Summary
Primary neuroendocrine carcinoma of the breast (NEBC) is a very rare occurrence accounting for less than 0.1% of all breast cancers. Typically, the tumor presents with ER- and PgR-positive and HER-2-negative status. Despite its luminal type, NEBC is associated with a more aggressive clinical course and poorer prognosis compared to the other types of invasive breast cancer. Clinical and radiological findings are nonspecific. The most common clinical manifestation is a palpable mass whereas in mammography the tumor most commonly appears as a round or oval mass without spiculated margins. Herein, a very rare case of NEBC is described in an asymptomatic patient who presented with an area of architectural distortion and the presence of microcalcifications that was incidentally detected on a screening mammography. A review of the literature has also been conducted. The diagnosis of NEBC requires a thorough investigation to exclude the possibility of a metastatic neuroendocrine tumor from another site because the two entities require different treatment approaches. Due to the rarity of the disease, the optimal therapeutic approach has not been clearly defined. Surgical resection is the mainstay of treatment. Further research is needed to better understand the molecular characteristics of NEBC and identify novel targeted therapies.
Keywords: neuroendocrine, carcinoma, breast
1. Introduction
Neuroendocrine neoplasms are rare heterogeneous tumors originating from neuroendocrine cells throughout the body. They are most commonly seen arising from the gastrointestinal and respiratory tracts (1). Although foci of neuroendocrine differentiation can be detected in up to 30% of the cases of invasive ductal carcinomas of the breast (2), primary NEBC represents a distinct, and very rare entity accounting for less than 0.1% of all breast cancers (1,3-6) and less than 1% of all neuroendocrine tumors (7). NEBCs exhibit similar morphological and phenotypic features to their counterparts arising in the gastrointestinal and respiratory tracts (8,9).
Their exact incidence is difficult to determine because neuroendocrine markers are not routinely used in breast cancer diagnostics (3,4). Park et al. (2), reported that of 12,945 patients with breast cancer diagnosed over 27-years, only 120 (1%) were found to have NEBC. In an epidemiologic based study from the SEER database it was found that among 381,644 cases of stage I-IV breast carcinoma diagnosed within six-years, only 142 (< 0.1%) were NEBC (6). In 2003 WHO defined as neuroendocrine breast carcinoma a tumor of epithelial origin with positive staining of one or more neuroendocrine markers in at least 50% of the tumor cells (10). However, in the revised 2012 WHO classification it was acknowledged that the 50% cut-off for diagnosis was arbitrary and therefore no specific threshold of tumor cell expression is currently required for a diagnosis of NEBC. Therefore, breast carcinomas with neuroendocrine features were classified into three categories: neuroendocrine tumor well-differentiated, resembling carcinoid tumors originating at other sites, poorly differentiated neuroendocrine carcinoma, or small cell carcinoma which is morphologically identical to small lung carcinoma, and invasive breast carcinoma with neuroendocrine differentiation (11).
NEBCs usually run a more aggressive clinical course and tend to have a higher propensity for local and distant recurrence when compared to other types of invasive breast carcinoma (2,12). NEBCs do not have specific clinical and radiological features. Besides, their optimal treatment has not been clearly defined. We present a case of NEBC with both rare clinical and imaging features along with a review of the literature.
2. Case Report
A 50-year-old Caucasian woman presented for further evaluation of a mammographic finding that was incidentally detected on a screening mammogram. She denied any symptoms. Her medical history was unremarkable and she had no history of breast or ovarian cancer.
Physical examination revealed an area of asymmetry rather than a discrete mass in the upper inner quadrant of the right breast. There was no palpable axillary and supraclavicular lymphadenopathy. The mammogram showed an area of architectural distortion along with the presence of confluent microcalcifications in the upper inner quadrant of the right breast (Figure 1). Ultrasound revealed two adjacent hypoechoic masses with irregular margins measuring 2.8 cm without prominent acoustic enhancement (Figure 2).
Figure 1.
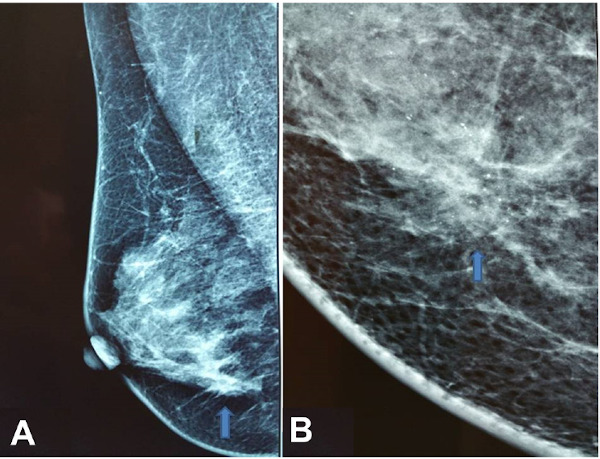
(A) Right mediolateral oblique mammogram showing an area of architectural distortion (arrow); (B) Magnification view showing in detail the area of architectural distortion along with confluent microcalcifications (arrow).
Figure 2.
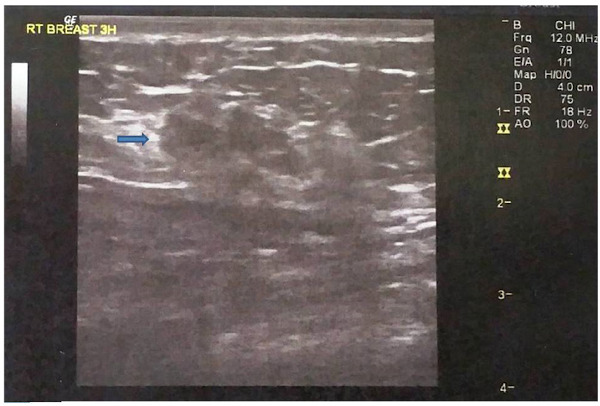
Ultrasonography showing two adjacent hypoechoic nodules with irregular margins (arrow).
The patient underwent a core needle biopsy under ultrasonographic guidance, which revealed a breast adenocarcinoma with neuroendocrine differentiation. Based on these findings the possibility of an NEBC was considered. Following the core biopsy the patient underwent a thorough staging investigation including hematologic and biochemical evaluation, computed tomography of the chest and abdomen, and bone scintigraphy. All of the above were unremarkable. She then underwent a modified radical mastectomy because the two sentinel lymph nodes were found to harbor metastatic disease in the intraoperative frozen section.
Histological evaluation of the mastectomy specimen showed primary neuroendocrine breast carcinoma of grade II according to the Bloom-Richardson grading system (Figure 3). On gross appearance the tumor measured 3 × 2.7 × 2.5 cm and was solid whitish and elastic. On immunohistochemical evaluation the tumor cells stained strongly positive for estrogen receptors, focally positive for progesterone receptors whereas the expression for Her2 was negative (Figure 4). All tumor cells were strongly positive for synaptophysin and negative for chromogranin A and CD56. Ki-67 proliferation index was 15-20%. Ductal carcinoma in situ (DCIS) was also detected associated with comedo necroses and microcalcifications. Metastatic disease was detected in 2 of the 14 removed axillary lymph nodes. The patient received adjuvant chemotherapy followed by radiotherapy and is currently under hormonal therapy. She is well without any evidence of recurrence 44 months after surgery.
