Summary
Background
Content‐valid and clinically meaningful instruments are required to evaluate outcomes of therapeutic interventions in alopecia areata (AA).
Objectives
To develop an Investigator's Global Assessment (IGA) to interpret treatment response in AA treatment studies.
Methods
Qualitative interviews were conducted in the USA with expert dermatologists and with patients with AA who had experienced ≥ 50% scalp‐hair loss. Thematic data analysis identified critical outcomes and evaluated the content validity of the new IGA.
Results
Expert clinicians (n = 10) judged AA treatment success by the amount of scalp‐hair growth (median 80% scalp hair). Adult (n = 25) and adolescent (n = 5) patients participated. Scalp‐hair loss was the most bothersome AA sign/symptom for most patients. Perceived treatment success – short of 100% scalp hair – was the presence of ~ 70–90% scalp hair (median 80%). Using additional clinician and patient insights, the Alopecia Areata Investigator Global Assessment (AA‐IGA™) was developed. This clinician‐reported outcome assessment is an ordinal, static measure comprising five severity categories of scalp‐hair loss. Nearly all clinicians and patients in this study agreed that, for patients with ≥ 50% scalp‐hair loss, successful treatment would be hair regrowth resulting in ≤ 20% scalp‐hair loss.
Conclusions
We recommend using the Severity of Alopecia Tool to assess the extent (0–100%) of scalp‐hair loss. The AA‐IGA is a robust ordinal measure providing distinct and clinically meaningful gradations of scalp‐hair loss that reflects patients’ and expert clinicians’ perspectives and treatment expectations.
What is already known about this topic?
The Severity of Alopecia Tool is widely used to assess the extent of scalp‐hair loss in patients with alopecia areata.
Guidelines define treatment success as a 50% improvement in scalp hair, and clinical trials have used dynamic thresholds of 50% and 90%.
However, there is no clinical consensus on these endpoints, and patient perspectives on treatment success are unknown.
What does this study add?
Through qualitative interviews with 10 expert dermatologists and 30 patients with alopecia areata who had experienced ≥ 50% scalp‐hair loss, we developed the Alopecia Areata Investigator Global Assessment (AA‐IGA™) to measure five clinically meaningful gradations of alopecia areata scalp‐hair loss that reflects patients’ and clinicians’ perspectives and expectations of treatment success in alopecia areata treatment studies.
What are the clinical implications of this work?
The AA‐IGA is a robust ordinal measure that can inform clinical evaluation of alopecia areata treatment outcomes.
The AA‐IGA can be used to determine clinically meaningful treatment success for alopecia areata, with success defined by patients and clinicians as reaching ≤ 20% scalp‐hair loss.
Linked Comment: Blome. Br J Dermatol 2020; 183:609.
Short abstract
Linked Comment: Blome. Br J Dermatol 2020; 183:609.
Plain language summary available online
To evaluate alopecia areata (AA) treatments, clinicians, regulators and pharmaceutical developers require valid, clinically meaningful outcome measures. In the era of patient‐focused drug development, patients’ experiences, perspectives and priorities must be meaningfully incorporated into the evaluation of medical products.1, 2, 3, 4, 5 The Food and Drug Administration (FDA) have noted the need to develop better endpoints for clinical trials to measure those aspects of AA that are important to patients.6 This should include well‐defined and reliable clinician‐reported and patient‐reported outcomes measures that capture clinicians’ and patients’ evaluations of treatment success.
The Severity of Alopecia Tool (SALT) is widely used to assess the extent of scalp‐hair loss in AA.7, 8 Recent AA treatment trials have used widely varying dynamic thresholds of 50% and 90% improvement from baseline in SALT scores to define treatment success,9, 10, 11, 12, 13 and 50% improvement (SALT50) is described in assessment guidelines.7, 14 However, patient perspectives on a success threshold remain unknown.
