1/SIOPEN): a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19:1617‐1629. 10.1016/S1470-2045(18)30578-3. [DOI] [PubMed] [Google Scholar]
- 23. Rounbehler RJ, Li W, Hall MA, Yang C, Fallahi M, Cleveland JL. Targeting ornithine decarboxylase impairs development of MYCN‐amplified neuroblastoma. Cancer Res. 2009;69:547‐553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yang XH, Tang F, Shin J, Cunningham JM. A c‐Myc‐regulated stem cell‐like signature in high‐risk neuroblastoma: a systematic discovery (target neuroblastoma ESC‐like signature). Sci Rep. 2017;7:41 10.1038/s41598-017-00122-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Laukaitis CM, Gerner EW. DFMO: targeted risk reduction therapy for colorectal neoplasia. Best Pract Res Clin Gastroenterol. 2011;25:495‐506. 10.1016/j.bpg.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during the NMTRC003/003b study are available in the clinicaltrials.gov repository: https://clinicaltrials.gov/ct2/show/NCT02395666. The data obtained via the BCC001 retrospective review that support the findings of our study are available from the corresponding author upon reasonable request.