Figure 3.
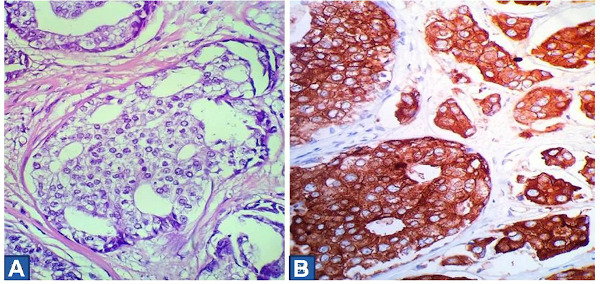
(A) High power photomicrograph, displaying medium-sized, mildly pleomorphic tumor cells, with clear cytoplasm, medium-sized round to oval nuclei with granular chromatin and inconspicuous nucleoli. Mitoses are relatively common. (Hematoxylin & Eosin ×400); (B) Tumor cells expressing strong cytoplasmic positivity for synaptophysin (Syn ×400).
Figure 4.
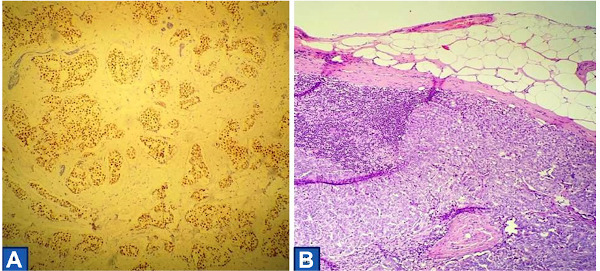
(A) Almost all tumor cells expressing strong nuclear positivity for estrogen receptors (ER ×100); (B) Sentinel lymph node with metastasis, which was visible during gross description (Hematoxylin & Eosin ×100).
3. Discussion
Primary neuroendocrine tumors of the breast were originally reported in 1977 by Cubilla and Woodruff (13), who described eight patients with a painless breast mass that histologically resembled carcinoids of other sites containing argyrophilic granules.
The exact histogenesis of NEBC is unclear. One theory suggests that the tumor arises from endocrine differentiation of preexisting endocrine cells in the breast. The second theory suggests that the tumor arises from divergent differentiation of neoplastic stem cells into epithelial and endocrine cell lines during early carcinogenesis (4,7).
Histologically NEBC is characterized by alveolar structures of solid sheets of cells with a tendency to produce peripheral palisading (10). The tumor is more likely to be of luminal subtype, positive for ER and PR expression, and negative for Her2 expression (1,3,9,12,14-16). Wei et al. (12), reported 92% positivity for ER and 69% positivity for PR, in patients with NEBC whereas in patients with other types of breast cancer the relevant rates were 72% and 57% respectively. Synaptophysin and chromogranin A are currently the most specific immunohistochemical markers for the evaluation of NEBC whereas Neurone-specific enolase and CD56 are less sensitive and specific (4,16).
The clinical presentation of patients with NEBC is similar to that in other types of invasive breast cancer (4,10,16). The tumor most commonly occurs in postmenopausal women in their sixth and seventh decades of life (3-6,9,14,17,18) who have reported being significantly older than patients with conventional invasive breast cancer (6). Premenopausal patients have been reported in up to 15% of the cases (12). Extremely rare cases have been reported affecting male patients (2). The most common clinical manifestation is a painless palpable retro areolar lump (19). Nipple retraction, skin alterations, and bloody nipple discharge may be present (19). Nipple discharge has been reported in 54.5% of the cases (5), and should therefore alert the clinician to the possibility of an underlying NEBC.
Locally advanced NEBCs have been reported in 4.7- 9.3% of the cases (3,8,12), whereas axillary metastases are detected in 43-47% of the cases at presentation (2,20). Lee et al. (8), reported a case of neuroendocrine breast carcinoma manifesting as inflammatory breast cancer.
The median tumor size at presentation is 3.1 cm (2), and has been reported to be significantly larger than that of other types of breast cancer (6). Multifocality and multicentricity have been reported in 6.9% and 9.2% of the cases respectively (2). Patients with NEBC present at a higher clinical stage and higher histologic grade compared to patients with conventional breast cancer (6). Well-differentiated and poorly defined NEBCs have been reported in 45% and 40% of the cases respectively (21).
The radiological findings of NEBCs are nonspecific (15,16,21). On a mammogram, the tumor most commonly appears as a high-density round or oval mass with circumscribed margins (5,10). Spiculated margins and the presence of microcalcifications have been reported in 18% and 26.4% of the cases respectively (2). The mammographic appearance, however, may mimick benign entities such as fibroadenomas or intramammary lymph nodes (5).
On ultrasonography, NEBC appears as a hypoechoic, irregular mass with indistinct margins and increased vascularity (4,5). Posterior enhancement is infrequently reported. Jeon et al. (5) reported the absence of acoustic enhancement in 82% of the cases. MRI findings include an irregular mass with heterogeneous rapid enhancement and washed out pattern (2,8,16). In 43% of the cases with multiple lesions reported by Park et al. (2), multicentricity or multifocality was detected only with MRI.
The diagnosis of NEBC is established by core needle biopsy (15,16), although a precise diagnosis may not be possible in up to 40% of the cases (5). Fine needle aspiration may not be adequate for the diagnosis (16), because the cytology findings, of NEBC, may overlap with those of invasive ductal carcinoma and intraductal papilloma (19).
The differential diagnosis of NEBC includes metastatic neuroendocrine tumors to the breast, Merkel cell carcinoma, melanoma, and lymphoma (16). Differentiating between primary and metastatic breast neuroendocrine tumors is essential since the two entities require different therapeutic approaches (4,14,20,21). The presence of an intraductal component with similar cytologic features is suggestive of a primary breast tumor (10,15,16) since it has been reported in 68% of the cases (2). The most specific immunohistochemical markers indicative of a primary breast tumor are GATA3, mammaglobin, and GCDFP15 which are stained negative in metastatic tumors (4). The absence of an intraductal component, negativity for ER, PR, and absence of axillary metastases are suggestive of a metastatic tumor (16). Metastases to the breast account for less than 1% of breast tumors whereas metastatic neuroendocrine tumors account for 1-2% of the metastases to the breast (4). Thorough imaging investigation of the patient with CT scans and PET CT is mandatory to exclude any other primary site. A detailed clinical history and complete physical examination are essential in the assessment of the patient with NEBC.
Perry e t a l . (22), reported 18 metastatic neuroendocrine tumors to the breast of whom 62% were of gastrointestinal tract origin, 28% were from the lungs and 10% were of intermediate origin. Interestingly, 44% of these cases were initially misdiagnosed as primary breast carcinomas. All the metastatic tumors stained positive for synaptophysin and chromogranin and 83% were stained positive for NSE or CD56 (22).
Metastases from NEBC may occur many years after the initial treatment and thus a long-term follow-up is mandatory. Most common metastatic sites include the liver, bones, lungs, soft tissues, pleura, brain, mediastinal lymph nodes, adrenal glands, ovaries, and pancreas (9,12,16).
There is no standard treatment protocol for NEBC and the therapeutic approach is similar to that for other types of breast cancer (5).
