Abstract
A growing number of studies on coronavirus disease 2019 (COVID-19) are becoming available, but a synthesis of available data focusing on the critically ill population has not been conducted. We performed a scoping review to synthesize clinical characteristics, treatment, and clinical outcomes among critically ill patients with COVID-19. Between January 1, 2020, and May 15, 2020, we identified high-quality clinical studies describing critically ill patients with a sample size of greater than 20 patients by performing daily searches of the World Health Organization and LitCovid databases on COVID-19. Two reviewers independently reviewed all abstracts (2785 unique articles), full text (218 articles), and abstracted data (92 studies). The 92 studies included 61 from Asia, 16 from Europe, 10 from North and South America, and 5 multinational studies. Notable similarities among critically ill populations across all regions included a higher proportion of older males infected and with severe illness, high frequency of comorbidities (hypertension, diabetes, and cardiovascular disease), abnormal chest imaging findings, and death secondary to respiratory failure. Differences in regions included newly identified complications (eg, pulmonary embolism) and epidemiological risk factors (eg, obesity), less chest computed tomography performed, and increased use of invasive mechanical ventilation (70% to 100% vs 15% to 47% of intensive care unit patients) in Europe and the United States compared with Asia. Future research directions should include proof-of-mechanism studies to better understand organ injuries and large-scale collaborative clinical studies to evaluate the efficacy and safety of antivirals, antibiotics, interleukin 6 receptor blockers, and interferon. The current established predictive models require further verification in other regions outside China.
Abbreviations and Acronyms: ACEI, angiotensin-converting enzyme inhibitor; APACHE, Acute Physiology and Chronic Health Evaluation; ARB, angiotensin receptor blocker; ARDS, acute respiratory distress syndrome; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; CT, computed tomography; EUA, emergency use authorization; HFNC, high-flow nasal cannula oxygen therapy; ICU, intensive care unit; IF, impact factor; IL, interleukin; IMV, invasive mechanical ventilation; LOS, length of stay; NIMV, noninvasive mechanical ventilation; PE, pulmonary embolism; RCT, randomized clinical trial; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SOFA, Sequential Organ Failure Assessment
Article Highlights.
-
•
This scoping review is the first of its kind to synthesize the current literature focusing on patients with coronavirus disease 2019 and critical illness by analyzing 92 included studies from Asia, Europe, and North and South America.
-
•
Scoping reviews are valuable for summarizing existing knowledge, highlighting gaps in our understanding, and guiding future research directions.
-
•
Notable similarities among critically ill populations across all regions included a higher proportion of older males infected and with severe illness, high frequency of comorbidities, abnormal chest imaging findings, and death secondary to respiratory failure. Differences in regions included newly identified complications (eg, pulmonary embolism) and epidemiological risk factors (eg, obesity), and a longer stay in the intensive care unit, less chest computed tomography performed, and increased use of mechanical ventilation (70% to 100% vs 15% to 47% of intensive care unit patients) in Europe and the United States compared with China.
-
•
Future research directions include a need for studies to confirm the mechanism of organ injuries and large-scale collaborative clinical studies to evaluate the efficacy and safety of drugs. The current established predictive models require further verification in other regions outside China.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), causing coronavirus disease 2019 (COVID-19), is the novel coronavirus first detected in Wuhan, China, in December 20191 , 2 that continues to spread globally, with more than 13 million cases confirmed as of July 15, 2020.3 The clinical spectrum of COVID-19 ranges widely from asymptomatic infection to critical illness with a high risk of mortality.4, 5, 6 Based on the evidence to date, there is a high probability of requirement for intensive care unit (ICU) level of care among patients with COVID-19, leading to a substantial increase in the demand for critical care beds. Understanding the disease trajectory and those most likely to benefit from critical care will be vital for ICU clinicians as they grapple with this new illness and understand how best to manage patients within potentially resource-constrained settings.
Because there are limited antiviral treatment options for COVID-19 and the severity of disease is closely related to the prognosis,5 it is also essential to triage patients with COVID-19 and identify early from among the many mild cases the few who will have progression to critical illness to effectively prioritize resources for patients with the highest risk, keep them under continuous close monitoring, and intervene as needed to prevent the progression of disease.
As a growing body of literature on COVID-19 is becoming available, a small number of systematic and scoping reviews have been published since February 2020. However, because of the relatively short time since the outbreak of this novel disease, most reviews have either focused on a single aspect of COVID-19 (eg, imaging findings,7 comorbidities,8 treatment9) or included a very limited number of studies with minimal quantitative data synthesis,10 or primarily described disease features among the general population11 and often included studies from a single country.12 , 13 Initial research emerged from Asia, but more COVID-19 studies from Europe and North America have now been published. What is missing from the current literature, however, is a synthesis of the worldwide literature on critically ill patients specifically. Given the urgency of the COVID-19 epidemic and the need to understand information about critically ill patients with COVID-19, a scoping review was considered suitable and useful for mapping the available evidence to identify knowledge gaps.14 This study had several objectives. First, we wanted to synthesize the current and emerging literature describing the clinical features, treatment, outcomes, and risk factors associated with poor prognosis among critically ill patients with COVID-19 during the early months of the pandemic. Second, we wanted to provide frontline clinicians with insights for the effective management of severely ill patients with COVID-19. Finally, we aimed to identify knowledge gaps to guide future research endeavors.
Methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) checklist in reporting findings.15
Data Source and Search Strategy
We identified eligible studies daily by searching the World Health Organization COVID-19 database16 and the LitCovid database17 , 18 for COVID-19 literature published in English from January 1, 2020, to May 15, 2020. Both of these databases track and provide daily updates on COVID-19 scientific studies. To focus our efforts, we included peer-reviewed human studies in clinical journals with an impact factor (IF) of greater than 5. We did not include studies on animals or those examining molecular disease mechanisms. We imported the identified articles into Covidence systematic review software19 for the title, abstract, and full-text screening.
Study Selection
Studies that met all of the following criteria were included: (1) analysis included full cohort or subgroup of critically ill adult patients with COVID-19; defined in our study as patients admitted to the ICU, those categorized with severe disease by guidelines, end-organ injury/dysfunction, and patients who died from COVID-19; (2) reporting at least one of the following types of information: clinical characteristics, treatment, or use of multivariate analysis of risk factors associated with higher disease severity or poor prognosis; (3) study designs: randomized clinical trials (RCT), prospective or retrospective observational studies; and (4) sample size of 20 or more individual patients.
Studies were excluded on the basis of one or more of the following: (1) participants younger than 18 years, pregnant women, no analysis of critically ill patients; (2) study designs were research letters without patient data, news reports, editorials, commentaries, case reports, mathematical modeling studies, recommendations, guidelines, or review articles; and (3) non-English articles.
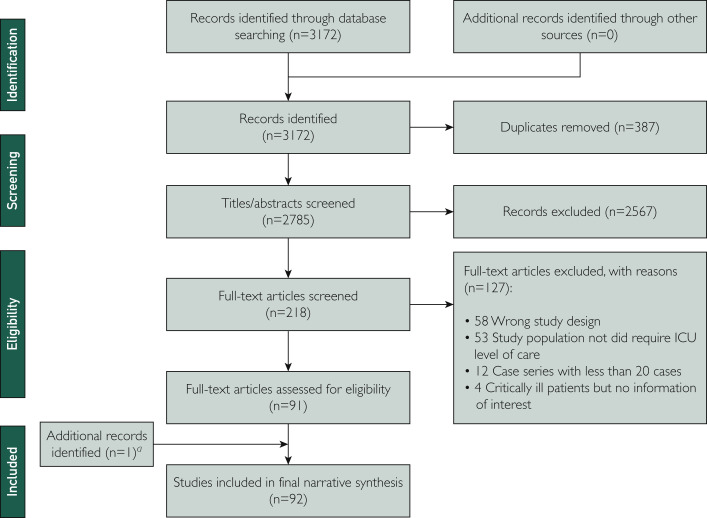
All titles and abstracts screened in the first phase, as well as full-text articles in the second phase, were reviewed independently by 2 investigators (C.H., J.S., S.H., Y.P., or K.P.). Discrepancies were resolved by a senior team member (Y.D. or A.K.B.) in each phase. Reasons for exclusion are reported in the PRISMA-ScR flowchart (Figure ). During the process of gathering and reviewing information, we modified the inclusion and exclusion criteria as new ideas emerged and ensured that the previous steps complied with the change in protocol.
Figure.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram for the scoping review process. aAfter May 15, 2020, when we had discontinued daily updates, we included one study describing a randomized clinical trial meeting inclusion criteria. ICU, intensive care unit.
Data Extraction and Synthesis
Data abstraction on each of the included articles was performed by 2 independent reviewers (C.H., J.S., S.H., Y.P., or K.P.) using a tailored Excel (Microsoft) form, which was piloted beforehand. Data abstracted included the following: literature characteristics (eg, journal, publication date, title, first author), characteristics related to study method (eg, article type, study design, country where the study was conducted, sample size, subgroups divided), variables related to patient clinical characteristics (eg, demographic characteristics, comorbidities, symptoms, laboratory findings, imaging assessments, severity of illness), interventions (eg, drug treatments, oxygen supplies, mechanical ventilation), clinical outcomes (eg, discharge, severity of disease, mortality, hospital length of stay [LOS]), risk factors associated with severity of illness or prognosis as identified using multivariate analysis, and key findings. As the data extraction process unfolded, we also enhanced the data extraction form, with new or more precise categories as needed. Reviewers also had regular meetings to address any challenges and to ensure concordance with their abstraction methods.
For data analysis, we descriptively summarized and qualitatively synthesized the data. The findings are presented in a narrative form, including tables and figures, to aid in data presentation and interpretation where appropriate.
Results
Literature Characteristics
The literature search generated 3172 records. Of these, 387 were duplicates and 2567 were excluded. A full-text review was conducted on 218 studies, and 127 were excluded because they did not meet our criteria. One additional relevant RCT study was included for data synthesis. A total of 92 studies were included for data abstraction (Figure).
