Abstract
This review discusses evidence for population‐based screening with contemporary screening tools. In Europe, prostate‐specific antigen (PSA)‐based screening led to a relative reduction of prostate cancer (PCa) mortality, but also to a substantial amount of overdiagnosis and unnecessarily biopsies. Risk stratification based on a single variable (a clinical variable or based on the presence of a lesion on prostate imaging) or based on multivariable approaches can aid in reducing unnecessary prostate biopsies and overdiagnosis by selecting men who can benefit from further clinical assessment. Multivariable approaches include clinical variables, and biomarkers, often combined in risk calculators or nomograms. These risk calculators can also incorporate the result of MRI imaging. In general, as compared to a purely PSA based approach, the combination of relevant prebiopsy information results in superior selection of men at higher risk of harboring clinically significant prostate cancer. Currently, it is not possible to draw any conclusions on the superiority of these multivariable risk‐based approaches since head‐to‐head comparisons are virtually lacking. Recently initiated large population‐based screening studies in Finland, Germany and Sweden, incorporating various multivariable risk stratification approaches will hopefully give more insight in whether the harm‐benefit ratio can be improved, that is, maintain (or improving) the ability to reduce metastatic disease and prostate cancer mortality while reducing harm caused by unnecessary testing and overdiagnosis including related overtreatment.
Keywords: clinical decision making, medical overuse, prostatic neoplasms, prostate‐specific antigen, risk assessment
Abbreviations
- 4K
4‐kallikrein
- CAP
Cluster Randomized Trial of PSA Testing for Prostate Cancer
- csPCa
clinical significant prostate cancer
- DRE
digital rectal examination
- EAU
European Association of Urology
- ERSPC
European Randomized Study of Screening for Prostate Cancer
- G2
Göteborg prostate cancer screening 2
- hK2
Kallikrein‐related peptidase 2
- ISUP
International Society of Urological Pathology
- miRNA
microRNAs
- mpMRI
multiparametric MRI
- PCA
prostate cancer
- PCA3
prostate cancer gene 3
- PCPTRC
Prostate Cancer Prevention Trial Risk Calculator
- PHI
Prostate Health Index
- PLCO
Prostate Lung, Colorectal and Ovarian Cancer Screening Trial
- PRECISION
Prostate Evaluation for Clinically Important Disease: Sampling Using Image Guidance or Not?
- PROBASE
Prostate Early Detection Study Based on a “Baseline” PSA Value in Young Men
- PROMIS
Prostate MR Imaging Study
- PSA
prostate‐specific antigen
- PSMA
prostate‐specific membrane antigen
- RPCRC
Rotterdam Prostate Cancer Risk Calculator
- SNP
single nucleotide polymorphisms
- STHLM3
Stockholm 3
- TRUS
transrectal ultrasonography
1. PROSTATE‐SPECIFIC ANTIGEN‐BASED SCREENING, ONE SIZE FITS ALL
In the mid‐1990s a decrease in prostate cancer (PCa) mortality was observed. 1 , 2 , 3 This reduction can be attributed to both advances in treatment modalities and early detection, that is, prostate‐specific antigen (PSA) testing. A modeling study showed that the rate of PCa mortality would increase without PSA testing while it would decrease with PSA testing. 4 PSA testing accounted for 45% to 70% of the reduction in PCa mortality, implying an improvement in PCa‐specific survival and more favorable tumor characteristics in PCa cases detected with PSA testing. The effect of PSA‐based screening and PCa specific mortality was evaluated in several randomized trials. One of such randomized trials, the European Randomized Study of Screening for Prostate Cancer (ERSPC), demonstrated with a follow‐up of 16 years a 20% relative PCa mortality reduction in favor of men screened, translating in an absolute difference in PCa mortality of approximately 18 less PCa deaths per 10 000 men screened. 5 To prevent one man dying of PCa, 570 men needed to be invited for screening, and as compared to a no‐screening situation 18 additional PCa cases needed to be diagnosed. The above numbers relate to the intention‐to‐treat analysis, adjusting for noncompliance and PSA contamination (screening in the control arm) showed an even larger PCa mortality reduction in favor of screening ranging from 22% to 32% within different ERSPC centers. 6 , 7 , 8
The counterpart of the ERSPC in the United States (the Prostate Lung, Colorectal and Ovarian Cancer Screening Trial [PLCO]) did not show a reduction in PCa mortality. 9 However, this trial was criticized due to the high PSA contamination in the control arm. 10 , 11 , 12 , 13 Modeling studies suggest that with higher biopsy compliance and less contamination in the PLCO trial, it would most likely showed comparable results to the ERSPC trial. 10 , 14
The ERSPC study also confirmed the hypothesis that with active screening more cancer would be detected. 5 , 12 , 15 , 16 It must however be noted that a lot of these screen‐detected cancers were low‐grade tumors defined as Gleason 3 + 3 or International Society of Urological Pathology (ISUP) grade group 1 PCa cases that, due to their very low potential of causing harm to the patient can be considered as being overdiagnosed. The rate of overdiagnosis has been estimated based on lead time (ie, the time that screening advances diagnosis compared to the time of the diagnosis without screening), the excess incidence between screened men and nonscreened men, and the presence of low‐grade tumor on either biopsy, radical prostatectomy or autopsy. 17 These different approaches resulted in estimates of rates of overdiagnosis between 1.7% and 67% for men with screen‐detected PCa. To elaborate, based on radical prostatectomy studies the rate of overdiagnosis ranged from 2% up to 47% and from autopsy studies the rate of overdiagnosis ranged from 19% to 43%. In a small pilot study of ERSPC Rotterdam with currently a median follow‐up of 19 years a total of 55% of the diagnoses in the screening arm were graded as Gleason 3 + 3 (ISUP grade group 1), of which a considerable part can safely be considered as overdiagnosis. 18
From the long‐running and high‐quality ERSPC study, 19 several lessons could be learned. The relative PCa mortality reduction of 20% was only observed when applying multiple screening interventions, 20 potentially explaining why no reduction in PCa mortality was observed in the Cluster Randomized Trial of PSA Testing for Prostate Cancer (CAP) trial. 21 PSA‐based screening is associated with a considerable rate of overdiagnosis, unnecessary PSA testing (only 23% of all PSA measurements were considered as a positive test) and unnecessary biopsies (only 24% of all prostate biopsies triggered by an elevated PSA level were positive). 5 So, while the data support the fact that there are life‐threatening prostate cancers that with early detection and subsequent treatment can be cured or inhibited in their progression the way to selectively identify those cancers remains subject of many ongoing research projects worldwide. Taken into account all data above showing that some—but not all—men can benefit of screening and the fact that this screening coincides with high rates of overdiagnosis strongly suggests that the “one size fits all” approach does not hold for PSA‐based screening.
The European Association of Urology (EAU) recently published a statement in which they favor PSA‐based screening, but with a critical note that overdiagnosis, unnecessary testing and overtreatment should be reduced. 22 The goal of nowadays PSA‐based screening (eg, PSA tests at the general practitioner) should be in detecting the cancers that have the potential to metastasize or to grow outside the prostatic gland. In other words, PSA‐based screening should not be aimed at detecting as much as PCa as possible but selectively focus on detecting those cancers that can harm if left undetected and untreated. This reduction in unnecessary testing, overdiagnosis and coinciding subsequent overtreatment could be achieved with personalized risk stratification with the aim to identify those men that will very likely benefit from screening.
2. HOW SHOULD WE SCREEN TO KEEP THE BENEFIT AND REDUCE THE HARM
2.1. Risk stratification
Risk stratification is a method to quantify the risk that a patient has for the event of interest, for example, the probability of detecting PCa or clinically significant (cs)PCa on prostate biopsy. In this review, we defined csPCa as Gleason 3 + 4 or ISUP grade group 2 and indicated if otherwise defined in the study discussed in this review. The goal of risk stratification is to select only those men that are considered to have an increased risk for further assessment with often (more) invasive procedures. However, it is essential to differentiate between population‐based screening studies and studies in the clinical, daily practice setting (often referred to as opportunistic screening). The latter type of screening starts with risk stratification at, for example, the general practitioner and often include PSA but certainly also other characteristics like urinary complains and comorbidities. Subsequently, after referral to the urologist a second risk stratification is often done leading to a higher risk population as compared to the population‐based setting as described in the currently available literature. In these population‐based trials, all men within a certain age range are invited and then risk stratified on the basis of only PSA. Those with elevated PSA are immediately eligible for biopsy resulting in a population with a lower a priori (before biopsy) risk on having PCa (and csPCa) as compared to the population referred for biopsy in the opportunistic/clinical setting. More contemporary population‐based screening studies now include additional risk stratification tools to increase this a priori risk and will be discussed in a separate section of this manuscript.