Surgery is the mainstay treatment in patients with NEBC (8,16). The type of surgery depends on the tumor location and clinical stage and can be either lumpectomy or mastectomy with sentinel node biopsy, or modified radical mastectomy in cases with metastatic sentinel nodes. Lack of surgical treatment, along with higher tumor stage, larger tumor, and negative ER, PR status has been associated with shorter overall survival in NEBC patients compared to that of patients with invasive breast carcinoma (6). There are limited data on oncoplastic conservation and immediate reconstruction in patients with NEBC. As the tumor may develop a pagetoid pattern of spread, the assessment of surgical margins may be difficult especially in the intraoperative frozen section (20).
Chemotherapy can be used either in the adjuvant setting in patients with a high risk of relapse or as a neoadjuvant treatment in patients with locally advanced disease not amenable to surgery (16,18). It can also be used for downstaging large tumors to allow for breast conservation treatment.
However, the optimal chemotherapy regimen has not been clearly defined and the current consensus is to treat NEBC with the chemotherapeutic regimens that are used in the treatment of conventional breast cancer and pulmonary small-cell carcinoma (1,18,20). Several regimens including anthracyclines and or taxanes that are used for other types of breast cancer and a combination of platinum and etoposide are commonly administered (16,19). Suhani et al. (15) reported good results in four patients with NEBC who were treated with Cyclophosphamide, Adriamycin, and 5-Fluorouracil (CAF) based adjuvant chemotherapy, irradiation of chest wall, and hormonal therapy. Interestingly, in some series the patients who received chemotherapy appeared to have both shorter overall survival and disease-free survival than those who did not receive chemotherapy. This difference, however did not reach statistical difference likely due to the small number of patients, and the fact that chemotherapy is used depending on the clinical stage and tumor histological characteristics (12,18). The poor response to chemotherapy in patients with NEBC may be attributed either to the chemoresistance commonly seen in neuroendocrine tumors in other sites or to the lack of an optimal chemotherapeutic regimen (4).
Hormonal therapy should be given in cases with positive hormonal status (2,15). Patients who received endocrine therapy have been associated with longer overall survival and distant recurrence-free survival (12).
There are conflicting reports for prognosis in patients with NEBC (21). Most authors report significantly worse outcomes for overall survival, local recurrence-free survival, and distant recurrence-free survival in patients with NEBC compared to the matched group of patients with invasive ductal cancer (4,6,9,12). On the contrary, Jeon et al. (5) reported that all 11 patients of their study showed favorable prognosis and were free of locoregional disease 21-76 months after treatment. A 15% and 34% risk for local and distant recurrence has been respectively reported at five years (12).
The 5-year overall survival has been reported from 70-80% (17,21). In a literature review by Lu et al. (18), including 86 primary NEBCs the overall survival at 48 months was 83.5%. A more favorable prognosis has been reported for tumors detected at an early stage (5). Large tumors, high tumor stage, negative hormonal status, regional metastases, and ki-67 > 14% have been associated with worse overall survival and disease-free survival (12,16). In the multivariate analysis of the population-based study from the SEER database, it was found that older age and positive lymph node status were independent prognostic factors for overall survival in patients with NEBC tumors, whereas positive lymph nodes, negative PR status and lack of surgical treatment were independent prognostic factors for disease-specific survival (6). In the same study it was shown that radiation therapy did not prolong survival (6). Patients with early NEBC without axillary dissection are associated with better overall survival compared to advanced-stage patients treated with mastectomy and axillary dissection (18). The presence of mucinous differentiation has been reported as a favorable prognostic factor (10).
The published data on molecular characteristics of NEBCs is scarce. Molecular analysis of 47 NEBCs showed that these tumors are part of the spectrum of luminal carcinomas. An equal distribution between A and B subtypes was observed. In addition, only three (7%) of the cases were found harboring a PIK3CA mutation and 7% were harboring TP53 mutations (9). Despite its luminal phenotype NEBC is associated with an aggressive clinical course and poor prognosis (23).
In an effort for the development of novel targeted therapies in patients with NEBC, Vranic et al. (23), identified several potential targets for novel therapies including farletuzumab and mirvetuximab soravtansine (FOLR1), sacituzumab govitecan (TROP-2) and HDAC inhibitors (H3K36Me3). Novel therapeutic approaches should further be explored (6,12).
After conducting a literature review, we were able to find twenty-five retrospective reviews and case series (1-3,5-7,14,15,18,19,24-38) and thirty-one case reports (20,21,39-67) of neuroendocrine breast carcinoma published since 2000. The clinicopathological characteristics are summarized in Table 1 and Table 2.
Table 1. Summary of clinicopathological findings of primary neuroendocrine breast carcinoma published in retrospective reviews and case series since 2000.
| Author/Year (Ref.), Study design |
Patients/sex | Age | Size (mm) | Pathological, Immunohistochemical Findings |
Treatment | Follow-up (months) | Outcome |
|---|---|---|---|---|---|---|---|
| OZDIRIK/2020 | 5 F | 67 (49-73) | 12 (9-53) | ER+: 60%, PR +: 80%, | BCS+ALND: 20% | 45.4 (11-130) | Alive: 60% |
| (19), CS | HER2 - : 60% | BCS+SNLB: 40% | |||||
| ER +, PR -, HER2 - : 100% | No Surgery: 40% | Died :40% | |||||
| Syn+: 100%, CgA+ : 80% | Chemo : 40% | ||||||
| N+: 20%, N/A: 40% | RT: 40%, ET:20% | ||||||
| CANBAK/2020 | 11 F | 68 (49-86) | N/A | ER+, PR+: 90% N/A:10% | BCS: 64% | N/A | Alive: 90% |
| (14), RR | HER2 - :100% | ||||||
| Syn+:, CgA+: 100% | MT: 36% | Died :10% | |||||
| N: N/A | |||||||
| HEJJANE/2020 | 2 F | 71 | 22 | ER+, PR+, HER2- | MRM + ALND | 21 | Alive |
