Summary
Primary leiomyoma of the liver (PLL) is a rare benign tumor occurring in immunosuppressed people. From 1926 less than fifty cases are reported in the scientific literature and about half are in immunocompetent patients. Etiology of this kind of lesion is not yet well known. We report a case of primary hepatic leiomyoma in a 60-year-old immunocompetent woman. The patient presented with lipothymia with unexpected vomiting. She underwent an ultrasound (US), and a computed tomography (CT) scan that revealed the presence of a single, solid lesion about 9 cm located between the S5 and S8 segment of the liver. It showed a well-defined, heterogeneous hypodensity with internal and peripheral enhancement and various central hypoattenuating areas and no wash-out in the portal and the late phases. Because of her symptoms and the risk of malignancy, the patient underwent a surgical liver resection. Histological diagnosis was primary leiomyoma of the liver. The patient had an uneventful recovery and was discharged after 7 days. At 30 months follow-up there were no symptoms and no evidence of disease. Leiomyoma of the liver is a rare benign neoplasm of which clinical symptoms are nonspecific and the exact radiological diagnosis still remains a challenge for radiologists. Etiology is still unclear and usually PLL represents an incidental diagnosis. Surgery plays a primary role not only in the treatment algorithm, but also in the diagnostic workout.
Keywords: liver resection, liver leiomyoma, liver neoplasm, rare liver diseases
1. Introduction
Primary leiomyoma of the liver (PLL) is a rare benign tumor occurring in immunosuppressed patients and arising from the muscularis of the gut or the media of the blood vessels, usually in the urogenital and gastrointestinal tracts (1,2). However it can originate from any organ or tissue such as the biliary tract and large vessels of the liver (3). Its occurrence in immunocompetent patients is extremely rare. Less than fifty cases are reported in literature; twenty-three of them in immunocompetent patients (4,5). This is the 24th described. Etiology is still unclear and usually PLL represents an incidental diagnosis. Surgery plays a primary role not only in the treatment algorithm, but also in the diagnostic workout. We report a case of a resected hepatic mass with an unexpected diagnosis of PLL in an immunocompetent patient.
2. Case Report
A 60-year-old woman with abdominal pain and nausea was referred to our institute because of an incidental finding of liver neoplasm detected by US examination performed in the Emergency Department of another hospital for lipothymia with unexpected vomiting. An hypoechoic and heterogenic liver mass about 9 cm diameter between V-VIII segments of the liver was revealed (Figure 1). The patient had a history of hypertension and type 2 diabetes mellitus. Neither liver disease nor immunosuppression were reported. Upon admission, liver function and routine blood exams were within normal limits and viral marker tests like hepatitis B virus (HBV), hepatitis C virus (HCV), human immunodeficiency virus (HIV), Epstein-Barr virus (EBV) were negative. Tumor markers showed normal values: alphafetoprotein (AFP) 1.35 ng/mL, carcinoembryonic antigen (CEA) 1.21 ng/mL, carbohydrate antigen 19-9 (CA199) 17 U/mL, and cancer antigen 125 (CA125) 24 U/mL. Further diagnostic triphasic CT confirmed a single solid lesion of about 9 cm (Figure 2) without characteristics of malignancy or clear signs of benign liver neoplasms. The patient underwent a percutaneous US-guided biopsy and diagnosis was unspecified myopericytoma. Because of her symptoms and the risk of malignancy, the patient was scheduled for liver resection. Anatomical bisegmentectomy of V-VIII liver segments by kellyclasia technique was performed (Figure 3). After an uneventful recovery, the patient was discharged on postoperative day 7. At histological examination the liver specimen showed spindle cells with eosinophilic cytoplasm forming a pattern of interlacing bundles, separated by sclero-hyaline tissue with no mitoses or atypia. Immunohistochemical examination revealed staining positivity for Smooth-Muscle-Actin, Vimentin and Desmin, and negativity for CD31 and CD34 (Figure 4). Diagnosis was PLL. At 30 months follow-up there is no evidence of disease.