The result of the risk stratification process can be used in the decision making process in the form of a so‐called decision aid where individually calculated probabilities are presented with recommendations on how to continue. Available research has shown that the implementation of decision aids in clinical practice lead to improved decision quality. 23 , 24 , 25 , 26 A simple risk stratification in the pathway potentially leading to the detection of csPCa can be achieved by assessing a single PSA measurement at age 40 to 55 27 or at age 60. 28 For example, applying a cut‐off for further assessment (detection and treatment) for men aged 60 with a first PSA > 1.06 ng/mL could potentially have avoided 91% of all metastatic disease that surfaced during the 25 year observation period (missing 0.37% of the total of 3.7% of men that were diagnosed with metastatic disease over 25 year). A total of 95% of all deathly cases could have been detected earlier (missing 0.15% of the total of 3.0% of men dying of PCa over 25 year). At the same time this data suggest that men aged 60 with a PSA level ≤ 1 ng/mL could refrain from any further screening since the probability of being confronted with a life‐threatening PCa is low. Another study showed that the 13‐year cumulative incidence for csPCa of men aged 55 to 60 with a first PSA between 0.50 and 0.99 ng/mL was as low as 1.5%, which increased to 5.4% for men with a first PSA between 1.00 and 1.99 ng/mL. These data also suggest that men within this age category and a PSA < 1.00 ng/mL are at very low risk of harboring csPCa and could consider to refrain from further screening. 29 In addition, other studies investigating PSA kinetics, for example, PSA velocity, in the field of early detection could not confirm that PSA kinetics were more predictive than the absolute level for the diagnosis of PCa. 30 , 31 , 32
Next to risk stratification based on a single clinical variable, multivariable model‐based approaches estimate the relation between relevant clinical variables and the outcome (eg, PCa on biopsy) for men with clinical suspicion of PCa. There are numerous of these so‐called prediction models to detect csPCa. 33 , 34 , 35 The difference between all predictions models is the selection of (clinical) variables. However, for the generalizability of a prediction model, it is essential to test the performance of the model outside the development setting. This so‐called external validation is often lacking. 34 , 36 , 37 Two well‐validated risk calculators which are also being recommended by the EAU 38 , 39 are the Rotterdam Prostate Cancer Risk Calculator (RPCRC) 40 , 41 and the Prostate Cancer Prevention Trial Risk Calculator (PCPTRC) 2.0. 32 , 42 , 43 For the RPCRC, data of the Dutch part of the ERSPC study were used to develop a model to predict the presence of PCa at prostate biopsy using data of relevant clinical prebiopsy variables. 44 In this model, PSA and prostate volume were the strongest predictors for the detection of PCa, followed by digital rectal examination (DRE) and transrectal ultrasonography (TRUS). However, models should differentiate between men with prior biopsy or without previous biopsy as it was demonstrated that the relation between PSA level and the presence of PCa is different in men with a prior biopsy. 45 These findings are combined in the RPCRC to allow individualized predictions for the probability of detecting PCa and csPCa at biopsy. 40 , 41 This individual risk‐based strategy as compared to a strategy of biopsying all with a PSA ≥ 3.0 ng/mL showed a reduction of 33% of all biopsies for men biopsied for the first time while 14% of all PCa and 7% of all csPCa would not be detected. 41 For men with previous biopsy, 37% of biopsies would be avoided while 16% of all PCa and 9% all csPCa would not have been detected. Next to the RPCRC, another widely used prediction model is the risk calculator from the PCPTRC 2.0. 32 This risk calculator calculates the probability of finding no PCa, any PCa, and csPCa based on PSA, age, race, family history of PCa, DRE and the presence of a previous biopsy. An external validation of these two risk calculators did not show much difference for the prediction of csPCa: the RPCRC showed a slightly better discrimination and a slightly higher net benefit, while the PCPTRC 2.0 showed a slightly better calibration. 46 The difference between these and the Finne, Chin, Karakiewicz, Sunnybrook, Prostataclass and PCPT 1.0 risk calculators were further studied in a head‐to‐head comparison. 47 In this study, the authors did not find any difference in discrimination in the prediction of any PCa. However, in the prediction of csPCa the RPCRC showed the most superior discrimination and the highest net benefit followed by the PCPTRC 2.0. The authors also showed that offering biopsies if the model‐based probability of csPCa was ≥4%, applying the RPCRC would lead to reduction of 32% of all biopsies while 5% of all csPCa would not have been detected. For the PCPTRC 2.0, 16% of biopsies would have been reduced while 3% of all csPCa would not have been detected.
Next to risk stratification using model‐based approaches, other risk stratification tools involve the use of an MRI in case of clinical suspicion of PCa. However, the role of the MRI as a triage test for the detection of PCa remains debated. Some authors proposed that a negative MRI (ie, PI‐RADS < 3) of the prostate can be used to refrain from prostate biopsies. 48 , 49 Men in the Prostate Evaluation for Clinically Important Disease: Sampling Using Image Guidance or Not? (PRECISION) trial did not receive a biopsy in case of a negative MRI, which led to a reduction of 28% of all biopsies. 50 In this trial, 29% of all positive PI‐RADS lesions were defined as PI‐RADS score 3, 40% as PI‐RADS score 4 and 31% as PI‐RADS score 5. However, follow‐up data of these men is yet not available so it remains unclear if these men are actually free from csPCa or whether detection remains in time. Another study found that 20% of indolent PCa and 3% of csPCa would not have been detected if biopsies were only offered to men with a positive MRI. 51 In this trial, 13% of all positive PI‐RADS lesions were defined as PI‐RADS score 3, 43% as PI‐RADS score 4 and 44% as PI‐RADS score 5. The so‐called Prostate MR imaging study (PROMIS) showed that if the MRI would be used as a triage test, 27% of biopsies could be avoided on basis of a negative MRI at the cost of not detecting 7% of all Gleason ≥4 + 3 PCa (ISUP grade group ≥ 3) or PCa with a maximum cancer core length of ≥6 mm. 52 In this trial, 39% of all positive PI‐RADS lesions were defined as PI‐RADS score 3, 29% as PI‐RADS score 4 and 32% as PI‐RADS score 5. While these trials showed a considerable amount of reduction in biopsies, MRI cannot be currently used as the sole triage test, currently available data come from very different study settings which lead to highly variable performance characteristics. 53 In addition, interobserver variability and the fact that MRI is likely to miss tumors of smaller size, with a lower PSA density, a lower Gleason score, a multifocal appearance, and nonindex tumors warrants further research. 54 While previous studies focused on finding (cs)PCa, another study suggested that MRI can also be used to identify indolent PCa. 55
Another promising imaging modality which might have a role in de detection of PCa is the prostate‐specific membrane antigen (PSMA) PET/CT. A retrospective cohort of men with a negative or contraindications on MRI showed that the PSMA PET/CT was positive in 56% of all men, of which 44% was diagnosed with any PCa of which 36% was csPCa. 56 However, it should be noted that this study did not differentiate between men with a negative MRI or men with contraindications for undergoing MRI, so it cannot be concluded that the PSMA PET/CT detects tumors missed by MRI. Further evaluation of the potential of PSMA PET/CT will be done in the prospective PRIMARY trial in which men will receive both the PSMA PET/CT and an MRI. 57 However, it should be mentioned that the relative short half‐life of the tracer and the relative higher cost of the image modality as compared to the MRI can limit the availability of the scan.
