Summary
Dupilumab is the first biologic registered for the treatment of atopic dermatitis (AD). We report on seven patients with AD presenting with a paradoxical head and neck erythema that appeared 10–39 weeks after the start of dupilumab treatment. The patients presented with a relatively sharply demarcated, patchy erythema in the head and neck area that showed no or less scaling compared with their usual eczema. Only one patient experienced symptoms of itch and burning, although this was notably different from his pre‐existent facial AD. Except for a notable ‘red face’, eczema on other body parts had greatly improved in six of the seven patients, with a mean numerical rating scale for treatment satisfaction of 9 out of 10 at the time of biopsy. Treatment of the erythema with topical and systemic drugs was unsuccessful. Despite the presence of this erythema, none of our patients discontinued dupilumab treatment. Lesional skin biopsies showed an increased number of ectatic capillaries, and a perivascular lymphohistiocytic infiltration in all patients. In addition, epidermal hyperplasia with elongation of the rete ridges was observed in four patients, resembling a psoriasiform dermatitis. Additional immunohistochemical stainings revealed increased numbers of plasma cells, histiocytes and T lymphocytes. Interestingly, spongiosis was largely absent in all biopsies. We report on patients with AD treated with dupilumab developing a paradoxical erythema in a head and neck distribution. Both clinically and histopathologically we found a heterogeneous response, which was most suggestive of a drug‐induced skin reaction.
Short abstract
What's already known about this topic?
Dupilumab has proven to be an efficacious and effective treatment for atopic dermatitis with an acceptable safety profile.
The most frequently observed side‐effects in patients with atopic dermatitis treated with dupilumab are conjunctivitis, herpes infections and injection‐site reactions.
What does this study add?
For the first time, we report on patients with atopic dermatitis treated with dupilumab who developed a paradoxical, mainly asymptomatic erythema in a head and neck distribution.
Histological examination of skin biopsies revealed a psoriasiform reaction pattern suggestive of a drug‐induced skin reaction.
Dupilumab is the first biologic registered for treatment of moderate‐to‐severe atopic dermatitis (AD). By binding to the interleukin (IL)‐4 receptor alpha chain, dupilumab blocks IL‐4 and IL‐13 signalling, thereby modulating the T helper (Th)2‐mediated inflammation in AD.1 In clinical trials, conjunctivitis, herpes infections and injection‐site reactions were found to be the most frequently observed side‐effects.1 Currently, only one case of paradoxical, refractory erythema in a head and neck distribution developing during dupilumab treatment has been reported.2
Here we describe a series of seven patients with AD who were treated with dupilumab and developed a paradoxical erythema in a head and neck distribution, differing from their usual AD lesions. We did not previously check systematically for this erythema in our dupilumab‐treated patients with AD (n > 150). However, we increasingly seem to observe this phenomenon. We recorded the medical history, patient‐ and physician‐reported outcome measures, and clinical symptoms, and obtained lesional skin biopsies for histological examination.
Case report
The patient characteristics are summarized in Table 1. The erythema appeared after at least 10 weeks of dupilumab treatment, and patients had been treated with dupilumab for 12–71 weeks at the time of biopsy. Concomitant treatment included topical corticosteroids and topical calcineurin inhibitors, but no systemic immunosuppressants. There were no relevant (nonatopic, dermatological) comorbidities and the patients did not use any other systemic drugs. Prior to dupilumab treatment, six of the seven patients had experienced erythematosquamous lesions in the head and neck area, associated with symptoms of itching and burning in five of these patients. The patients noted that the signs and symptoms of the paradoxical erythema were different from their regular eczema. Strikingly, six of the seven patients did not report any symptoms of itch and burning in the head and neck area during dupilumab treatment.
Table 1.
