Abstract
Morbidity and mortality associated with COVID-19 has increased exponentially, and patients with cardiovascular (CV) disease are at risk for poor outcomes. Several lines of evidence suggest a potential role for CV therapies in COVID-19 treatment. Characteristics of clinical trials of CV therapies related to COVID-19 registered on ClinicalTrials.gov have not been described.
Methods
ClinicalTrials.gov was queried on August 7, 2020 for COVID-19 related trials. Studies evaluating established CV drugs, other fibrinolytics (defibrotide), and extracorporeal membrane oxygenation were included. Studies evaluating anti-microbial, convalescent plasma, non-colchicine anti-inflammatory, and other therapies were excluded. Trial characteristics were tabulated from study-specific entries.
Results
A total of 2,935 studies related to COVID-19 were registered as of August 7, 2020. Of these, 1,645 were interventional studies, and the final analytic cohort consisted of 114 studies evaluating 10 CV therapeutic categories. Antithrombotics (32.5%; n = 37) were most commonly evaluated, followed by pulmonary vasodilators (14.0%; n = 16), renin-angiotensin-aldosterone system-related therapies (12.3%; n = 14), and colchicine (8.8%; n = 10). Trials evaluating multiple CV therapy categories and CV therapies in combination with non-CV therapies encompassed 4.4% (n = 5) and 9.6% (n = 11) of studies, respectively. Most studies were designed for randomized allocation (87.7%; n = 100), enrollment of less than 1000 participants (86.8%; n = 99), single site implementation (55.3%; n = 63), and had a primary outcome of mortality or a composite including mortality (56.1%; n = 64). Most study populations consisted of patients hospitalized with COVID-19 (81.6%; n = 93). At the time of database query, 28.9% (n = 33) of studies were not yet recruiting and the majority were estimated to be completed after December 2020 (67.8%; n = 78). Most lead sponsors were located in North America (43.9%; n = 50) or Europe (36.0%; n = 41).
Conclusions
A minority (7%) of clinical trials related to COVID-19 registered on ClinicalTrials.gov plan to evaluate CV therapies. Of CV therapy studies, most were planned to be single center, enroll less than 1000 inpatients, sponsored by European or North American academic institutions, and estimated to complete after December 2020. Collectively, these findings underscore the need for a network of sites with a platform protocol for rapid evaluation of multiple therapies and generalizability to inform clinical care and health policy for COVID-19 moving forward.
The coronavirus disease 2019 (COVID-19) pandemic, which has affected over 27 million individuals and caused over 899,000 deaths globally as of September 10, 2020,1 has motivated a laudable global effort to rapidly evaluate novel and established therapies that may improve outcomes in COVID-19 patients. At present, a limited number of therapies have been shown to improve clinical outcomes in patients infected by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) via randomized controlled trial (RCT) data.2 , 3
Cardiovascular (CV) disease is widely prevalent and remains the leading cause of mortality globally.4 Patients with CV comorbidities are more likely to have severe COVID-19 illness and in-hospital mortality.5., 6., 7. Moreover, hospitalized COVID-19 patients who develop acute cardiac injury, manifesting as elevation in troponin level, constitute a cohort with a particularly poor prognosis.5 , 7 , 8 These clinical observations are corroborated by evidence of various CV pathophysiologic mechanisms in COVID-19 patients.9., 10., 11. Additionally, both the renin-angiotensin-aldosterone system (RAAS)12 and thromboinflammation13 are implicated in COVID-19 pathogenesis. Collectively, these findings highlight a potential prominent role for CV therapies in the management of COVID-19. However, the characteristics of ongoing trials evaluating CV therapies for treatment of COVID-19 are not well described, and have implications for the clinical, investigative, and policy communities, as well as funding agencies. We therefore evaluated registered clinical trials of established CV therapies related to COVID-19 using the ClinicalTrials.gov database, which was established to improve monitoring and conduct of research studies.14
Methods
The ClinicalTrials.gov database was queried on August 7, 2020 using the search terms “COVID-19,” “SARS-CoV-2,” “2019-nCoV,” “severe acute respiratory syndrome coronavirus 2,” and “2019 novel coronavirus” (found at: https://clinicaltrials.gov/ct2/results?cond=COVID-19) for interventional studies. Studies in the following standardized, ClinicalTrials.gov-generated intervention categories were reviewed: “Anti-Arrhythmia Agents,” “Anticoagulants,” “Antihypertensive Agents,” “Cardiotonic Agents,” “Channel Blockers,” “Colchicine,” “Fibrinolytic Agents,” “Hypoglycemic Agents,” “Lipid Regulating Agents,” “Natriuretic Agents,” “Platelet Aggregation Inhibitors,” and “Vasodilator Agents.” Studies evaluating established cardiovascular drugs (e.g., antithrombotics, RAAS related therapies, nitric oxide) or other fibrinolytics (defibrotide) were included. Given the longstanding role of colchicine in the treatment of pericarditis15 and recent evidence demonstrating its benefit in patients with myocardial infarction16 and chronic coronary artery disease,17 it was considered a CV therapy. The database search was then reviewed for studies evaluating extracorporeal membrane oxygenation (ECMO), and these studies were also included for analysis. Trials evaluating CV therapies in combination with non-CV therapies were also included, as these often represent large, network-based, platform trials. Studies evaluating anti-microbial (e.g., hydroxychloroquine, remdesivir), convalescent plasma, non-colchicine anti-inflammatory (e.g., methylprednisolone, tocilizumab), experimental, or other therapies without established CV therapies were excluded, as were studies that were withdrawn or terminated. Three investigators (ASV, DEW, AK) independently reviewed studies for eligibility to ensure all included trials involved evaluation of established CV therapies. No extramural funding was used to support this work. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper, and its final contents.