Next to risk stratification using an MRI in case of clinical suspicion of PSA, it has been suggested to incorporate the result of the MRI into a multivariable prediction model 58 or to perform an upfront risk classification to reduce the number of MRIs. One study referred men with a risk above the RPCRC threshold of 20% for any PCa and/or 4% for csPCa and showed that 51% of the MRIs could have been avoided at the cost of not detecting 25% of all indolent PCa and 10% of all csPCa. 59 In another study including only biopsy naïve men, upfront risk stratification using the RPCRC would have avoided 37% of all multiparametric (mp)MRI while missing only 4% csPCa. 60 Next to upfront risk stratification to refer men for MRI, other groups included the PI‐RADS score in their prediction model to estimate the probability of PCa. One of those models used age, African American ethnicity, prior negative biopsy, results of the DRE, PSA and the PI‐RADS score to predict the presence of csPCa at biopsy. 61 They found that the discrimination of the model increased from 0.72 to 0.84 when including PI‐RADS score and MRI‐derived prostate volume. The authors also found that compared to biopsying all men with a positive MRI their model could have avoided 18% of biopsies without missing any csPCa. This was confirmed by other studies where discrimination increased with the inclusion of the MRI results in the prediction model. 62 , 63 , 64 , 65 One of those studied combined the RPCPC with the PI‐RADSv1.0 score for predicting csPCa, and found for biopsy naïve men that discrimination increased from 0.81 to 0.83. 63 In contrast, the increase in discrimination was larger for men with previous biopsy, showing an increase in discrimination from 0.66 to 0.81. Recently, the RPCRC has been updated to incorporate the results of MRI. 65 This model showed that for men with previous negative biopsy with a risk threshold of 5% csPCa, 27% of biopsies could have been reduced while 3% of all csPCa would not have been detected. A risk threshold of 10% csPCa would lead to a reduction of 36% biopsies while 4% of all csPCa would not have been detected. However, for biopsy naïve men this updated risk calculator might be questionable, since, with a threshold of 5%, only 2% of biopsies would be reduced while missing 15% of csPCa compared offering all men a biopsy with a PSA ≥ 3.0 ng/mL.
All taken together, individualized predictions as an aid at decision making can assist the physician to select those patients most likely to benefit from further clinical assessment 66 as opposed to the one size fits all approach. This individualized risk‐adapted strategy for the detection of PCa is recommended by EAU. 38 , 39 To ease interpretation of a model, it can be visualized in a so‐called nomogram 67 , 68 , 69 , 70 or in eHealth and mHealth applications, such as RPCRC 71 and the PCPTRC 2.0 72 to ease clinical decision making. In addition, models have been suggested which incorporate both the probability of aggressive cancer and the life expectancy to make a recommendation about referral to the urologist. 73 , 74
2.2. Biomarkers to aid in risk stratification
Proteomics like, for example, the PSA protein and its subforms can be detected in both blood and urine. One of those blood‐based biomarkers is the Prostate Health Index (PHI) which combines total, free and [−2]proPSA into one score. Several studies demonstrated that the PHI showed better discrimination for (cs)PCa than PSA 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 and even better discrimination was observed for PHI density. 83 For example, in one study, the discrimination for detecting csPCa was 0.71 for PHI, compared to 0.55 for total PSA. 81 Also, the same study showed that offering biopsy for men with a PHI cut‐off above 28.6 would have avoided 30% of biopsies while 5% of Gleason ≥ 7 (ISUP grade group ≥ 2) would not have been detected. In addition, a multicenter European and Asian study showed that the effect of PHI differed between cohorts: for European centers, the discrimination for predicting Gleason ≥3 + 4 (ISUP grade group ≥ 2) was 0.63 for PSA and 0.71 for PHI, while this was for Asian centers 0.54 for PSA and 0.84 for PHI. 75 That same study also showed that a similar sensitivity of 90% was reached in European centers with a PHI cut‐off of 40 in which 40% of biopsies could have been reduced while 10% csPCa would not have been detected. In Asian centers, this 90% sensitivity was reached with a PHI cut‐off of 30 in which 56% of biopsies could have been reduced while 11% csPCa would not have been detected, suggesting that regional differences should be taken into account. Other studies have shown that PHI can improve the discrimination of the RPCRC, 84 , 85 and the PCPTRC 2.0. 85 In the latter study, the discrimination of the PCPTRC 2.0 for csPCa increased from 0.58 to 0.70 with PHI; the discrimination of the RPCRC increased from 0.65 to 0.71. 85 Finally, studies have shown that PHI next to MRI have led to better discrimination for csPCa as shown in an Asian population 86 and in the United Kingdom. 87 The advantage of the PHI test is that it is based on a blood test without the need of clinical variables, and is relatively cheap with prices between 30 and 90 euro. 35 , 88
Next to PHI, another available biomarker panel for the detection of PCa is the 4‐kallikrein (4K) score which is next to the clinical variables age, and DRE based on total, free, and intact PSA, kallikrein‐related peptidase 2 (hK2), and was updated to include the history of previous biopsy. The discrimination of a model predicting any PCa including age, total PSA, and DRE increased from 0.72 to 0.84 with the addition of free PSA, intact PSA and hK2. 89 However, effects for csPCa were minimal as the discrimination increased from 0.87 to 0.90. In contrast, another study showed that discrimination of csPCa increased for screening naïve men from 0.68 to 0.80 with the addition of the 4K score in the model and from 0.72 to 0.83 for men with previous screening. 90 This model showed that for screening naïve men 74% of all biopsies could be reduced if biopsies were offered from a model‐based probability of 20% but this would lead to 26% csPCa not being detected; for previous screened men, 41% of biopsies could be reduced in which only 2% of csPCa would not be detected. While the former studies showed that the 4K score should be preferred over a model without the 4K score, other studies did not find that the 4K score showed a better discrimination than PHI 91 or the RPCRC including cribriform growth in the definition of csPCa. 92
Another type of blood‐based biomarkers for the detection of (cs)PCa is based on microRNAs (miRNA). There are over 50 miRNAs identified, but an essay is not yet implemented in clinical practice. 93 , 94 It was previously demonstrated that a model with both miRNAs and PSA improved prediction compared to PSA alone. 95 However, validation of miRNA studies are yet lacking and larger studies are needed before miRNA can be considered in the clinical setting. 96 In addition, head‐to‐head comparisons with other prediction models and biomarkers are needed to assess the superiority of miRNA essays.
Next to blood‐based biomarkers, urine‐based biomarkers are also available. One of those urine‐based biomarkers is prostate cancer gene 3 (PCA3) which is calculated as the ratio of the PCA3 mRNA and PSA mRNA in urine voided after DRE. The discrimination for any PCa pooled over 46 studies was 0.75, 97 which is better than total PSA, 98 , 99 , 100 but lower compared to PHI. 101 , 102 , 103 However, another study showed that there was no difference in discrimination for any PCa using only the PCA3 or only the PHI. 104 Discrimination of the model increased significantly from 0.71 to 0.77 with both PCA3 and PHI in the model.
Another urine‐based biomarker is SelectMDx which was initially based on the proteins HOXC6, TDRD1 and DLX1 and showed a discrimination for csPCa of 0.77, which was higher than PCA3 alone (0.68) or PSA alone (0.72). 105 Combing the HOXC6 and TDRD1 and DLX1 with PSA showed an increase discrimination of 0.81. A validation study of these markers showed that the protein TDRD1 actually did not improve discrimination, but that discrimination was improved when including information like PSA density and having had a previous biopsy. The discrimination increased significantly from 0.81 to 0.86. 106 In the same study, it was also shown that the SelectMDx score outperformed the PCPTRC 2.0 and the PCPTC 2.0 with PCA3. More recently, it was shown that the SelectMDx score was positively related to the PI‐RADS score, but unfortunately, the authors did not report the discriminative ability of a model containing both the information from SelectMDx and the PI‐RADS score. 107
One of the latest developed multivariable risk models is the so‐called Stockholm 3 (STHLM3) risk‐based model. This model predicts the probability of csPCa based on a combination of plasma protein biomarkers, genetic single nucleotide polymorphisms (SNPs), and clinical variables. 108 In that development study, they showed that the discrimination of csPCa with total PSA was 0.56, which increased with additional information from risk factors (age, family history, the presence of previous biopsies) to 0.58, and with the addition of biomarkers (one genetic score for all SNPs, beta‐microseminoprotein, macrophage inhibitory cytokine 1, free PSA, intact PSA and hK2), to 0.70, which further increased to 0.74 with the addition of DRE and prostate volume. This model showed that offering biopsies if the model probability was ≥10%, 32% for all biopsies would have been reduced, 44% of benign biopsies would have been detected, and 17% of indolent PCa would not have been detected without missing any csPCa compared to offering a biopsy in all men with PSA ≥3 ng/mL. However, all biomarkers were added to the model in one step which makes it impossible to disentangle the unique effects of every single biomarker. 109 The application of the STHLM3 in current clinical practice in Sweden showed that with this model based threshold of 10%, 53% of biopsies and 76% of benign biopsies would have been reduced. 110 Other research studied the effect of the STHLM3 and MRI. 111 In this study, they showed that men with a positive STHLM3 and a positive MRI with systematic biopsies, 38% of all biopsies could have been reduced in which 8% of all csPCa could have been missed compared to only MRI (including systematic biopsies) approach.