Figure 1.
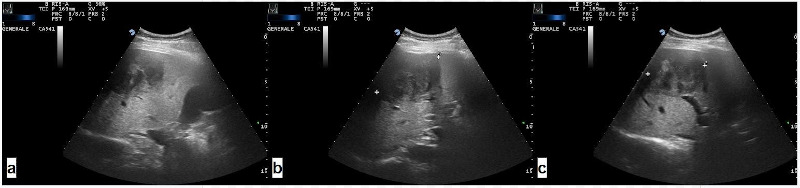
Ultrasound images: different slices of the hepatic leiomyoma.
Figure 2.
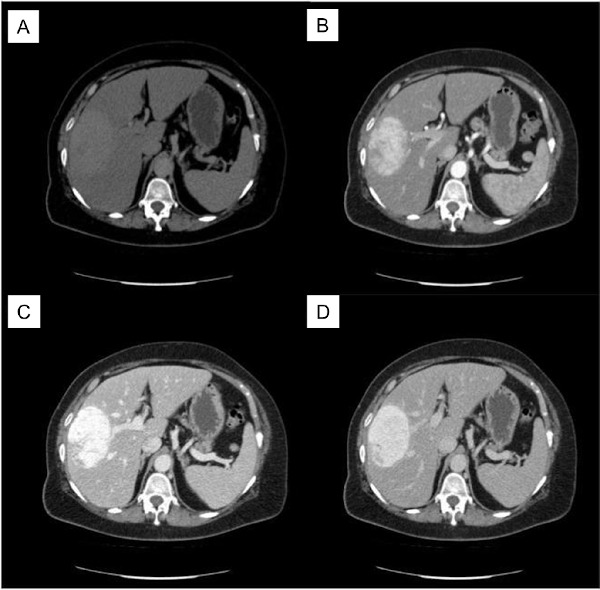
CT Scan. (A), basal phase; (B), arterial phase; (C), venous phase; (D), portal phase.
Figure 3.
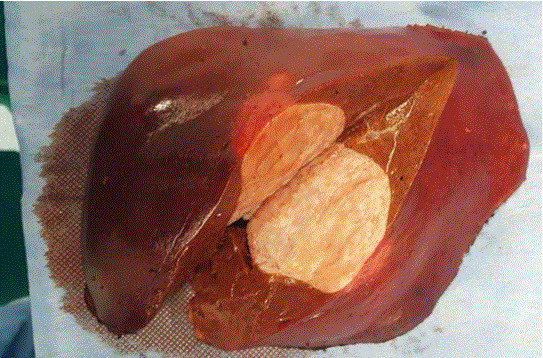
Specimen. Hepatic parenchyma within the center a whitish encapsulated liver mass.
Figure 4.
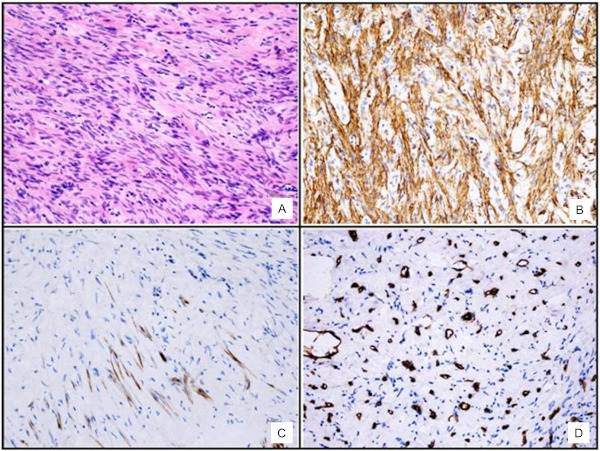
(A), Haematoxylin-Eosin staining. The hepatic tumor was composed of bundles of spindle-shaped cells with elongated nuclei and eosinophilic cytoplasm. No mitotic activity was seen; (B), Smooth Muscle Actin staining of tumor cells, showing diffuse positivity of spindle cells; (C), Desmin staining of tumor cells showing focal positivity; (D), CD34 staining of the tumor, showing positivity in blood vessels and negativity of tumor cells.