| (25), CS | N- | ET | |||||
| 48 | 40 | ER+, PR+, Syn+, CgA+ | BCS+ ALND | 28 | Alive with recurrence | ||
| HER2-, N+ | Chemo, ET | at 12 months | |||||
| ZHANG/2020 | 2 F | 46 | 65 | ER-, PR-, HER2 - | MRM + ALND | N/A | N/A |
| (24), CS | Syn+, CgA-, N:- | Chemo | |||||
| 34 | 30, 20 | ER+, PR+ ,HER2 + | N/A | N/A | N/A | ||
| Syn+, CgA+ N- | |||||||
| LI/2017 | 119 F | 53.2 (N/A) | T1:23% | ER+: 81%, PR+: 72.2%, | MT: 79.4% | 4-144 | Alive without tumor: 80.2% |
| (26), RR | 7 M | T2:31% | HER2+: 15.1%, | BCS: 14.3% | Alive with tumor:7.1% | ||
| T3:19% | N: +15.1%, N/A: 46% | Other: 5.5% | Died of disease: 5.6% | ||||
| T4:6.3% | ALND: 82.5% | Died of other causes:0.8% | |||||
| N/A:20.6% | Chemo: 43.7% | Lost to follow-up: 6.3% | |||||
| RT: 17.5% | |||||||
| ET: 63.5% | |||||||
| ROININEN/2017 | 43F | 66 | 25.3 (18.9-31.7%) | ER+: 97.7%, PR+: 58.1%, | MT+ALND: 44.2% | 35.4 (23.5-47.2) | Local recurrence: 7.6% |
| (3), RR | HER2+: 4.7%, | BCS+ ALND: 25.6% | |||||
| Syn+: 100%, CgA+: 69.8% | Other type: 14% | ||||||
| N+: 44.2% | Chemo: 30.3% | ||||||
| RT: 74.4% | |||||||
| ET:76.7% | |||||||
| YANG/2017 | 19 F | 59.2 (17-82) | ER+: 93.3%, PR+: 73.3%, | MT +ALND: 89% | 59.2 | Alive: 52.6% | |
| (1), RR | HER2+: 4.7%, HER2-: 93% | MT: 5.3% | (15.5-114) | Died: 10.5% | |||
| Syn+: 77.8% CgA+: 42.1% | MT+SLNB: 5.3% | N/A:32% | N/A: 36.8% | ||||
| N+: 22.2% | Chemo: 61% | ||||||
| RT: 16.6% | |||||||
| ET:44%, N/A: 44% | |||||||
| COLLADO-MESA/2017 | 2 F | 58 | 15, 8 | ER+, PR+, HER2- | BCS+SLNB (-) | N/A | N/A |
| (7), CS | Syn+, CgA+, PN- | ||||||
| 62 | 17 | ER+, PR+, HER2- | BCS+SLNB (-) | N/A | N/A | ||
| Syn+, CgA+, N- | |||||||
| LOCURTO/2016 | 5 F | 59.4 (50-75) | 29 (2.3-4.5) | ER+: 100%, PR+: 80% | MRM -ALND: 20% | N/A | Alive: 80% |
| (27), CS | HER2+: 100% | BCS- ALND :60% | Died: 20% | ||||
| Syn+: 80%, CgA+: 60% | BCS+SLNB: 20% (-) | ||||||
| N+: 60% | Chemo: 60%, | ||||||
| RT: 100%, ET:100% | |||||||
| LU/2014 | 85 F | 53.9 (25-83) | 27.5 (0-180) | ER+:59%, PR+: 66% | MRM : 50% | 38.1 | Alive with no evidence of recurrence 79%. |
| (18), LR | 1 M | HER2+: 27% | BCS: 50% | (3-99) | OS: 94%, 86%, 73% in I, II, III stages respectively | ||
| Syn+: 80%, CgA+: 60% | Chemo: 41.7%, | ||||||
| RT: 28.3%, ET:13.3% | |||||||
| PARK/2014 | 84 F | 62.9 (28-89) | 31 (6-110) | ER+:98.9%, PR+: 77% | N/A | N/A | N/A |
| (2), RR | 3 M | HER2+: 2.4% | |||||
| SUHANI/2014 | 4 F | 58 (50-65) | 51 (4-6.5) | ER+:100%, PR+: 80% | MRM: 100% | 27.7 | Alive:100% |
| (15), RR | HER2- : 100% | Chemo: 100% | (9-48) | ||||
| Syn+: 50%, CgA+: 75% | RT: 100% | ||||||
| N+: 75% | ET: 100% | ||||||
| WANG/2014 | 139 F | 64 (26-99) | 31.9 | ER+:54.2%, PR+: 37.3% | Surgery: 76.8% | N/A | Median survival:26 months |
| (6), RR, SEER | 3 M | HER2 : N/A | RT:35.9% | (12-48) | |||
| N+: 28.2% | Chemo: N/A | ||||||
| ET: N/A | |||||||
| JEON/2014 | 11 F | 54.7 (29-79) | 18 (0.5-4) | ER+:100%, PR+: 100% | BCS+SLNB: 55% | 38.6 | Alive free of disease: 100% |
| (5), RR | HER2 -: 55%, N/A in 5 cases | BCS: 9% | (21-76) | ||||
| N+: 28.2% | MRM+SLNB:27% | ||||||
| MRM:9% | |||||||
| Chemo: N/A, | |||||||
| RT: N/A, ET: N/A | |||||||
| ZHU/2013 | 22 F | 52.5 (29-77) | 22 (0.5-4) | ER+:91%, PR+: 95% | MRM:100% | 64.5 | Alive free of disease: 95% |
| (28), RR | HER2 -: 75%, | Chemo: 64% | (4-89) | Alive with recurrence: 5% | |||
| ET: 91% | |||||||
| RT: 0% | |||||||
| ROVERA/2013 | 61 F | 70 (42-87) | 2.05 (0.6-6) | ER+: 90%, PR+: 75% | BCS:49% | 88 | Local or systemic recurrence: 14.8% after a median of 53.7 months |
| (29), RR | HER2- :98% | MRM:48% | (4-242) | ||||
| N+: 21.3% | Chemo:5% | ||||||
| RT: 48% | |||||||
| ET: 72% | |||||||
| ZHANG/2012 | 107 F | 65 (25-95) | 8-50 | ER+: 94.3%, PR+: 85.05% | N/A | 27 | Overall survival: 85.1% |
| (31), RR | HER 2- :97.2% | (3-134) | Local recurrence: 3.7% | ||||
| N+:24.3% | Distant recurrence: 5% | ||||||
| WU/2012 | 13 F | 53 (36-78) | 25.5 (10-40) | ER+, PR+: 100% | N/A | 67.5 | Alive free of disease: 85% |
| (32), RR | HER2+: 100% | (41-89) | Died: 7.5% | ||||
| Syn+, CgA+: 54% | Lost: 7.5% | ||||||
| N+: 7.7% | |||||||
| MENENDEZ/2012 | 4 F | 44 | 20 | ER:+, PR:-, HER2: -, N:+ | BCS+ALND, Chemo,RT | ||
| (33), CS | CgA+, N+ | 48 | Alive disease free | ||||
| 68 | 36 | ER, RP, HER2:N/A, CgA+, | BCS+ALND, Chemo, RT, ET | 24 | Alive disease free | ||
| Syn:N/A, N+ | |||||||
| 58 | 10 | ER:+, PR:-, N: -, HER2:- | BCS+SLNB, Chemo, RT | 8 | Alive with liver metastasis | ||
| 69 | 14 | ER:+, PR:+, HER2:-, | |||||
| Syn:+, CgA: +, N: - | BCS+SLNB, Adjuvant treatment: N/A | 2 | Alive disease free | ||||
| BRASK/2013 | 13 F | 67.8 (42-89) | 15.1 (8-30) | ER+: 100%, PR: N/A, HER2-: 100%, | BCS: 77% | N/A | N/A |
| (30), RR | Syn+: 92%, CgA+:30% | MRM: 23% | |||||
| N+: 23% | Adjuvant treatment: N/A | ||||||
| KAWASAKI/2012 | 24 F | 47.8 (28-74) | 4.9 (1-25) | ER+, PR+, HER2-: 100%, | BCS: 63% | 83.7 | Alive free of disease:100% |
| (34), RR | Syn:+, CgA: +, N: - | MRM: 37% | (64-101) | ||||
| Adjuvant treatment: N/A | |||||||
| GHANEM/2011 | 7 F | 56 (50-82) | 40 | ER+, PR+: 85% | BCS+ALND: 29% | 28 | Alive free of disease: 86% |
| (35), RR | (14-130) | HER2-: 100%, | MRM +ALND: 71% | (0-38) | |||
| Syn+, CgA+: 100%, | Chemo:66%, RT: 86% | Alive with recurrence: 14% | |||||
| N +: 71% | ET: 71% | ||||||
| ADEGBOLA/2005 | 3F | 46 | 10 | ER:-, PR:-, HER2: - | BCS, Chemo, RT | 48 | Alive free of disease |
| (36), CS | Syn:+, CgA: +, PN: - | ||||||
| 60 | 17 | ER:-, PR:-, HER2: - | BCS, Chemo, RT | 20 | Died | ||
| Syn:+, CgA: +, PN: - | |||||||
| 61 | 17 | ER:-, PR:-, HER2: - | BCS, Chemo, RT | 6 | Alive with disease | ||
| Syn: -, CgA: +, N: + | |||||||
| ZEKIOGLU/2003 | 12F | 66.5 (43-79) | 23.5 (8-70) | Syn+, CgA+: 66%, | BCS+ALND: 50% | 24-54 | Alive free of disease |
| (38), RR | MT+ALND:50% | ||||||
| SHIN/2000 | 9F | 54.1 (43-70) | 26 (13-50) | ER+, PR+: 92% | BCS:67% MT: 33% | 3-35 | Metastases:22% |
| (37), RR | HER2-: N/A, | ALND: 89% | |||||
| Syn+:42%, CgA+: 92%, | Chemo:78%, RT:44% | All alive | |||||
| N+: 50% | ET:22% |
CS: case series, RR: retrospective review, LR: literature review, F: female, M: male, ER: estrogen receptors, PR: progesterone receptors, Syn: synaptophysin, CgA: chromogranin A, MT: mastectomy, BCS: breast conserving surgery, SLNB: sentinel lymph node biopsy, ALND: axillary lymph node dissection, N: nodes, Chemo: chemotherapy, RT: radiation therapy, ET: endocrine therapy, N/A: not available.