All taken together, there are several biomarkers available. At the moment, it is impossible to draw conclusions on superiority for the detection of csPCa since head‐to‐head comparisons comprising both multivariable models and biomarkers are lacking. The conclusion we can, however, make from these studies is the fact that all multivariable approaches outperform a PSA‐based strategy in reducing unnecessary biopsies and overdiagnosis, see Figure 1 and Table 1. According to Figure 1, a men with clinical suspicion can either undergo univariable risk stratification including a PSA measurement or risk stratification solely on MRI, or multivariable risk stratification including risk calculators with or without MRI or proteomics and genomics. This clinical suspicion can originate from urinary complains, family history of PCa or the wish of the patient. We favor the use of multivariable risk stratification over an univariable approach with the currently available tools. Men with low risk according to the risk stratification should be referred to clinical follow‐up or should be refrained from further clinical follow‐up when they are at very low risk of harboring csPCa. Men with elevated risk according to the risk stratification should receive targeted and/or systematic biopsies. If no cancer is found, men should be referred to clinical follow‐up, while men diagnosed with PCa should be referred to treatment including active surveillance.
FIGURE 1.
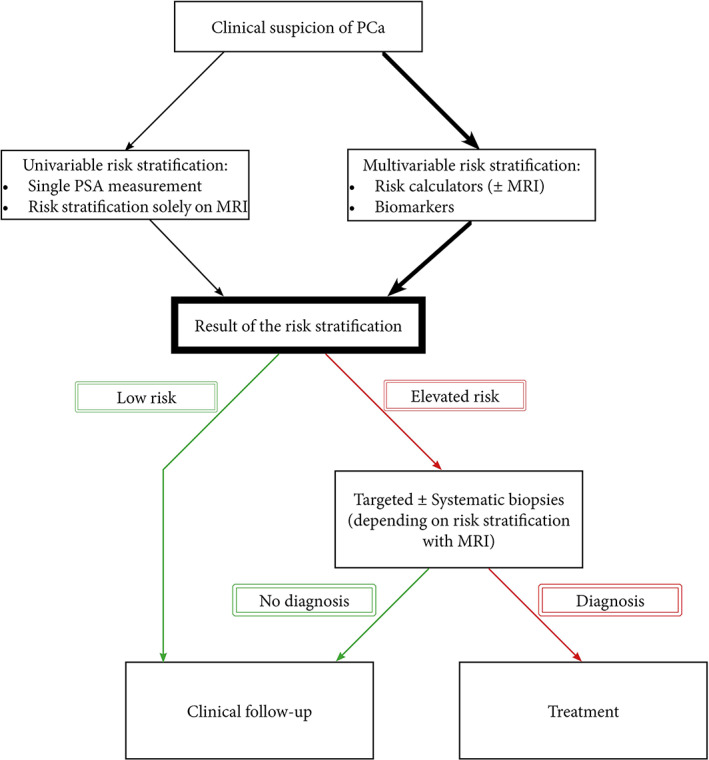
Flowchart of men with suspicion of PCa combined with risk stratification [Color figure can be viewed at wileyonlinelibrary.com]
TABLE 1.
Summary of key results of various decision‐making approaches
| Risk stratification tools (cut‐off) | Setting | References | Reduced biopsies (%) | Reduced indolent PCa diagnosis (%) | Missed csPCa (%) |
|---|---|---|---|---|---|
| Univariable | |||||
| PSA test (≥3.0 ng/mL) a | Population based screening | 5, 9, 12, 15, 16 | N/A | N/A | N/A |
| MRI in triage setting (≥PI‐RADS 3) | Clinical suspicion | 50‐52 | 27‐29 | 20 | 3‐7 |
| Multivariable | |||||
| RPCRC (compared to PSA ≥3.0 ng/mL) | Clinical suspicion + biopsy naive | 41 | 33 | 14 | 7 |
| RPCRC (compared to PSA ≥3.0 ng/mL) | Clinical suspicion + prior negative biopsy | 41 | 37 | 16 | 9 |
| RPCRC (≥4%) | Clinical suspicion | 47 | 32 | 25 | 5 |
| PCPTRC 2.0 (≥4%) | Clinical suspicion | 47 | 16 | 15 | 3 |
| RPCRC + MRI (≥5%) | Clinical suspicion + biopsy naive | 65 | 2 | 10 | 15 |
| RPCRC + MRI (≥5%) | Clinical suspicion + prior negative biopsy | 65 | 27 | 14 | 3 |
| PHI (90% sensitivity) | Clinical suspicion | 75, 81 | 30‐56 | 31‐33 | 5–11 |
| 4K score (≥20%) | Clinical suspicion + biopsy naive | 90 | 74 | 38 | 26 |
| 4K score (≥20%) | Clinical suspicion + prior negative biopsy | 90 | 41 | 73 | 2 |
| STHLM3 risk‐based model (≥10%) | Clinical suspicion | 108, 110 | 32‐53 | 17‐76 | 0 |
Note: Head to head comparisons cannot be made based on the data in this table and performance of risk stratification tools should be confirmed in an external validation.
In the population‐based screening studies there were no biopsies performed if the PSA was lower than 3.0 ng/mL. Therefore, it is not possible to assess the missed cancers following this strategy.
2.3. Which men are most likely to benefit from screening
Considering the natural history of PCa, it is clear that screening a man in his 80s or 90s when comorbidity is an issue is not the way to go. In a modeling study, it was simulated that a single screening at age 55 would result in 27% overdiagnosis which doubled to 56% for a single screening at age 75. 112 However, elderly men should not automatically be excluded from screening simply based on age. It has been shown that men screened for the first, and last time at age 70 to 74 can still be confronted with the diagnosis of csPCa. The risk is small (approximately 3% with a maximum follow‐up of 24 years) but still despite the high age, 26% of all these men die of PCa. 113 This suggests that age is not the only and perhaps not the correct factor to decide to continue or start screening. To selectively identify elderly men that are at high risk of being diagnosed and die of PCa, again multivariable risk stratification including taking into account life expectancy and comorbidities is advised. 74
Next to the issue of screening or not screening elderly men, there is also the question on when to start screening. The Prostate Early Detection Study Based on a “Baseline” PSA Value in Young Men (PROBASE) trial randomized 50 000 men aged 45 at an immediate screening arm or a delayed screening arm at age 50 with screening intervals based on their “baseline” PSA level. 114 The first results of this trial show that only 14% complied with the invitation and of the men randomized to immediate screening, only 1.8% showed a PSA ≥3.0 ng/mL, of which 43% based on a confirmatory second PSA test had a PSA ≥3.0 ng/mL. 115 Updated results of this trial show similar results; 1.5% of men randomized to immediate screening showed PSA ≥3.0 ng/mL, of which 53% is confirmed at repeat PSA testing. 116 Of the men with confirmed PSA ≥3.0 ng/mL, 33% of men were diagnosed with PCa, of which 68% was csPCa. These findings suggest that early detection at age 45 in men with relatively high PSA values considering their age and thus the absence of benign prostatic hyperplasia is indicated, especially since almost 70% of PCa is considered csPCa. Longer follow‐up data will show whether the delay of 5 years is acceptable or not and whether early detection and subsequent treatment will indeed reduce suffering and dying from PCa. With the latter preferably resulting in a higher mortality reduction as is currently seen in the randomized trials that all started at a higher age.
3. FUTURE OF SCREENING
We should also mention there are challenges for pathologists to improve the grading of the PCa. 117 In addition, aggressive cancer is usually defined as ISUP grade group 2, but the aggressiveness of these tumors is under debate. 118 , 119 Other challenges at the moment are involved with the use of biparametric MRI as opposed to mpMRI, although the sensitivity and specificity of the modalities are similar. 120 At the moment, there are ongoing several population‐based screening studies next to the previous discussed PROBASE trial in Germany. In Sweden, two trials are recruiting. The Göteborg prostate cancer screening 2 (G2) trial recruits men from September 2015 till the end of 2019. 121 , 122 In this trial, over 40 000 men aged 50 to 60 are randomized between a screening and a control group. In the screening group, men are randomly assigned into three arms. Men assigned to the first arm and with a PSA ≥ 3.0 ng/mL will receive standard biopsies, DRE, and mpMRI; for men with positive MRI targeted biopsies will be offered. Men with a PSA is below this threshold will not receive further testing and will be re‐invited. Men assigned to the second arm and a PSA ≥ 3.0 ng/mL will only receive targeted biopsies in case of a positive MRI and no systematic biopsies; men with a negative MRI will be re‐invited. Men assigned to the third arm and a PSA cut‐off ≥1.8 ng/mL will only receive targeted biopsies and no systematic biopsies. All men assigned to the second and third arm with a PI‐RADS 5 will undergo both systematic biopsies and targeted biopsies. Re‐invitation interval is based on the PSA level: men with a PSA < 0.6 ng/mL will be re‐invited 8 years later, men with a PSA between 0.6 and 1.19 ng/mL will be re‐invited 4 years later and men with a PSA between 1.2 and 2.99 ng/mL will be invited after 2 years (see recent changes in amendment 2, https://www.g2screening.se/wp-content/uploads/2019/12/Amendment-2.pdf). Men will be invited twice, after which men with a PSA ≤ 0.59 ng/mL will be screened until they reach the age of 62, men with a PSA between 0.60 and 1.19 ng/mL will be screened until they reach the age of 65, men with a PSA between 1.2 and 1.79 ng/mL will be screened until they reach the age of 70, and men with a PSA above 1.8 ng/mL will be screened until they reach the age 75 (see recent changes in amendment 10, https://www.g2screening.se/wp-content/uploads/2020/02/Amendment-10.pdf). Nonattenders will be re‐invited after 3 months and after 9 months. The primary outcome of this trial is the rate of overdiagnosis, defined as small insignificant tumors that would never be detected within one's life without screening.