3. Discussion
Leiomyoma is relatively common, tends to originate from the muscularis of the gut or the media of the blood vessels, and usually develops in the urogenital and gastrointestinal tracts (1-3). However it can originate from any organ or tissue such as the biliary tract and large vessels of the liver (6,7). PLL is rare and has its own particular clinical and biological features (1). Hepatic leiomyomas can be multiple or solitary lesions and can grow to a large size sometimes exceeding 20 cm in diameter with a low tendency for necrosis and hemorrhage (6). On the cut surface they appear yellowish to whitish in color and may be fibrous in texture (Figure 3). Some authors described in their papers that PLL may contain cystic parts (8-10).
The first case was described in 1926 in a 42-year-old woman (11). Ninety years after the first case of primary leiomyoma of the liver was reported, to the best knowledge of this author, 44 cases of primary leiomyoma of the liver have been reported in the literature. The age of presentation is very variable. The mean age of diagnosis is 41 years (range 3-87). Primary leiomyomas of the liver have been reported to have female sex predilection. Familial predispositions have not been reported.
To diagnose primary hepatic leiomyoma Hawkins et al. proposed two criteria. First, the tumour must be composed of leiomyocytes. Second, the presence of leiomyoma in other intraabdominal sites, especially in urogenital tissue, must be excluded (12). Both benign and malignant smooth-cell liver neoplasms are often found in immunosuppressed (child and adult) patients, like EBV-infected, HIV-infected and organ transplant recipients. However, they also occur in immunocompetent patients, as in our case (13-16). There is strong evidence for the role that some viruses exercise in the pathogenesis of certain human neoplasms. Patients with an immune deficiency condition are at risk for virus-associated malignancies (17). Kaposi Sarcoma and smooth-muscle tumors might arise from a common stem cell under the influence of some unknown factor produced during HIV infection. This relationship was demonstrated in 1990 in cultured cells (18,19). Epstein-Barr virus shows a near consistent association with smooth muscle tumors of the liver. This association has been reported several times; so much that some authors have proposed to term these lesions EBV-associated smooth muscle tumors (EBV-SMT) (7,20-21). The marked expression of EBV early antigen (EBER) in the nuclei of numerous smooth muscle cell tumours has been well documented since 1993. EBER in the nuclei was first detected in neoplastic smooth-muscle cells in three cases of post-transplant spindle cell tumor (7,22). Then some researchers detected EBER using in situ hybridization/ISH, which is the gold standard for detection and localization of latent EBV in tissues in both adults and children (along with quantitative polymerase chain reaction). Nonetheless, EBV infection is not necessary nor sufficient for the development of primary liver leiomyoma (14,16,23).
Usually, in immunosuppressed patients, these lesions are detected during routine follow-up for the underlying disease, so tumors tend to be found at a smaller size when compared with tumors found in patients without immunosuppressive disorders. Instead, in immunocompetent patients, because symptoms are induced by compression of the surroundings organs, they are generally discovered when they are greater than 10 cm in diameter (24). Microscopically PLL generally shows a proliferation of spindle cells with eosinophilic cytoplasm forming a pattern of interlacing bundles, often separated by sclero-hyaline tissue. No mitoses are observed. Some authors suggested the limit of 10 mitoses per HPF as one of the criteria to discern leiomyomas from leiomyosarcomas. Atypia, if present, is slight. Immunohystochemical examination reveals positive staining for SMA and Vimentin, less often for Desmin, and negative for CD31, CD34, S-100 and HMB-45. The proliferation activity, based on Ki-67, was lower than 5% in all the papers we reviewed. The most common symptoms, when present, are abdominal, epigastric, or right upper quadrant pain, abdominal mass, abdominal discomfort, dyspepsia, vomiting and liver dysfunction, listed in order of decreasing frequency. Blood liver function tests and tumor markers are generally negative.