Characteristics of Included Studies
Table 1 summarizes the main characteristics of the 92 included studies, 54 (59%) of which were original articles and 35 (38%) were research letters. Study designs were primarily retrospective observational studies (83 [90%]), followed by RCTs (5 [5%]) and prospective observational studies (4 [4%]). Nearly half of them were multicenter studies. Geographically, the articles largely originated in Asian countries (61 [66%]), followed by European countries (16 [17%]), North or South American countries (10 [11%]), and multinational regions (5 [5%]). The sample size ranged from 20 to 44,672 patients, with over one-half of the studies including more than 100 patients. Of the 92 studies, 28 [30%] analyzed a full cohort, and the other studies included 1 or 2 subgroups of critically ill patients with COVID-19. The definition of what constituted critical illness varied in the studies, characterized as patients admitted to the ICU (23 studies [25%]),4 , 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41 severe COVID-19 defined by Chinese guidelines (25 studies [27%])42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66 or American Thoracic Society guidelines (3 studies [3%]),67, 68, 69 patients with organ injury or dysfunction (23 studies [25%]),70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92 and those who died of COVID-19 (18 studies [20%]).5 , 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109
Table 1.
Characteristics of the 92 Included Studiesa
| Characteristic | No. (%) of studiesb |
|---|---|
| Journal impact factor | |
| >50 | 19 (21) |
| 20-50 | 8 (9) |
| 5-20 | 65 (71) |
| Article type | |
| Original article | 54 (59) |
| Research letter | 35 (38) |
| Other (brief report or communication) | 3 (3) |
| Study design | |
| Retrospective observational | 83 (90) |
| RCT | 5 (5) |
| Prospective observational | 4 (4) |
| Study center | |
| Single-center | 51 (55) |
| Multicenter | 39 (42) |
| Unknown | 2 (2) |
| Geographic area | |
| Asia | 61 (66) |
| Europe | 16 (17) |
| North/South America | 10 (11) |
| Multicountry | 5 (5) |
| Sample size | |
| 20-100 | 36 (39) |
| 100-1000 | 45 (49) |
| >1000 | 11 (12) |
| Critically ill population | |
| Subgroup | 64 (70) |
| Full cohort | 28 (30) |
| Critical illness categories | |
| Patients admitted to the ICU | 23 (25) |
| Severe (Chinese guidelines) | 25 (27) |
| Severe (American guidelines) | 3 (3) |
| Organ injury or dysfunction | 23 (25) |
| Patients who died of COVID-19 | 18 (20) |
| Research domain | |
| Clinical characteristics | 52 (57) |
| Risk factors | 29 (32) |
| Treatment | 11 (12) |
COVID-19, coronavirus disease 2019; ICU, intensive care unit; RCT, randomized clinical trial.
Percentages may not total 100 because of rounding.
Clinical Characteristics and Outcomes of Critically Ill Patients With COVID-19
The main clinical characteristics and outcomes from 52 studies of critically ill patients with COVID-19 are summarized in Table 2 (not including laboratory findings). Among those who died, it appears that those in European countries95 , 103 were older than those in Asian countries.98 , 99 , 101 , 102 , 104 , 105 Generally, the median/mean age of nonsurvivors was higher compared with survivors in Europe. The fatality rate of male patients was significantly higher than that of females in all studies. Males were more likely to have organ injuries, experience progression to severe status, and be admitted to the ICU in almost all the studies. Two US studies reported that race/ethnicity did not significantly differ between nonsevere and severe COVID-19 groups.76 , 88 Studies found that 21% to 84% of critically ill patients had development of neurologic symptoms including central nervous system symptoms.23 , 38 , 69 This population often had comorbidities, with 38% to 90% having at least one coexisting condition. The prevalence of chronic obstructive pulmonary disease (20% to 21%95 , 103 vs 2% to 5%99 , 102 , 104), diabetes (47% to 65%95 , 103 vs 18% to 35%98, 99, 100, 101, 102 , 104), chronic kidney disease (30% to 53%95 , 103 vs 3% to 18%98 , 99 , 101 , 102 , 104), cancer (15% to 37%95 , 103 vs 2% to 7%98 , 100, 101, 102 , 104), smoking (34%95 , 103 vs 8%98) among patients who died in European countries or the United States appears to be higher than in Asian countries. Obesity76 , 95 and obstructive sleep apnea,24 , 32 , 103 which were seldom reported in Asian countries, are common among critically ill patients infected with SARS-CoV-2 in Western countries.
Table 2.
Clinical Characteristics, Interventions, and Outcomes of Critically Ill Patients With COVID-19
| Characteristics | Asian (30 studies) | European and American (21 studies) |
|---|---|---|
| Age range (y), median | ||
| Overall | 49-714,26,27,29, 30, 31, 32, 33,42,44, 45, 46, 47, 48, 49, 50, 51,53,64,66,67,69,74,77 | 58-7022, 23, 24, 25,32,35,37,38,40,41,76,82,86,88,90 |
| Fatal cases | 66-7398,99,101,102,104,105 | 72 (Italy)95; 77 (UK)103 |
| Male | ||
| Overall | 46%-91%4,26,27,29, 30, 31,42,44, 45, 46, 47, 48, 49, 50, 51,53,64,66,67,69,74,77 | 52%-90%22, 23, 24, 25,32,35,37,40,41,75,88,90 |
| Fatal cases | 64%-85%96,98, 99, 100, 101, 102,104,105 | 90% (Italy)95; 60% (UK)103 |
| Symptoms | ||
| Common | Fever, 46%-100%; cough, 22%-85%; dyspnea, 30%-100%; fatigue, 10%-100%; myalgia, 2%-50%; sputum, 20%-61%; diarrhea, 6%-31%4,26,27,29, 30, 31,44,46,48,50,51,53,64,66,67,69,77,98,99,101,102,104 | Fever, 35%-100%; cough, 30%-88%; dyspnea/SOB, 24%-91%; fatigue, 19%-67%; myalgia, 5%-55%; sputum, 42%; diarrhea, 12%-28%24,25,32,40,75,76,88,103 |
| Neurologic | 46% (China)69 | 84% (France)38; 21% (Turkey)23 |
| Comorbidities | ||
| Overall | Any: 38%-85%4,26,27,29, 30, 31,46,50,51,53,67,69 Respiratory, 1%-8%29,30 (COPD, 8%4,27); diabetes, 8%-27%4,26,27,29,30; hypertension, 15%-65%4,26,27,30; cardiovascular, 10%-32%4,26,27,29,30; neurologic, 14%-17%27,29; CKD, 4%-6%26,27,30; cancer, 4%-11%27,29; smoking, 4%-50%29, 30, 31 |
Any: 68%-86%22,24,75 Respiratory, 7%-18%25,32,35,40 (COPD, 4%-33%22,24); diabetes, 17%-58%22,24,25,32,35,40,41; hypertension, 33%-51%22,25,40,41; cardiovascular, 4%-48%22,25,35,40; CKD, 3%-48%22,24,25,32,35,40; cancer, 5%-8%22,25,35,40; smoking, 22%-34%22,32; obesity, 43%76; OSA, 21%-29%24,32 |
| Fatal cases | Any: 63%-77%98,99,101,102,104,105; COPD, 2%-5%99,102,104; diabetes, 18%-35%98, 99, 100, 101, 102,104; hypertension, 38%-65%98, 99, 100, 101, 102,104,105; cardiovascular, 12%-37%98, 99, 100, 101, 102,104,105; neurologic, 4%-18%98,99,101,102,104; CKD, 3%-18%98,99,101,102,104; cancer, 2%-7%98,100, 101, 102,104; smoking, 8%98 |
Up to 90%95,103: COPD, 20%-21%; diabetes, 47%-65%; hypertension, 55%-68%; cardiovascular, up to 90%; neurologic, 15%; CKD, 30%-53%; cancer, 15%-37%; smoking, 34%; obesity, 28%; OSA, 10%103 |
| Chest imaging | ||
| Bilateral infiltrates | 100% (x-ray31,98); 82%-100% (CT4,27,46,51,67,99,102) | 50%-100% (x-ray25,32,40,76,103) |
| Severity on ICU admission | ||
| APACHE II score | 11-1727,29,30 | 1837 |
| SOFA score | 2-527,30,31 | 835,37 |
| Time from symptom onset (d), median/mean | ||
| To hospital admission | 4-104,26,27,31,48,51,98,99,102,105 | 4-824,32,37,40,76 |
| To ICU admission | 6-1027,29,31 | 323 |
| Complications | ||
| ARDS | 58%-100% (overall)4,27,29, 30, 31,74; 74%-100% (fatal cases)98,99,101,102,104,105 | 75%-100% (overall)24,32,35,38,40 |
| Acute cardiac injury | 8%-31% (overall)4,27,29,46,50; 42%-77% (fatal cases)98,99,101,102,105 | 8% (patients receiving IMV)76; cardiomyopathy in 33% (overall)24 |
| Acute kidney injury | 6%-37% (overall)4,27,29,46,50,74; 25%-88% (fatal cases)98,99,101,105 | 19% with acute kidney failure (overall)24; 68%-84% (fatal cases)108 |
| Acute liver injury | 6%-29% (overall)29,46,50,74; 9%-77% (fatal cases)98,101,102,105 | 14% (overall)24; 7%-19% (fatal cases)108 |
| Pulmonary embolism | NA | 17%-21% (overall) (France)35,39 |
| Sepsis | 33%-100% (fatal cases)98,102 | NA |
| Shock | 23%-31% (overall)4,27; 41%-81% (fatal cases)98,101,102 | NA |
| Secondary infection | Bacterial: 2%-35% (overall)4,29,46,50,51,64; 16%-82% (fatal cases)99,101,104,105 Viral: 11% (fatal cases)99 |
Bacterial: 5%-12% (overall)24,76 Viral: 14% (overall)24 |
| Intervention | ||
| HFNC | 10%-68%27,29, 30, 31,50,51,98,104 | 2%-15% (US)24,25,40,76,88; 42% (early stage in the US)32 |
| NIMV | 11%-72% (overall)4,27,29,30,50,51,67,98,101,102,104,105 | 0%-30% (overall)22, 23, 24, 25,32,37,40,76,103 |
| IMV | 15%-47% (ICU patients)4,27,29,30; 15%-84% (fatal cases)98,99,101,102,104,105 | 70%-100% (ICU patients)22, 23, 24, 25,32,35,37,40; 15%-51% (fatal cases)103,108 |
| ECMO | 1%-15%4,27,29, 30, 31,46,50,51,66,67,98,99,101,104,105 | 0%-8%22,32,35,40,88 |
| Prone ventilation | 4%-12%29,104 | 27%-47%22,24,32,40 |
| Vasopressor | 35%-95%29,31 | 67%-95%24,32,37,40,76 |
| CRRT | 3%-30%4,27,29,31,50,67,98,102,104,105 | 13%-35%35,37,40,76,108 |
| Clinical outcomes | ||
| Hospital LOS (d), median range | 10-24 (overall)42,48,51,64,67; 5-14 (fatal cases)98,99,101,104 | 12-22 (overall)32,37,82; 3-8 (fatal cases)103,108 |
| ICU LOS (d), median range | 7-948 | 9-1022,32,35,37; 18 (patients receiving IMV)40 |
| Mortality | 8%-83% for severe disease4,30,42,44,46, 47, 48,50,51,64,67,74,77 | 9%-52% (overall)22,24,25,35,37,40,41; 88% (patients receiving IMV)108 |
APACHE, Acute Physiology and Chronic Health Evaluation; ARDS, acute respiratory distress syndrome; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; CRRT, continuous renal replacement therapy; CT, computed tomography; ECMO, extracorporeal membrane oxygenation; HFNC, high-flow nasal cannula oxygen therapy; ICU, intensive care unit; IMV, invasive mechanical ventilation; LOS, length of stay; NA, not applicable; NIMV, noninvasive mechanical ventilation; OSA, obstructive sleep apnea; SOB, short of breath; SOFA, Sequential Organ Failure Assessment; US, United States.