The first step in the development of a new outcome measure is to establish the content validity of the measure in the intended population of interest. Content validity is the extent to which the instrument measures the concept of interest and is supported by evidence from qualitative studies that demonstrate the measure is appropriate, relevant, easily understood and clinically meaningful both to clinicians and to patients within the population of interest.1, 3
Therefore, the objectives of this noninterventional, cross‐sectional, qualitative interview study were (i) to understand clinicians’ and patients’ perspectives and expectations of a clinically meaningful treatment outcome and (ii) to develop a content‐valid Investigator's Global Assessment (IGA) to measure distinct and clinically relevant gradations of scalp‐hair loss support the definition of treatment success in AA treatment studies.
Patients and methods
This study was designed to elicit qualitative evidence, which was generated from clinician and patient input, to develop a new content‐valid IGA through methodology aligned with the FDA guidance for industry, and the International Society for Pharmacoeconomics and Outcomes Research Good Practice for Clinician Reported Outcome Assessments of Treatment Benefit.1, 3
Clinician interviews
Leading AA clinical experts practising in the United States were identified by their contemporary research involvement and expertise in treating patients with AA. A semistructured interview guide was developed to guide enquiry systematically. Open‐ended questions explored clinicians’ insights into the diagnosis and treatment of patients with AA and their opinions on the amount of scalp hair that would be a clinically meaningful threshold for treatment success (concept elicitation).3
To develop the IGA content, clinicians were provided with draft item wording and a five‐category ordinal response scale with hair loss descriptors (e.g. ‘None’, ‘Limited’, ‘Moderate’, ‘Severe’, ‘Complete’) and blank percentage ranges to correspond with these descriptors. Cognitive interviewing techniques were utilized to ask clinicians to review the item wording and descriptors and provide corresponding percentage ranges of scalp‐hair loss.3 Finally, clinicians were asked to identify the categories (and percentage ranges) that could indicate treatment success. The interviews also explored several other outcome measures; development and validation of these measures are to be reported elsewhere.
Interviews lasted 60 min and were audio recorded and transcribed verbatim. Transcripts were analysed in ATLAS.ti (version 7·5; Scientific Software Development GmbH, Berlin, Germany). Open‐ended data were thematically analysed:15 firstly, the transcripts were read by the analysts, and overarching ideas were identified; secondly, descriptive codes were generated and assigned to quotes within the transcripts; thirdly, the codes were grouped into themes, which were compared and contrasted to identify relationships; and, finally, key concepts and representative quotes were extracted. Cognitive interview data were subject to framework analysis;16 predefined codes were applied to explore the relevance and appropriateness of the IGA.
Small panel meeting
Any discrepancies in findings were reviewed in a small panel meeting with two expert clinicians. By reviewing the key quotes in the study data and incorporating their clinical expertise in AA, these clinicians reached consensus and finalized a draft IGA prior to testing with patients.
Patient interviews
To further evaluate the content validity of the IGA, patients’ input was sought. The protocol (2016‐4951) was reviewed and approved by the Western Institutional Review Board (reference number 20171820).
Eligible patients with AA who had experienced ≥ 50% scalp‐hair loss (Figure 1) were identified by clinicians at Yale University and the University of California, Irvine, and invited to participate in 90‐min, one‐to‐one, face‐to‐face, semistructured interviews. Purposive sampling targeted the overenrolment of patients with eyebrow and/or eyelash involvement, and patients who had been successfully treated for their AA with oral Janus kinase (JAK) inhibitors, to understand their perception of treatment success. Additional a priori sampling quotas sought diverse age, sex, race/ethnicity and education‐level representation across the targeted patient sample size (n = 30 patients), which reflects recent recommendations for clinical outcome assessment development.17
Figure 1.