The following structured data fields were extracted and tabulated in Microsoft Excel (Microsoft Corporation, Redmond, WA) from study-specific ClinicalTrials.gov entries: interventional therapy, study allocation and masking strategy, study phase, primary study purpose (treatment vs. prevention), number of arms, participant inclusion and exclusion criteria, exclusive enrollment of healthcare workers (HCW), desired study enrollment size, primary outcome measures, control group type, estimated or actual study start date, recruitment status, estimated or actual study completion date, and lead sponsor type and location. Study population was categorized as outpatient, inpatient, or both. Studies enrolling inpatients were further categorized as enrolling only intensive care unit (ICU) patients, only non-ICU patients, or all inpatients. Primary endpoints were categorized as mortality only, composite endpoints including mortality, clinical endpoints not including mortality, or surrogate endpoints. Control groups were categorized as placebo, active comparator, usual care, or none. Lead sponsors were categorized as academic, government, industry, or other. Categorical variables are presented as counts and percentages. Maps displaying study lead sponsor locations and COVID-19 prevalence were generated using Tableau Desktop Professional Edition (Tableau Software Inc., Seattle, WA).
Results
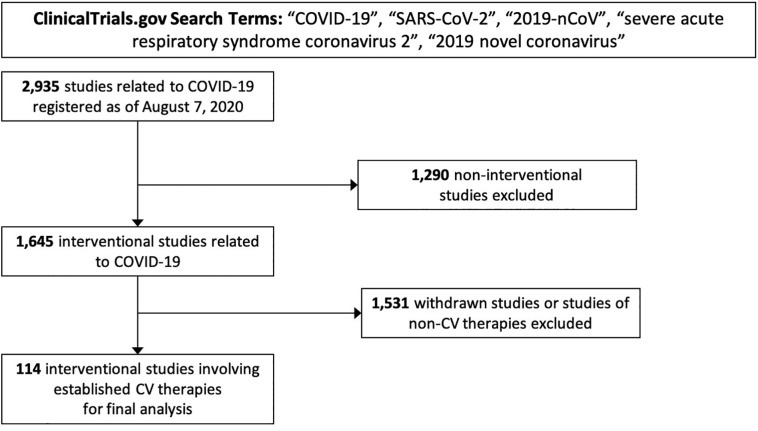
The initial ClinicalTrials.gov query yielded 2,935 registered studies related to COVID-19 registered on or before August 7, 2020. Of these, 1,645 (56.0%) had an interventional study design. Among interventional studies, 114 (6.9%) involved established CV therapies (Figure 1 ).
Figure 1.
Derivation of 114 interventional studies of cardiovascular therapies related to COVID-19 registered on ClinicalTrials.gov for analysis. Abbreviations: COVID-19, coronavirus disease 2019; CV, cardiovascular.
Trial characteristics
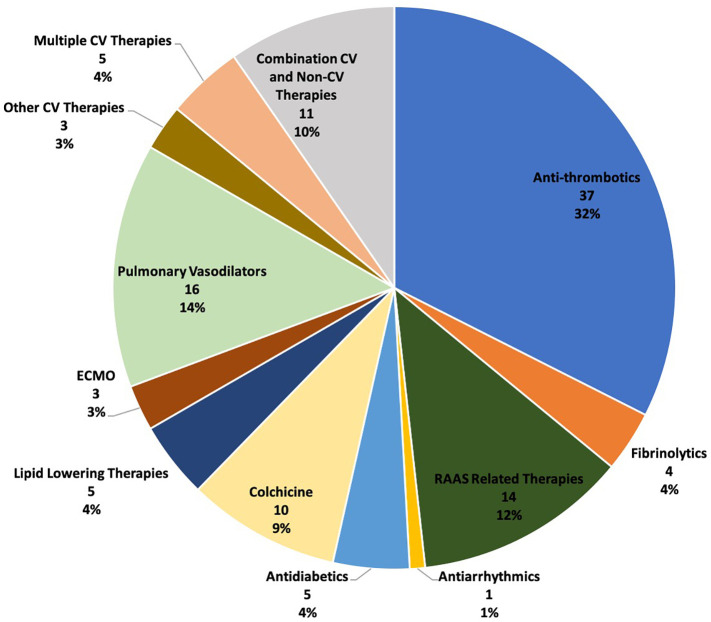
A total of 10 therapeutic categories were represented among trials of CV therapies related to COVID-19 (Figure 2 ). Trials evaluating antithrombotics were the most common (32.5%; 37/114), followed pulmonary vasodilators (14.0%; 16/114), RAAS-related therapies (12.3%; 14/114), and colchicine (8.8%; 10/114). Multiple CV therapies will be assessed in 4.4% (5/114) of studies, and 9.6% (11/114) will evaluated CV therapies in combination with non-CV therapies. Detailed trial characteristics are presented in Table I . Randomized allocation was planned in 87.7% (100/114), and 60.5% (69/114) of trials were open label. The most common control group type was usual care (50.9%; 58/114) and placebo-controlled trials made up 23.7% (27/114) of the analytic study cohort. Most trials were registered as phase 2 or beyond (86.8%; 99/114). The majority of studies planned to enroll less than 1000 participants (86.8%; 99/114) and were registered as single site (55.3%; 63/114). Of the 15 trials planned to enroll at least 1000 participants, 6 (40.0%) were evaluating antithrombotics, 3 (20%) were evaluating CV therapies in combination with non-CV therapies, 2 each (13.3%) were evaluating multiple CV therapies and colchicine, and 1 (6.7%) trial involved icosapent ethyl. Lead sponsors were academic institutions and industry in 79.8% (91/114) and 7.0% (8/114) of studies, respectively.