Most critically ill patients had abnormalities on chest radiography or computed tomography (CT), characterized by bilateral infiltrates and multiple ground-glass opacities. Computed tomography was frequently performed for most patients with COVID-19 in China (89%),45 , 67 and 82% to 100% of severe COVID-19 cases had evidence of bilateral infiltrate pneumonia on chest CT.4 , 27 , 46 , 51 , 67 , 99 , 102 In contrast, the United States and European countries did not perform CT initially during diagnosis but preferred chest radiography instead, with 50% to 100% of critical patients having radiographic evidence of bilateral infiltrates.25 , 32 , 40 , 76 , 103 Xu et al45 found that critically ill patients with COVID-19 more commonly had involvement of 4 to 5 lobes and particularly bilateral lower and upper lobes on CT compared with the general population, with features of mostly patchy ground-glass opacities in the peripheral areas under the pleura with partial consolidation.
Patients with severe COVID-19 had more prominent laboratory abnormalities, including lymphocytopenia and leukopenia,67 and exhibited a significant decrease of T lymphocyte subset counts (CD3+, CD4+, CD8+ T-cell markers), which was associated with a higher risk of mortality.64 Evidence of inflammatory cytokine abnormalities (tumor necrosis factor α, interleukin [IL] 1, and IL-6) was notable,53 with increased IL-6 recorded in 87% of severe cases.61 The establishment of an inflammatory state as evidenced by elevated C-reactive protein (CRP) levels was also noted in 76% to 100% of critically ill patients,59 , 61 , 62 , 68 , 89 , 109 and the median concentration of CRP was nearly 10-fold higher than in mildly ill patients.64 Procalcitonin elevation indicating an inflammatory response was observed in 14% to 47% of patients with severe disease,59 , 61 , 62 , 67 75% of ICU patients,27 and 34% to 100% of nonsurvivors20 , 93 , 101 in Chinese studies but in only 25% of patients receiving invasive mechanical ventilation (IMV) in the United States.76 Dramatic elevation of D-dimer values was found in 50% to 87% of critically ill patients in China,20 , 59 , 67 , 68 , 109 43% in the United States,76 and more than 95% in France.35
SARS-CoV-2 infection can cause systemic inflammation, characterized as multiple organ dysfunction and immune dysfunction in critically ill patients.51 Common complications observed in all severe COVID-19 cases included acute respiratory distress syndrome (ARDS), acute cardiac injury, acute kidney injury, acute liver injury, sepsis, and shock. The main cause of death was respiratory failure.43 , 92 , 104 , 107 The median time from the first symptoms to ARDS was 7 to 9 days as reported in China.27 , 30 , 99 , 102 Ruan et al104 first reported that the infection of SARS-CoV-2 might cause fulminant myocarditis. A high rate of cardiomyopathy (33%) and congestive heart failure (43%) was also observed as a cardiac complication of SARS-CoV-2 infection among patients with critical illness in the United States.24 A Portuguese study reported that liver injury was prevalent in ICU patients but was generally transient and nonsevere.37
Notably, pulmonary embolism (PE) was reported in 17% to 21% of ICU patients in France.35 , 39 Recently, several studies in Europe have raised awareness of the increased incidence of COVID-19–related life-threatening thrombotic complications in critically ill patients (up to 43%) despite prophylactic anticoagulation, which was seldom reported previously in Asia.35 , 39 , 41 , 71 , 82 , 90 Patients in Europe and the United States had higher Acute Physiology and Chronic Health Evaluation (APACHE) II scores and Sequential Organ Failure Assessment (SOFA) scores on ICU admission35 , 37 compared with China and South Korea.27 , 29, 30, 31 The APACHE II score was found to be independently associated with hospital mortality and performed better to predict mortality in patients with COVID-19 than the SOFA score, and an APACHE II score of 17 or greater served as an early warning indicator of death.20 The CRB-65 (confusion, respiratory rate, blood pressure, age 65 years and older) score was considered better to identify patients with COVID-19 at risk for intensive respiratory or vasopressor support than the quick sepsis-related organ failure assessment score.78
It appeared that patients in the United States and Europe had a longer stay in the ICU than patients in China (9 to 18 days22 , 32 , 35 , 37 , 40 vs 7 to 9 days48). There is wide variability in case-fatality ratios among critically ill patients, partially because of differences in length of follow-up. For example, in 2 studies, the reported mortality was 7% and 8%, with 89% still hospitalized at the time of data analysis.44 , 67 Feng et al51 reported the mortality of critical cases was 41%, with 19% still in the hospital. A very high mortality rate (50% to 67%) was reported in mechanically ventilated patients with COVID-19 in both China and the United States,24 , 25 , 29 , 84 reaching up to 88% among patients receiving IMV during the initial pandemic in the United States.108
Treatment for Critically Ill Patients With COVID-19
High-flow nasal cannula oxygen therapy (HFNC) and noninvasive mechanical ventilation (NIMV) were used frequently for patients in China, particularly with limited resources early27 , 29, 30, 31 , 50 , 51 , 98 , 104 and also during the initial stages of the pandemic in the United States.32 Generally, however, several US institutions adopted an early intubation strategy with limited use of HFNC or NIMV for respiratory failure,40 , 76 which may have resulted in more critically ill patients receiving IMV in the United States (71% to 100%)24 , 25 , 32 , 40 than in China (15% to 47%).4 , 27 , 29 , 30 The use of prone ventilation was reported in ICU patients with COVID-19 in China, Italy, and the United States.22 , 24 , 29 , 32 , 40 , 104 Treatment with extracorporeal membrane oxygenation was used among 5% to 15% of ICU patients with COVID-194 , 27 , 29 , 30 , 35 , 40 and among 1% to 12% of patients who died of COVID-19.5 , 98 , 99 , 101 , 104 , 105 , 109 Up to 95% of mechanically ventilated patients in the United States, South Korea, and Portugal needed vasopressor support,31 , 37 , 40 , 76 and up to 30% of ICU patients across the world received continuous renal replacement therapy.29 , 31 , 35 , 40 Notably, a French study found that a high incidence of circuit clotting (97%) occurred among patients with COVID-19–induced ARDS who received continuous renal replacement therapy35 (Table 2).
Characteristics of pharmacological treatment for severe COVID-19 are displayed in Table 3 . Antivirals were provided to 44% to 100% of critically ill patients in Asia and Europe.4 , 27 , 29, 30, 31 , 35 , 46 , 48 , 50 , 51 , 66 , 67 , 75 , 77 , 98 , 99 , 101 , 104 An early RCT found no benefit from lopinavir-ritonavir treatment for clinical improvement or reducing mortality.87 Three studies have focused on the efficacy and safety of remdesivir therapy among severely ill patients with COVID-19.58 , 79 , 83 One early RCT in China found that remdesivir did not provide significant clinical or antiviral effects.58 Recently, however, preliminary results of an ongoing multinational RCT showed that the 10-day course of remdesivir was superior to placebo in shortening the median time to recovery (11 vs 15 days) while adverse events did not increase.83 A multinational prospective observation study also reported that 68% of patients with severe COVID-19 treated with remdesivir had clinical improvement in oxygen support status.79 Of note, the United States did not rely on the antiviral agent in the initial phases of the pandemic (only in 4% to 29% of critical cases40 , 76 , 88), preferring hydroxychloroquine in approximately 90% of critically ill patients with COVID-19.40 , 88 Two observational studies involving large samples in the United States found that treatment with hydroxychloroquine was not associated with reduced in-hospital mortality or intubation.80 , 92 Furthermore, adverse events among patients receiving hydroxychloroquine were common, including arrhythmia, QT prolongation, and even cardiac arrest. It was reported that QTc intervals increased by 93% in ICU patients after hydroxychloroquine therapy.21 The phase IIb RCT of chloroquine diphosphate in severe COVID-19 in Brazil found no improved mortality outcomes from chloroquine.33
Table 3.
