Abstract
Tezepelumab, a human immunoglobulin G2 monoclonal antibody against thymic stromal lymphopoietin, is currently under clinical development for the treatment of severe, uncontrolled asthma. This phase 1, randomized, placebo‐controlled, single‐ascending‐dose study assessed the safety, tolerability, pharmacokinetics, and immunogenicity of subcutaneous tezepelumab in healthy Japanese men. Participants were assigned to 1 of 3 tezepelumab dose cohorts (35, 105, or 280 mg; n = 8 per cohort) and randomized (6:2) to receive a single subcutaneous dose of tezepelumab or placebo, with a follow‐up period of 84 to 112 days. The overall incidences and severities of treatment‐emergent adverse events were similar across tezepelumab doses and between the tezepelumab and placebo groups. Tezepelumab was absorbed slowly, reaching a maximum serum concentration (mean, 5.2‐39.7 µg/mL) after 7 to 10 days. Area under the concentration‐time curve (mean, 207.2‐1612.0 µg · day /mL) increased in an approximate dose‐proportional manner. Tezepelumab had a long terminal serum half‐life (mean, 23.9‐26.3 days) and a small apparent distribution volume, suggesting limited distribution into peripheral tissues. No participants developed anti‐tezepelumab antibodies. Single‐dose, subcutaneous administration of tezepelumab 35 to 280 mg resulted in an acceptable safety profile with linear pharmacokinetics in healthy Japanese men. No clear differences in tezepelumab safety and pharmacokinetics between Japanese and non‐Japanese populations were identified.
Keywords: asthma, pharmacokinetics, phase 1, safety, tezepelumab, thymic stromal lymphopoietin
Asthma is a chronic inflammatory disease of the airways, characterized by symptoms of wheezing, shortness of breath, chest tightness, and cough.1 It is one of the most common respiratory diseases, affecting approximately 358 million people worldwide.2 The prevalence of asthma in Japan is approximately 7%.3 Five percent to 10% of patients with asthma have severe disease that remains uncontrolled despite treatment with medium‐ to high‐dose inhaled corticosteroids and long‐acting β‐agonists.4 Patients with uncontrolled asthma have a substantially impaired quality of life and a significantly higher risk of comorbidities (principally, allergic rhinitis and rhinosinusitis1, 5), exacerbations, hospitalizations, and death compared with patients with good disease control.4, 6
Efforts to improve disease control in patients with severe asthma have been hampered by heterogeneous patient responses to recommended treatments.7 This is indicative of the complexity of asthma pathogenesis, which includes both type 2 (T2) and non‐T2 inflammatory pathways.8, 9, 10, 11 Thymic stromal lymphopoietin (TSLP) is a key upstream cytokine, termed an alarmin, which is released by airway epithelial cells in response to allergens, pollutants, or pathogens.12 TSLP activates dendritic cells and stimulates the differentiation of T‐naïve cells into type 2 helper T cells, thereby driving the release of T2 cytokines including interleukin (IL)‐4, IL‐5, and IL‐13.12 Along with the other epithelial alarmins, IL‐25 and IL‐33, TSLP activates many types of inflammatory cells, including group 2 innate lymphoid cells, mast cells, basophils, and macrophages.13, 14, 15, 16 TSLP can also drive type 17 helper T cell expansion, which promotes neutrophil recruitment.12 TSLP expression levels have been shown to be higher in the airways of patients with asthma than in those of healthy controls.17, 18 Taken together, these data identify TSLP as an important therapeutic target for asthma, encompassing both T2 and non‐T2 inflammatory pathways.19
Tezepelumab is a human immunoglobulin G2 monoclonal antibody against TSLP that is under clinical development for the treatment of severe, uncontrolled asthma. A proof‐of‐concept study demonstrated that tezepelumab suppressed bronchoconstriction and biomarkers of T2 inflammation after inhaled allergen challenge in adult patients with mild, atopic asthma.20 In a subsequent phase 2b trial in adults with uncontrolled moderate to severe asthma, treatment with tezepelumab resulted in significantly lower rates of asthma exacerbations, together with improved lung function, asthma control, and quality of life, compared with placebo. These treatment benefits were observed irrespective of baseline blood eosinophil counts or T2 inflammation status.21 Previous clinical pharmacokinetic (PK) and safety studies of tezepelumab have demonstrated linear PK and a long terminal serum half‐life (t½,z).22 No severe adverse events (AEs), treatment‐emergent adverse events (TEAEs), serious TEAEs, or AEs leading to study discontinuation were reported.23, 24 To date, all clinical studies evaluating the safety, tolerability, and PK of tezepelumab have been performed in Caucasian participants. Here, we report the results of a phase 1, randomized, placebo‐controlled study to assess the safety, tolerability, PK, and immunogenicity of tezepelumab in healthy Japanese male volunteers.