The exact radiological diagnosis of liver neoplasms (and rare tumors overall), which have often variable imaging behaviors and still unknown patterns, is still a challenge for radiologists. By US, hepatic leiomyomas have been repeatedly described as well defined hypoechoic lesions with variable degrees of heterogeneity (2,25,26). Some authors reported an echo-poor halo surrounding the lesion (27). The CT findings that have been described are similar to those of other smooth muscle tumors in the body (28). By CT scan, most authors reported hypodense lesions with brisk enhancement in arterial phase, occasionally mainly peripheral, and a persistent enhancement in portal and delayed phase (27-29).
By angiography, marginal, diffuse or irregular hypervascularization were variously reported. Nevertheless, in all cases reported the authors concluded that the angiography study was nondiagnostic, but could perhaps play a role in a therapeutic process, as in the rare case of acute bleeding.
On MRI, these lesions were hypointense in Tl-weighted images, partially hyperintense in T2- weighted sequences, and showed marked gadolinium enhancement in both early and equilibrium phases. Gadobenatedimeglumine is a liver-specific, gadolinium-based MR contrast agent with a vascular-interstitial distribution in the first minutes after bolus injection, followed by delayed hepatobiliary excretion. In a study published in 2008 (30), the most common imaging pattern and the imaging protocol that the authors used is very well described. On pre-contrast T2- and T1- weighted images, the lesion showed isointensity and marked hypointensity respectively to the surrounding liver. As with the previous CT examination, the lesion showed intense enhancement on T1-weighted VIBE images acquired during the arterial phase, followed by persistent and homogeneous enhancement during the hepatic venous and equilibrium phases. During the delayed hepatobiliary phase, the lesion showed lack of contrast retention, and thus, was identified as a well-defined, hypointense focus against the highly enhanced background liver (30). Despite that, there are primary leiomyomas of the liver characterized by hypointensity in the T2-weighted MRI image that may be related to its dense fusocellular nature, composed by intramuscular actin, myosin and collagen, that decrease extracellular fluid compared with surrounding tissue (26).
The feasibility of an accurate preoperative diagnosis by fine needle aspiration (FNA) versus biopsy is still a matter of debate. Some authors reported the inadequacy of FNA for preoperative diagnosis of PLL because of the difficulty of getting an adequate sample (31) and favoured biopsy (26), defined more appropriate in cases of smooth muscle neoplasm samples (13,28,32). In our case, like most reported, the preoperative imaging tests and the biopsy were inconclusive (4).
When preoperative tests do not present a clear diagnosis, surgery has a primary role not only in the treatment algorithm, but also in the diagnostic process. As for other benign lesions of the liver, surgery should be performed in all symptomatic PLL, in those with dimensions > 10 cm with a higher risk to develop malignant changes, and for those in which radiological diagnosis can not be reached. Surgery should be performed in institutes and units that with surgical skills for these kind of procedures. Laparoscopic resections have been described by some authors (24,33). Mininvasive liver surgery, including robotic surgery, for benign lesions is feasible and safe for well-selected patients, and may play a role in patients with immuno-suppressive conditions in which abdominal incisions carry a higher risk of wound complications (24).
In conclusion, leiomyoma of the liver is a rare neoplasm which can also grow in immunocompetent patients. Clinical symptoms are nonspecific and the exact radiological diagnosis of these liver neoplasms is still a challenge for radiologists. Due to that, we think that surgery may play a key role in the diagnostic process and in the treatment algorithm.
Funding: No grants or funding have been received for the drawing up of the present paper.
Conflict of Interest: The authors have no conflict of interest to disclose.