Patient and clinical characteristics (n = 7)
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Patient characteristics | |||||||
| Sex | Male | Female | Male | Male | Male | Male | Male |
| Age (years) | 28 | 29 | 26 | 19 | 58 | 35 | 46 |
| Disease duration (years) | 16 | 29 | 26 | 19 | 52 | 35 | 46 |
| Fitzpatrick skin type | II | III | II | II | III | II | II |
| Asthma/allergic rhinoconjunctivitis | +/+ | +/+ | −/+ | +/+ | −/+ | +/+ | +/+ |
| ACDa | + | − | − | + | NA | NA | − |
| Occupation, hobbies | Office job | Office job | Biologic crop control, soccer | Student | Warehouse worker | PE teacher, tennis coach | Salesman |
| Previous systemic immunosuppressants | CsA, MMF, AZA, MTX, APR, UST, prednisone | CsA, prednisone | CsA, prednisone | CsA, AZA, prednisone | CsA, MTX, prednisone | CsA, MTX, prednisone | CsA, MTX, prednisone |
| Clinical characteristics | |||||||
| Onset of erythema (weeks of dupilumab treatment) | 39 | 28 | 16–29 | 16–28 | 10–22 | 20 | 11 |
| Treatment duration at biopsy (weeks) | 49 | 53 | 42 | 51 | 22 | 71 | 12 |
| Symptoms before dupilumab | |||||||
| Erythema | + | + | + | + | − | + | + |
| Scaling | + | + | + | + | − | + | + |
| Itch | + | − | + | + | − | + | + |
| Burning sensation | + | − | + | + | − | + | + |
| Symptoms of paradoxical erythema | |||||||
| Erythema | + | + | + | + | + | + | + |
| Scaling | + | ± | − | − | − | − | − |
| Itch | +b | − | − | − | − | − | − |
| Burning sensation | +b | ± | − | − | − | − | − |
| Influence of: | |||||||
| Ultraviolet | − | − | + | − | − | − | − |
| Alcohol | − | NA | − | − | − | − | − |
| Smoking | − | NA | NA | NA | NA | − | − |
| Retesting ACDc | NA | − | − | NA | NA | + | NA |
| Topicals prescriptionsd , e | Class 3–4 TCS, TCI, emollients, ivermectin | Class 3–4 TCS, emollients | Class 3–4 TCS, emollients, fusidic acid | Class 3–4 TCS, emollients | Class 3–4 TCS, emollients | Class 3 TCS, TCI, emollients | Class 5 TCS |
| Systemic prescriptionsd | Prednisone | Prednisone | Antifungals, antibiotics, antihistamine | Antihistamine | None | None | None |
ACD, allergic contact dermatitis; APR, apremilast; AZA, azathioprine; CsA, ciclosporin A; MMF, mycophenolate mofetil; MTX, methotrexate; NA, not applicable; PE, physical education; TCI, topical calcineurin inhibitors; TCS, topical corticosteroids; UST, ustekinumab. aEpicutaneous patch‐proven ACD, tested prior to dupilumab treatment. bSymptoms were notably different from the pre‐existing situation. cEpicutaneous patch‐proven ACD, (re)tested after onset of erythema. dPrescribed for head and neck erythema. eWorld Health Organization classification for topical corticosteroids.
During dupilumab treatment, the patients presented with a relatively sharply demarcated, patchy erythema in the head and neck area that showed less or no scaling compared with their usual eczema (Fig. 1a, b). Follicular accentuation was absent. Only one patient who presented with scaling lesions experienced symptoms of itch and burning, although they were notably different from the pre‐existent symptoms. Treatment of the erythema with topical and systemic drugs, including potent topical corticosteroids, topical calcineurin inhibitors, emollients, antifungal medication, antibiotics, antihistamines and oral corticosteroids, was unsuccessful (Table 1). Although the lesions did not respond to treatment all patients continued dupilumab treatment.
Figure 1.