Figure 2.
Prevalence of cardiovascular therapeutic classes evaluated in analytic trials cohort. Abbreviations: CV, cardiovascular; ECMO, extracorporeal membrane oxygenation; RAAS, renin-angiotensin-aldosterone system.
Table I.
Characteristics of interventional studies related to COVID-19 evaluating cardiovascular therapies registered on ClinicalTrials.gov as of August 7, 2020 (n = 114)
| Characteristic | N (%) |
|---|---|
| Allocation strategy | |
| Randomized | 100 (87.7) |
| Single group | 9 (7.9) |
| Sequential | 2 (1.8) |
| Other | 3 (2.6) |
| Masking | |
| Open label | 69 (60.5) |
| Single | 18 (15.8) |
| Double | 6 (5.3) |
| Triple | 8 (7.0) |
| Quadruple | 13 (11.4) |
| Control group | |
| Placebo | 27 (23.7) |
| Active comparator | 17 (14.9) |
| Usual care | 58 (50.9) |
| None | 11 (9.6) |
| Sham | 1 (0.9) |
| Trial phase | |
| Phase 1 | 3 (2.6) |
| Phase 1/2 | 3 (2.6) |
| Phase 2 | 42 (36.8) |
| Phase 2/3 | 7 (6.1) |
| Phase 3 | 33 (28.9) |
| Phase 4 | 17 (14.9) |
| NA | 9 (7.9) |
| Number of arms | |
| 1 | 10 (8.8) |
| 2 | 87 (76.3) |
| ≥3 | 17 (14.9) |
| Desired enrollment | |
| < 51 | 24 (21.1) |
| 51-100 | 16 (14.0) |
| 101-200 | 21 (18.4) |
| 201-999 | 38 (33.3) |
| ≥1000 | 15 (13.2) |
| Lead sponsor location | |
| Africa | 3 (2.6) |
| Europe | 41 (36.0) |
| North America | 50 (43.9) |
| South America | 9 (7.9) |
| Asia | 10 (8.9) |
| Australia | 1 (0.9) |
| Lead sponsor type | |
| Academic | 91 (79.8) |
| Industry | 8 (7.0) |
| Government | 3 (2.6) |
| Health system | 7 (6.1) |
| Other | 5 (4.4) |
| Number of sites | |
| Single | 63 (55.3) |
| Multiple | 48 (42.1) |
| Unknown / not listed | 3 (2.6) |
| Study population | |
| Outpatient | 17 (14.9) |
| Inpatient | 93 (81.6) |
| ICU only | 15 (16.1)⁎ |
| Non-ICU only | 15 (16.1)⁎ |
| Other or not listed | 4 (3.5) |
| Healthcare workers only | 3 (2.6) |
| Primary outcome | |
| Mortality only | 10 (8.8) |
| Composite including mortality | 54 (47.4) |
| Clinical without mortality | 25 (21.9) |
| Surrogate | 25 (21.9) |
| Nature of non-mortality clinical outcomes | |
| Pulmonary | 30 (26.3) |
| Cardiovascular | 10 (8.8) |
| Ordinal scale | 15 (13.2) |
| Safety | 3 (2.6) |
| Other | 27 (23.7) |
| Primary purpose | |
| Treatment | 107 (93.9) |
| Prevention | 6 (5.3) |
| Other | 1 (0.9) |
| Recruitment status | |
| Recruiting | 79 (69.3) |
| Not yet recruiting | 33 (28.9) |
| Completed | 1 (0.9) |
| Suspended | 1 (0.9) |
Abbreviations: ICU, intensive care unit; NA, not applicable.
% out of studies evaluating inpatients.
Most study populations consisted of patients hospitalized with COVID-19 (81.6%; 93/114). Of 15 trials enrolling ICU patients only, 26.7% (4/15) involved pulmonary vasodilators, 20% (3/15) involved ECMO strategies, and 20% (3/15) involved antithrombotics. Two trials enrolling outpatient HCW only will evaluate inhaled nitric oxide therapy; one trial will evaluate icosapent ethyl in HCW. A total of 14.9% (17/114) of trials will enroll outpatients only, and several therapy classes are represented among these studies.
The most common primary outcome category was a composite including a mortality component (47.4%; 54/114). Non-mortality clinical outcomes (21.9%; 25/114) and surrogate outcomes (21.9%; 25/114) were the next most frequent primary outcome categories, followed by mortality alone (8.8%; 10/114). Pulmonary complications (e.g., need for mechanical ventilation) were most common non-mortality clinical outcome type (26.3%; 30/114) and CV outcomes were the primary outcome type in 8.8% (10/114) of studies. Most trials were designed with a primary purpose of informing COVID-19 treatment (93.9%; 107/114), while 5.3% (6/114) had a primary purpose of informing COVID-19 prevention. At the time of the database search, 69.3% (79/114) of studies were recruiting, one study was completed, one study was suspended, and the remainder (28.9%; 33/114) were not yet recruiting. See eTables 1-9 in the Supplement for additional detailed trial-specific information.