References
- 1. Luo XZ, Ming CS, Chen XP, Gong NQ. Epstein-Barr virus negative primary hepatic leiomyoma: case report and literature review. World J Gastroenterol. 2013; 19:4094-4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hollands MJ, Jaworski R, Wong KP, Little JM. A leiomyoma of the liver. HPB Surg. 1989; 1:337-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fong JA, Ruebner BH. Primary leiomyosarcoma of the liver. Hum Pathol. 1974; 5:115-119. [DOI] [PubMed] [Google Scholar]
- 4. Jia B, Jin Z, Gao P, Liu Y. Primary hepatic leiomyoma in a Chinese female patient without underlying disease: a case report. BMC Surg. 2019; 19:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Omiyale AO. Primary leiomyoma of the liver: a review of a rare tumour. HPB Surg. 2014; 2014:959202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Belli G, Ciciliano F, Iannelli A, Marano I. Hepatic resection for primary giant leiomyoma of the liver. HPB (Oxford). 2001; 3:11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang MH, Wu CT, Hung CC, Liang JD, Chen PJ. Hepatic leiomyomatous neoplasm associated with Epstein Barr virus infection in an adult with acquired immunodeficiency syndrome. J Formos Med Assoc. 2000; 99:873-875. [PubMed] [Google Scholar]
- 8. Urizono Y, Ko S, Kanehiro H, Hisanaga M, Aomatsu Y, Nagao M, Ikeda N, Yamada T, Nakajima Y. Primary leiomyoma of the liver: report of a case. Surg Today. 2006; 36:629-632. [DOI] [PubMed] [Google Scholar]
- 9. Sclabas GM, Maurer CA, Wente MN, Zimmermann A, Büchler MW. Case report: Hepatic leiomyoma in a renal transplant recipient. Transplant Proc. 2002; 34:3200-3202. [DOI] [PubMed] [Google Scholar]
- 10. Yoon GS, Kang GH, Kim OJ. Primary myxoid leiomyoma of the liver. Arch Pathol Lab Med. 1998; 122:1112-1115. [PubMed] [Google Scholar]
- 11. Demel R. Ein operierter fall von leber-myom. Virchows Archiv für Pathologische Anatomie und Physiologie und für Klinische Medizin. 1926; 261:881-884. [Google Scholar]
- 12. Hawkins EP, Jordan GL, McGavran MH Primary leiomyoma of the liver. Successful treatment by lobectomy and presentation of criteria for diagnosis. Am J Surg Pathol. 1980; 4:301-304. [PubMed] [Google Scholar]
- 13. Ha C, Haller JO, Rollins NK. Smooth muscle tumors in immunocompromised (HIV negative) children. Pediatr Radiol. 1993; 23:413-414. [DOI] [PubMed] [Google Scholar]
- 14. Prévot S, Néris J, de Saint Maur PP. Detection of Epstein Barr virus in an hepatic leiomyomatous neoplasm in an adult human immunodeficiency virus 1-infected patient. Virchows Arch. 1994; 425:321-325. [DOI] [PubMed] [Google Scholar]
- 15. Kanazawa N, Izumi N, Tsuchiya K, et al. A case of primary leiomyoma of the liver in a patient without evidence of immunosuppression. Hepatol Res. 2002; 24:80-88. [DOI] [PubMed] [Google Scholar]
- 16. Lee ES, Locker J, Nalesnik M, Reyes J, Jaffe R, Alashari M, Nour B, Tzakis A, Dickman PS. The association of Epstein-Barr virus with smooth-muscle tumors occurring after organ transplantation. N Engl J Med. 1995; 332:19-25. [DOI] [PubMed] [Google Scholar]
- 17. Safai B, Diaz B, Schwartz J. Malignant neoplasms associated with human immunodeficiency virus infection. CA Cancer J Clin. 1992; 42:75-95. [DOI] [PubMed] [Google Scholar]