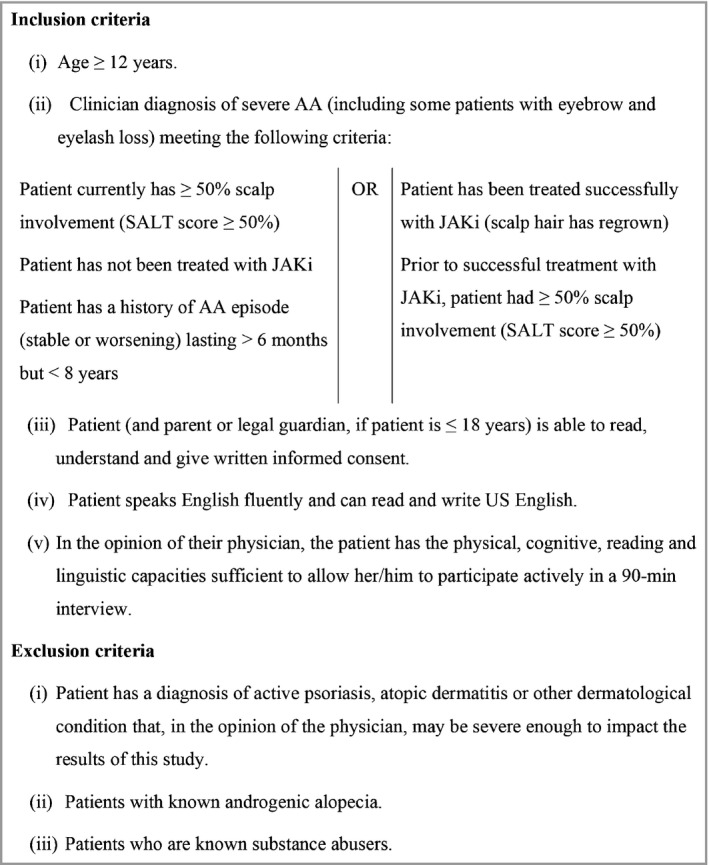
Patient eligibility criteria. AA, alopecia areata; JAKi, Janus kinase inhibitors; SALT, Severity of Alopecia Tool.
Insights from the clinicians informed a semistructured interview guide, developed to systematically explore patients’ experience of AA signs and symptoms and the amount and type of improvements they would consider clinically meaningful during treatment (concept elicitation). Time permitting, the guide also explored patients’ acceptance, opinions and interpretation of several AA outcome measures, including the newly developed IGA. Interviews were conducted by an experienced qualitative researcher trained in techniques for clinical outcome assessment development. Recording, transcription and analyses were undertaken for patient interviews as described for the clinician interviews.
Researcher characteristics/reflexivity and techniques to reduce bias
K.W.W., F.P.N., Y.D., N.M., J.M.K. and B.A.K. have considerable experience and expertise in AA because of their past research and/or clinical experience. N.M., J.M.K. and B.A.K. were interviewed for this study, J.M.K. and B.A.K. comprised the small panel, and B.A.K. and N.M. were involved in patient recruitment. Development of the interview guide, interview conduct and primary analysis were undertaken by H.K., N.V.J.A., S.K. and J.M., as a separate, multidisciplinary team, who could employ an inductive approach because of less prior AA knowledge.
Results
Clinician interview results
Sample
Ten expert dermatologists from specialist clinics and university hospitals participated in the telephone interviews. The clinicians regularly treated patients with AA; the AA experience of the clinicians ranged from 9 years to 45 years (mean 23·3).
Characterizing scalp‐hair loss
Clinicians characterized AA by scalp‐hair loss to varying extents. Clinicians noted that many patients with AA experience scalp‐hair loss only and highlighted the profound importance of this symptom, including the psychosocial impact.
Clinician perceptions of clinically meaningful treatment success
Clinicians noted that, in nearly every case, they ‘treated to the scalp’, with other areas (e.g. eyebrows, eyelashes and body‐hair loss) targeted only if the absence of hair was bothersome to the patient. Clinicians emphasized that clinically meaningful treatment success was a combination of the amount of scalp‐hair growth, density, location and quality, with an emphasis on amount. When asked to describe the amount of scalp hair indicative of treatment success, responses were 90% (n = 1 clinician), 80% (n = 5 clinicians) and 75% (n = 3 clinicians), while one clinician strongly preferred a ≥ 50% change (dynamic) metric vs. a static amount. All clinicians noted that only the amount of terminal hair regrowth, not vellus hair, would be considered.
Iterative development of the Alopecia Areata Investigator Global Assessment
Clinicians reviewed a draft IGA (‘Please rate the patient's scalp‐hair loss, as it looks today’) with five response categories (‘None’ = 0, ‘Limited’ = 1, ‘Moderate’ = 2, ‘Severe’ = 3, and ‘Complete’ = 4) and described their reactions to the item wording, recall period and response categories. When asked whether ‘scalp‐hair loss’ was the correct measurement concept of interest (vs. ‘scalp‐hair growth’), most clinicians (n = 8 clinicians) considered ‘loss’ more easily assessed and a reflection of disease activity. All clinicians agreed that a static assessment of the patient ‘today’ was appropriate.