Table 2. Summary of clinicopathological findings of case reports of primary neuroendocrine breast carcinoma published since 2000.
| Author/Year (Ref.) |
Age | Size (mm) | Clinical Findings | Imaging Findings Mammogram/US | Pathological Immunohistochemical Findings |
Treatment | Follow-up | Outcome |
|---|---|---|---|---|---|---|---|---|
| SALEMIS/2020 | 50 | 30 | None | Architectural distortion, Calcifications | ER+, PR+, HER2- | MT+ALND | 44 | AWD |
| (present case) | Hypoechoic mass. Irregular margins | Syn+, CgA-, N+ | Chemo, RT, ET | |||||
| VALENTE/2020 | 69 | 15 | Palpable mass, nipple, | Nodule with calcifications | ER-, PR-, HER2- | BCS+ALND | 96 | AWRD |
| (39) | areolar erosions | CgA+, N+ | Chemo, RT, ET | |||||
| Nodule with irregular margins and posterior shadowing | Concomitant invasive lobular carcinoma | |||||||
| PULAT/2019 | 73 | 40 | Pain, swelling, skin | Macrolobular mass | ER+, PR+, HER2- | MT+ALND | 48 | AWD |
| (40) | changes | Irregular, hypoechoic mass | N- | ET | ||||
| TREMELLING/2017 | 65 | 50 | Palpable mass | Irregular mass with increased vascularity | ER-, PR-, HER2-, | Chemo, RT | 3 | AWD |
| (41) | Syn+, CgA+ | |||||||
| N+ | ||||||||
| SOE/2017 | 57 | 40 | Palpable mass | Oval, lobulated | ER+,PR+, Syn+,CgA+ | BCS+Chemo | 18 | AWRD |
| (42) | dense with mass calcifications | |||||||
| Hypoechoic lobulated mass with marked posterior enhancement | ||||||||
| BERGSTROM/2017 | 53 | 80 | Palpable mass | Irregular mass | ER+, PR-, HER2- | Chemo | N/A | N/A |
| (43) | Syn+, CgA+ | |||||||
| VATS/2017 | 32 | 60 | Palpable mass | BIRADS-5 | ER+, PR+, HER2+ | Chemo, RT | 12 | AWRD |
| (44) | Syn+, CgA+, N+ | |||||||
| MEKIAROVA/2016 | 42 | 32 | Palpable mass | Circumscribed mass with calcifications | ER-, PR-, HER2-, | BCS+SLNB | 36 | AWD |
| (45) | Hypoechoic mass with enhancement | Syn+, CgA+ | ||||||
| MARINOVA/2016 | 42 | 35 | None | Distinctive mass with microcalcifications | ER+, PR+, HER2-, N+ | BT+ALND | 12 | AWD |
| (46) | Chemo, RT, ET | |||||||
| YOSHIMURA/2015 | 34 | N/A | Palpable mass | Microlobulated, hyperdense mass | ER+, PR+, | MT+ALND. | 48 | AWD |
| (47) | HER2-, N+ | Refused | ||||||
| adjuvant | ||||||||
| treatment | ||||||||
| WRONSKI/2015 | 45 | 22 | None | N/A | ER+, PR+, HER2- | BCS+ALND | N/A | N/A |
| (48) | Syn+, CgA+ , N+ | |||||||
| TATO-VARELA/2015 | 62 | 20 | Palpable mass | Increased trabeculation | ER+, PR+, | MT+ALND | N/A | AWD |
| (21) | HER2- | Chemo, RT | ||||||
| Syn+, CgA+, N+ | ||||||||
| WEI/2014 | 43 | 83 | Palpable mass | Irregular mass | ER+, PR-, HER2+ | MT+ALND | N/A | N/A |
| (49) | Lobulated lump | Syn+, N+ | Chemo | |||||
| Hypoechoic mass | ||||||||
| PANJVANI/2015 | 70 | 25 | Breast swelling | Hypoechoic mass with increased vascularity | Syn+ | MT+ALND | N/A | N/A |
| (50) | ||||||||
| LEE/2015 | 48 | 24 | Breast enlargement, | Diffuse skin thickening | ER+, PR+, HER2- | Chemo, | N/A | AWRD |
| (51) | eythema | Hypoechoic irregular mass | Syn+, N+ | RT, ET | ||||
| JANOSKY/2015 | 34 | 43 | Palpable mass | Irregular mass with calcifications | ER-, PR-, HER2- | Chemo, MT, RT | N/A | AWRD |
| (52) | CgA+, Syn+ | |||||||
| ALVA/2015 | 53 | 50 | Palpable mass | Irregular lump | ER-, PR-, HER2- | MT, Chemo | N/A | AWD |
| (53) | CgA+, Syn+, N- | |||||||
| VALENTIM/2014 | 75 | 19 | None | Ovoid, well defined mass | CgA+, Syn+ | BCS | N/A | N/A |
| (54) | Hypoechoic irregular mass without posterior enhancement | |||||||
| PAGANO/2014 | 51 | 35 | Palpable lump | Irregular mass | ER+, PR+, HER2- | MT+ALND | 126 | AWD |
| (55) | CgA+, Syn+, N+ | Chemo, ET | ||||||
| BOZKURT/2014 | 75 | 31 | Palpable mass | Spiculated Mass | ER+, PR+, HER2- | MT,Chemo, RT | N/A | AWD |
| (56) | Hypoechoic mass with regular borders | CgA+, Syn+, | ||||||
| LINGAPPA/2014 | 80 | 100 | Palpable mass | Well-defined mass | CgA+, N+ | MT+ALND | N/A | N/A |
| (57) | Chemo | |||||||
| TAJIMA/2013 | 78 | 20 | None | N/A | ER+, PR+, HER2- | BCS+ALND, | N/A | N/A |
| (58) | CgA+, Syn+, N+ | ET | ||||||
| MURTHY/2013 | 34 | 25 | Palpable mass | Ill defined-mass | ER+, PR+, HER2- | MT+ALND | 6 | AWD |
| (59) | CgA+, Syn+, N- | Chemo, TR,ET | ||||||
| HANNA/2013 | 60 | 20 | Axillary mass | None | ER+, PR+, HER2- | BCS+ALND | N/A | N/A |
| (60) | Enlarged axillary node | Chemo | ||||||
| ANGARITA/2013 | 51 | 32 | Breast lump | Spiculated mass with calcifications | ER+, PR-, | Chemo, RT, MT+ALND, | 13 | AWRD |
| (20) | HER2- | ET | ||||||
| CgA+, Syn+, | ||||||||
| WATROWSKI/2012 | 56 | 17 | Palpable mass | Hypoechoic mass with angular margins and | ER+, PR-, | BCS, Chemo, | 15 | AWD |
| (61) | posterior shadowing | HER2-, Syn+ | RT, ET | |||||
| N- | ||||||||
| SANGUINETTI/2012 | 63 | 65 | Bulky mass, skin | Mass with suspicious characteristics | ER+, PR+, | Chemo, MT+ALND, ET | 6 | AWRD |
| (62) | changes | HER2-, Syn+ | ||||||
| CgA+, N+ | ||||||||
| PSOMA/2012 | 46 | 80 | Palpable mass | Asymetric Hyperdense Microlobulated Area | CgA+, Syn+ | MT+ALND, | 6 | AWD |
| (63) | Hypoechoic irregular mass with posterior enhancement | Paget disease. | RT | |||||
| NAWAWI/2012 | 22 | 24 | Palpable mass | Hypoechoic mass | N/A | Chemo | N/A | Died |
| (64) | ||||||||
| GRACA/2012 | 83 | 85 left | Palpable lump | Nodule with Benign characteristics. | ER+, PR+, Syn+ | BCS+SLNB | N/A | AWD |
| (65) | Heterogeneous benign nodule | ET | ||||||
| ZHANG/2011 | 29 | 20 right | Bilateral Nipple | Bilateral Suspicious lesions | ER+, PR+, | Bilateral BCS+ Axillary sampling, Chemo | N/A | N/A |
| (66) | discharge | HER2-, Syn+ | ||||||
| N- | ||||||||
| GHANEM/2011 | 64 | 80 | Palpable mass | Suspicious characteristics | ER+, PR+, | Chemo, MT+ALND, ET | 6 | AWRD |