Trial timelines and geography
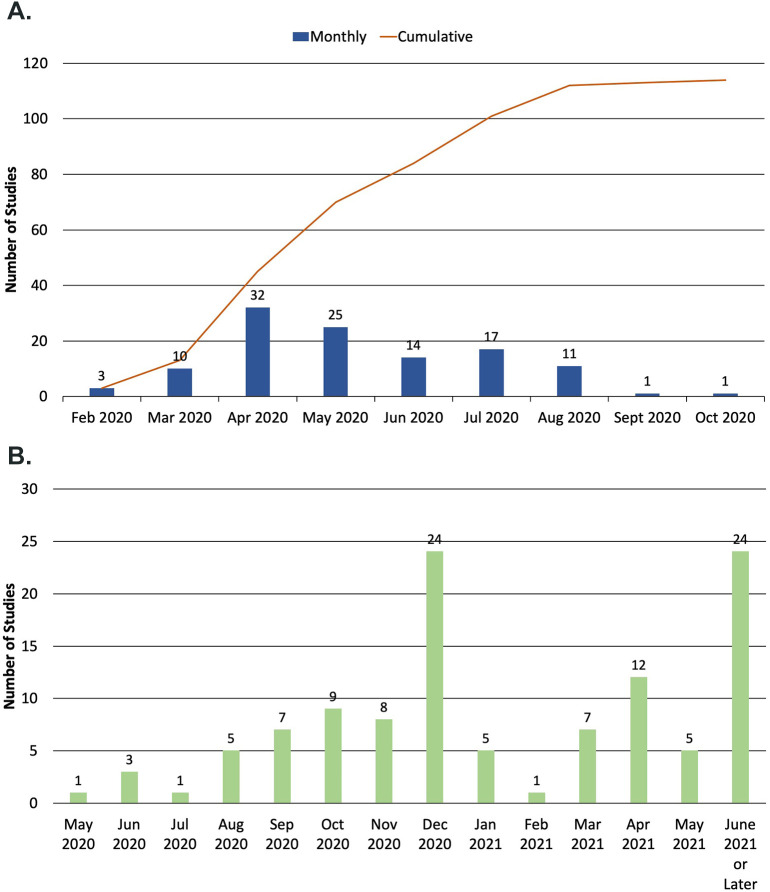
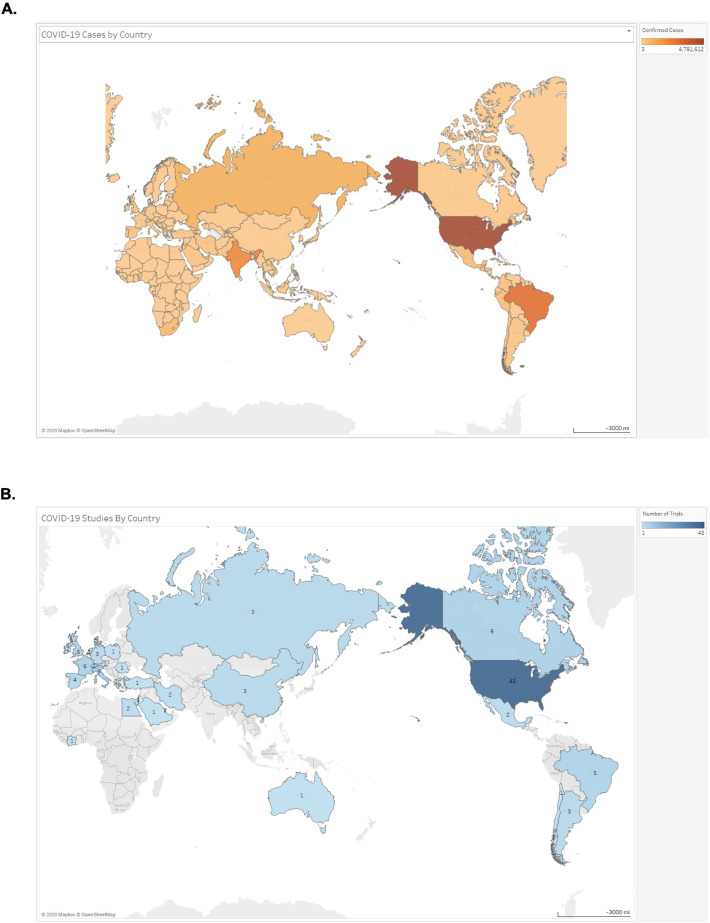
At the time of the ClinicalTrials.gov query, 98.2% (112/114) of trials were planned to begin during or prior to August 2020 (Figure 3A). Most studies were estimated to be complete after December 2020 (67.8%; 78/114) (Figure 3B). Trials planned to end in June 2021 or later comprised 21.1% (24/114) of the analytic cohort. The majority of lead sponsors were located in either North America (43.9%; 50/114) or Europe (36.0%; 41/114) (Figure 4 ). Lead sponsors from Asia, South America, Africa, and Australia were less common.
Figure 3.
A, Monthly estimated or actual start dates of studies in analytic cohort. B, Monthly estimated end dates of studies in analytic cohort.
Figure 4.