- 18. AE W, Mitchell C, Armstrong G, et al. Propagation and properties of Kaposis sarcoma-derived cell lines obtained from patients with AIDS: similarity of cultured cells to smooth muscle cells. AIDS. 1991; 5:1485-1493. [DOI] [PubMed] [Google Scholar]
- 19. Mueller BU, Butler KM, Higham MC, Husson RN, Montrella KA, Pizzo PA, Feuerstein IM, Manjunath K. Smooth muscle tumors in children with human immunodeficiency virus infection. Pediatrics. 1992; 90:460-463. [PubMed] [Google Scholar]
- 20. Le Bail B, Morel D, Mérel P, Comeau F, Merlio JP, Carles J, Trillaud H, Bioulac-Sage P. Cystic smooth-muscle tumor of the liver and spleen associated with Epstein-Barr virus after renal transplantation. Am J Surg Pathol. 1996; 20:1418-1425. [DOI] [PubMed] [Google Scholar]
- 21. Davidoff AM, Hebra A, Clark BJ 3rd, Tomaszewski JE, Montone KT, Ruchelli E, Lau HT. Epstein-Barr virus-associated hepatic smooth neoplasm in a cardiac transplant recipient. Transplantation. 1996; 61:515-517. [DOI] [PubMed] [Google Scholar]
- 22. Lee E, Dickman PS, Jaffe R, Alashari M, Tzakis A, Reyes J. Post-transplant spindle cell tumor (PTST): an entity associated with Epstein-Barr virus. Mod Pathol. 1993;6:127A Abstract. [Google Scholar]
- 23. McClain KL, Leach CT, Jenson HB, Joshi VV. Association of Epstein-Barr Virus with leiomyosarcomas in young people with AIDS. N Engl J Med. 1995; 332:12-18. [DOI] [PubMed] [Google Scholar]
- 24. Perini MV, Fink MA, Yeo DA, Carvalho CA, Morais CF, Jones RM, Christophi C. Primary liver leiomyoma: a review of this unusual tumour. ANZ J Surg. 2013; 83:230-233. [DOI] [PubMed] [Google Scholar]
- 25. Beuzen F, Roudie J, Moali I, Maitre S, Barthelemy P, Smadja C. Primary leiomyoma of the liver: a rare benign tumor. Gastroenterol Clin Biol. 2004; 28:1169-1172. (in French) [DOI] [PubMed] [Google Scholar]
- 26. Sousa HT, Portela F, Semedo L, Furtado E, Marinho C, Cipriano MA, Leitão MC. Primary leiomyoma of the liver: accurate preoperative diagnosis on liver biopsy. BMJ Case Rep. 2009; 2009:bcr09.2008.0898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wachsberg RH, Cho KC, Adekosan A. Two leiomyomas of the liver in an adult with AIDS: CT and MR appearance. J Comput Assist Tomogr. 1994; 18:156-157. [DOI] [PubMed] [Google Scholar]
- 28. Sadler M, Mays WL, Albert P, Javors B. Hepatic leiomyomas in two adult patients with AIDS: intravenous contrast-enhanced CT and MR imaging. Emerg Radiol. 2002; 9:175-177. [DOI] [PubMed] [Google Scholar]
- 29. Santos I, Valls C, Leiva D, Serrano T, Martinez L, Ruiz S. Primary hepatic leiomyoma: case report. Abdom Imaging. 2011; 36:315-317. [DOI] [PubMed] [Google Scholar]
- 30. Marin D, Catalano C, Rossi M, Guerrisi A, Di Martino M, Berloco P, Passariello R. Gadobenate dimeglumine-enhanced magnetic resonance imaging of primary leiomyoma of the liver. J Magn Reson Imaging. 2008; 28:755-758. [DOI] [PubMed] [Google Scholar]
- 31. Guy CD, Yuan S, Ballo MS. Spindle-cell lesions of the liver: diagnosis by fine-needle aspiration biopsy. Diagn Cytopathol. 2001; 25:94-100. [DOI] [PubMed] [Google Scholar]
- 32. Doyle H, Tzakis AG, Yunis E, Starzl TE. Smooth muscle tumor arising de novo in a liver allograft: A case report. Clin Transplant. 1991; 5:60-62. [PMC free article] [PubMed] [Google Scholar]
- 33. Vyas S, Psica A, Watkins J, Yu D, Davidson B. Primary hepatic leiomyoma : unusual cause of an intrahepatic mass. Ann Transl Med. 2015; 3:73. [DOI] [PMC free article] [PubMed] [Google Scholar]