| (67) | HER2-, Syn+ | |||||||
| N+ |
CS: case series, RR: retrospective review, LR: literature review, ER: estrogen receptors, PR: progesterone receptors, Syn: synaptophysin, CgA: chromogranin A, MT: mastectomy, BCS: breast conserving surgery, SLNB: sentinel lymph node biopsy, ALND: axillary lymph node dissection, N: nodes, Chemo: chemotherapy, RT: radiation therapy, ET: endocrine therapy, AWD: alive without disease, AWRD: alive with recurrent disease, N/A: not available
Similar to the majority of the published cases, our patient had an ER and PR positive and Her2 negative tumor. Regarding the clinical presentation findings, in the vast majority of the published cases the patients presented with a palpable breast mass. On the contrary, our patient was completely asymptomatic and the tumor was incidentally detected on a screening mammogram. The asymptomatic presentation of neuroendocrine breast carcinoma is an exceedingly rare manifestation.
Besides, the mammographic appearance of architectural distortion, as observed in our case, is very rare as well. The presence of architectural distortion and calcifications, as seen in our case, is an extremely uncommon mammographic manifestation and has been reported in only 2.3% of the cases (2).
In conclusion, NEBC is a very rare breast malignancy with unclear histogenesis, which is associated with a more aggressive clinical course compared to other types of invasive breast cancer. Due to the rarity of the tumor the optimal treatment has not been clearly defined and is currently treated similarly to conventional breast cancer. Surgery is the mainstay of treatment. The distinction of primary from metastatic neuroendocrine breast tumors is crucial as these two entities require different therapeutic approaches. Further research is needed to understand the molecular profile of the tumor and identify novel targeted therapies.
Acknowledgements
The author would like to thank Dr. Ilias Katikaridis from the Pathology Department for providing the histology slides.
References
- 1. Yang X, Cao Y, Chen C, Liu L, Wang C, Liu S. Primary neuroendocrine breast carcinomas: a retrospective analysis and review of literature. Onco Targets Ther. 2017; 10:397-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Park YM, Wu Y, Wei W, Yang WT. Primary neuroendocrine carcinoma of the breast: clinical, imaging, and histologic features. AJR Am J Roentgenol. 2014; 203:W221-230. [DOI] [PubMed] [Google Scholar]
- 3. Roininen N, Takala S, Haapasaari KM, Jukkola- Vuorinen A, Mattson J, Heikkilä Karihtala P. Primary neuroendocrine breast carcinomas are associated with poor local control despite favourable biological profile: a retrospective clinical study. BMC Cancer. 2017; 17:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rosen LE, Gattuso P. Neuroendocrine tumors of the breast. Arch Pathol Lab Med. 2017; 141:1577-1581. [DOI] [PubMed] [Google Scholar]
- 5. Jeon CH, Kim SM, Jang M, Yun BL, Ahn HS, Kim SW, Kang E, Park SY. Clinical and radiologic features of neuroendocrine breast carcinomas. J Ultrasound Med. 2014; 33:1511-1518. [DOI] [PubMed] [Google Scholar]
- 6. Wang J, Wei B, Albarracin CT, Hu J, Abraham SC, Wu Y. Invasive neuroendocrine carcinoma of the breast: a population-based study from the surveillance, epidemiology and end results (SEER) database. BMC Cancer. 2014; 14:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Collado-Mesa F, Net JM, Klevos GA, Yepes MM. Primary neuroendocrine carcinoma of the breast: report of 2 cases and literature review. Radiol Case Rep. 2017; 12:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee DH, Park AY, Seo BK, Kim YS, Lee KY, Cha SH. Primary neuroendocrine carcinoma of the breast with clinical features of inflammatory breast carcinoma: a case report and literature review. J Breast Cancer. 2015; 18:404-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lavigne M, Menet E, Tille JC, Lae M, Fuhrmann L, Bonneau C, Deniziaut G, Melaabi S, Ng CCK, Marchiò C, Rouzier R, Bièche I, Vincent-Salomon A. Comprehensive clinical and molecular analyses of neuroendocrine carcinomas of the breast. Mod Pathol. 2018; 31:68-82. [DOI] [PubMed] [Google Scholar]
- 10. Tavassoli FA, Devilee P. Pathology and genetics. In: Tumors of the breast and female genital organs. WHO Classification of Tumors Series. Lyon, France, 2003; pp.32-34. [Google Scholar]
- 11. Bussolati G, Badve S. Carcinomas with neuroendocrine features. In: Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, van der Vijver MJ, eds. WHO Classification of Tumours of the Breast. Lyon, France, 2012; pp.62-63. [Google Scholar]
- 12. Wei B, Ding T, Xing Y, Wei W, Tian Z, Tang F, Abraham S, Nayeemuddin K, Hunt K, Wu Y. Invasive neuroendocrine carcinoma of the breast: a distinctive subtype of aggressive mammary carcinoma. Cancer. 2010; 116:4463-4473. [DOI] [PubMed] [Google Scholar]
- 13. Cubilla AL, Woodruff JM. Primary carcinoid tumour of the breast: a report of eight patients. Am J Surg Pathol. 1977; 4:283-292. [Google Scholar]
- 14. Canbak T, Acar A, Tolan HK, Ozbagriacik M, Ezberci F. Primary neuroendocrine carcinoma of the breast: a 5-year experiences. Ann Ital Chir. 2020; 91:23-26. [PubMed] [Google Scholar]
- 15. Suhani, Ali S, Desai G, Thomas S, Aggarwal L, Meena K, Kumar J, Jain M, Tudu SK. Primary neuroendocrine carcinoma breast: our experience. Breast Dis. 2014; 34:95-99. [DOI] [PubMed] [Google Scholar]