A, Geographic prevalence of COVID-19 cases as of August 7, 2020 (data from WHO COVID-19 Situation Report). B, Geographic prevalence of lead sponsors of trials of cardiovascular therapies related to COVID-19 registered on ClinicalTrials.gov as of August 7, 2020.
Discussion
This analysis of clinical trials of CV therapies for COVID-19 registered on ClinicalTrials.gov has the following notable findings: 1) a minority of interventional studies related to COVID-19 registered on ClinicalTrials.gov plan to evaluate CV therapies or CV specific outcomes; 2) most studies are planned to be single center, enroll less than 1000 inpatients, sponsored by European or North American academic institutions; and 3) are estimated to complete after December 2020. Given that the CV system is strongly implicated in COVID-19 pathogenesis and patients with CV comorbidities have increased risk for adverse outcomes with COVID-19, these findings have the potential to inform future investigations of possible COVID-19 therapies. This analysis also provides a quantitative basis for the need for thoughtful application of trial results to clinical practice and policy development by considering important trial design characteristics and limitations.
Characteristics of RCTs of CV therapies related to COVID-19
Approximately 7% of registered clinical trials related to COVID-19 sought to evaluate CV therapies. COVID-19 is caused by SARS-CoV-2 infection and disease-related morbidity primarily manifests as acute respiratory distress syndrome. Thus, it is plausible that most registered trials seek to evaluate anti-microbial and pulmonary therapies. However, CV comorbidities confer a high burden of morbidity and mortality in COVID-19 patients, and the RAAS, thromboinflammation, and CV complications (ST segment elevation myocardial infarction,18 stroke,19 and myocarditis20) have a prominent role in COVID-19 pathogenesis. It is therefore possible that studies evaluating CV therapies are underrepresented in the current COVID-19 trials portfolio. The reasons for this are likely myriad, including evolving insights into COVID-19 disease stages and complications,21 a paucity of granular pathophysiologic data characterizing distinct phenotypes of COVID-19 related cardiac injury, and greater focus on anti-microbial, anti-inflammatory, and convalescent plasma-based therapies among interventional studies.
Studies in the analytic cohort plan to evaluate an array of CV therapies, each of which has varying levels of plausibility for efficacy in COVID-19. RAAS-related therapies, which have widespread use in patients with CV comorbidities, were commonly evaluated. Given that SARS-CoV-2 enters epithelial cells via angiotensin-converting enzyme 2 (ACE2), leads to subsequent downregulation of ACE2 expression,22 and serum angiotensin II levels have been shown to correlate with SARS-CoV-2 viral load and severity of respiratory failure,23 the focus on these therapies among CV drugs is appropriate. Severe COVID-19 is also characterized by abnormalities in hemostatic markers24 and potentially endotheliitis.11 As such, it is apt that most registered studies are designed to evaluate antithrombotic strategies in COVID-19 patients. Colchicine, which reduces levels of inflammatory mediators25 that are correlated with worse outcomes in COVID-19 patients,7 is also appropriately being evaluated. Despite the known endothelial-stabilizing, anti-inflammatory, and beneficial CV effects of statins,26 there are few trials evaluating the potential role of these commonly used and generally well-tolerated drugs in COVID-19 patients.
This analysis also demonstrates that registered CV therapeutic trials are limited in scale and generalizability. Of note, multiple distinct trials seek to evaluate the same therapy, but have differing primary outcomes. Most trials were open label, a placebo control arm was present in less than one quarter, and about 10% of trials had no control group. Such designs may introduce systematic biases27 and results from these trials should be interpreted in the appropriate context before clinical application. Despite widespread COVID-19 prevalence and multiple studies examining the same or similar therapies, most studies aimed to enroll less than 1000 patients and were planned to be single-center.
Findings from this analysis also delineate the timeline of when potentially beneficial CV therapies for COVID-19 may be identified and underscore the need for measured approaches to public policy changes that take into account the time required for meaningful clinical evidence to be generated. While almost all studies in the analytic cohort were scheduled to begin enrollment before August 2020, it is noteworthy that almost one-third of studies were not yet recruiting at the time of the database query. Additionally, over two-thirds of studies are estimated to be completed after December 2020.
The vast majority of lead sponsors of studies analyzed were from either Europe or North America, which is consistent with reported COVID-19 disease prevalence globally.28 However, lack of adequate testing in low income countries may under-estimate the true prevalence of COVID-19 in many regions of the world. Resource-limited countries are particularly vulnerable to devastating ramifications from spread of SARS-CoV-2,29 and studies conducted in Europe and North America may have limited generalizability to these regions based on differences in population prevalence of co-morbidities, environmental factors, and healthcare infrastructure. Therefore, planned RCTs should seek to enroll patients in low income countries when feasible and trials should be established to inform care specifically in these regions. Lastly, if beneficial CV therapies are identified for COVID-19 patients, a global supply chain that could facilitate rapid production, distribution, and utilization of such treatments should be expediently established to maximize therapy access and effectiveness.