Response categories and associated percentages
Nine clinicians agreed that the response category descriptors were appropriate and represented distinct levels of scalp involvement; one clinician questioned whether ‘Severe’ and ‘Complete’ were clinically different categories. Several clinicians spontaneously noted that including percentages would improve interrater reliability and reduce subjectivity because it would provide quantitative guidance for each level. Nine clinicians then provided their initial percentage ranges of scalp‐hair loss for each category descriptor (Figure 2).
Figure 2.
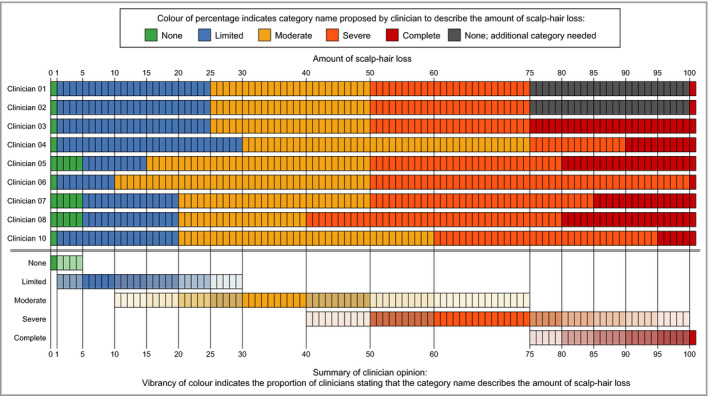
Initial clinician‐proposed percentage ranges for each of the draft Alopecia Areata Investigator Global Assessment category descriptors (note that Clinician 09 did not complete this task).
Clinicians then reviewed proposed percentage ranges based on prior clinical input (‘None’, 0%; ‘Limited’, 1–20%; ‘Moderate’, 21–49%; ‘Severe’, 50–99%; and ‘Complete’, 100%) and provided their feedback. Consistency was observed in the revised percentage ranges of scalp‐hair loss provided for the ‘None’, ‘Limited’ and ‘Moderate’ categories, and there was full agreement that the ‘Severe’ category should include patients with at least 50% scalp‐hair loss (Figure 3).
Figure 3.
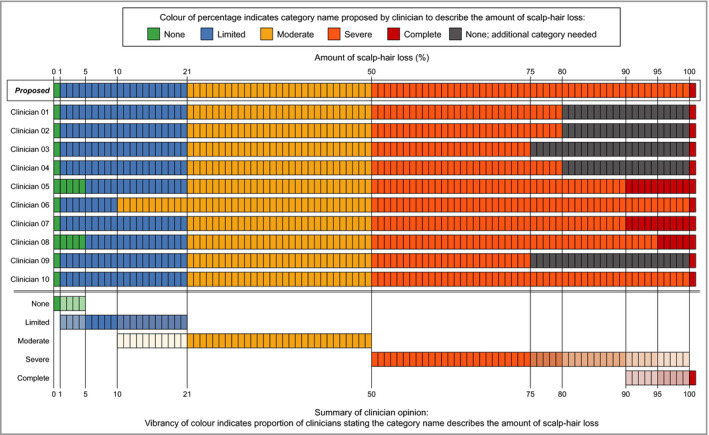
Revised clinician opinions on the percentage ranges proposed for inclusion in the draft Alopecia Areata Investigator Global Assessment.
Small panel review of the draft Alopecia Areata Investigator Global Assessment
Two expert clinicians reviewed the detailed clinician data on the proposed category descriptors and associated percentages, with a focus on the larger hair‐loss range, where the greatest inconsistencies were observed (Figure 3). The clinicians proposed that the fifth category descriptor of the IGA be ‘Very Severe’ and also include nearly complete scalp‐hair loss (95–99% hair loss), a patient presentation that is clinically very similar to 100% scalp‐hair loss.18 These changes resulted in the final version of the Alopecia Areata Investigator Global Assessment (AA‐IGA) (Figure 4).