- 16. Inno A, Bogina G, Turazza M, Bortesi L, Duranti S, Massocco A, Zamboni G, Carbognin G, Alongi F, Salgarello M, Gori S. Neuroendocrine carcinoma of the breast: current evidence and future perspectives. Oncologist. 2016; 21:28-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Feki J, Fourati N, Mnif H, Khabir A, Toumi N, Khanfir A, Boudawara T, Amouri H, Daoud J, Frikha M. Primary neuroendocrine tumors of the breast: a retrospective study of 21 cases and literature review. Cancer Radiother.. 2015; 19:308-312. (in French). [DOI] [PubMed] [Google Scholar]
- 18. Lu CS, Huang SH, Ho CL, Chen JH, Chao TY. Primary neuroendocrine carcinoma of the breast. J BUON. 2014; 19:419-429. [PubMed] [Google Scholar]
- 19. Özdirik B, Kayser A, Ullrich A, Savic LJ, Reiss M, Tacke F, Wiedenmann B, Jann H, Roderburg C. Primary neuroendocrine neoplasms of the breast: case Series and literature review. Cancers (Basel). 2020; 12:733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Angarita FA, Rodríguez JL, Meek E, Sánchez JO, Tawil M, Torregrosa L. Locally-advanced primary neuroendocrine carcinoma of the breast: case report and review of the literature. World J Surg Oncol. 2013; 11:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tato-Varela S, Albalat-Fernández R, Pabón-Fernández S, Zarco ER, Calle-Marcos ML. Primary neuroendocrine tumour of the breast: a case report and review of the literature. Ecancermedicalscience. 2015; 9:607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Perry KD, Reynolds C, Rosen DG, Edgerton ME, T Albarracin C, Gilcrease MZ, Sahin AA, Abraham SC, Wu Y. Metastatic neuroendocrine tumour in the breast: a potential mimic of in-situ and invasive mammary carcinoma. Histopathology. 2011; 59:619-630. [DOI] [PubMed] [Google Scholar]
- 23. Vranic S, Palazzo J, Sanati S, Florento E, Contreras E, Xiu J, Swensen J, Gatalica Z. Potential novel therapy targets in neuroendocrine carcinomas of the breast. Clin Breast Cancer. 2019; 19:131-136. [DOI] [PubMed] [Google Scholar]
- 24. Zhang Q, He L, Lv W, Wang N. Neuroendocrine carcinoma of the breast with hyperprolactinemia: report of two cases and a minireview. Int J Clin Exp Pathol. 2020; 13:1457-1462. [PMC free article] [PubMed] [Google Scholar]
- 25. Hejjane L, Oualla K, Bouchbika Z, Bourhafour M, Lhlou Mimi A, Boubacar E, Benider A, Benbrahim Z, Aarifi S, Mellas N. Primary neuroendocrine tumors of the breast: two case reports and review of the literature. J Med Case Rep. 2020; 14:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li Y, Du F, Zhu W, Xu B. Neuroendocrine carcinoma of the breast: a review of 126 cases in China. Chin J Cancer. 2017; 36:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Locurto P, Antona AD, Grillo A, Ciulla A, Martorana S, Cipolla C, Graceffa G, Vieni S. Primary neuroendocrine carcinoma of the breast A single Center experience and review of the literature. Ann Ital Chir. 2016; 87:S2239253X1602658X. [PubMed] [Google Scholar]
- 28. Zhu Y, Li Q, Gao J, He Z, Sun R, Shen G, Zhang H, Xia W, Xu J. Clinical features and treatment response of solid neuroendocrine breast carcinoma to adjuvant chemotherapy and endocrine therapy. Breast J. 2013; 19:382-387. [DOI] [PubMed] [Google Scholar]
- 29. Rovera F, Lavazza M, La Rosa S, Fachinetti A, Chiappa C, Marelli M, Sessa F, Giardina G, Gueli R, Dionigi G, Rausei S, Boni L, Dionigi R. Neuroendocrine breast cancer: retrospective analysis of 96 patients and review of literature. Int J Surg. 2013; 11 Suppl 1:S79-83. [DOI] [PubMed] [Google Scholar]
- 30. Brask JB, Talman ML, Wielenga VT. Neuroendocrine carcinoma of the breast - a pilot study of a Danish population of 240 breast cancer patients. APMIS. 2014; 122:585-592. [DOI] [PubMed] [Google Scholar]
- 31. Zhang Y, Chen Z, Bao Y, Du Z, Li Q, Zhao Y, Tang F. Invasive neuroendocrine carcinoma of the breast: a prognostic research of 107 Chinese patients. Neoplasma. 2013; 60:215-222. [DOI] [PubMed] [Google Scholar]
- 32. Wu J, Yang QX, Wu YP, Wang DL, Liu XW, Cui CY, Wang L, Chen Y, Xie CM, Zhang R. Solid neuroendocrine breast carcinoma: mammographic and sonographic features in thirteen cases. Chin J Cancer. 2012; 31:549-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Menéndez P, García E, Rabadán L, Pardo R, Padilla D, Villarejo P. Primary neuroendocrine breast carcinoma. Clin Breast Cancer. 2012; 12:300-303. [DOI] [PubMed] [Google Scholar]
- 34. Kawasaki T, Mochizuki K, Yamauchi H, et al. High prevalence of neuroendocrine carcinoma in breast lesions detected by the clinical symptom of bloody nipple discharge. Breast. 2012; 21:652-656. [DOI] [PubMed] [Google Scholar]
- 35. Ghanem S, Kabaj H, Naciri S, Glaoui M, Ismaili N, Benjaafar N, Errihani H. Primary neuroendocrine carcinoma of the breast: A rare and distinct entity. J Cancer Res Exper Oncol. 2011; 3:50-54. [Google Scholar]
- 36. Adegbola T, Connolly CE, Mortimer G. Small cell neuroendocrine carcinoma of the breast: a report of three cases and review of the literature. J Clin Pathol. 2005; 58:775-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shin SJ, DeLellis RA, Ying L, Rosen PP. Small cell carcinoma of the breast: a clinicopathologic and immunohistochemical study of nine patients. Am J Surg Pathol. 2000; 24:1231-1238. [DOI] [PubMed] [Google Scholar]
- 38. Zekioglu O, Erhan Y, Ciriş M, Bayramoglu H. Neuroendocrine differentiated carcinomas of the breast: a distinct entity. Breast. 2003; 12:251-257. [DOI] [PubMed] [Google Scholar]
- 39. Valente I, Tringali G, Martella EM, Pallavera L, D'Aloia C. Primary neuroendocrine carcinoma of the breast: A case report of liver and lymph node metastases after eight years from diagnosis. Breast J. 2020; 26:505-507. [DOI] [PubMed] [Google Scholar]