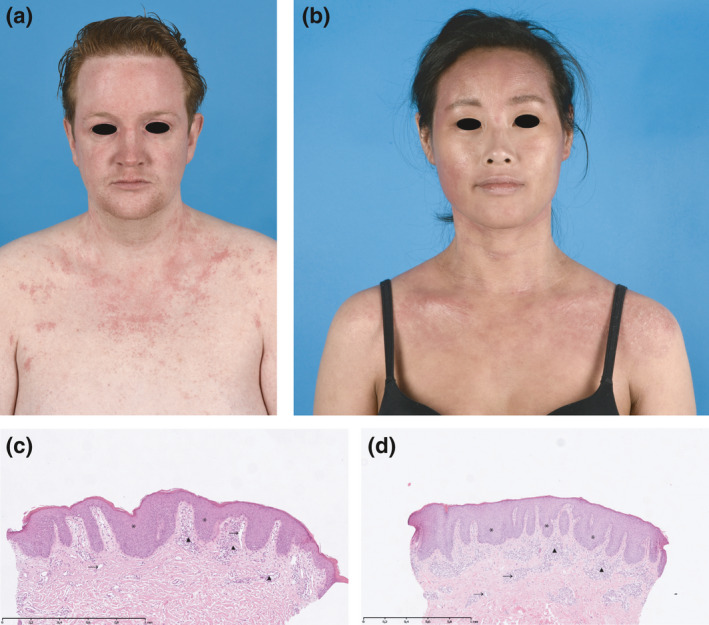
(a, b) Clinical pictures of paradoxical erythema in a head and neck distribution with (c, d) corresponding histological haematoxylin and eosin staining of lesional biopsies. (a) Patient 1: a 28‐year‐old male patient showing a relatively sharply demarcated, minimally scaling, patchy erythema of the face and neck (scalp not affected), which was associated with burning and itching, but was notably different from his usual eczema. (b) Patient 2: a 29‐year‐old female patient showing a minimally scaling, patchy erythema of the face and neck (scalp not affected), which was asymptomatic. (c, d) Histological examination of lesional skin biopsies of patients 1 (c) and 2 (d), both obtained from the neck, revealed psoriasiform epidermal hyperplasia with bulbous elongated rete ridges (*), increased numbers of ectatic capillaries in the papillary dermis (→) and a moderate perivascular lymphocytic infiltrate (▲). Interestingly, spongiosis was largely absent in all biopsies.
All patients experienced a clinically relevant reduction of the total Eczema Area and Severity Index (EASI) score (Table S1; see Supporting Information). However, in all seven patients, the disease severity scores increased again after 10–39 weeks of treatment. In the same period, all patients gradually developed an erythema specifically in the head and neck region, which was reflected by a disproportional increase of the head and neck EASI subscore (Table S1). Despite the development of this erythema, the mean numerical rating scale for treatment satisfaction rated by the patients at time of biopsy was still 9 on a 10‐point scale.
Histopathological examination of lesional skin biopsies (n = 7) included haematoxylin and eosin (Fig. 1c, d) and immunohistochemical stainings for CD3, CD20, CD68, CD138 and CD117 (Table S2; see Supporting Information). Histopathology showed epidermal hyperplasia with bulbous elongated rete ridges, increased numbers of ectatic capillaries in the papillary dermis and a moderate perivascular lymphocytic inflammation reminiscent of a psoriasiform dermatitis in four patients (Fig. 1c, d; Table S2). Biopsies from the other three patients also showed increased ectatic capillaries and perivascular lymphocytic exocytosis, but no epidermal hyperplasia. Overall, small numbers of eosinophils were found, and neutrophils and melanophages were largely absent. We found normal numbers of mast cells (CD117), T cells (CD3) and histiocytes (CD68). There were variable numbers of plasma cells (CD138), but B cells (CD20) were largely absent. Surprisingly, spongiosis was largely absent in all biopsies.