Pharmacological Treatment for Critically Ill Patients With COVID-19
| Category | Study design | Conclusion |
|---|---|---|
| Antiviral | Observational | 44%-100% in Asia4,27,29, 30, 31,46,48,50,51,66,67,77,98,99,101,104; 4%-29% in the US40,76,88; 65%-100% in Europe35,75 |
| Lopinavir-ritonavir | RCT | No benefit of lopinavir-ritonavir treatment in clinical improvement or reducing 28-day mortality87 |
| Remdesivir | 2 RCTs; 1 prospective observational study | Remdesivir did not provide significant clinical or antiviral effects in severe COVID-1958 Remdesivir was superior to placebo in shortening the median time to recovery83 68% of severe COVID-19 patients treated with compassionate-use remdesivir had clinical improvement in oxygen support status79 |
| Hydroxychloroquine | Observational | 89%-91% in the US40,88; 33% in France35 Treatment with hydroxychloroquine was not associated with significantly reduced in-hospital mortality or intubation. Adverse events: abnormal ECG findings (27%), particularly arrhythmia (16%) and QT prolongation (14%)80,92 |
| Chloroquine diphosphate | RCT | No apparent benefit of chloroquine was seen regarding lethality; the high dosage of chloroquine (600 mg twice daily for 10 days) should not be recommended for critically ill patients because of its association with more toxic effects and higher mortality33 |
| Antibacterial | Observational | 43%-100% in China4,5,20,29,48,50,51,54,64,66,67,72,74,77,84,87,98,99,101,102,104,109; 75%-98% in the US40,80; 88%-100% in Europe37,75 |
| Corticosteroid | Observational | 33%-100% in Asia4,27,29, 30, 31,46,48,50,51,64,66,67,73,74,77,84,98,99,101,102,104,105; 8%-25% in the US25,40,76 |
| Immunoglobulin | Observational | 14%-76% in Asia20,29, 30, 31,46,48,50,64,66,67,74,98,101,102 None reported in Europe or America Early treatment with immunoglobulin reduced hospital and ICU LOS and 28-day mortality34 |
| Interferon | Observational | 22%-63% in Asia30,48,58,89,98,102 |
COVID-19, coronavirus disease 2019; ECG, electrocardiographic; ICU, intensive care unit; LOS, length of stay; RCT, randomized clinical trial; US, United States.
Despite no concurrent observed bacterial infection, antibacterial therapy was widely used among patients with severe COVID-19 in China (43% to 100%),4 , 5 , 20 , 29 , 48 , 50 , 51 , 54 , 64 , 66 , 67 , 72 , 74 , 77 , 84 , 87 , 98 , 99 , 101 , 102 , 104 , 109 in the United States (75% to 98%),40 , 80 and also in Europe (88% to 100%).37 , 75 Currently, glucocorticoids have been used empirically in 33% to 100% of cases of severe COVID-19 in Asia.4 , 27 , 29, 30, 31 , 46 , 48 , 50 , 51 , 64 , 66 , 67 , 74 , 77 , 98 , 99 , 101 , 102 , 104 , 105 It appeared that US hospitals were less likely to use corticosteroids, even in patients receiving IMV.25 , 40 , 76 Studies reported that methylprednisolone treatment might be beneficial for COVID-19 patients with ARDS by reducing the risk of death,84 and low-dose methylprednisolone in severe cases did not delay viral clearance.57 Immunoglobulin is also commonly applied as an adjuvant treatment in up to 75% of critically ill cases in Asian countries.29, 30, 31 , 46 , 48 , 50 , 64 , 66 , 67 , 74 , 98 , 101 , 102 Early treatment with immunoglobulin reduced hospital duration, ICU LOS, and 28-day mortality.34 Other therapeutic options under investigation include the use of interferon30 , 58 , 89 , 98 , 102 and selective cytokine blockade such as tocilizumab and antioxidant.80 , 84
Risk Factor Analysis
Of the 92 studies included, 31 explored risk factors related to clinical prognosis using multivariate methods, mainly with logistic regression models (42%) and Cox proportional hazards models (35%). In total, over 50 risk factors were found to be associated with disease severity or worse outcomes, including factors related to demographic characteristics, symptoms, comorbidities, complications, laboratory results, chest imaging, severity scores assessment, and treatment (Table 4 ). Among these studies, advanced age (defined as >65 years in most studies) was the most frequently identified independent factor (n=18), followed by elevated lactate dehydrogenase (n=6), male sex (n=5), hypoxia (n=5), lymphopenia (n=5) and presence of any comorbidity (n=4), hypertension (n=4), and increased CRP (n=4). Furthermore, antiviral treatment (lopinavir-ritonavir, 200/50 mg, plus hydroxychloroquine, 200 mg, twice a day) was found to be one of the predictors for lower odds of discharge from the hospital for patients with COVID-19 in an Italian study.75 Moreover, 6 clinical or laboratory models were developed to predict COVID-19 progression or in-hospital mortality in China, and some of them were validated in patients from centers (Table 4).55 , 65 , 68 , 81 , 97
Table 4.
Risk Factor Identification With Multivariate Analysis (31 Studies)a
| Risk factor | Associated with: | No. of studies and references |
|---|---|---|
| Demographic-related | ||
| Advanced age | Death; disease progression; unfavorable outcomesb; development of ARDS; myocardial injury; lower odds of hospital discharge | 185,28,43,54,55,59,63,65,68,72,75,81,84,93,94,97,99,109 |
| Male | Death; disease progression; development of severe respiratory failure; disease refractoriness | 555,63,85,89,99 |
| Symptom-related | ||
| Dyspnea | Death; development of critical illness | 368,72,109 |
| Hemoptysis | Development of critical illness | 168 |
| Unconsciousness | Development of critical illness | 168 |
| Anorexia on admission | Disease refractoriness | 189 |
| Fever on admission | A protective factor for disease refractoriness | 189 |
| Hypoxia | Death; disease progression; SpO2-FiO2 ratio negatively correlated with the incidence of ARDS | 528,61,72,93,97 |
| Comorbidity-related | ||
| Presence of any comorbidity (not specific) | Death; disease progression | 428,54,63,81 |
| No. of comorbidities | Development of critical illness | 168 |
| Hypertension | Death; disease progression; myocardial injury | 443,55,60,97 |
| Diabetes | Unfavorable outcomes | 159 |
| Cardiovascular disease (not specific) | Death | 1106 |
| Coronary heart disease | Death; myocardial injury | 394,97,109 |
| Chronic heart failure | Death; myocardial injury | 243,94 |
| Cardiac arrhythmia | Death | 194 |
| Cerebrovascular disease | Death | 1109 |
| COPD | Death; myocardial injury | 343,94,106 |
| Chronic renal failure | Myocardial injury | 143 |
| Cancer | Development of critical illness | 168 |
| Smoking | Death; unfavorable outcomes | 259,94 |
| Complication-related | ||
| ARDS | Death | 1106 |
| PE | Invasive mechanical ventilation | 190 |
| Organ dysfunction (not specific) | Death; development of ARDS | 284,93 |
| Critical disease status | Unfavorable outcomes | 159 |
| Laboratory-related | ||
| Leukocytosis | Unfavorable outcomes | 159 |
| Lymphopenia | Death; disease progression; higher lymphocyte count associated with decreasing mortality | 528,60,62,81,93 |
| Decreased CD4 cell count | Disease progression | 161 |
| Neutrophilia | Death; development of ARDS; unfavorable outcomes | 359,84,97 |
| Neutrophil- lymphocyte ratio | Disease progression | 263,68 |
| Elevated CRP | Death; development of critical illness; myocardial injury | 443,60,65,97 |
| Elevated procalcitonin | Death | 1109 |
| Elevated IL-6 | Disease progression | 252,56 |
| Elevated AST | Death | 297,109 |
| Direct bilirubin | Development of critical illness | 265,68 |
| Elevated troponin | Death; cardiac ejection fraction (TTE); unfavorable outcomes | 343,59,70 |
| Elevated CK-MB | Death | 143 |
| Elevated LDH | Death; development of critical illness | 665,68,75,81,93,97 |
| Elevated D-dimer | Death; development of ARDS | 35,84,97 |
| Serum amyloid A | Disease progression | 162 |
| suPAR | Development of severe respiratory failure | 185 |
| Detectable serum SARS-CoV-2 viral load | Poor prognosis | 152 |
| GFR | Death; log hs-TnT was independently associated with estimated GFR | 270,97 |
| Higher BUN | Disease progression | 165 |
| Lower albumin | Disease progression | 165 |
| RDW | Disease progression | 165 |
| Imaging-related | ||
| Chest radiographic abnormality | Development of critical illness | 168 |
| Visual or software quantification of extent of CT lung abnormality | Predictors of ICU admission or death: %V-WAL <73%; %S-WAL <71%; VOLWAL <2.9 L | 191 |
| Severity score–related | ||
| High SOFA score | Death | 25,20 |
| High APACHE II score | Death | 120 |
| Treatment-related | ||
| ACEI/ARB | Log hs-TnT independently associated with the use of ACEI/ARB | 170 |
| Antiviral treatment | Lower odds of hospital discharge | 175 |
| Early time from illness onset to antiviral treatment | Improvement of prognosis | 154 |
| Administration of hypnotics | Protective effects on patient outcomes | 159 |
| Predictive models | ||
| Host risk score | Severe COVID-19: older age, male, presence of hypertension | 155 |
| Risk nomogram | Severe COVID-19: older age, higher LDH, CRP, RDW, DBIL, and BUN, and lower ALB | 165 |
| Clinical risk score (Web-based calculator) | Development of critical illness: chest radiographic abnormality, age, hemoptysis, dyspnea, unconsciousness, number of comorbidities, cancer history, neutrophil- lymphocyte ratio, lactate dehydrogenase, and direct bilirubin | 168 |
| CALL score | Disease progression: older age, comorbidity, lymphopenia, and increased LDH (>500 U/L)c | 181 |
| Clinical predictors | In-hospital mortality: age, history of hypertension and coronary heart disease | 197 |
| Laboratory predictors | In-hospital mortality: age, hs-CRP, SpO2, neutrophil and lymphocyte count, D-dimer, AST and GFR | 197 |
ACEI, angiotensin-converting enzyme inhibitor; ALB, albumin; APACHE, Acute Physiology and Chronic Health Evaluation; ARB, angiotensin receptor blocker; ARDS, acute respiratory distress syndrome; AST, aspartate aminotransferase; BUN, serum urea nitrogen; CALL, comorbidity, age, lymphocytes, and lactate dehydrogenase; CK-MB, creatine kinase myocardial band; COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; CT, computed tomography; DBIL, direct bilirubin; FIO2, fraction of inspired oxygen; GFR, glomerular filtration rate; hs-CRP, high-sensitivity CRP; hs-TnT, high-sensitivity troponin T; ICU, intensive care unit; IL-6, interleukin 6; LDH, lactate dehydrogenase; PE, pulmonary embolism; RDW, coefficient of variation of red blood cell distribution width; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SOFA, Sequential Organ Failure Assessment; SpO2, peripheral capillary oxygen saturation; suPAR, soluble urokinase plasminogen activator receptor; %S-WAL, software-based assessment of well-aerated lung percentage; TTE, transthoracic echocardiogram; VOLWAL, open-source software assessment of well-aerated lung absolute volume; %V-WAL, visual assessment of well-aerated lung percentage.