Proposed solutions and policy implications for clinical research
Even in the midst of a global pandemic resulting in unprecedented levels of disruption in healthcare and society, it is clear that the CV and broader medical communities remain deeply committed to improving patient outcomes. Large scale, coordinated trial efforts related to COVID-19 are emerging, including the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) initiative by the National Institutes of Health and the recently presented BRACE-CORONA trial, which involved 34 sites and was sponsored by D'Or Institute for Research and Education. However, findings from this analysis highlight the need for continued development of pragmatic, centralized RCT infrastructures that can facilitate efficient, multi-center trials. Integration of research into routine clinical care30 or established registries31 focusing on specific disease states, therapies, or patient populations could help establish such infrastructures.32 Additionally, utilization of adaptive trial designs,33 real-world data, and technology-based platforms for trial enrollment and execution has the potential to substantially decrease trial costs and complexity.34 While such trials are often open label, the benefits of efficient evidence generation using these mechanisms may outweigh the potential for bias introduction, especially if efforts are made to transparently acknowledge pertinent trial limitations upon reporting.
The COVID-19 pandemic also represents an important opportunity to enhance patient engagement in clinical research. Specifically, given the contagious nature of the disease, enabling virtual patient trial participation and endpoint adjudication may facilitate safer and more streamlined enrollment of COVID-19 outpatients. Some of these principles have been applied to COVID-19 related research in HCW35 , 36 and in the Randomised Evaluation of COVID-19 Therapy (RECOVERY) trial.37 However, additional mechanisms for efficient evidence generation are urgently needed to enable evaluation of potential COVID-19 disease-modifying therapies in trials with clinically meaningful endpoints of adequate size and statistical power.38
While important COVID-19 related research efforts have been rapidly funded by local and national mechanisms, the scale of this funding is likely inadequate for the conduct of large, clinically informative RCTs of potential COVID-19 therapies. An alternate approach of funding national or international clinical trials networks dedicated to rapid evaluation of candidate COVID-19 therapies may facilitate a more coordinated and streamlined approach to large-scale RCT execution. This approach may help diminish inefficient trial redundancy. For instance, this analysis found ten registered RCTs evaluating colchicine in hospitalized patients with COVID-19. None of these trials have the same primary outcome, and in aggregate they will likely provide mechanistic insights on the potential role of colchicine in COVID-19 treatment but are unlikely to convincingly establish a therapeutic stance. In the context of a clinical trials network or collaboratory, these RCTs could still be permitted to function independently, but could have established a priori an agreement to pool patient-level data upon trial conclusion and assess clinically meaningful outcomes. Such a strategy could facilitate more efficient evidence generation that influences clinical care and patient outcomes on the rapid time scale necessary.
Moreover, this analysis found limited industry sponsorship of trials of CV therapies in COVID-19 patients. Given the strain the COVID-19 pandemic has placed on the global economy and existing public research infrastructures, an important opportunity exists to expand industry engagement in evidence generation. Outside of traditional sponsorship of patented pharmaceutical trials, corporations should consider funding trials of generic therapies in order to identify patient cohorts that may be relevant for future studies of novel therapeutics, establish collaborations with trial networks and investigators, gain insights into non-pharmacologic aspects of healthcare delivery (e.g., implementation strategies), and raise social capital. Additionally, companies that focus predominantly on medical equipment and sensors have an opportunity to deploy new devices and technologies in such trials and learn valuable information regarding device safety and efficacy, even if the intervention formally being evaluated is a generic medication.
Limitations
This analysis should be interpreted in the context of several limitations. First, the COVID-19 related trials portfolio is rapidly evolving, and this cross-sectional analysis reflects the state of the portfolio on August 7, 2020. It is anticipated that numerous trials not represented in this analysis will be registered on ClinicalTrials.gov as the pandemic progresses and that the characteristics of analyzed trials will change. Second, although registration on ClinicaTrials.gov is required by the US Food and Drug Administration, National Institutes of Health, European Union, World Health Organization, and International Committee of Medical Journal Editors for certain types of clinical trials,39 this database may not reflect all ongoing clinical trials related to COVID-19. Specifically, trials in Asia, Africa, and South America may have been underrepresented and may be registered with other clinical trial registries. Third, extracted data relied on accurate and timely information entered for each trial on respective ClinicalTrials.gov entries. Protocols or published study designs were not available for review for verification. Fourth, although lead sponsor types were tabulated from trial-specific entries, ClinicalTrials.gov does not require detailed reporting of trial funding sources. Given that the majority of lead sponsors were academic medical centers, many of which receive financial support from government entities, this may have led to underappreciation of government funding sources. Fifth, while lead sponsor location is available from ClinicalTrials.gov, not all study entries included detailed information regarding trial sites. Therefore, the geographic distribution of lead sponsor locations may not necessarily reflect the locations of study enrollment and implementation. In addition, the presence of one study location was interpreted as a trial being single site unless a study entry specifically indicated a trial was multi-center. However, it is possible that some trials may be multi-center despite listing only a single study location. Lastly, while interventional studies involving pharmacologic and device therapies are registered, certain types of interventional studies, including health system research studies, implementation science studies, and quality improvement research, may not require ClinicalTrials.gov registration. Thus, these types of studies may be underreported in our analysis.
Conclusions
The unprecedented COVID-19 pandemic has led to an impressive, rapid initiation of numerous clinical trials evaluating potential COVID-19 therapies. Despite a significant role of the CV system in COVID-19 pathophysiology and outcomes, a minority of clinical trials related to COVID-19 registered on ClinicalTrials.gov plan to evaluate CV therapies. Most trials are planned to be single center, enroll less than 1000 inpatients, sponsored by European or North American academic institutions, and estimated to complete after December 2020. Trial results should be applied to clinical practice and policy development by considering important study limitations and design characteristics. Collectively, these findings underscore the need for a network of sites with a platform protocol for rapid evaluation of multiple therapies and generalizability to inform clinical care and health policy for COVID-19 moving forward.