Another population‐based screening study in Sweden is the STHLM3‐MR Phase 2 trial. 123 In this trial, 25 000 men aged 50‐74 will be invited and men with an elevated risk will be randomized. The PSA and STHLM‐3 test define elevated risk: a PSA ≥3 ng/mL or a PSA ≥1.5 ng/mL with STHLM3 > 11%. Men will be randomized in a 2 (control arm): 3 (experimental) ratio. Men in the control arm will receive only systematic biopsies and men in the experimental arm will receive an MRI. Men with a positive MRI will receive both targeted and systematic biopsies; men with a negative MRI will receive systematic biopsies if the STHLM3 test is ≥25%, or no biopsies if the STHLM3 < 25%. Primary outcome in this trial is the number of detected csPCa and indolent PCa between the diagnostic pathways.
In Finland, the ProScreen trial which started in 2018 randomized 67 000 men aged 55‐67. 124 Randomization is in a 1 (screening arm): 3 (control arm) ratio. Men in the screening arm will be offered PSA test; men with a PSA ≥ 3.0 ng/mL will receive an additional 4K test. Men with increased risk (ie, 4K score ≥ 7.5) will undergo mpMRI. Biopsies will only be taken in men with a positive MRI. Men with a positive screening (ie, PSA ≥3.0 ng/mL) will be re‐invited every 2 years until they complete five screening rounds. Men with a negative screening and a PSA between 1.5 and 3.0 ng/mL will be re‐invited after 5 to 6 years and after 9 to 10 years. Men with PSA below 1.5 ng/mL will be re‐invited after 7 to 8 years. The primary outcome of this trial is PCa mortality.
4. CONCLUSION
In Europe, PSA‐based screening showed a relative reduction of PCa mortality of 20%. However, this was accompanied by a substantial amount of overdiagnosis and unnecessary biopsies. In the past years, a large variety of tools has been developed to allow an individualized approach to select patients who would benefit from further clinical assessment. These risk stratification tools have shown to be able to reduce the number of biopsies and overdiagnosis, but head‐to‐head comparisons are lacking making it impossible to draw conclusions on the superiority of these tools. Current population‐based screening studies are using a multivariable individualized approach with the aim to maintain reduction of PCa mortality and to reduce the number of biopsies and overdiagnosis. These trials will lead to new insights in the field of population‐based screening for PCa. It is however not advisable to await these results and continue opportunistic screening activities based on PSA alone. Current guidelines based on contemporary knowledge should be implemented in clinical practice and decision making without delay. 125
CONFLICT OF INTEREST
The authors do not report any conflicts of interest.
Remmers S, Roobol MJ. Personalized strategies in population screening for prostate cancer. Int. J. Cancer. 2020;147:2977–2987. 10.1002/ijc.33045
This article was published online on 3 June 2020. A typographical error was subsequently identified in page 3. This notice is included in the online and print versions to indicate that both have been corrected on 12 June 2020.
Funding information Publication fees were covered by Stichting Wetenschappelijk Onderzoek Prostaatkanker (SWOP)
REFERENCES
- 1. Kelly SP, Rosenberg PS, Anderson WF, et al. Trends in the incidence of fatal prostate cancer in the United States by race. Eur Urol. 2017;71:195‐201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dickinson J, Shane A, Tonelli M, et al. Trends in prostate cancer incidence and mortality in Canada during the era of prostate‐specific antigen screening. CMAJ Open. 2016;4:E73‐E79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Collin SM, Martin RM, Metcalfe C, et al. Prostate‐cancer mortality in the USA and UKin 1975–2004: an ecological study. Lancet Oncol. 2008;9:445‐452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Etzioni R, Tsodikov A, Mariotto A, et al. Quantifying the role of PSA screening in the US prostate cancer mortality decline. Cancer Causes Control. 2008;19:175‐181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hugosson J, Roobol MJ, Mansson M, et al. A 16‐yr follow‐up of the European randomized study of screening for prostate cancer. Eur Urol. 2019;76:43‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kilpeläinen TP, Tammela TLJ, Malila N, et al. The Finnish prostate cancer screening trial: analyses on the screening failures. Int J Cancer. 2015;136:2437‐2443. [DOI] [PubMed] [Google Scholar]
- 7. Kerkhof M, Roobol MJ, Cuzick J, et al. Effect of the correction for noncompliance and contamination on the estimated reduction of metastatic prostate cancer within a randomized screening trial (ERSPC section Rotterdam). Int J Cancer. 2010;127:2639‐2644. [DOI] [PubMed] [Google Scholar]
- 8. Roobol MJ, Kerkhof M, Schröder FH, et al. Prostate cancer mortality reduction by prostate‐specific antigen‐based screening adjusted for nonattendance and contamination in the European randomised study of screening for prostate cancer (ERSPC). Eur Urol. 2009;56:584‐591. [DOI] [PubMed] [Google Scholar]
- 9. Andriole GL, Crawford ED, Grubb RL 3rd, et al. Prostate cancer screening in the randomized prostate, lung, colorectal, and ovarian cancer screening trial: mortality results after 13 years of follow‐up. J Natl Cancer Inst. 2012;104:125‐132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gulati R, Tsodikov A, Wever EM, et al. The impact of PLCO control arm contamination on perceived PSA screening efficacy. Cancer Causes Control. 2012;23:827‐835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pinsky PF, Black A, Kramer BS, Miller A, Prorok PC, Berg C. Assessing contamination and compliance in the prostate component of the prostate, lung, colorectal, and ovarian (PLCO) cancer screening trial. Clin Trials. 2010;7:303‐311. [DOI] [PubMed] [Google Scholar]
- 12. Schröder FH, Roobol MJ. ERSPC and PLCO prostate cancer screening studies: what are the differences? Eur Urol. 2010;58:46‐52. [DOI] [PubMed] [Google Scholar]
- 13. Shoag JE, Mittal S, Hu JC. Reevaluating PSA testing rates in the PLCO trial. N Engl J Med. 2016;374:1795‐1796. [DOI] [PubMed] [Google Scholar]
- 14. Palma A, Lounsbury DW, Schlecht NF, Agalliu I. A system dynamics model of serum prostate‐specific antigen screening for prostate cancer. Am J Epidemiol. 2015;183:227‐236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate‐cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320‐1328. [DOI] [PubMed] [Google Scholar]
- 16. Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate cancer mortality: results of the European randomised study of screening for prostate cancer (ERSPC) at 13 years of follow‐up. The Lancet. 2014;384:2027‐2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Loeb S, Bjurlin MA, Nicholson J, et al. Overdiagnosis and overtreatment of prostate cancer. Eur Urol. 2014;65:1046‐1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Osses DF, Remmers S, Schröder FH, van der Kwast T, Roobol MJ. Results of prostate cancer screening in a unique cohort at 19yr of follow‐up. Eur Urol. 2019;75:374‐377. [DOI] [PubMed] [Google Scholar]
- 19. Roobol MJ, Carlsson SV. The ERSPC study: quality takes time and perseverance. Clin Chem. 2019;65:208‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pakarainen T, Nevalainen J, Talala K, et al. The number of screening cycles needed to reduce prostate cancer mortality in the Finnish section of the European randomized study of prostate cancer (ERSPC). Clin Cancer Res. 2019;25:839‐843. [DOI] [PubMed] [Google Scholar]
- 21. Martin RM, Donovan JL, Turner EL, et al. Effect of a low‐intensity PSA‐based screening intervention on prostate cancer mortality: the CAP randomized clinical trial. JAMA. 2018;319:883‐895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gandaglia G, Albers P, Abrahamsson P‐A, et al. Structured population‐based prostate‐specific antigen screening for prostate cancer: the European Association of Urology position in 2019. Eur Urol. 2019;76:142‐150. [DOI] [PubMed] [Google Scholar]