Unfavorable clinical outcome included death, progression, and/or maintenance of severity status.
SI conversion factor: To convert LDH value to μkat/L, multiply by 0.0167.
Discussion
In this scoping review, we synthesized the emerging global evidence on critically ill patients with COVID-19, specifically clinical characteristics, treatment, and risk factors associated with poor prognosis. This review aimed to provide insights for effective management of severe COVID-19 and identify gaps in knowledge to guide future research. Some clinical findings were similar for all regions—for example, the frequency of ARDS. However, some of the findings differed by region, likely due to different patient population characteristics, medical resources, and management strategies. These comparative analyses are helpful for frontline health care professionals to guide our understanding of the disease and what patient and population characteristics may be more influential in determining clinical outcomes, thereby guiding decision making about care intensity.
Globally, older patients are more likely to have progression to critical illness, which may be associated with a higher frequency of comorbidities or age-related immune dysfunction resulting from low-grade chronic inflammation.27 Most studies found that male patients were more susceptible to development of critical illness or death than females, potentially because of the protection of the X chromosome and sex hormones, which play an essential role in innate and adaptive immunity.110
In our scoping review, 2 studies that documented race and ethnicity in the United States reported that race/ethnicity did not relate to the severity of COVID-19.76 , 88 Of note, outside the United States, how race and ethnicity are classified differs from the US approach, making assumptions from the demographic data included in our scoping review challenging and inconclusive. In recent months, however, notable disparities in outcomes for racial and ethnic minorities in the United States and the United Kingdom have become apparent when the information has been accurately collected.111, 112, 113
Clinical manifestations are similar in all countries. Frontline clinicians should be aware of the possible neurologic involvement of SARS-CoV-2 in critically ill patients23 , 38 , 69 and some atypical extrapulmonary symptoms such as gastrointestinal manifestations, which may lead to diagnostic delay.48 Of note, only 74% of patients with refractory disease presented with fever on admission (significantly lower than general patients), and fever was found to be a protective factor for recovery, suggesting that patients with a slow or meager response to the virus were more likely to have severe disease.89 Elevated lactate dehydrogenase levels and lymphopenia were frequently identified as markers of severity. A meta-analysis study supported these findings.13 These similar patterns of age or sex differences in COVID-19 severity or mortality and similar clinical manifestations in various countries allow us to map the global epidemiology of the disease.
Notably, there is variation in coexisting chronic medical conditions geographically, indicating that prediction models from Asia may not be reliable in the United States and Europe where the impact of other risk factors such as obesity on disease severity should be thoroughly explored. For example, when compared with multiple Asian studies, European and US populations receiving IMV were more likely to have obesity,76 a risk factor for worse prognosis in patients with COVID-19.114 Given the prevalence of obesity and the association between obesity and decreased pulmonary function, these findings are not surprising.
The presence of hypertension is thought to be an independent risk factor for poor prognosis.43 , 55 , 60 , 97 It is unclear if this association is related to the pathogenesis of hypertension or to antihypertensive treatment. Angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs) were identified as being associated with cardiac injury,70 but some other studies found that long-term ACEI/ARB use was not associated with worse outcomes.115 , 116 These associations should be considered with extreme caution because the studies’ results have not been adjusted for age, comorbidities, or other confounding variables. Currently, clinical data support continuing to follow existing professional society guidelines and not discontinuing ACEIs/ARBs in the setting of the COVID-19 pandemic.42 , 117 , 118
Using the markers for early identification of severe COVID-19 may help health care professionals prioritize the need to provide aggressive therapeutics and guide clinical management. This issue is critically important for risk stratification for optimal resource allocation, particularly in settings with limited critical care resources. Remarkably, several studies evaluating risk factors used univariate analysis instead of multivariate analysis, and thus the conclusions should be interpreted with caution. A systematic review on risk factors for severe COVID-19 was conducted, but all the included studies were retrospective studies from China and thus the findings cannot necessarily be applied to patients worldwide.13 Unlike that work,13 this scoping review includes high-quality studies that evaluated potential risk factors of illness severity or poor prognosis in multivariate models.
The higher incidence of PE in critically ill patients with COVID-19 in European countries may be associated with the prevalence of obesity in the patient group.39 Increased D-dimer suggesting hypercoagulability is a nonspecific biomarker in patients with COVID-19. In patients who have limited disease but require supplementary oxygen, an additional contrast-enhanced CT should be performed to rule out PE per the European Society of Radiology and European Society of Thoracic Imaging guidelines.119 Alternatively, if the severity of respiratory failure cannot be explained by the current unenhanced CT findings, CT pulmonary angiography should be considered (European Society of Cardiology).120 Clinicians should recognize that those with COVID-19 are a high-risk population and therefore consideration of early thromboprophylaxis is essential.35
The comparisons of clinical outcomes should be interpreted with caution because criteria for hospitalization, ICU admission, discharge, and health care infrastructures are likely to be different between countries. The case to fatality ratios of severe COVID-19 vary across regions and in different time points, mainly because of differences in testing approaches and capacity (in some regions, patients with mild disease were not tested, resulting in a higher case-fatality ratio). Other contributors to outcome differences include characteristics of the health care system infrastructure (mortality rates were higher in health systems with fewer medical resources and limited access to critical care resources), the impact of policies changing over time (implementation of physical distancing measures and testing speed), accuracy in reporting total cases, and mechanisms for representing mortality from COVID-19 (in data that calculated the mortality for mechanically ventilated patients from New York, the denominators excluded people who were still in the ICU on a ventilator108).
We should note that during the early outbreaks in some regions such as Wuhan and New York, inadequate capacity in the health system may have contributed to the high rate of mortality of mechanically ventilated patients. In South Korea where medical resources were adequate with a small outbreak, it was observed that 30-day mortality in mechanically ventilated patients with COVID-19 was 25%, similar to those with seasonal influenza–related respiratory failure.31 Additionally, patients in the United States and Europe appeared to be older, and sicker as indicated by higher APACHE II and SOFA scores, and had longer ICU LOS than Asian patients, and these factors may have contributed to the higher mortality.
Management of acute respiratory failure is key to the treatment of seriously ill patients. Studies from China revealed that for patients with ARDS or extensive pulmonary effusion on CT, HFNC or NIMV was recommended to maintain positive end-expiratory pressure and prevent alveolar collapse even among patients who did not have refractory hypoxemia.121 As recent data from the international VIRUS COVID-19 Registry indicates,122 neither HFNC nor NIMV (15% and 11% of hospitalized patients, respectively) were adopted frequently in the other 17 countries. Currently, however, there is still no evidence that patients would benefit more from NIMV or an early intubation strategy. Experience from both China and the United States has revealed that prone positioning improved oxygenation and pulmonary heterogeneity for COVID-19–induced respiratory failure compared with the supine position.40 , 121
Clinical trials of antiviral agents evaluating efficacy and safety are ongoing. The very first RCT of remdesivir did not find any significant benefit.58 However, this trial was terminated early and thus was statistically underpowered. Recent evidence from multicountry trials has shown the efficacy of remdesivir for shortening the time to recovery and improving oxygen support status.79 , 83 The US Food and Drug Administration has issued an emergency use authorization (EUA) for remdesivir to treat COVID-19 in adults and children hospitalized with severe disease.123 On March 30, 2020, when clinical trials were unavailable, the Food and Drug Administration issued an EUA for chloroquine and hydroxychloroquine in hospitalized patients. However, due to the lack of evidence of clinical efficacy combined with serious toxic adverse effects in RCTs,33 , 124 this EUA was revoked on June 15, 2020.
The use of corticosteroids to reduce possible immune-mediated organ damage remains controversial because higher doses were associated with a higher subsequent plasma viral load in patients with severe acute respiratory syndrome125 Although observational studies in China revealed potential benefits of corticosteroids for patients with COVID-19,57 , 84 they must be carefully balanced against the risks. The United States and Europe did not adopt corticosteroids as often as China. Fortunately, a preliminary, unpublished analysis from a multicenter RCT in the United Kingdom revealed that dexamethasone (6 mg once per day) reduced mortality by up to one-third in hospitalized patients with severe respiratory complications of COVID-19.126
Globally, very few studies mentioned a specific indication for the use of antibiotics, and rates of secondary bacterial infections reported in severe COVID-19 appear to be low.4 , 24 , 29 , 46 , 50 , 51 , 64 , 76 However, the use of broad-spectrum antimicrobial therapy was widely reported. The frequent prescription of empirical antimicrobials in patients with COVID-19 is worrisome because of potential increasing bacterial resistance. Additionally, there were no differences in mortality among those who did or did not receive antimicrobial drugs.101
Study Strengths and Limitations
To our knowledge, this is the first scoping review focusing on critically ill patients with COVID-19 mapping the clinical characteristics, treatment, and risk factors associated with prognosis. At the beginning of the study, we consulted with a specialist librarian and confirmed that the World Health Organization and LitCovid databases on COVID-19 were appropriate to capture the breadth of research on this novel topic. Furthermore, we focused on journals with an IF of 5 or higher so we could quickly review and synthesize high-quality studies.