Acknowledgments
Acknowledgements
None
Funding/support
No external funding
Author disclosures/conflicts of interest
Anubodh S. Varshney: T32 postdoctoral training grant from the National Heart, Lung, and Blood Institute (T32HL007604-35). Advisory board for Broadview Ventures.
David E. Wang: None
Ankeet S. Bhatt: T32 postdoctoral training grant from the National Heart, Lung, and Blood Institute (T32HL007604-35). Honorarium from Sanofi Pasteur and participation in a clinical endpoints committee for a trial sponsored by the National Institutes of Health (NIH).
Alexander Blood: T32 postdoctoral training grant from the National Heart, Lung, and Blood Institute (T32HL007604-35).
Musa A. Sharkawi: None
Hasan K. Siddiqi: None
Muthiah Vaduganathan: supported by the KL2/Catalyst Medical Research Investigator Training award from Harvard Catalyst (NIH/NCATS Award UL 1TR002541) and serves on advisory boards for Amgen, AstraZeneca, Baxter Healthcare, Bayer AG, Boehringer Ingelheim, Cytokinetics, and Relypsa.
Peter P. Monteleone: Advisory boards for Medtronic, Biotronik. Institutional grant support from Medtronic, Abbott.
Manesh R. Patel: No direct conflicts pertinent to the development of this paper. Research Grants: Bayer, Janssen, National Heart, Lung, and Blood Institute, Heartflow; Advisory board: Bayer, Janssen, Heartflow.
W. Schuyler Jones: None
Renato D. Lopes: Research support from Bristol-Myers Squibb, GlaxoSmithKline, Medtronic, Pfizer; Consulting fees from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi-Sankyo, GlaxoSmithKline, Medtronic, Merck, Pfizer, Portola.
Deepak L. Bhatt: Advisory Board: Cardax, Cereno Scientific, Elsevier Practice Update Cardiology, Level Ex, Medscape Cardiology, PhaseBio, PLx Pharma, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care, TobeSoft; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Vice-Chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Medtelligence/ReachMD (CME steering committees), Level Ex, MJH Life Sciences, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Abbott, Afimmune, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Cardax, Chiesi, CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Idorsia, Ironwood, Ischemix, Lexicon, Lilly, Medtronic, Pfizer, PhaseBio, PLx Pharma, Regeneron, Roche, Sanofi Aventis, Synaptic, The Medicines Company; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); Site Co-Investigator: Biotronik, Boston Scientific, CSI, St. Jude Medical (now Abbott), Svelte; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Merck, Novo Nordisk, Takeda.
Mandeep R. Mehra: no direct conflicts pertinent to the development of this paper. Other general conflicts include consulting relationships with Abbott (paid to Brigham and Women’s Hospital), Janssen, Mesoblast, Portola, Bayer, NupulseCV, FineHeart, Leviticus, Baim Institute for Clinical Research, Roivant (paid to Brigham and Women’s Hospital) and Triple Gene.
Ajar Kochar: None
Footnotes
Padma Kaul, PhD served as guest editor for this article.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ahj.2020.10.065.
Appendix A. Supplementary data
Supplementary tables
References
- 1.COVID-19 situation reports [Internet]. [cited 2020 May 3]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports.
- 2.RECOVERY Collaborative Group. Horby P., Lim W.S., et al. Dexamethasone in hospitalized patients with COVID-19—Preliminary Report. N Engl J Med. 2020 Jul 17 [Google Scholar]
- 3.Beigel J.H., Tomashek K.M., Dodd L.E., et al. Remdesivir for the treatment of COVID-19—preliminary report. N Engl J Med. 2020 May;22 doi: 10.1056/NEJMc2022236. [DOI] [PubMed] [Google Scholar]
- 4.GBD 2017 Causes of Death Collaborators Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018 10;392(10159):1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo T., Fan Y., Chen M., et al. Cardiovascular implications of fatal outcomes of patients with Coronavirus Disease 2019 (COVID-19) JAMA Cardiol. 2020 Mar;27 doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guan W.-J., Ni Z.-Y., Hu Y., et al. Clinical characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020 Feb;28 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 Mar;11 doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi S., Qin M., Shen B., et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020 Mar 25;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atri D., Siddiqi H.K., Lang J., et al. COVID-19 for the cardiologist: a current review of the virology, clinical epidemiology, cardiac and other clinical manifestations and potential therapeutic strategies. JACC Basic Transl Sci. 2020 Apr 10;5:518–536. doi: 10.1016/j.jacbts.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varga Z., Flammer A.J., Steiger P., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020 Apr;20 doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Libby P., Lüscher T. COVID-19 is, in the end, an endothelial disease. Eur Heart J. 2020 01;41(32):3038–3044. doi: 10.1093/eurheartj/ehaa623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaduganathan M., Vardeny O., Michel T., et al. Renin–angiotensin–aldosterone system inhibitors in patients with COVID-19. New England Journal of Medicine. 2020 Mar 30;0(0) doi: 10.1056/NEJMsr2005760. null. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Connors J.M., Levy J.H. Thromboinflammation and the hypercoagulability of COVID-19. J Thromb Haemost. 2020 Apr;17 doi: 10.1111/jth.14849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCray A.T., Ide N.C. Design and implementation of a national clinical trials registry. J Am Med Inform Assoc. 2000 Jun;7(3):313–323. doi: 10.1136/jamia.2000.0070313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiabrando J.G., Bonaventura A., Vecchié A., et al. Management of acute and recurrent pericarditis. JACC State-of-the-Art Review. J Am Coll Cardiol. 2020 Jan 7;75(1):76–92. doi: 10.1016/j.jacc.2019.11.021. [DOI] [PubMed] [Google Scholar]