- 23. Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017CH001431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Belkora J, Chan JM, Cooperberg MR, et al. Development and pilot evaluation of a personalized decision support intervention for low risk prostate cancer patients. Cancer Med. 2020;9:125‐132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Salkeld G, Cunich M, Dowie J, et al. The role of personalised choice in decision support: a randomized controlled trial of an online decision aid for prostate cancer screening. PLoS One. 2016;11:e0152999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stacey D, Légaré F, Lewis KB. Patient decision aids to engage adults in treatment or screening decisions. JAMA. 2017;318:657‐658. [DOI] [PubMed] [Google Scholar]
- 27. Vickers AJ, Ulmert D, Sjoberg DD, et al. Strategy for detection of prostate cancer based on relation between prostate specific antigen at age 40‐55 and long term risk of metastasis: case‐control study. BMJ. 2013;346:f2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vickers AJ, Cronin AM, Björk T, et al. Prostate specific antigen concentration at age 60 and death or metastasis from prostate cancer: case‐control study. BMJ. 2010;341:c4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kovac E, Carlsson SV, Lilja H, et al. Association of baseline prostate‐specific antigen level with long‐term diagnosis of clinically significant prostate cancer among patients aged 55 to 60 years: a secondary analysis of a Cohort in the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial. JAMA Netw Open. 2020;3:e1919284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vickers AJ, Savage C, O'Brien MF, Lilja H. Systematic review of pretreatment prostate‐specific antigen velocity and doubling time as predictors for prostate cancer. J Clin Oncol. 2009;27:398‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Roobol MJ, Kranse R, de Koning HJ, Schröder FH. Prostate‐specific antigen velocity at low prostate‐specific antigen levels as screening tool for prostate cancer: results of second screening round of ERSPC (ROTTERDAM). Urology. 2004;63:309‐313. [DOI] [PubMed] [Google Scholar]
- 32. Thompson IM, Ankerst DP, Chi C, et al. Assessing prostate cancer risk: results from the prostate cancer prevention trial. J Natl Cancer Inst. 2006;98:529‐534. [DOI] [PubMed] [Google Scholar]
- 33. Louie KS, Seigneurin A, Cathcart P, Sasieni P. Do prostate cancer risk models improve the predictive accuracy of PSA screening? A meta‐analysis. Ann Oncol. 2014;26:848‐864. [DOI] [PubMed] [Google Scholar]
- 34. Shariat SF, Karakiewicz PI, Roehrborn CG, Kattan MW. An updated catalog of prostate cancer predictive tools. Cancer. 2008;113:3075‐3099. [DOI] [PubMed] [Google Scholar]
- 35. Osses DF, Roobol MJ, Schoots IG. Prediction medicine: biomarkers, risk calculators and magnetic resonance imaging as risk stratification tools in prostate cancer diagnosis. Int J Mol Sci. 2019;20:1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Collins GS, de Groot JA, Dutton S, et al. External validation of multivariable prediction models: a systematic review of methodological conduct and reporting. BMC Med Res Methodol. 2014;14:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bandini M, Fossati N, Briganti A. Nomograms in urologic oncology, advantages and disadvantages. Curr Opin Urol. 2019;29:42‐51. [DOI] [PubMed] [Google Scholar]
- 38. Mottet N, Briers E, Bolla M, et al. EAU‐ESTRO‐ESUR‐SIOG Guidelines on Prostate Cancer. EAU Guidelines Office: Arnhem, The Netherlands; 2018. [Google Scholar]
- 39. Mottet N, Bellmunt J, Bolla M, et al. EAU‐ESTRO‐SIOG guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2017;71:618‐629. [DOI] [PubMed] [Google Scholar]
- 40. Roobol MJ, van Vugt HA, Loeb S, et al. Prediction of prostate cancer risk: the role of prostate volume and digital rectal examination in the ERSPC risk calculators. Eur Urol. 2012;61:577‐583. [DOI] [PubMed] [Google Scholar]
- 41. Roobol MJ, Steyerberg EW, Kranse R, et al. A risk‐based strategy improves prostate‐specific antigen–driven detection of prostate cancer. Eur Urol. 2010;57:79‐85. [DOI] [PubMed] [Google Scholar]
- 42. Grill S, Fallah M, Leach Robin J, et al. Incorporation of detailed family history from the Swedish family cancer database into the PCPT risk calculator. J Urol. 2015;193:460‐465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ankerst DP, Hoefler J, Bock S, et al. Prostate cancer prevention trial risk calculator 2.0 for the prediction of low‐ vs high‐grade prostate cancer. Urology. 2014;83:1362‐1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kranse R, Beemsterboer P, Rietbergen J, Habbema D, Hugosson J, Schröder FH. Predictors for biopsy outcome in the European randomized study of screening for prostate cancer (Rotterdam region). Prostate. 1999;39:316‐322. [DOI] [PubMed] [Google Scholar]
- 45. Vickers AJ, Cronin AM, Roobol MJ, et al. The relationship between prostate‐specific antigen and prostate cancer risk: the prostate biopsy collaborative group. Clin Cancer Res. 2010;16:4374‐4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Poyet C, Nieboer D, Bhindi B, et al. Prostate cancer risk prediction using the novel versions of the European randomised study for screening of prostate cancer (ERSPC) and prostate cancer prevention trial (PCPT) risk calculators: independent validation and comparison in a contemporary European cohort. BJU Int. 2016;117:401‐408. [DOI] [PubMed] [Google Scholar]
- 47. Pereira‐Azevedo N, Verbeek JFM, Nieboer D, Bangma CH, Roobol MJ. Head‐to‐head comparison of prostate cancer risk calculators predicting biopsy outcome. Transl Androl Urol. 2018;7:18‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bjurlin MA, Mendhiratta N, Wysock JS, Taneja SS. Multiparametric MRI and targeted prostate biopsy: improvements in cancer detection, localization, and risk assessment. Cent Eur J Urol. 2016;69:9‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ahmed HU, Kirkham A, Arya M, et al. Is it time to consider a role for MRI before prostate biopsy? Nat Rev Clin Oncol. 2009;6:197‐206. [DOI] [PubMed] [Google Scholar]
- 50. Kasivisvanathan V, Rannikko AS, Borghi M, et al. MRI‐targeted or standard biopsy for prostate‐cancer diagnosis. N Engl J Med. 2018;378:1767‐1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. van der Leest M, Cornel E, Israël B, et al. Head‐to‐head comparison of transrectal ultrasound‐guided prostate biopsy versus multiparametric prostate resonance imaging with subsequent magnetic resonance‐guided biopsy in biopsy‐naive men with elevated prostate‐specific antigen: a large prospective multicenter clinical study. Eur Urol. 2019;75:570‐578. [DOI] [PubMed] [Google Scholar]
- 52. Ahmed HU, El‐Shater Bosaily A, Brown LC, et al. Diagnostic accuracy of multi‐parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. The Lancet. 2017;389:815‐822. [DOI] [PubMed] [Google Scholar]
- 53. Moldovan PC, Van den Broeck T, Sylvester R, et al. What is the negative predictive value of multiparametric magnetic resonance imaging in excluding prostate cancer at biopsy? A systematic review and meta‐analysis from the European Association of Urology prostate cancer guidelines panel. Eur Urol. 2017;72:250‐266. [DOI] [PubMed] [Google Scholar]
- 54. Johnson DC, Raman SS, Mirak SA, et al. Detection of individual prostate cancer foci via multiparametric magnetic resonance imaging. Eur Urol. 2019;75:712‐720. [DOI] [PubMed] [Google Scholar]
- 55. Shukla‐Dave A, Hricak H, Akin O, et al. Preoperative nomograms incorporating magnetic resonance imaging and spectroscopy for prediction of insignificant prostate cancer. BJU Int. 2012;109:1315‐1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lopci E, Saita A, Lazzeri M, et al. 68Ga‐PSMA positron emission tomography/computerized tomography for primary diagnosis of prostate cancer in men with contraindications to or negative multiparametric magnetic resonance imaging: a prospective observational study. J Urol. 2018;200:95‐103. [DOI] [PubMed] [Google Scholar]