- 40. Pülat H, Sabuncuoğlu MZ, Karaköse O, Benzin MF, Eroğlu HE, Kemal Kürşat Bozkurt KKB. A rare breast tumor: primary neuroendocrine carcinoma. Turk J Surg. 2018; 35:236-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tremelling A, Samuel S, Murray M. Primary small cell neuroendocrine carcinoma of the breast - A case report and review of the literature. Int J Surg Case Rep. 2017; 38:29-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Soe AM, Joseph G, Guevara E, Xiao P. Primary Neuroendocrine Carcinoma of the Breast Metastatic to the Bones, Which Chemotherapy? Breast J. 2017; 23:589-593. [DOI] [PubMed] [Google Scholar]
- 43. Bergstrom C, Porembka J, Fang Y, Sarode V, Syed S. Primary neuroendocrine carcinoma of the breast. Breast J. 2019; 25:519-520. [DOI] [PubMed] [Google Scholar]
- 44. Vats M, Sachan V, Prajapati S, Mandal S. Triple receptor-positive primary neuroendocrine carcinoma of breast in a young patient. BMJ Case Rep. 2018; 2018:bcr2017223280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mečiarová I, Sojáková M, Mego M, Mardiak J, Pohlodek K. High-grade neuroendocrine carcinoma of the breast with focal squamous differentiation. Int J Surg Pathol. 2016; 24:738-742. [DOI] [PubMed] [Google Scholar]
- 46. Marinova L, Malinova D, Vicheva S. Primary neuroendocrine carcinoma of the breast: histopathological criteria, prognostic factors, and review of the literature. Case Rep Pathol. 2016; 2016:6762085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yoshimura N, Sasada T, Yonehara S. Primary large-cell neuroendocrine carcinoma of the breast occurring in a pre-menopausal woman. Breast Care (Basel). 2015; 10:281-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wronski K, Zechowicz M, Frackowiak L, Koda M. Primary woman neuroendocrine breast tumor - case report and review of the literature. New Med. 2015; 19:9-12. [Google Scholar]
- 49. Wei X, Chen C, Xi D, Bai J, Huang W, Rong L, Wu M, Zhang G. A case of primary neuroendocrine breast carcinoma that responded to neo-adjuvant chemotherapy. Front Med. 2015; 9:112-116. [DOI] [PubMed] [Google Scholar]
- 50. Panjvani SI, Gandhi MB, Chaunhari BR, Sarvaiya AN. A rare solid variant of primary neuroendocrine carcinoma of breast. Annals of Pathology and Laboratory Medicine. 2015; 2:c101-c105. [Google Scholar]
- 51. Lee DH, Park AY, Seo BK, Kim YS, Lee KY, Cha SH. Primary neuroendocrine carcinoma of the breast with clinical features of inflammatory breast carcinoma: a case report and literature review. J Breast Cancer. 2015; 18:404-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Janosky M, Bian J, Dhage S, Levine J, Silverman J, Jors K, Moy L, Cangiarella J, Muggia F, Adams S. Primary large cell neuroendocrine carcinoma of the breast, a case report with an unusual clinical course. Breast J. 2015; 21:303-307. [DOI] [PubMed] [Google Scholar]
- 53. Alva KA, Tauro LF, Shetty P, Saldanha E. Primary neuroendocrine carcinoma of the breast: a rare and distinct entity. Indian J Cancer. 2015; 52:636-637. [DOI] [PubMed] [Google Scholar]
- 54. Valentim MH, Monteiro V, Marques JC. Primary neuroendocrine breast carcinoma: a case report and literature review. Radiol Bras. 2014; 47:125-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pagano M, Asensio SN, Zanelli F, Lococo F, Cavazza A, Damiani S, Rapicetta C, Gnoni R, Boni C. Is there a role for hormonal therapy in neuroendocrine carcinoma of the breast? A Paradigmatic case report. Clin Breast Cancer. 2014; 14:e99-e101. [DOI] [PubMed] [Google Scholar]
- 56. Bozkurt MA, Kocataş A, Özkan Y, Kalaycı MU, Alış H. A Rare Entity of Breast Cancer: Primary Neuroendocrin Carcinoma. J Breast Health. 2014; 10:242-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lingappa HA, Indushekar V, Chamarthy NP, Soni A. Primary neuroendocrine carcinoma of male breast: a cytologically diagnosed rare entity. J Cytol. 2014; 31:105-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tajima S, Horiuchi H. Neuroendocrine tumor, well differentiated, of the breast: a relatively high-grade case in the histological subtype. Case Rep Pathol. 2013; 2013:204065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Murthy V, Geethamala K, Kumar B, Sudharao M. Primary neuroendocrine carcinoma of breast: a rare case report. Ann Med Health Sci Res. 2013; 3(Suppl 1):S35-S37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hanna MY, Leung E, Rogers C, Pilgrim S. Primary large-cell neuroendocrine tumor of the breast. Breast J. 2013; 19:204-206. [DOI] [PubMed] [Google Scholar]
- 61. Watrowski R, Jäger C, Mattern D, Horst C. Neuroendocrine carcinoma of the breast - diagnostic and clinical implications. Anticancer Res. 2012; 32:5079-5082. [PubMed] [Google Scholar]
- 62. Sanguinetti A, Santoprete S, Lucchini R, Triola R, Loreti F, Avenia N. A rare breast tumor: solid neuroendocrine carcinoma. Ann Ital Chir. 2013; 84:81-85. [PubMed] [Google Scholar]
- 63. Psoma E, Nikolaidou O, Stavrogianni T, Mavromati A, Lytras K, Xirou P, Koumbanaki M, Panagiotopoulou D. A rare case report of a primary large-cell neuroendocrine carcinoma of the breast with coexisting Paget disease. Clin Imaging. 2012; 36:599-601. [DOI] [PubMed] [Google Scholar]
- 64. Nawawi O, Ying Goh K, Rahmat K. A rare case of primary infiltrating neuroendocrine carcinoma of the breast. Iran J Radiol. 2012; 9:212-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Graça S, Esteves J, Costa S, Vale S, Maciel J. Neuroendocrine breast cancer. BMJ Case Rep. 2012; 2012:bcr1220115343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zhang JY, Chen WJ. Bilateral primary breast neuroendocrine carcinoma in a young woman: report of a case. Surg Today. 2011; 41:1575-1578. [DOI] [PubMed] [Google Scholar]
- 67. Ghanem SS, Glaoui M, Naciri S, Khoyaali S, Kabbaj M, Khanoussi B, Errihani H. A rare tumor of the breast: solid neuroendocrine carcinoma. WebmedCentral. 2011; 2:WMC001591. [Google Scholar]