Figure 4.
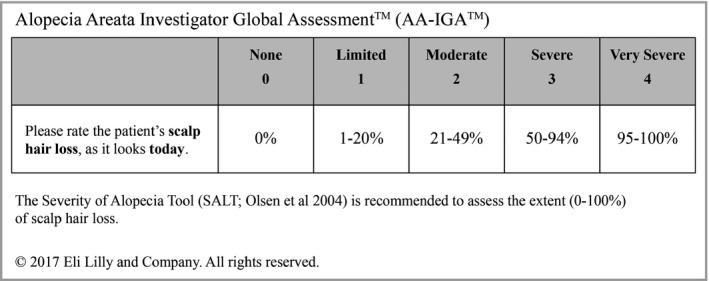
The Alopecia Areata Investigator Global Assessment scale (final version; reviewed in patient interviews).
Patient interview results
Sample
Thirty patients participated [57% female, mean age 35·2 years (range 15–72), mean SALT scores 57·9 (range 0–100)]. Most patients (n = 24 patients; 80%) had eyebrow and/or eyelash involvement. Eighteen patients (60%) were currently being treated with oral JAK inhibitors or had previously been treated with them. Thirteen patients (43%) were receiving no AA treatment at the time of interview.
Patient perceptions of scalp‐hair loss
Although patients with eyebrow and/or eyelash involvement were purposely overenrolled, scalp‐hair loss was the most bothersome AA sign/symptom for 77% (n = 23 patients) of the sample because it affected their appearance and confidence, and limited their ability to participate in some activities, due to fear of their wig being dislodged and scalp being revealed. Patients with SALT scores < 100% (n = 19 patients) were asked to describe their current scalp hair. Of these, some patients included descriptions of hair density (n = 9 patients) and/or length (n = 6 patients) but, overall, their primary consideration was the amount of scalp hair (n = 18 patients).
Patient perceptions of clinically meaningful treatment success
With the goal of understanding a meaningful treatment benefit, all patients were asked what amount of scalp hair – short of 100% – they would consider a treatment success (Figure 5). Most patients perceived treatment success as having ~ 70–90% of their scalp hair (median 80%). Within key patient subgroups, perceived treatment success was 75% scalp hair for patients who had previously been treated with JAK inhibitors, 85% scalp hair for patients who had not previously been treated with JAK inhibitors, 78% for adults and 85% for adolescents (all median values).
Figure 5.
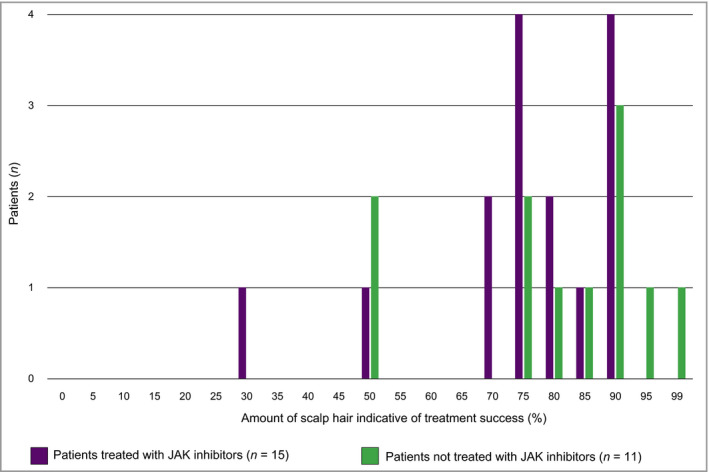
Patients’ perceived thresholds of treatment success (note that four patients did not spontaneously provide an answer when queried). JAK, Janus kinase.
Twenty patients were queried about the location of hair growth following treatment. Twelve patients felt location was important; some commented that, if the amount was enough in some locations, they could cover any missing patches in others. However, eight patients did not consider location important and were focused on achieving an acceptable amount of scalp hair.
Patient review of the Alopecia Areata Investigator Global Assessment
All patients confirmed the appropriateness of the AA‐IGA (Figure 4) and the specific gradations. Nineteen patients were queried on the importance and relevance of this clinician assessment, and all confirmed it was an important question. Six patients spontaneously commented how clinicians could provide a more accurate answer than patients could because the former are trained in assessing scalp‐hair loss and can view the whole head.