- 57. Amin A, Blazevski A, Thompson J, et al. PRIMARY Trial—a prospective multi‐centre cross‐sectional study of the additive diagnostic value of gallium‐68 prostate specific membrane antigen (PSMA) PET/CT to multiparametric (mp) MRI in the diagnostic setting for men being investigated for prostate cancer: clinical trial protocol. BJU Int. 2020;125:515‐524. [DOI] [PubMed] [Google Scholar]
- 58. Schoots IG, Padhani AR. Personalizing prostate cancer diagnosis with multivariate risk prediction tools: how should prostate MRI be incorporated? World J Urol. 2019;38:531‐545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Alberts AR, Schoots IG, Bokhorst LP, van Leenders GJ, Bangma CH, Roobol MJ. Risk‐based patient selection for magnetic resonance imaging‐targeted prostate biopsy after negative Transrectal ultrasound‐guided random biopsy avoids unnecessary magnetic resonance imaging scans. Eur Urol. 2016;69:1129‐1134. [DOI] [PubMed] [Google Scholar]
- 60. Mannaerts CK, Gayet M, Verbeek JF, et al. Prostate cancer risk assessment in biopsy‐naïve patients: the Rotterdam prostate cancer risk calculator in multiparametric magnetic resonance imaging‐Transrectal ultrasound (TRUS) fusion biopsy and systematic TRUS biopsy. Eur Urol Oncol. 2018;1:109‐117. [DOI] [PubMed] [Google Scholar]
- 61. Mehralivand S, Shih JH, Rais‐Bahrami S, et al. A magnetic resonance imaging–based prediction model for prostate biopsy risk stratification. JAMA Oncol. 2018;4:678‐685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Dwivedi DK, Kumar R, Dwivedi AK, et al. Prebiopsy multiparametric MRI‐based risk score for predicting prostate cancer in biopsy‐naive men with prostate‐specific antigen between 4–10 ng/mL. J Magn Reson Imaging. 2018;47:1227‐1236. [DOI] [PubMed] [Google Scholar]
- 63. Radtke JP, Wiesenfarth M, Kesch C, et al. Combined clinical parameters and multiparametric magnetic resonance imaging for advanced risk modeling of prostate cancer—patient‐tailored risk stratification can reduce unnecessary biopsies. Eur Urol. 2017;72:888‐896. [DOI] [PubMed] [Google Scholar]
- 64. van Leeuwen PJ, Hayen A, Thompson JE, et al. A multiparametric magnetic resonance imaging‐based risk model to determine the risk of significant prostate cancer prior to biopsy. BJU Int. 2017;120:774‐781. [DOI] [PubMed] [Google Scholar]
- 65. Alberts AR, Roobol MJ, Verbeek JFM, et al. Prediction of high‐grade prostate cancer following multiparametric magnetic resonance imaging: improving the Rotterdam European randomized study of screening for prostate cancer risk calculators. Eur Urol. 2019;75:310‐318. [DOI] [PubMed] [Google Scholar]
- 66. Vickers AJ. Prediction models in cancer care. CA Cancer J Clin. 2011;61:315‐326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhu X, Albertsen PC, Andriole GL, Roobol MJ, Schröder FH, Vickers AJ. Risk‐based prostate cancer screening. Eur Urol. 2012;61:652‐661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Shariat SF, Capitanio U, Jeldres C, Karakiewicz PI. Can nomograms be superior to other prediction tools? BJU Int. 2009;103:492‐497. [DOI] [PubMed] [Google Scholar]
- 69. Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16:e173‐e180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Shariat SF, Kattan MW, Vickers AJ, Karakiewicz PI, Scardino PT. Critical review of prostate cancer predictive tools. Future Oncol. 2009;5:1555‐1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Pereira‐Azevedo N, Osório L, Fraga A, Roobol MJ. Rotterdam prostate cancer risk calculator: development and usability testing of the Mobile phone app. JMIR Cancer. 2017;3:e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. http://www.riskcalc.org/PCPTRC/.
- 73. Nam RK, Satkunasivam R, Chin JL, et al. Next‐generation prostate cancer risk calculator for primary care physicians. Can Urol Assoc J. 2017;12:E64‐E70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Verbeek FMJ, Nieboer D, Parker C, Kattan WM, Steyerberg WE, Roobol JM. A tool for shared decision making on referral for prostate biopsy in the primary care setting: integrating risks of cancer with life expectancy. J Person Med. 2019;9:E19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Chiu PKF, Ng C‐F, Semjonow A, et al. A multicentre evaluation of the role of the prostate health index (PHI) in regions with differing prevalence of prostate cancer: adjustment of PHI reference ranges is needed for European and Asian settings. Eur Urol. 2019;75:558‐561. [DOI] [PubMed] [Google Scholar]
- 76. Jansen FH, van Schaik RHN, Kurstjens J, et al. Prostate‐specific antigen (PSA) isoform p2PSA in combination with Total PSA and free PSA improves diagnostic accuracy in prostate cancer detection. Eur Urol. 2010;57:921‐927. [DOI] [PubMed] [Google Scholar]
- 77. Foley RW, Gorman L, Sharifi N, et al. Improving multivariable prostate cancer risk assessment using the prostate health index. BJU Int. 2016;117:409‐417. [DOI] [PubMed] [Google Scholar]
- 78. Zhu Y, Han C‐T, Zhang G‐M, et al. Development and external validation of a prostate health index‐based nomogram for predicting prostate cancer. Sci Rep. 2015;5:15341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lazzeri M, Lughezzani G, Haese A, et al. Clinical performance of prostate health index in men with tPSA>10ng/ml: Results from a multicentric European study. Urol Oncol Semin Original Investig. 2016;34:415.e13‐e19. [DOI] [PubMed] [Google Scholar]
- 80. Tosoian JJ, Druskin SC, Andreas D, et al. Use of the prostate health index for detection of prostate cancer: results from a large academic practice. Prostate Cancer Prostatic Dis. 2017;20:228‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Loeb S, Sanda Martin G, Broyles Dennis L, et al. The prostate health index selectively identifies clinically significant prostate cancer. J Urol. 2015;193:1163‐1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Chiu PKF, Roobol MJ, Teoh JY, et al. Prostate health index (PHI) and prostate‐specific antigen (PSA) predictive models for prostate cancer in the Chinese population and the role of digital rectal examination‐estimated prostate volume. Int Urol Nephrol. 2016;48:1631‐1637. [DOI] [PubMed] [Google Scholar]
- 83. Druskin SC, Tosoian JJ, Young A, et al. Combining prostate health index density, magnetic resonance imaging and prior negative biopsy status to improve the detection of clinically significant prostate cancer. BJU Int. 2018;121:619‐626. [DOI] [PubMed] [Google Scholar]
- 84. Roobol MJ, Vedder MM, Nieboer D, et al. Comparison of two prostate cancer risk calculators that include the prostate health index. Eur Urol Focus. 2015;1:185‐190. [DOI] [PubMed] [Google Scholar]
- 85. Loeb S, Shin SS, Broyles DL, et al. Prostate health index improves multivariable risk prediction of aggressive prostate cancer. BJU Int. 2017;120:61‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Hsieh P‐F, Li W‐J, Lin W‐C, et al. Combining prostate health index and multiparametric magnetic resonance imaging in the diagnosis of clinically significant prostate cancer in an Asian population. World J Urol. 2019;38:1207‐1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Gnanapragasam VJ, Burling K, George A, et al. The prostate health index adds predictive value to multi‐parametric MRI in detecting significant prostate cancers in a repeat biopsy population. Sci Rep. 2016;6:35364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Mathieu R, Castelli C, Fardoun T, et al. Cost analysis of prostate cancer detection including the prostate health index (phi). World J Urol. 2019;37:481‐487. [DOI] [PubMed] [Google Scholar]