Discussion
We describe a paradoxical erythema in a head and neck distribution that developed in patients with AD during dupilumab treatment. Histopathology showed a psoriasiform hyperplasia in four of seven patients, with ectatic capillaries in six of seven. Interestingly, spongiosis was absent in five of the seven patients. Although the histological findings could represent an atypical manifestation of chronic AD, the clinical manifestation with a sharply demarcated, patchy erythema and absence of itch was not typical for AD.3 Although these patients were very satisfied with their overall treatment result, it has been shown that involvement of the face or neck is associated with higher patient‐perceived importance of almost or complete skin clearance.4
The histopathology of acute AD lesions is characterized by spongiosis and perivascular lymphocytic and eosinophilic infiltrates.3 Subacute and chronic lesions show acanthosis, sometimes in a psoriasiform pattern; hyper‐ and parakeratosis; fibrosis; spongiosis; dense infiltrates of mononuclear cells; eosinophils and increased mast cells.3 Interestingly, we found that most of these histopathological hallmarks of AD were missing in the biopsies. Spongiosis and mononuclear, mast cell and eosinophilic infiltrates were mostly absent, confirming our clinical observation that these are not typical AD lesions.
AD has been hypothesized to be a biphasic T‐cell‐driven disease, with a predominance of Th2 cytokines in the acute phase and increased expression of the Th1, Th17 and Th22 cytokines in the chronic phase.5 As dupilumab targets the IL‐4 receptor alpha chain, it blocks the key signalling pathways for Th2 T‐cell differentiation.1 Blocking the Th2 pathway could hypothetically result in a shift towards a more Th1‐, Th17‐ and Th22‐dominated response, resulting in the psoriasiform reaction pattern that we observed.6 Fowler et al. recently reported the development of psoriatic lesions during dupilumab treatment in two patients,7 but in contrast to our findings, they describe typical psoriasis lesions that were not located in the head and neck region.
Because we found this paradoxical erythema only in a typical head and neck distribution, we also considered allergic contact dermatitis (ACD), Malassezia furfur‐associated head and neck dermatitis, Demodex‐associated rosacea‐like dermatosis, and a drug‐induced photosensitivity reaction. Patch testing for ACD was performed in three patients during dupilumab treatment, but only one patient showed positive patch tests, to lanolin and cocamidopropyl betaine. However, avoidance of the allergens did not improve this erythema and the histopathological findings were not suggestive of ACD.8
It has been suggested that (co)sensitization to the human and/or fungal (including Malassezia spp.) enzyme superoxide dismutase might play an important role in the pathogenesis of AD and associated head and neck dermatitis.9, 10 In murine models it was recently shown that Malassezia induces Th17‐driven inflammation.11 In patients with AD treated with dupilumab, IL‐4 receptor blockade might facilitate a Th17‐dominated response. However, in these mouse models, Malassezia also triggered massive infiltration of neutrophils and monocytes in the skin, which we did not find in the biopsies of our patients. Increased Th17 cytokine expression could also be in favour of Demodex colonization, which is associated with rosacea.12 However, the clinical presentation in our patients was not typical for rosacea. In addition, histological features of rosacea were absent.13
The distribution of the lesions in our patients was also suggestive of a drug‐induced photosensitivity reaction, but none of our patients was using a known photosensitive drug, and influence of ultraviolet radiation on the erythema was denied.14 Furthermore, the most common histological pattern found in drug‐induced photosensitivity reactions is a vacuolar interface dermatitis,15 which was not seen in our patients.
We speculate that this paradoxical head and neck erythema is a dupilumab‐induced skin reaction. From personal communication with other experts in AD we know that others have also observed this phenomenon. This emphasizes the importance of daily‐practice registries to gain better insights in the incidences of this phenomenon. Also, further research is needed to elucidate the underlying pathophysiological process.
Supporting information
Table S1 Disease severity (sub)scores at baseline and during dupilumab treatment.
Table S2 Histopathological findings in lesional skin biopsies.
Funding sources None.