Clinically meaningful change
Nine patients were asked whether achieving the ‘Limited (1–20% hair loss)’ category on the IGA, following treatment lasting up to 9 months, would indicate the treatment was successful, to understand their perception of treatment success. All nine felt that reaching 20% hair loss would be successful; two patients noted that 100% of scalp hair would be true success but considered 20% loss as ‘mostly successful’. No notable response differences were observed between the patients who had been treated with JAK inhibitors (n = 4 patients) and those who had not (n = 5 patients).
Discussion
The development and publication in 2004 of the SALT was a key milestone in the AA field, providing a standardized method to derive a 0–100% scalp‐hair loss score.7 Building on this achievement, the AA‐IGA provides an ordinal, static measure with five distinct clinical gradations of SALT scores. The AA‐IGA is a meaningful clinician‐reported measure of scalp‐hair loss, reflecting patients' and expert clinicians' perspectives and treatment expectations. Nearly all clinicians and patients in this study confirmed that successful treatment of patients with ≥ 50% scalp‐hair loss would be hair regrowth resulting in ≤ 20%.
Creating the AA‐IGA required clinician and patient input to derive the key gradations in the scale.18 The first level (‘None’, 0%) indicates the absence of any form of scalp‐hair loss. The second level (‘Limited’, 1–20%) required in‐depth exploration and, in spite of the very broad range of possibilities, there was great consistency both within and between clinicians and patient groups on a meaningful treatment benefit level. Moreover, nonsystemic control of disease is possible with topical treatments and/or intralesional corticosteroid injections at this level. The lower limit of the fourth level (‘Severe’, 50–94%) is ubiquitous in the AA scientific literature and treatment guidelines, in which severe disease has consistently been noted at the ≥ 50% scalp‐hair loss level,8, 11, 14, 18, 19, 20, 21, 22, 23 thus defining the third level (‘Moderate’, 21–49%). Changing ‘Complete’ (100%) to ‘Very Severe’ (95–100%) resolved the discordance in the clinician estimates between the fourth and fifth levels. The proposed ‘Very Severe’ category describes the clinical presentation of patients who have lost all or nearly all of their scalp hair, and avoids the ‘alopecia totalis’ and ‘alopecia universalis’ classification terminology, which has been used in the literature to describe various degrees of hair loss.18
Using the SALT to assess the extent (0–100%) of scalp‐hair loss is recommended, as noted in Figure 4.7 The AA‐IGA, in turn, provides distinct and clinically meaningful gradations of scalp‐hair loss. Because the AA‐IGA score is a directly mapped function of the SALT score, repeatability of SALT scores across clinicians (assessing the same patients) and at different timepoints by the same clinician (reassessing stable patients) yields perfect AA‐IGA inter‐ and intrarater reliability, addressing a key psychometric consideration for clinician‐reported outcome assessments.
There are some limitations of this study. Firstly, all participants lived in the USA, and most patients were adults; therefore, the findings may not be immediately generalizable to other countries or age groups without further confirmation. Secondly, patients with successful treatment with JAK inhibitors were overenrolled, to allow the researchers to understand the patient perception of treatment benefit in the context of actual experience of successful regrowth. Thirdly, the proportion of patients with eyebrow and/or eyelash involvement may not be representative of the overall AA population, as these patients were overenrolled to understand fully the importance of scalp‐hair loss among patients with additional AA signs/symptoms. Fourthly, personal biases can influence qualitative interpretation, although this was mitigated by (i) having a multidisciplinary research team and (ii) using structured tasks that lessened elements of interpretation bias. Finally, the AA‐IGA does not have an accompanying photo guide, which is often provided with new dermatologic measures, owing to the developers’ recommendation to conduct a SALT assessment of scalp‐hair loss to determine the AA‐IGA score. This is because there are an infinite number of possible patient presentations of scalp‐hair loss at any level across the SALT 1–99% range.