- 89. Vickers AJ, Cronin AM, Aus G, et al. A panel of kallikrein markers can reduce unnecessary biopsy for prostate cancer: data from the European randomized study of prostate cancer screening in Göteborg. Sweden BMC Med. 2008;6:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Vickers AJ, Cronin AM, Aus G, et al. Impact of recent screening on predicting the outcome of prostate cancer biopsy in men with elevated prostate‐specific antigen. Cancer. 2010;116:2612‐2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Nordström T, Vickers A, Assel M, Lilja H, Grönberg H, Eklund M. Comparison between the four‐kallikrein panel and prostate health index for predicting prostate cancer. Eur Urol. 2015;68:139‐146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Verbeek JFM, Bangma CH, Kweldam CF, et al. Reducing unnecessary biopsies while detecting clinically significant prostate cancer including cribriform growth with the ERSPC Rotterdam risk calculator and 4Kscore. Urol Oncol Semin Original Investig. 2019;37:138‐144. [DOI] [PubMed] [Google Scholar]
- 93. Kanwal R, Plaga AR, Liu X, Shukla GC, Gupta S. MicroRNAs in prostate cancer: functional role as biomarkers. Cancer Lett. 2017;407:9‐20. [DOI] [PubMed] [Google Scholar]
- 94. Fabris L, Ceder Y, Chinnaiyan AM, et al. The potential of MicroRNAs as prostate cancer biomarkers. Eur Urol. 2016;70:312‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Roberts MJ, Chow CWK, Schirra HJ, et al. Diagnostic performance of expression of PCA3, Hepsin and miR biomarkers inejaculate in combination with serum PSA for the detection of prostate cancer. Prostate. 2015;75:539‐549. [DOI] [PubMed] [Google Scholar]
- 96. Kelly BD, Miller N, Healy NA, Walsh K, Kerin MJ. A review of expression profiling of circulating microRNAs in men with prostate cancer. BJU Int. 2013;111:17‐21. [DOI] [PubMed] [Google Scholar]
- 97. Cui Y, Cao W, Li Q, et al. Evaluation of prostate cancer antigen 3 for detecting prostate cancer: a systematic review and meta‐analysis. Sci Rep. 2016;6:25776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Nygård Y, Haukaas SA, Eide GE, et al. Prostate cancer antigen‐3 (PCA3) and PCA3‐based nomograms in the diagnosis of prostate cancer: an external validation of Hansen's nomogram on a Norwegian cohort. Scand J Urol. 2015;49:8‐15. [DOI] [PubMed] [Google Scholar]
- 99. Hansen J, Auprich M, Ahyai SA, et al. Initial prostate biopsy: development and internal validation of a biopsy‐specific nomogram based on the prostate cancer antigen 3 assay. Eur Urol. 2013;63:201‐209. [DOI] [PubMed] [Google Scholar]
- 100. Chun FK, de la Taille A, van Poppel H, et al. Prostate cancer gene 3 (PCA3): development and internal validation of a novel biopsy nomogram. Eur Urol. 2009;56:659‐668. [DOI] [PubMed] [Google Scholar]
- 101. Seisen T, Rouprêt M, Brault D, et al. Accuracy of the prostate health index versus the urinary prostate cancer antigen 3 score to predict overall and significant prostate cancer at initial biopsy. Prostate. 2015;75:103‐111. [DOI] [PubMed] [Google Scholar]
- 102. Scattoni V, Lazzeri M, Lughezzani G, et al. Head‐to‐head comparison of prostate health index and urinary PCA3 for predicting cancer at initial or repeat biopsy. J Urol. 2013;190:496‐501. [DOI] [PubMed] [Google Scholar]
- 103. Ferro M, Bruzzese D, Perdonà S, et al. Prostate health index (phi) and prostate cancer antigen 3 (PCA3) significantly improve prostate cancer detection at initial biopsy in a total PSA range of 2–10 ng/ml. PLoS One. 2013;8:e67687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Perdonà S, Bruzzese D, Ferro M, et al. Prostate health index (phi) and prostate cancer antigen 3 (PCA3) significantly improve diagnostic accuracy in patients undergoing prostate biopsy. Prostate. 2013;73:227‐235. [DOI] [PubMed] [Google Scholar]
- 105. Leyten GHJM, Hessels D, Smit FP, et al. Identification of a candidate gene panel for the early diagnosis of prostate cancer. Clin Cancer Res. 2015;21:3061. [DOI] [PubMed] [Google Scholar]
- 106. Van Neste L, Hendriks RJ, Dijkstra S, et al. Detection of high‐grade prostate cancer using a urinary molecular biomarker‐based risk score. Eur Urol. 2016;70:740‐748. [DOI] [PubMed] [Google Scholar]
- 107. Hendriks RJ, van der Leest MMG, Dijkstra S, et al. A urinary biomarker‐based risk score correlates with multiparametric MRI for prostate cancer detection. Prostate. 2017;77:1401‐1407. [DOI] [PubMed] [Google Scholar]
- 108. Grönberg H, Adolfsson J, Aly M, et al. Prostate cancer screening in men aged 50–69 years (STHLM3): a prospective population‐based diagnostic study. Lancet Oncol. 2015;16:1667‐1676. [DOI] [PubMed] [Google Scholar]
- 109. Carlsson SV, Kattan MW. The STHLM3 prostate cancer diagnostic study: calibration, clarification, and comments. Nat Rev Clin Oncol. 2016;13:394. [DOI] [PubMed] [Google Scholar]
- 110. Eklund M, Nordström T, Aly M, et al. The Stockholm‐3 (STHLM3) model can improve prostate cancer diagnostics in men aged 50–69 yr compared with current prostate cancer testing. Eur Urol Focus. 2018;4:707‐710. [DOI] [PubMed] [Google Scholar]
- 111. Grönberg H, Eklund M, Picker W, et al. Prostate cancer diagnostics using a combination of the Stockholm3 blood test and multiparametric magnetic resonance imaging. Eur Urol. 2018;74:722‐728. [DOI] [PubMed] [Google Scholar]
- 112. Draisma G, Boer R, Otto SJ, et al. Lead times and Overdetection due to prostate‐specific antigen screening: estimates from the European randomized study of screening for prostate cancer. J Natl Cancer Inst. 2003;95:868‐878. [DOI] [PubMed] [Google Scholar]
- 113. Roobol MJ, Remmers S. PSA‐testen bij mannen ouder dan 70 jaar: zwart‐wit of toch grijs? [PSA testing in men older than 70 years: black and white or grey?]. Tijdschrift Urol. 2019;9(suppl 1):4‐5. [Google Scholar]
- 114. Arsov C, Becker N, Hadaschik BA, et al. Prospective randomized evaluation of risk‐adapted prostate‐specific antigen screening in young men: the PROBASE trial. Eur Urol. 2013;64:873‐875. [DOI] [PubMed] [Google Scholar]
- 115. Arsov C, Becker N, Herkommer K, et al. The German risk‐adapted PCA screening trial (PROBASE)—first results. Eur Urol Suppl. 2015;14:e23. [Google Scholar]
- 116. Arsov C, Becker N, Herkommer K, et al. The German risk‐adapted prostate cancer screening trial (PROBASE): first results after recruitment of 30.000 men. Eur Urol Suppl. 2018;17:e372‐e373. [Google Scholar]
- 117. Kweldam CF, Nieboer D, Algaba F, et al. Gleason grade 4 prostate adenocarcinoma patterns: an interobserver agreement study among genitourinary pathologists. Histopathology. 2016;69:441‐449. [DOI] [PubMed] [Google Scholar]
- 118. Zumsteg ZS, Spratt DE, Pei I, et al. A new risk classification system for therapeutic decision making with intermediate‐risk prostate cancer patients undergoing dose‐escalated external‐beam radiation therapy. Eur Urol. 2013;64:895‐902. [DOI] [PubMed] [Google Scholar]
- 119. Richard PO, Timilshina N, Komisarenko M, et al. The long‐term outcomes of Gleason grade groups 2 and 3 prostate cancer managed by active surveillance: results from a large population‐based cohort. Can Urol Assoc J. 2020;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Woo S, Suh CH, Kim SY, Cho JY, Kim SH, Moon MH. Head‐to‐head comparison between Biparametric and multiparametric MRI for the diagnosis of prostate cancer: a systematic review and meta‐analysis. Am J Roentgenol. 2018;211:W226‐W241. [DOI] [PubMed] [Google Scholar]
- 121. https://www.g2screening.se/.
- 122. Hugosson J. The GÖTEBORG prostate cancer screening 2 trial. ISRCTN. 2017. [Google Scholar]
- 123. Nordström T, Jäderling F, Carlsson S, Aly M, Grönberg H, Eklund M. Does a novel diagnostic pathway including blood‐based risk prediction and MRI‐targeted biopsies outperform prostate cancer screening using prostate‐specific antigen and systematic prostate biopsies? ‐ protocol of the randomised study STHLM3MRI. BMJ Open. 2019;9:e027816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Auvinen A, Rannikko A, Taari K, et al. A randomized trial of early detection of clinically significant prostate cancer (ProScreen): study design and rationale. Eur J Epidemiol. 2017;32:521‐527. [DOI] [PubMed] [Google Scholar]
- 125. Roobol M. Perspective: Enforce the clinical guidelines. Nature. 2015;528:S123. [DOI] [PubMed] [Google Scholar]