This review also has some limitations. First, it is possible that the use of additional literature databases, the involvement of journals with IFs of 5 or lower, and articles published in non-English sources would have yielded relevant articles, giving us a more comprehensive understanding of critically ill patients with COVID-19. Second, a majority of patients with COVID-19 were still hospitalized at the end point of studies, potentially causing inaccurate clinical outcomes and underestimating mortality. Third, many of the included studies were observational in nature, which may limit inferences that can be drawn from their findings. Fourth, disparities in outcomes for racial and ethnic minorities were not apparent based on our work, although this issue has become a fundamental aspect of critical illness outcomes in the United States.127 , 128 Fifth, the vast differences in societal structures, health care systems, and policies made a globally valid report challenging given that those with the worst performance were also likely to contribute the least published data. As COVID-19 continues to spread globally, the epidemiology as well as mortality and morbidity outcomes may be changing based on improved management strategies and our understanding.
Research Gap and Future Directions
Our review revealed several gaps in our knowledge of COVID-19 within critical illness.
Hypertension has been identified as a host risk factor for severe COVID-19 infection. However, the conflicting findings about associations between ACEI/ARB use with clinical outcomes and the relationship between hypertension and the involvement of the renin-angiotensin system in the pathogenesis of the disease55 call for further research to explore the relevant pathways and clarify mechanisms in order to accurately inform our understanding of individual risk and guide antihypertension treatment during the COVID-19 pandemic.
Most of the studies reporting PE used prophylactic anticoagulation,35 , 39 but the development of life-threatening thrombotic complications was still frequent, indicating the hypercoagulation status of critically ill patients and highlighting the need to modify dosing of antithrombotic agents in high-risk patients with SARS-Cov-2 infection. Large-scale clinical data on COVID-19–related PE frequency and optimal management strategies are needed.
There is an urgent need to fund scientific investments into advancing novel therapeutic interventions for SARS-CoV-2 infections. Promising signals from observational data must be rigorously confirmed in high-quality RCTs. Some studies have suggested that COVID-19 severity might be due to virus-activated inflammatory cytokine abnormalities,52 , 53 , 56 , 61 leading to organ injuries. In the future, large-scale collaborative clinical studies, proof-of-mechanism studies, and autopsy studies are needed to better understand organ injuries. In the meantime, whether anticytokine therapies or immunomodulators such as IL-6 receptor blockers (eg, tocilizumab and sarilumab) to control overactive cytokine responses could be effective therapeutic drugs for COVID-19 requires further exploration.52 , 129 Given that a multicenter RCT found that early antiviral therapy combined with interferon beta-1b was superior to antiviral drugs alone in shortening the duration of viral shedding and hospital stay in patients with nonsevere COVID-19,130 future studies should also consider focusing on the role of interferon in treatment. Additionally, whether critically ill patients with COVID-19 can benefit from antibiotic therapy still needs to be confirmed by well-designed prospective studies with sufficient follow-up duration.
The established predictive models,55 , 65 , 68 , 81 , 97 although validated in different populations, were all limited to some specific region or country, and thus there is uncertainty about the generalizability of the risk scores to other areas of the world. Further verification is required in populations in other regions and should be adjusted by specific demographic risk factors.
Conclusion
In this scoping review, we assessed the current evidence related to critically ill patients with COVID-19 to summarize the clinical manifestations, management, and risk factors associated with poor prognosis to inform clinicians and identify future research directions. Some early clinical research findings from China and Europe can be applied to US practice; however, there are notable differences in coexisting chronic disease, complications, and intervention strategies geographically as well as the application of predictive models. In the future, it would be useful to consider large adaptive platform trials to pool data for demonstrating efficacy and safety for novel treatments.
Acknowledgments
We thank Danielle J. Gerberi, a librarian working at Mayo Clinic, Rochester, Minnesota, for her guidance about relevant databases and literature searching strategies and Drs Ognjen Gajic and Vitaly Herasevich for their critical review of and comments on the submitted manuscript.
Footnotes
Grant Support: This work was supported in part by grant R18HS026609 from the Agency for Healthcare Research and Quality and by a Discovery Grant from the Society of Critical Care Medicine.
Potential Competing Interests: Dr Pickering has received fees for board membership, has patents (planned, pending, or issued, funds paid to his institution), has received royalties, and has stock/stock options (all not related to the current work) from Ambient Clinical Analytics. The other authors report no competing interests.
References
- 1.Zhu N., Zhang D., Wang W. China Novel Coronavirus Investigating and Research Team. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 3.Center for Systems Science and Engineering. COVID-19 dashboard. https://www.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6
- 4.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China [published correction appears in Lancet. 2020;395(10223):496] Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study [published corrections appear in Lancet. 2020;395(10229):1038] Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19) https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf Published February 28, 2020.
- 7.Salehi S., Abedi A., Balakrishnan S., Gholamrezanezhad A. Coronavirus disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients. AJR Am J Roentgenol. 2020;215(1):87–93. doi: 10.2214/AJR.20.23034. [DOI] [PubMed] [Google Scholar]
- 8.Yang J., Zheng Y., Gou X. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vijayvargiya P., Esquer Garrigos Z., Castillo Almeida N.E., Gurram P.R., Stevens R.W., Razonable R.R. Treatment considerations for COVID-19: a critical review of the evidence (or lack thereof) Mayo Clin Proc. 2020;95(7):1454–1466. doi: 10.1016/j.mayocp.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun P., Qie S., Liu Z., Ren J., Li K., Xi J. Clinical characteristics of hospitalized patients with SARS-CoV-2 infection: a single arm meta-analysis. J Med Virol. 2020;92(6):612–617. doi: 10.1002/jmv.25735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adhikari S.P., Meng S., Wu Y.-J. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infect Dis Poverty. 2020;9(1):29. doi: 10.1186/s40249-020-00646-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herman C., Mayer K., Sarwal A. Scoping review of prevalence of neurologic comorbidities in patients hospitalized for COVID-19. Neurology. 2020;95(2):77–84. doi: 10.1212/WNL.0000000000009673. [DOI] [PubMed] [Google Scholar]
- 13.Zheng Z., Peng F., Xu B. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect. 2020;81(2):e16–e25. doi: 10.1016/j.jinf.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Munn Z., Peters M.D.J., Stern C., Tufanaru C., McArthur A., Aromataris E. Systematic review or scoping review? guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. 2018;18(1):143. doi: 10.1186/s12874-018-0611-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tricco A.C., Lillie E., Zarin W. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization COVID-19: global literature on coronavirus disease. https://search.bvsalud.org/global-research-on-novel-coronavirus-2019-ncov/ Accessed June 25, 2020.
- 17.Chen Q., Allot A., Lu Z. Keep up with the latest coronavirus research [letter] Nature. 2020;579(7798):193. doi: 10.1038/d41586-020-00694-1. [DOI] [PubMed] [Google Scholar]
- 18.National Center for Biotechnology Information LitCovid database. https://www.ncbi.nlm.nih.gov/research/coronavirus/#data-download Accessed June 25, 2020.
- 19.Covidence database. https://www.covidence.org/ Accessed July 10, 2020.
- 20.Zou X., Li S., Fang M. Acute Physiology and Chronic Health Evaluation II score as a predictor of hospital mortality in patients of coronavirus disease 2019. Crit Care Med. 2020;48(8):e657–e665. doi: 10.1097/CCM.0000000000004411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bessière F., Roccia H., Delinière A. Assessment of QT intervals in a case series of patients with coronavirus disease 2019 (COVID-19) infection treated with hydroxychloroquine alone or in combination with azithromycin in an intensive care unit. JAMA Cardiol. 2020;5(9):1067–1069. doi: 10.1001/jamacardio.2020.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grasselli G., Zangrillo A., Zanella A. COVID-19 Lombardy ICU Network. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323(316):1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kandemirli S.G., Dogan L., Sarikaya Z.T. Brain MRI findings in patients in the intensive care unit with COVID-19 infection. Radiology. 2020;297(1):E232–E235. doi: 10.1148/radiol.2020201697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arentz M., Yim E., Klaff L. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020;323(16):1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Myers L.C., Parodi S.M., Escobar G.J., Liu V.X. Characteristics of hospitalized adults with COVID-19 in an integrated health care system in California. JAMA. 2020;323(321):2195–2198. doi: 10.1001/jama.2020.7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen L., Li S., Zhu Y. Clinical and laboratory-derived parameters of 119 hospitalized patients with coronavirus disease 2019 in Xiangyang, Hubei Province, China. J Infect. 2020;81(1):147–178. doi: 10.1016/j.jinf.2020.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(311):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y., Lu X., Li Y. Clinical course and outcomes of 344 intensive care patients with COVID-19. Am J Respir Crit Care Med. 2020;201(11):1430–1434. doi: 10.1164/rccm.202003-0736LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study [published correction appears in Lancet Respir Med. 2020;8(4):e26] Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang X., Du R.-H., Wang R. Comparison of hospitalized patients with ARDS caused by COVID-19 and H1N1. Chest. 2020;158(1):195–205. doi: 10.1016/j.chest.2020.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee J., Lee Y.H., Chang H.-H. Comparison of short-term mortality between mechanically ventilated patients with COVID-19 and influenza in a setting of sustainable healthcare system. J Infect. 2020;81(2):e76–e78. doi: 10.1016/j.jinf.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhatraju P.K., Ghassemieh B.J., Nichols M. Covid-19 in critically ill patients in the Seattle region - case series. N Engl J Med. 2020;382(21):2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borba M.G.S., Val F.F.A., Sampaio V.S., CloroCovid-19 Team Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Netw Open. 2020;3(4):e208857. doi: 10.1001/jamanetworkopen.2020.8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xie Y., Cao S., Dong H. Effect of regular intravenous immunoglobulin therapy on prognosis of severe pneumonia in patients with COVID-19. J Infect. 2020;81(2):318–356. doi: 10.1016/j.jinf.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Helms J., Tacquard C., Severac F., CRICS TRIGGERSEP Group (Clinical Research in Intensive Care and Sepsis Trial Group for Global Evaluation and Research in Sepsis) High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu C.-N., Xia L.-Z., Li K.-H. High-flow nasal-oxygenation-assisted fibreoptic tracheal intubation in critically ill patients with COVID-19 pneumonia: a prospective randomised controlled trial. Br J Anaesth. 2020;125(1):e166–e168. doi: 10.1016/j.bja.2020.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cardoso F.S., Pereira R., Germano N. Liver injury in critically ill patients with COVID-19: a case series. Crit Care. 2020;24(1):190. doi: 10.1186/s13054-020-02924-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Helms J., Kremer S., Merdji H. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382(23):2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poissy J., Goutay J., Caplan M., Lille ICU Haemostasis COVID-19 Group Pulmonary embolism in patients with COVID-19: awareness of an increased prevalence. Circulation. 2020;142(2):184–186. doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- 40.Ziehr D.R., Alladina J., Petri C.R. Respiratory pathophysiology of mechanically ventilated patients with COVID-19: a cohort study. Am J Respir Crit Care Med. 2020;201(12):1560–1564. doi: 10.1164/rccm.202004-1163LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Criel M., Falter M., Jaeken J. Venous thromboembolism in SARS-CoV-2 patients: only a problem in ventilated ICU patients, or is there more to it? Eur Respir J. 2020;56(1):2001201. doi: 10.1183/13993003.01201-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li J., Wang X., Chen J., Zhang H., Deng A. Association of renin-angiotensin system inhibitors with severity or risk of death in patients with hypertension hospitalized for coronavirus disease 2019 (COVID-19) infection in Wuhan, China. JAMA Cardiol. 2020;5(7):825–830. doi: 10.1001/jamacardio.2020.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shi S., Qin M., Cai Y. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. Eur Heart J. 2020;41(22):2070–2079. doi: 10.1093/eurheartj/ehaa408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tian S., Hu N., Lou J. Characteristics of COVID-19 infection in Beijing. J Infect. 2020;80(4):401–406. doi: 10.1016/j.jinf.2020.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu Y.-H., Dong J.-H., An W.-M. Clinical and computed tomographic imaging features of novel coronavirus pneumonia caused by SARS-CoV-2. J Infect. 2020;80(4):394–400. doi: 10.1016/j.jinf.2020.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen G., Wu D., Guo W. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ma J., Yin J., Qian Y., Wu Y. Clinical characteristics and prognosis in cancer patients with COVID-19: a single center's retrospective study. J Infect. 2020;81(2):318–356. doi: 10.1016/j.jinf.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pan L., Mu M., Yang P. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. Am J Gastroenterol. 2020;115(5):766–773. doi: 10.14309/ajg.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou B., She J., Wang Y., Ma X. The clinical characteristics of myocardial injury in severe and very severe patients with 2019 novel coronavirus disease. J Infect. 2020;81(1):147–178. doi: 10.1016/j.jinf.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen L., Zhang B., Ti M.-Y.-D.-L.N.-J., Yang K., Zou Y., Zhang S. Clinical course of severe and critically ill patients with coronavirus disease 2019 (COVID-19): a comparative study. J Infect. 2020;81(2):e82–e84. doi: 10.1016/j.jinf.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Feng Y., Ling Y., Bai T. COVID-19 with different severities: a multicenter study of clinical features. Am J Respir Crit Care Med. 2020;201(11):1380–1388. doi: 10.1164/rccm.202002-0445OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen X., Zhao B., Qu Y. Detectable serum severe acute respiratory syndrome coronavirus 2 viral load (RNAemia) is closely correlated with drastically elevated interleukin 6 level in critically ill patients with coronavirus disease 2019. Clin Infect Dis. 2020;71(8):1937–1942. doi: 10.1093/cid/ciaa449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qin C., Zhou L., Hu Z. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71(15):762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu J., Li W., Shi X. Early antiviral treatment contributes to alleviate the severity and improve the prognosis of patients with novel coronavirus disease (COVID-19) J Intern Med. 2020;288(1):128–138. doi: 10.1111/joim.13063. [DOI] [PubMed] [Google Scholar]
- 55.Shi Y., Yu X., Zhao H., Wang H., Zhao R., Sheng J. Host susceptibility to severe COVID-19 and establishment of a host risk score: findings of 487 cases outside Wuhan. Crit Care. 2020;24(1):108. doi: 10.1186/s13054-020-2833-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang C., Fei D., Li X., Zhao M., Yu K. IL-6 may be a good biomarker for earlier detection of COVID-19 progression. Intensive Care Med. 2020;46(7):1475–1476. doi: 10.1007/s00134-020-06065-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fang X., Mei Q., Yang T. Low-dose corticosteroid therapy does not delay viral clearance in patients with COVID-19. J Infect. 2020;81(1):147–178. doi: 10.1016/j.jinf.2020.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang Y., Zhang D., Du G. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial [published correction appears in Lancet. 2020;395(10238):1694] Lancet. 2020;395(10236):1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hu L., Chen S., Fu Y. Risk Factors associated with clinical outcomes in 323 coronavirus disease 2019 (COVID-19) hospitalized patients in Wuhan, China. Clin Infect Dis. 2020;71(16):2089–2098. doi: 10.1093/cid/ciaa539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu X., Zhou H., Zhou Y. Risk factors associated with disease severity and length of hospital stay in COVID-19 patients. J Infect. 2020;81(1):e95–e97. doi: 10.1016/j.jinf.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wei Y.-Y., Wang R.-R., Zhang D.-W. Risk factors for severe COVID-19: evidence from 167 hospitalized patients in Anhui, China. J Infect. 2020;81(1):e89–e92. doi: 10.1016/j.jinf.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li H., Xiang X., Ren H. Serum amyloid A is a biomarker of severe coronavirus disease and poor prognosis. J Infect. 2020;80(6):646–655. doi: 10.1016/j.jinf.2020.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Long L., Zeng X., Zhang X. Short-term outcomes of COVID-19 and risk factors for progression. Eur Respir J. 2020;55(5):2000990. doi: 10.1183/13993003.00990-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu B., Fan C.-Y., Wang A.-L. Suppressed T cell-mediated immunity in patients with COVID-19: a clinical retrospective study in Wuhan, China. J Infect. 2020;81(1):e51–e60. doi: 10.1016/j.jinf.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gong J., Ou J., Qiu X. A tool for early detection of severe coronavirus disease 2019 (COVID-19): a multicenter study using the risk nomogram in Wuhan and Guangdong, China [published online ahead of print, 2020 Apr 16] Clin Infect Dis. 2020;71(15):833–840. doi: 10.1093/cid/ciaa443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zheng S., Fan J., Yu F. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. BMJ. 2020;369:m1443. doi: 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guan W.-J., Ni Z.-Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liang W., Liang H., Ou L., China Medical Treatment Expert Group for COVID-19 Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Intern Med. 2020;180(8):1081–1089. doi: 10.1001/jamainternmed.2020.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mao L., Jin H., Wang M. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wei J.-F., Huang F.-Y., Xiong T.-Y. Acute myocardial injury is common in patients with COVID-19 and impairs their prognosis. Heart. 2020;106(15):1154–1159. doi: 10.1136/heartjnl-2020-317007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Léonard-Lorant I., Delabranche X., Séverac F. Acute pulmonary embolism in patients with COVID-19 at CT angiography and relationship to D-dimer levels. Radiology. 2020:201561. doi: 10.1148/radiol.2020201561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xie J., Covassin N., Fan Z. Association between hypoxemia and mortality in patients with COVID-19. Mayo Clin Proc. 2020;95(6):1138–1147. doi: 10.1016/j.mayocp.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shi S., Qin M., Shen B. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guo T., Fan Y., Chen M. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(7):811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Colaneri M., Sacchi P., Zuccaro V., COVID19 IRCCS San Matteo Pavia Task Force Clinical characteristics of coronavirus disease (COVID-19) early findings from a teaching hospital in Pavia, North Italy, 21 to 28 February 2020. Euro Surveill. 2020;25(16):2000460. doi: 10.2807/1560-7917.ES.2020.25.16.2000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Goyal P., Choi J.J., Pinheiro L.C. Clinical characteristics of Covid-19 in New York City. N Engl J Med. 2020;382(24):2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang Z., Yang B., Li Q., Wen L., Zhang R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. 2020;71(15):769–777. doi: 10.1093/cid/ciaa272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Su Y., Tu G.-W., Ju M.-J. Comparison of CRB-65 and quick sepsis-related organ failure assessment for predicting the need for intensive respiratory or vasopressor support in patients with COVID-19. J Infect. 2020;81(4):647–679. doi: 10.1016/j.jinf.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Grein J., Ohmagari N., Shin D. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med. 2020;382(24):2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Geleris J., Sun Y., Platt J. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;382(25):2411–2418. doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ji D., Zhang D., Xu J. Prediction for progression risk in patients with COVID-19 pneumonia: the CALL score. Clin Infect Dis. 2020;71(6):1393–1399. doi: 10.1093/cid/ciaa414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bompard F., Monnier H., Saab I. Pulmonary embolism in patients with COVID-19 pneumonia. Eur Respir J. 2020;56(1):2001365. doi: 10.1183/13993003.01365-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Beigel J.H., Tomashek K.M., Dodd L.E., ACTT-1 Study Group Members Remdesivir for the treatment of Covid-19 - preliminary report. N Engl J Med. 2020;383(19):1813–1826. doi: 10.1056/NEJMc2022236. [DOI] [PubMed] [Google Scholar]
- 84.Wu C., Chen X., Cai Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rovina N., Akinosoglou K., Eugen-Olsen J., Hayek S., Reiser J., Giamarellos-Bourboulis E.J. Soluble urokinase plasminogen activator receptor (suPAR) as an early predictor of severe respiratory failure in patients with COVID-19 pneumonia. Crit Care. 2020;24(1):187. doi: 10.1186/s13054-020-02897-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stefanini G.G., Montorfano M., Trabattoni D. ST-elevation myocardial infarction in patients with COVID-19: clinical and angiographic outcomes. Circulation. 2020;141(25):2113–2116. doi: 10.1161/CIRCULATIONAHA.120.047525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cao B., Wang Y., Wen D. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pereira M.R., Mohan S., Cohen D.J. COVID-19 in solid organ transplant recipients: initial report from the US epicenter. Am J Transplant. 2020;20(7):1800–1808. doi: 10.1111/ajt.15941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mo P., Xing Y., Xiao Y. Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. https://doi.org/10.1093/cid/ciaa270 [published online ahead of print March 16, 2020]. Clin Infect Dis.
- 90.Grillet F., Behr J., Calame P., Aubry S., Delabrousse E. Acute pulmonary embolism associated with COVID-19 pneumonia detected with pulmonary CT angiography. Radiology. 2020;296(3):E186–E188. doi: 10.1148/radiol.2020201544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Colombi D., Bodini F.C., Petrini M. Well-aerated lung on admitting chest CT to predict adverse outcome in COVID-19 pneumonia. Radiology. 2020;296(2):E86–E96. doi: 10.1148/radiol.2020201433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rosenberg E.S., Dufort E.M., Udo T. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York State. JAMA. 2020;323(24):2493–2502. doi: 10.1001/jama.2020.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang K., Zhang Z., Yu M., Tao Y., Xie M. 15-Day mortality and associated risk factors for hospitalized patients with COVID-19 in Wuhan, China: an ambispective observational cohort study. Intensive Care Med. 2020;46(7):1472–1474. doi: 10.1007/s00134-020-06047-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mehra M.R., Desai S.S., Kuy S., Henry T.D., Patel A.N. Cardiovascular disease, drug therapy, and mortality in Covid-19 [retracted in: N Engl J Med. 2020;382(26):2582] N Engl J Med. 2020;382(25):e102. doi: 10.1056/NEJMoa2007621. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 95.Inciardi R.M., Adamo M., Lupi L. Characteristics and outcomes of patients hospitalized for COVID-19 and cardiac disease in Northern Italy. Eur Heart J. 2020;41(19):1821–1829. doi: 10.1093/eurheartj/ehaa388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li W., Zhang J., Xiao S., Sun L. Characteristics of deaths amongst health workers in China during the outbreak of COVID-19 infection. J Infect. 2020;81(1):147–178. doi: 10.1016/j.jinf.2020.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang K., Zuo P., Liu Y. Clinical and laboratory predictors of in-hospital mortality in patients with coronavirus disease 2019: a cohort study in Wuhan, China. Clin Infect Dis. 2020;71(16):2079–2088. doi: 10.1093/cid/ciaa538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen T., Wu D., Chen H. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study [published correction appears in BMJ. 2020;368:m1295] BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang D., Yin Y., Hu C. Clinical course and outcome of 107 patients infected with the novel coronavirus, SARS-CoV-2, discharged from two hospitals in Wuhan, China. Crit Care. 2020;24(1):188. doi: 10.1186/s13054-020-02895-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Deng G., Yin M., Chen X., Zeng F. Clinical determinants for fatality of 44,672 patients with COVID-19. Crit Care. 2020;24(1):179. doi: 10.1186/s13054-020-02902-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cao J., Tu W.-J., Cheng W. Clinical features and short-term outcomes of 102 patients with coronavirus disease 2019 in Wuhan, China] Clin Infect Dis. 2020;71(15):748–755. doi: 10.1093/cid/ciaa243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Du Y., Tu L., Zhu P. Clinical features of 85 fatal cases of COVID-19 from Wuhan: a retrospective observational study. Am J Respir Crit Care Med. 2020;201(11):1372–1379. doi: 10.1164/rccm.202003-0543OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tomlins J., Hamilton F., Gunning S., Sheehy C., Moran E., MacGowan A. Clinical features of 95 sequential hospitalised patients with novel coronavirus 2019 disease (COVID-19), the first UK cohort. J Infect. 2020;81(2):e59–e61. doi: 10.1016/j.jinf.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China [published correction appears in Intensive Care Med. 2020;46(6):1294-1297] Intensive Care Med. 2020;46(5):846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tu W.-J., Cao J., Yu L., Hu X., Liu Q. Clinicolaboratory study of 25 fatal cases of COVID-19 in Wuhan. Intensive Care Med. 2020;46(6):1117–1120. doi: 10.1007/s00134-020-06023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang L., He W., Yu X. Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up. J Infect. 2020;80(6):639–645. doi: 10.1016/j.jinf.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Webb G.J., Moon A.M., Barnes E., Barritt A.S., Marjot T. Determining risk factors for mortality in liver transplant patients with COVID-19. Lancet Gastroenterol Hepatol. 2020;5(7):643–644. doi: 10.1016/S2468-1253(20)30125-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Richardson S., Hirsch J.S., Narasimhan M. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area [published correction appears in JAMA. 2020;323(20):2098] JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen R., Liang W., Jiang M., Medical Treatment Expert Group for COVID-19 Risk factors of fatal outcome in hospitalized subjects with coronavirus disease 2019 from a nationwide analysis in China. Chest. 2020;158(1):97–105. doi: 10.1016/j.chest.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gal-Oz S.T., Maier B., Yoshida H. ImmGen report: sexual dimorphism in the immune system transcriptome. Nat Commun. 2019;10(1):4295. doi: 10.1038/s41467-019-12348-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wu K.J. Study of 17 million identifies crucial risk factors for coronavirus deaths. New York Times website. https://www.nytimes.com/2020/07/08/health/coronavirus-risk-factors.html Published July 8, 2020. Updated October 2, 2020. Accessed July 12, 2020.
- 112.Yancy C.W. COVID-19 and African Americans. JAMA. 2020;323(19):1891–1892. doi: 10.1001/jama.2020.6548. [DOI] [PubMed] [Google Scholar]
- 113.Webb Hooper M., Nápoles A.M., Pérez-Stable E.J. COVID-19 and racial/ethnic disparities. JAMA. 2020;323(24):2466–2467. doi: 10.1001/jama.2020.8598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sanchis-Gomar F., Lavie C.J., Mehra M.R., Henry B.M., Lippi G. Obesity and outcomes in COVID-19: when an epidemic and pandemic collide. Mayo Clin Proc. 2020;95(7):1445–1453. doi: 10.1016/j.mayocp.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vaduganathan M., Vardeny O., Michel T., McMurray J.J.V., Pfeffer M.A., Solomon S.D. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020;382(17):1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mancia G., Rea F., Ludergnani M., Apolone G., Corrao G. Renin-angiotensin-aldosterone system blockers and the risk of Covid-19. N Engl J Med. 2020;382(25):2431–2440. doi: 10.1056/NEJMoa2006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sanchis-Gomar F., Lavie C.J., Perez-Quilis C., Henry B.M., Lippi G. Angiotensin-converting enzyme 2 and antihypertensives (angiotensin receptor blockers and angiotensin-converting enzyme inhibitors) in coronavirus disease 2019. Mayo Clin Proc. 2020;95(6):1222–1230. doi: 10.1016/j.mayocp.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mehta N., Kalra A., Nowacki A.S. Association of use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with testing positive for coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(9):1020–1026. doi: 10.1001/jamacardio.2020.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Revel M.-P., Parkar A.P., Prosch H., European Society of Radiology (ESR) and the European Society of Thoracic Imaging (ESTI) COVID-19 patients and the radiology department - advice from the European Society of Radiology (ESR) and the European Society of Thoracic Imaging (ESTI) Eur Radiol. 2020;30(9):4903–4909. doi: 10.1007/s00330-020-06865-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.European Society of Cardiology ESC guidance for the diagnosis and management of CV disease during the COVID-19 pandemic. https://www.escardio.org/Education/COVID-19-and-Cardiology/ESC-COVID-19-Guidance Updated June 10, 2020. Accessed June 20, 2020.
- 121.Sun Q., Qiu H., Huang M., Yang Y. Lower mortality of COVID-19 by early recognition and intervention: experience from Jiangsu Province. Ann Intensive Care. 2020;10(1):33. doi: 10.1186/s13613-020-00650-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Society of Critical Care Medicine VIRUS COVID-19 Registry. https://sccmcovid19.org/ Updated December 10, 2020. Accessed July 13, 2020.
- 123.US Food and Drug Administration Coronavirus (COVID-19) update: FDA issues emergency use authorization for potential COVID-19 treatment [press release] https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-emergency-use-authorization-potential-covid-19-treatment Published May 1, 2020. Accessed June 10, 2020.
- 124.Boulware D.R., Pullen M.F., Bangdiwala A.S. A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19. N Engl J Med. 2020;383(6):517–525. doi: 10.1056/NEJMoa2016638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lee N., Allen Chan K.C., Hui D.S. Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients. J Clin Virol. 2004;31(4):304–309. doi: 10.1016/j.jcv.2004.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Low-cost dexamethasone reduces death by up to one third in hospitalised patients with severe respiratory complications of COVID-19: statement from the Chief Investigators of the Randomised Evaluation of COVid-19 thERapY (RECOVERY) Trial on dexamethasone. RECOVERY Trial website. https://www.recoverytrial.net/news/low-cost-dexamethasone-reduces-death-by-up-to-one-third-in-hospitalised-patients-with-severe-respiratory-complications-of-covid-19 Published June 16, 2020.
- 127.Morse M., Loscalzo J. Creating real change at academic medical centers - how social movements can be timely catalysts. N Engl J Med. 2020;383(3):199–201. doi: 10.1056/NEJMp2002502. [DOI] [PubMed] [Google Scholar]
- 128.Laurencin C.T., McClinton A. The COVID-19 pandemic: a call to action to identify and address racial and ethnic disparities. J Racial Ethn Health Disparities. 2020;7(3):398–402. doi: 10.1007/s40615-020-00756-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J., HLH Across Speciality Collaboration, UK COVID-19: consider cytokine storm syndromes and immunosuppression [letter] Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hung I.F.-N., Lung K.-C., Tso E.Y.-K. Triple combination of interferon beta-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395(10238):1695–1704. doi: 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]