While patients have confirmed that clinicians are important and informed raters of scalp‐hair loss, it is also valuable for patients to provide their own scalp‐hair assessments; therefore, a corresponding patient‐reported outcome (Scalp Hair Assessment PRO™) has been developed from the full interview findings.24 Moreover, several other key signs/symptoms were noted by patients and clinicians as important outcomes to measure in AA treatment trials (e.g. eyebrow involvement, eyelash involvement, eye irritation and nail damage). Further publications will describe the development of these clinician‐reported outcome and patient‐reported outcome measures.
In conclusion, the AA‐IGA is a robust ordinal measure comprising five categories of SALT scores that represent represents a distinct and clinically meaningful gradations of AA severity. Reflecting clinicians’ and patients’ perspectives and treatment expectations, this clinician‐reported outcome assessment provides an outcome measure of successful treatment for AA treatment studies.
Acknowledgments
The authors thank the expert clinicians who provided their thoughtful input during this study; these include, in alphabetical order, George Cotsarelis, Maria Hordinsky, Julian Mackay‐Wiggan, Amy Paller, Janet Roberts and Antonella Tosti, as well as one other clinician who chose to remain anonymous.
The authors also thank the patients interviewed in this study, who shared their alopecia areata symptoms and life impacts with great clarity and courage and who embraced the challenges of providing a quantitative estimate of treatment success and reviewing the AA‐IGA categorizations of scalp‐hair loss. Finally, the authors thank the site coordinators and staff at the Department of Dermatology, University of California, Irvine, and at the Yale University School of Medicine, who facilitated the recruitment of and access to these patient interviewees.
Funding Sources Eli Lilly and Company, a pharmaceutical company, funded DRG Abacus to conduct this study. DRG Abacus is a provider of health economics, outcomes research and market access services to the pharmaceutical and medical device industry.
Conflicts of interest H.K., S.K., N.V.J.A. and J.M. are employees of DRG Abacus, a health economic and outcomes research consultancy that consults with various pharmaceutical companies. F.P.N. and Y.D. are employees and stockholders at Eli Lilly and Company. K.W.W. was an employee and stockholder at Eli Lilly and Company when this research was conducted; she is now an employee and stockholder at Pfizer Inc. B.A.K. has served on advisory boards and is a consultant and clinical trial investigator for Eli Lilly and Company; he is a consultant for Aclaris Therapeutics, Inc., Eli Lilly and Company, Concert Pharmaceuticals, Inc., Pfizer Inc. and Dermavant Sciences, Inc. J.M.K. is a consultant for Eli Lilly and Company. Eli Lilly and Company have commissioned DRG Abacus, J.M.K. and B.A.K. to consult on clinical outcomes assessment strategies for alopecia areata.
Plain language summary available online
References
- 1. US Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research, Center for Devices and Radiological Health . Guidance for Industry Patient‐Reported Outcome Measures: Use in Medical Product Development To Support Labeling Claims. Food and Drug Administration, 2009. Available at: http://www.fda.gov/media/77832/download (last accessed 2 February 2020). [Google Scholar]
- 2. US Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research . Patient‐Focused Drug Development: Collecting Comprehensive and Representative Input. Guidance for Industry, Food and Drug Administration Staff, and Other Stakeholders. Draft Guidance. Food and Drug Administration, 2018. Available at: http://www.fda.gov/media/113653/download (last accessed 2 February 2020). [Google Scholar]
- 3. Powers JH, Patrick DL, Walton MK et al Clinician‐reported outcome assessments of treatment benefit: report of the ISPOR Clinical Outcome Assessment Emerging Good Practices Task Force. Value Health 2017; 20:2–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Walton MK, Powers JH 3rd, Hobart J et al Clinical outcome assessments. Conceptual foundation: report of the ISPOR Clinical Outcomes Assessment‐Emerging Good Practices for Outcomes Research Task Force. Value Health 2015; 18:741–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. US Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research . Qualification Process for Drug Development Tools: Guidance for Industry and FDA Staff. Draft Guidance. Food and Drug Administration, 2019. Available at: http://www.fda.gov/media/133511/download (last accessed 2 February 2020). [Google Scholar]
- 6. US Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research . The Voice of the Patient: A Series of Reports from the U.S. Food and Drug Administration's (FDA's) Patient‐Focused Drug Development Initiative. Alopecia Areata. Food and Drug Administration, 2018. Available at: http://www.fda.gov/media/112100/download (last accessed 2 February 2020). [Google Scholar]
- 7. Olsen EA, Hordinsky MK, Price VH et al Alopecia areata investigational assessment guidelines: part II. J Am Acad Dermatol 2004; 51:440–7. [DOI] [PubMed] [Google Scholar]
- 8. Renert‐Yuval Y, Guttman‐Yassky E. The changing landscape of alopecia areata: the therapeutic paradigm. Adv Ther 2017; 34:1594–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mackay‐Wiggan J, Jabbari A, Nguyen N et al Oral ruxolitinib induces hair regrowth in patients with moderate‐to‐severe alopecia areata. JCI Insight 2016; 1:e89790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kennedy Crispin M, Ko JM, Craiglow BG et al Safety and efficacy of the JAK inhibitor tofacitinib citrate in patients with alopecia areata. JCI Insight 2016; 1:e89776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu LY, Craiglow BG, Dai F, King BA. Tofacitinib for the treatment of severe alopecia areata and variants: a study of 90 patients. J Am Acad Dermatol 2017; 76:22–8. [DOI] [PubMed] [Google Scholar]
- 12. Craiglow BG, Liu LY, King BA. Tofacitinib for the treatment of alopecia areata and variants in adolescents. J Am Acad Dermatol 2017; 76:29–32. [DOI] [PubMed] [Google Scholar]
- 13. Guttman‐Yassky E, Nia JK, Hashim PW et al Efficacy and safety of secukinumab treatment in adults with extensive alopecia areata. Arch Dermatol Res 2018; 310:607–14. [DOI] [PubMed] [Google Scholar]
- 14. Olsen EA, Roberts J, Sperling L et al Objective outcome measures: collecting meaningful data on alopecia areata. J Am Acad Dermatol 2018; 79:470–8.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Braun V, Clarke V, Terry G. Thematic analysis. Qual Res Clin Health Psychol 2014; 24:95–114. [Google Scholar]
- 16. Ritchie J, Lewis J, McNaughton Nicholls C, Ormston R, eds. Qualitative Research Practice: A Guide for Social Science Students and Researchers, 2nd edn Los Angeles: SAGE Publications, 2013. [Google Scholar]
- 17. Turner‐Bowker DM, Lamoureux RE, Stokes J et al Informing a priori sample size estimation in qualitative concept elicitation interview studies for clinical outcome assessment instrument development. Value Health 2018; 21:839–42. [DOI] [PubMed] [Google Scholar]
- 18. Wambier CG, King BA. Rethinking the classification of alopecia areata. J Am Acad Dermatol 2019; 80:e45. [DOI] [PubMed] [Google Scholar]
- 19. Shapiro J. Current treatment of alopecia areata. J Investig Dermatol Symp Proc 2013; 16:S42–4. [DOI] [PubMed] [Google Scholar]
- 20. You HR, Kim S‐J. Factors associated with severity of alopecia areata. Ann Dermatol 2017; 29:565–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Olsen E, Hordinsky M, McDonald‐Hull S et al Alopecia areata investigational assessment guidelines. National Alopecia Areata Foundation. J Am Acad Dermatol 1999; 40:242–6. [DOI] [PubMed] [Google Scholar]
- 22. Strober BE, Siu K, Alexis AF et al Etanercept does not effectively treat moderate to severe alopecia areata: an open‐label study. J Am Acad Dermatol 2005; 52:1082–4. [DOI] [PubMed] [Google Scholar]
- 23. Liu LY, King BA, Craiglow BG. Alopecia areata is associated with impaired health‐related quality of life: a survey of affected adults and children, and their families. J Am Acad Dermatol 2018; 79:556–8. [DOI] [PubMed] [Google Scholar]
- 24. Wyrwich K, Kitchen H, Knight S et al Development of the Scalp Hair Assessment Patient‐Reported Outcome™ measure for alopecia areata. Br J Dermatology 2020; 10.1111/bjd.19024 [DOI] [PMC free article] [PubMed] [Google Scholar]