Conflicts of interest D.J.H. has been an investigator for AbbVie, LEO Pharma, MedImmune/AstraZeneca, Novartis and Sanofi/Regeneron; and performed consultancies for Sanofi/Regeneron, LEO Pharma, MedImmune/AstraZeneca, Novartis, Incyte, Janssen and Pfizer. The other authors declare that they have no conflicts of interest.
References
- 1. Beck LA, Thaçi D, Hamilton JD et al Dupilumab treatment in adults with moderate‐to‐severe atopic dermatitis. N Engl J Med 2014; 371:130–9. [DOI] [PubMed] [Google Scholar]
- 2. Albader SS, Alharbi AA, Alenezi RF et al Dupilumab side‐effect in a patient with atopic dermatitis: a case report study. Biologics 2019; 13:79–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weidinger S, Beck LA, Bieber T et al Atopic dermatitis. Nat Rev Dis Primers 2018; 4:1. [DOI] [PubMed] [Google Scholar]
- 4. Egeberg A, Thyssen JP. Factors associated with patient‐reported importance of skin clearance among adults with psoriasis and atopic dermatitis. J Am Acad Dermatol 2019; 81:943–9. [DOI] [PubMed] [Google Scholar]
- 5. Gittler JK, Shemer A, Suarez‐Farinas M et al Progressive activation of TH2/TH22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J Allergy Clin Immunol 2012; 130:1344–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Greb JE, Goldminz AM, Elder JT et al Psoriasis. Nat Rev Dis Primers 2016; 2:16082. [DOI] [PubMed] [Google Scholar]
- 7. Fowler E, Silverberg JI, Fox JD et al Presentation of psoriasiform dermatitis after initiation of treatment with dupilumab for atopic dermatitis. Dermatitis 2019; 10.1097/DER.0000000000000481 [DOI] [PubMed] [Google Scholar]
- 8. So JK, Hamstra A, Calame A et al Another great imitator: allergic contact dermatitis differential diagnosis, clues to diagnosis, histopathology, and treatment. Curr Treat Opt Allergy 2015; 2:333–48. [Google Scholar]
- 9. Sugita T, Boekhout T, Velegraki A et al Epidemiology of Malassezia‐related skin diseases In: Malassezia and the Skin: Science and Clinical Practice (Boekhout T, Guého E, Mayser P, Velegraki A, eds). Berlin, Heidelberg: Springer, 2010; 65–119. [Google Scholar]
- 10. Schmid‐Grendelmeier P, Fluckiger S, Disch R et al IgE‐mediated and T cell‐mediated autoimmunity against manganese superoxide dismutase in atopic dermatitis. J Allergy Clin Immunol 2005; 115:1068–75. [DOI] [PubMed] [Google Scholar]
- 11. Sparber F, De Gregorio C, Steckholzer S et al The skin commensal yeast Malassezia triggers a type 17 response that coordinates anti‐fungal immunity and exacerbates skin inflammation. Cell Host Microbe 2019; 25:389–403. [DOI] [PubMed] [Google Scholar]
- 12. Thyssen JP. Could conjunctivitis in patients with atopic dermatitis treated with dupilumab be caused by colonization with Demodex and increased interleukin‐17 levels? Br J Dermatol 2018; 178:1220. [DOI] [PubMed] [Google Scholar]
- 13. Cribier B. Rosacea under the microscope: characteristic histological findings. J Eur Acad Dermatol Venereol 2013; 27:1336–43. [DOI] [PubMed] [Google Scholar]
- 14. Kim WB, Shelley AJ, Novice K et al Drug‐induced phototoxicity: a systematic review. J Am Acad Dermatol 2018; 79:1069–75. [DOI] [PubMed] [Google Scholar]
- 15. Weyers W, Metze D. Histopathology of drug eruptions – general criteria, common patterns, and differential diagnosis. Dermatol Pract Concept 2011; 1:33–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Disease severity (sub)scores at baseline and during dupilumab treatment.
Table S2 Histopathological findings in lesional skin biopsies.