- 16.Tardif J.-C., Kouz S., Waters D.D., et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med. 2019 26;381(26):2497–2505. doi: 10.1056/NEJMoa1912388. [DOI] [PubMed] [Google Scholar]
- 17.Nidorf S.M., Fiolet A.T.L., Mosterd A., et al. Colchicine in patients with chronic coronary disease. N Engl J Med. 2020;31 doi: 10.1056/NEJMoa2021372. [DOI] [PubMed] [Google Scholar]
- 18.Bangalore S., Sharma A., Slotwiner A., et al. ST-segment elevation in patients with COVID-19—a case series. N Engl J Med. 2020 Apr;17 doi: 10.1056/NEJMc2009020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oxley T.J., Mocco J., Majidi S., et al. Large-vessel stroke as a presenting feature of COVID-19 in the young. N Engl J Med. 2020 Apr;28 doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inciardi R.M., Lupi L., Zaccone G., et al. Cardiac involvement in a patient with Coronavirus Disease 2019 (COVID-19) JAMA Cardiol. 2020 Mar;27 doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siddiqi H.K., Mehra M.R. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. Journal of Heart Lung Transplantation. 2020;39:405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuba K., Imai Y., Rao S., et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005 Aug;11(8):875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y., Yang Y., Zhang C., et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63(3):364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang N., Li D., Wang X., et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leung Y.Y., Yao Hui L.L., Kraus V.B. Colchicine—update on mechanisms of action and therapeutic uses. Semin Arthritis Rheum. 2015 Dec;45(3):341–350. doi: 10.1016/j.semarthrit.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oesterle A., Laufs U., Liao J.K. Pleiotropic effects of statins on the cardiovascular system. Circ Res. 2017 Jan 6;120(1):229–243. doi: 10.1161/CIRCRESAHA.116.308537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schulz K.F., Grimes D.A. Blinding in randomised trials: hiding who got what. Lancet. 2002 Feb 23;359(9307):696–700. doi: 10.1016/S0140-6736(02)07816-9. [DOI] [PubMed] [Google Scholar]
- 28.COVID-19 Map [Internet]. Johns Hopkins Coronavirus Resource Center. [cited 2020 Apr 27]. Available from: https://coronavirus.jhu.edu/map.html.
- 29.Gilbert M., Pullano G., Pinotti F., et al. Preparedness and vulnerability of African countries against importations of COVID-19: a modelling study. Lancet. 2020 14;395(10227):871–877. doi: 10.1016/S0140-6736(20)30411-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simon G.E., Platt R., Hernandez A.F. Evidence from pragmatic trials during routine care—slouching toward a learning health system. N Engl J Med. 2020 Apr 16;382(16):1488–1491. doi: 10.1056/NEJMp1915448. [DOI] [PubMed] [Google Scholar]
- 31.James S., Rao S.V., Granger C.B. Registry-based randomized clinical trials—a new clinical trial paradigm. Nat Rev Cardiol. 2015 May;12(5):312–316. doi: 10.1038/nrcardio.2015.33. [DOI] [PubMed] [Google Scholar]
- 32.Fanaroff A.C., Califf R.M., Lopes R.D. New approaches to conducting randomized controlled trials. J Am Coll Cardiol. 2020 Feb 11;75(5):556–559. doi: 10.1016/j.jacc.2019.11.043. [DOI] [PubMed] [Google Scholar]
- 33.Bhatt D.L., Mehta C. Adaptive designs for clinical trials. N Engl J Med. 2016 Jul 7;375(1):65–74. doi: 10.1056/NEJMra1510061. [DOI] [PubMed] [Google Scholar]
- 34.Marquis-Gravel G., Roe M.T., Robertson H.R., et al. Rationale and Design of the Aspirin Dosing-A Patient-Centric Trial Assessing Benefits and Long-term Effectiveness (ADAPTABLE) trial. JAMA Cardiol. 2020 Mar:18. doi: 10.1001/jamacardio.2020.0116. [DOI] [PubMed] [Google Scholar]
- 35.HERO [Internet]. HERO Research. [cited 2020 Apr 27]. Available from: https://heroesresearch.org/.
- 36.Healthcare Worker Exposure Response and Outcomes of Hydroxychloroquine - Full Text View - ClinicalTrials.gov [Internet]. [cited 2020 May 3]. Available from: https://clinicaltrials.gov/ct2/show/NCT04334148.
- 37.Welcome — RECOVERY Trial [Internet]. [cited 2020 Apr 27]. Available from: https://www.recoverytrial.net/.
- 38.Wang X., Bhatt D.L. COVID-19: an unintended force for medical revolution? J Invasive Cardiol. 2020;32(4):E81–E82. [PubMed] [Google Scholar]
- 39.Why Should I Register and Submit Results? - ClinicalTrials.gov [Internet]. [cited 2020 Apr 27]. Available from: https://clinicaltrials.gov/ct2/manage-recs/background.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables