Abstract
Increased levels of interleukin‐6 (IL‐6) contribute to the development of polycystic ovary syndrome (PCOS); in renal cell carcinoma, the long non‐coding RNA (lncRNA) SRLR upregulates IL‐6. In this study, we demonstrated that the levels of the lncRNA SRLR were upregulated in PCOS patients with high expression of plasma IL‐6 compared with heathy females. The levels of the lncRNA SRLR in the plasma had a positive correlation with expression of IL‐6 in patients with PCOS but not in healthy females. Upregulation of the lncRNA SRLR in plasma could distinguish PCOS patients from healthy females. Overexpression of the lncRNA SRLR led to upregulation of IL‐6 and promoted apoptosis of human granulosa‐like tumour cells (KGN). Therefore, the lncRNA SRLR participated in PCOS by regulating cell apoptosis and IL‐6 expression.
Significance of the Study
The lncRNA SRLR mediates its effects on apoptosis and IL‐6 expression in PCOS and could be used to distinguish PCOS patients from healthy controls. Plasma circulating levels of the lncRNA SRLR may be a potential target for the treatment of PCOS.
Keywords: apoptosis, granulosa‐like tumour cells, interleukin‐6, lncRNA SRLR, polycystic ovary syndrome
1. INTRODUCTION
Polycystic ovary syndrome (PCOS) is an endocrine disease which is characterized by polycystic ovary morphology, hyperandrogenism, and ovulation dysfunction.1, 2 It is a very common disease worldwide, and it has been reported that approximately 5% to 20% of females will develop PCOS during their reproductive age.3 While PCOS is not life‐threatening, it is a leading cause of anovulatory infertility.4 In addition, patients with PCOS have a high risk of cardiovascular disease and diabetes mellitus.5, 6 Despite increased efforts to prevent and treat PCOS, the long‐term prognosis for improved fertility is usually poor in a considerable number of patients.
At present, the pathogenesis of PCOS has not been fully elucidated, which leads to treatment difficulties.7 Changes in apoptosis are the obviously pathology in the progress of PCOS, and the levels of apoptotic markers are used to detect and study this disease.8 It has been reported that increased expression of IL‐6 is linked to excess production of ovarian androgen, which is a major pathological process in PCOS.9 Another study reported that the long non‐coding RNA (lncRNA) SRLR mediates drug resistance by upregulating IL‐6 in renal cell carcinoma.10
Thus, we hypothesized that the lncRNA SRLR may also interact with IL‐6 in PCOS. In this study, we found that the lncRNA SRLR participated in PCOS by regulating apoptosis and IL‐6 expression.
2. MATERIALS AND METHODS
2.1. Subjects and granulosa cells
This study included 48 healthy women and 52 patients with PCOS, admitted to Wenling Hospital Affiliated with Wenzhou Medical University from October 2018 to April 2019. The selection of patients with PCOS depended on the diagnosis according to the Rotterdam Consensus (2004). The age of the patients ranged from 21 to 38 years, with an average age of 30.4 + 3.9 years. The control group was 23 to 36 years old, with an average age of 30.1 + 3.4 years. Age, body mass index, and other basic information between these two groups were not statistically different. Blood (3 mL) was collected from each participant, and all plasma samples were prepared and stored at 4°C. The Ethics Committee of Wenling Hospital affiliated with Wenzhou Medical University approved the study.
Human granulosa‐like tumour cells (KGN) were established according to the method described by Nishi11 and cultured in Dulbecco's modified Eagle medium (DMEM)/F12 (1:1; Life Technology) supplemented with 10% fetal bovine serum (FBS).
2.2. Total RNA extraction and real‐time quantitative PCR
For real‐time quantitative PCR (qRT‐PCR) analysis, total RNA was isolated using the GenElute total RNA purification kit (Sigma Aldrich) according to the manufacturer's protocol. A NanoDrop 2000c was used to measure RNA purity and concentration, then cDNA was prepared using the Applied Biosystems large‐capacity DNA reverse transcription kit. The SYBR green quantitative kit (Sigma‐Aldrich) was used for qRT‐PCR with GAPDH as the internal reference gene. The target gene lncRNA SRLR primers were designed and purchased from the Shanghai Sanggong Company. Threshold cycle (Ct) values were determined by the Applied Biosystems 7500 real‐time PCR system software.
2.3. Enzyme‐linked immunosorbent assay
Human IL‐6 was measured using a quantitative enzyme‐linked immunosorbent assay (ELISA) kit (D6050, R&D system). All operations were implemented strictly in accordance with the instructions provided by the manufacturer. The level of IL‐6 was standardized as pg/mL.
2.4. Cell transfection
The construction of the vector which expressed the lncRNA SRLR and the empty vector control were designed and purchased from Sangon (Shanghai, China), with the corresponding siRNA. KGN cells were cultured overnight, and the fusion rate reached 70% to 80% before transfection with the Lipofectamine 2000 reagent (Invitrogen, USA). All experiments were carried out according to the manufacturer's instructions. The carrier dose was 12 nM, and the siRNA dose was 40 nM. Empty vector or negative control siRNA was used as a negative control (NC); the cells which were only treated with Liposome 2000 were used as the control (C).
2.5. Detection of apoptotic cells
KGN cells were collected and seeded into a 6‐well plate with a density of 6 × 104 cells/mL 24 hours after the transfection; each well contained 3 mL of medium. After 48 hours of incubation, cells were detached with 0.25% trypsin and stained with Annexin V‐FITC and propidium iodide (PI). The apoptosis rate was detected by flow cytometry and was expressed as the total amount of Annexin‐V‐FITC+ cells.
2.6. Western blotting
Total protein was extracted from KGN cells using an extraction kit from Novus (NBP2‐37853). Protein samples were denatured at 100°C and separated by SDS‐PAGE using a 10% gel. Proteins were transferred to a polyvinylidene difluoride (PVDF) membrane, blocked with 5% evaporated milk for 1.5 hours at room temperature, and incubated with antibodies. The main antibodies used were rabbit anti‐human IL‐6 (1:2000, AB6672, Abcam) and anti‐GAPDH antibodies (1:1000, AB9485, Abcam). The blots were washed three times with PBS and incubated with goat anti‐rabbit IgG‐HRP secondary antibody (1:1000, MBS435036, MyBiosource). Protein bands were detected by X‐ray after incubation with enhanced chemiluminescence reagents (Sigma‐Aldrich, USA) for 3 minutes. Image J v1.46 software was used to detect the expression of IL‐6, which was standardized with GAPDH.
2.7. Statistical analysis
The data are expressed as mean ± SD. Each experiment was carried out three times. An unpaired t test was used for comparison between two groups. Univariate analysis of variance and graph‐based tests were used to compare differences between three groups. A Pearson correlation coefficient was used to analyse the correlation between the lncRNA SRLR and IL‐6. The diagnostic value of plasma lncRNA SRLR levels was evaluated using a receiver operating characteristic (ROC) curve, which analysed PCOS patients as truly positive and healthy women as truly negative. The difference was significant when P < .05, as shown with “*.”
3. RESULTS
3.1. Plasma levels of the lncRNA SRLR and IL‐6 in patients with PCOS were higher than those in healthy females
Through qRT‐PCR, we showed the plasma levels of the lncRNA SRLR in PCOS patients were obviously higher than those in healthy females (Figure 1A). In addition, ELISA results indicated that the plasma IL‐6 levels in PCOS patients were obviously higher than those in healthy females (Figure 1B).
Figure 1.
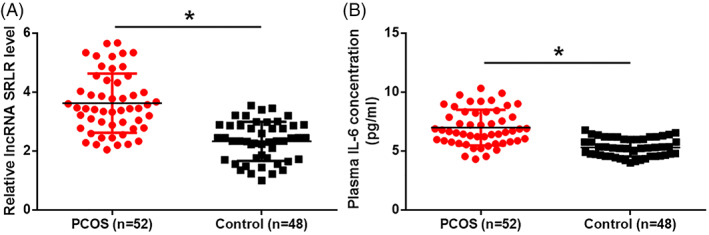
Plasma levels of the lncRNA SRLR and IL‐6 are both upregulated in PCOS patients compared with heathy females. Levels of the lncRNA SRLR (A) and IL‐6 (B) in plasma are significantly increased in patients with PCOS compared with healthy controls (*P < .05). PCOS, polycystic ovary syndrome
3.2. A significant positive correlation was found between plasma lncRNA SRLR and IL‐6 levels in patients with PCOS
To analyse the correlation between the lncRNA SRLR and IL‐6, we used the Pearson correlation coefficient to demonstrate a significant positive correlation between levels of the lncRNA SRLR and IL‐6 in plasma of PCOS patients (Figure 2A). There was no significant correlation between levels of the lncRNA SRLR and IL‐6 in plasma from healthy controls (Figure 2B).
Figure 2.
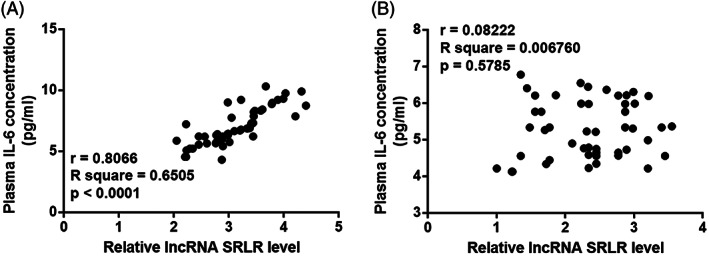
The lncRNA SRLR and IL‐6 are significantly and positively correlated in PCOS patients. The Pearson's correlation coefficient reveals a significant positive correlation between the plasma levels of the lncRNA SRLR and IL‐6 in PCOS patients (A) but not in healthy females (B). PCOS, polycystic ovary syndrome
3.3. Upregulation of levels of plasma lncRNA SRLR differentiated PCOS patients from healthy controls
Through ROC curve analysis, the diagnostic value of the lncRNA SRLR levels in plasma was evaluated in PCOS patients, which were considered as the true positive, while healthy females were considered as the true negative. We found the area under the curve was 0.8534, the SD was 0.03662, and the 95% confidence interval was 0.7816‐0.9252 (Figure 3, P < .0001). Therefore, upregulated plasma levels of the lncRNA SRLR could distinguish PCOS patients from healthy controls.
Figure 3.
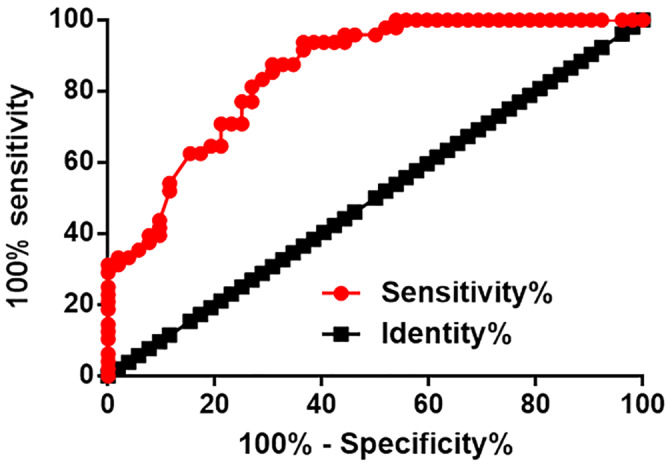
Upregulation of plasma lncRNA SRLR distinguishes PCOS patients from healthy controls. ROC curve analysis shows that upregulated plasma levels of the lncRNA SRLR distinguish PCOS patients from healthy controls. PCOS, polycystic ovary syndrome; ROC, receiver operating characteristic
3.4. Upregulation of the expression of the lncRNA SRLR resulted in upregulation of IL‐6 in KGN cells and promoted cell apoptosis
At 24 hours after transfection, the expression of the lncRNA SRLR was obviously increased in KGN cells (Figure 4A). In contrast with the control group (C), which had no treatment, and NC, the overexpression of the lncRNA SRLR upregulated expression of IL‐6 in KGN cells at both the mRNA and protein levels (Figure 4B). In addition, compared with the C and NC groups, KGN cells with overexpression of the lncRNA SRLR showed significantly increased cell apoptosis rates (Figure 4C).
Figure 4.
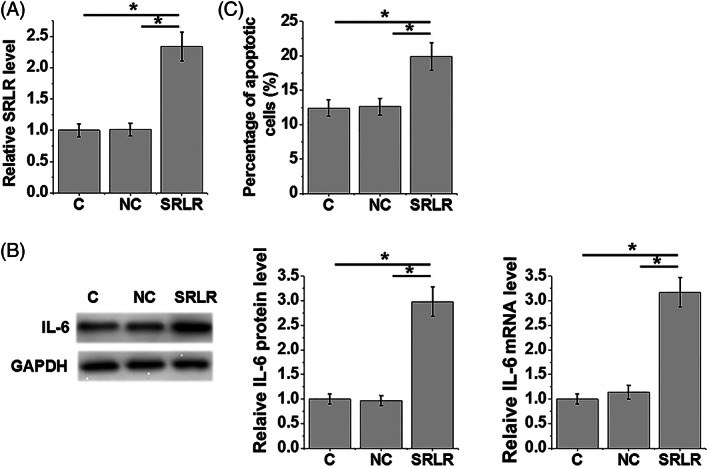
Overexpression of the lncRNA SRLR leads to upregulation of IL‐6 in KGN cells and promotes cell apoptosis. After 24‐h transfection, the expression of the lncRNA SRLR is significantly increased in KGN cells (A). Compared with the control (C) and negative control (NC) groups, overexpression of lncRNA SRLR leads to upregulated expression of IL‐6 in KGN cells (B), and increases the apoptotic rate of KGN cells (C) (*P < .05)
3.5. Silencing of the lncRNA SRLR resulted in the downregulation of IL‐6 and inhibition of apoptosis in KGN cells
The knockdown of the lncRNA SRLR was achieved in KGN cells 24 hours after transfection (Figure 5A). In contrast with the C and NC groups, the silencing of the lncRNA SRLR resulted in the downregulation of IL‐6 expression in KGN cells at both the level of protein and mRNA (Figure 5B). Additionally, the knockdown of the lncRNA SRLR and treatment with LMT‐28 (an IL‐6 antagonist, Sigma‐Aldrich, St. Louis, Missouri) significantly inhibited apoptosis of KGN cells compared with the C and NC groups (Figure 5C).
Figure 5.
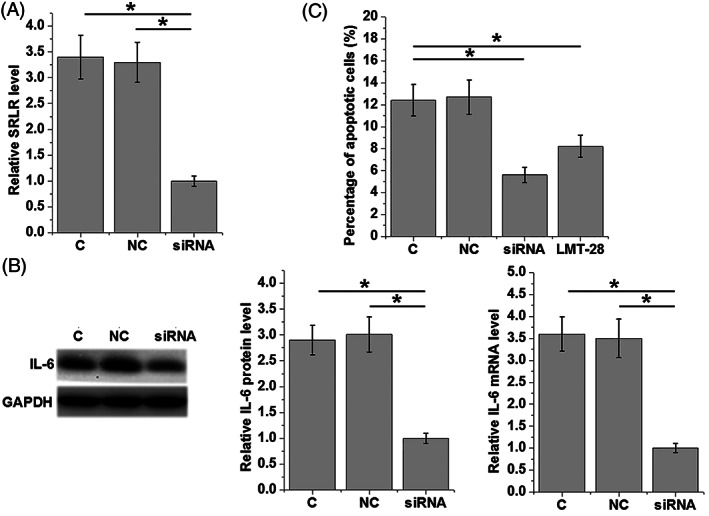
siRNA silencing of the lncRNA SRLR leads to downregulation of IL‐6 in KGN cells and inhibits cell apoptosis. After 24‐h transfection with siRNA, the knockdown of the lncRNA SRLR was achieved in KGN cells (A). LncRNA SRLR siRNA silencing leads to downregulated expression of IL‐6 in KGN cells at both the mRNA and protein levels (B). Compared with the C and NC groups, knockdown of the lncRNA SRLR and treatment with the IL‐6 antagonist LMT‐28 (Sigma‐Aldrich) leads to inhibition of the apoptosis of KGN cells (C) (*P < .05)
4. DISCUSSION
The lncRNA SRLR is a novel lncRNA which has a role as an oncogene in renal cell carcinoma.10 Nevertheless, the functionality of the lncRNA SRLR in other diseases remains unclear. The crux of this research was that upregulation of the lncRNA SRLR may also contribute to PCOS. The role of the lncRNA SRLR may be mediated by its effects on apoptosis and IL‐6 expression in PCOS.
Increasing the number of studies have shown that the development of PCOS is accompanied not only by changes in protein expression but also by changes in non‐coding RNA expression, such as lncRNA.12 Some differentially expressed lncRNAs, such as the lncRNA GAS5, have been shown to participate in the pathogenesis of PCOS and could be a potential target for treating PCOS.13 The lncRNA SRLR is a recently discovered lncRNA, which only has known expression patterns and functions in renal cell carcinoma.10 In this study, we first showed the upregulation of the lncRNA SRLR in PCOS. Actually, the upregulation of the lncRNA SRLR in plasma significantly distinguished PCOS patients from healthy controls. Consequently, plasma circulating levels of the lncRNA SRLR may be a potential target for the treatment of PCOS.
Altered apoptosis of granulosa cells is common in patients with PCOS.14, 15, 16 At present, multiple signalling pathways, such as Akt‐mTOR, BMP15/Smad1, and FoxO3, have been shown to participate in the regulation of granulosa cell apoptosis in PCOS. In a recent study, the lncRNA‐LET was also shown to induce apoptosis of the granulosa‐like tumour cell line KGN.17 Our study found that overexpression of the lncRNA SRLR promoted apoptosis of KGN cells. Therefore, the lncRNA‐LET may participate in PCOS by regulating apoptosis of KGN.
PCOS is accompanied with alterations in the expression of inflammatory factors in disease progression, indicating the nature of PCOS as an inflammatory disease.18 In a recent study, an excess production of IL‐6 was shown to be linked to excess production of ovarian androgen, which is a major pathological process in PCOS.9 In this research, we found the overexpression of the lncRNA SRLR upregulated IL‐6 expression in KGN cells, suggesting a positive interaction between the lncRNA SRLR and IL‐6. Meanwhile, the levels of the lncRNA SRLR and IL‐6 in PCOS patients' plasma were significantly positively correlated, as compared with those in the control group. Thus, the interplay between the lncRNA SRLR and IL‐6 may be mediated by pathological factors in KGN cells.
In summary, the lncRNA SRLR was upregulated in PCOS patients with high expression of IL‐6. The lncRNA SRLR may participate in PCOS by regulating cell apoptosis and IL‐6 expression.
CONFLICT OF INTERESTS
The authors declare that they have no competing interests, and all authors should confirm its accuracy.
ETHICAL APPROVAL
Ethical approval was obtained from the Ethics Committee of Wenling Hospital affiliated to Wenzhou Medical University.
INFORMED CONSENT
The study followed the tenets of the Declaration of Helsinki, and informed written consent was obtained from all patients and controls after we explained the nature and possible consequences of the study.
ACKNOWLEDGEMENTS
We thank the National Natural Science Foundation of China (Nos. 31571196 and 30801502 to Ling Wang) and the Fund for Young Scientists of the Shanghai Municipal Health and Family Planning Commission (No. 20184Y0218 to Lisha Li).
Li L, Zhu J, Ye F, et al. Upregulation of the lncRNA SRLR in polycystic ovary syndrome regulates cell apoptosis and IL‐6 expression. Cell Biochem Funct. 2020;38:880–885. 10.1002/cbf.3507
Funding information the Fund for Young Scientists of the Shanghai Municipal Health and Family Planning Commission, Grant/Award Number: 20184Y0218; National Natural Science Foundation of China, Grant/Award Numbers: 30801502, 31571196
DATA AVAILABILITY STATEMENT
The data used to support the findings of this study are available from the corresponding author upon request.
REFERENCES
- 1. McCartney Ch R, Marshall JC. Polycystic ovary syndrome. New Engl J Med. 2016;375:1398‐1399. [DOI] [PubMed] [Google Scholar]
- 2. Macut D, Bjekic‐Macut J, Rahelic D, Doknic M. Insulin and the polycystic ovary syndrome. Diabetes Res Clin Pract. 2017;130:163‐170. [DOI] [PubMed] [Google Scholar]
- 3. Azziz R, Carmina E, Chen Z, et al. Polycystic ovary syndrome. Nat Rev Dis Primers. 2016;2:16057. [DOI] [PubMed] [Google Scholar]
- 4. Balen AH, Morley LC, Misso M, et al. The management of anovulatory infertility in women with polycystic ovary syndrome: an analysis of the evidence to support the development of global WHO guidance. Hum Reprod Update. 2016;22:687‐708. [DOI] [PubMed] [Google Scholar]
- 5. Legro RS, Kunselman AR, Dodson WC, Dunaif A. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J Clin Endocrinol Metab. 1999;84:165‐169. [DOI] [PubMed] [Google Scholar]
- 6. Merz CN, Shaw LJ, Azziz R, et al. Cardiovascular disease and 10‐year mortality in postmenopausal women with clinical features of polycystic ovary syndrome. J Womens Health (Larchmt). 2016;25:875‐881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rosenfield RL, Ehrmann DA. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr Rev. 2016;37:467‐520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Uyanikoglu H, Sabuncu T, Dursun H, Sezen H, Aksoy N. Circulating levels of apoptotic markers and oxidative stress parameters in women with polycystic ovary syndrome: a case‐controlled descriptive study. Biomarkers. 2017;22:643‐647. [DOI] [PubMed] [Google Scholar]
- 9. Abdelhadi OA, Considine RV, Acton AJ, Gonzalez F. Increased lipid‐stimulated interleukin‐6 release from mononuclear cells is linked to excess ovarian androgen secretion in polycystic ovary syndrome. Fertil Steril. 2016;106:e33. [Google Scholar]
- 10. Xu Z, Yang F, Wei D, et al. Long noncoding RNA‐SRLR elicits intrinsic sorafenib resistance via evoking IL‐6/STAT3 axis in renal cell carcinoma. Oncogene. 2017;36:1965‐1977. [DOI] [PubMed] [Google Scholar]
- 11. Nishi Y, Yanase T, Mu Y, et al. Establishment and characterization of a steroidogenic human granulosa‐like tumor cell line, KGN, that expresses functional follicle‐stimulating hormone receptor. Endocrinology. 2001;142:437‐445. [DOI] [PubMed] [Google Scholar]
- 12. Huang X, Hao C, Bao H, Wang M, Dai H. Aberrant expression of long noncoding RNAs in cumulus cells isolated from PCOS patients. J Assist Reprod Genet. 2016;33:111‐121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lin H, Xing W, Li Y, Xie Y, Tang X, Zhang Q. Downregulation of serum long noncoding RNA GAS5 may contribute to insulin resistance in PCOS patients. Gynecol Endocrinol. 2018;34:784‐788. [DOI] [PubMed] [Google Scholar]
- 14. Cui X, Jing X, Wu X, et al. Abnormal expression levels of BMP15/Smad1 are associated with granulosa cell apoptosis in patients with polycystic ovary syndrome. Mol Med Rep. 2017;16:8231‐8236. [DOI] [PubMed] [Google Scholar]
- 15. Mikaeili S, Rashidi BH, Safa M, et al. Altered FoxO3 expression and apoptosis in granulosa cells of women with polycystic ovary syndrome. Arch Gynecol Obstet. 2016;294:185‐192. [DOI] [PubMed] [Google Scholar]
- 16. Song WJ, Shi X, Zhang J, Chen L, Fu SX, Ding YL. Akt‐mTOR signaling mediates abnormalities in the proliferation and apoptosis of ovarian granulosa cells in patients with polycystic ovary syndrome. Gynecol Obstet Invest. 2018;83:124‐132. [DOI] [PubMed] [Google Scholar]
- 17. Han Q, Zhang W, Meng J, Ma L, Li A. LncRNA‐LET inhibits cell viability, migration and EMT while induces apoptosis by up‐regulation of TIMP2 in human granulosa‐like tumor cell line KGN. Biomed Pharmacother. 2018;100:250‐256. [DOI] [PubMed] [Google Scholar]
- 18. Bahmani F, Karamali M, Shakeri H, Asemi Z. The effects of folate supplementation on inflammatory factors and biomarkers of oxidative stress in overweight and obese women with polycystic ovary syndrome: a randomized, double‐blind, placebo‐controlled clinical trial. Clin Endocrinol (Oxf). 2014;81:582‐587. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