Methods
Study Design and Eligibility
The study protocol was approved by the independent Hakata Clinic Institutional Review Board, Fukuoka, Japan. The study was performed in accordance with the ethical principles outlined in Good Clinical Practice guidelines and the Declaration of Helsinki. Written informed consent was obtained from all participants before initiation of the study.
This was a phase 1, single‐center, randomized, single‐blind, placebo‐controlled, single‐ascending dose study in healthy Japanese male adults (ClinicalTrials.gov identifier: NCT01913028). The study was conducted at SOUSEIKAI Fukuoka Mirai Hospital Clinical Research Center, Fukuoka, Japan, from August 3, 2013, to January 11, 2014.
Participants were required to be 20 to 45 years of age, with a body mass index of 18 to 27 kg/m2 at screening and no significant irregularities on physical examination, medical history, electrocardiogram (ECG), clinical chemistry, hematology, or urinalysis results. For participants to be considered ethnically Japanese, both parents and both sets of grandparents were required to be Japanese. Key exclusion criteria included a positive tuberculosis screening based on chest radiograph and a positive tuberculosis‐specific enzyme‐linked immunospot assay (T‐SPOT.TB, Oxford Immunotec Ltd, Oxford, UK) result25, 26; a history of malignancy of any type, other than surgically excised nonmelanomatous skin cancers, within 5 years before randomization; type 1 or type 2 diabetes; ECG abnormalities; evidence of bacterial, viral, fungal, or parasitic infection in the 30 days before randomization; serum positivity for HIV, hepatitis B, or hepatitis C; and use of systemic cytotoxic or systemic immunosuppressive medications (other than corticosteroids) in the 6 months before randomization. Apart from paracetamol or acetaminophen, no concomitant medication or therapy (including herbal remedies, vitamin supplements, and over‐the‐counter products) was permitted without the consent of the investigator.
Eligible volunteers were sequentially assigned to 1 of 3 tezepelumab dose cohorts (n = 8 per cohort): cohort 1, subcutaneous tezepelumab 35 mg; cohort 2, subcutaneous tezepelumab 105 mg; cohort 3, subcutaneous tezepelumab 280 mg. The dose levels for this study were based on the results from phase 1 clinical studies in Caucasian participants.22 Within each cohort, participants were randomized to receive subcutaneous tezepelumab or placebo in a 6:2 ratio. Following a screening period of up to 28 days, individuals received a single subcutaneous dose of tezepelumab or placebo on day 1. Progression to the next higher dose occurred only if the previous dose level was deemed to be safe and well tolerated by the Safety Review Committee, based on stopping criteria detailed in the study protocol (AstraZeneca, clinical study protocol: Study D5180C00003; unpublished data). The follow‐up period after dosing was 84 days for cohorts 1 and 2 and 112 days for cohort 3 (Figure 1).
Figure 1.
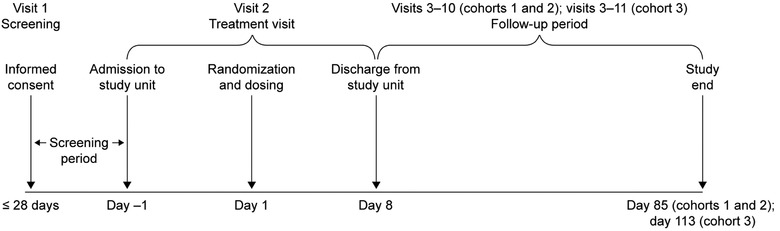
Study design.
Safety and Tolerability Assessments
Safety assessments included the incidence and severity of AEs. AEs were classified according to Preferred Term and System Organ Class using the Medical Dictionary of Regulatory Activities, Version 16.1. AE severity was graded according to the Common Terminology Criteria for Adverse Events, Version 4.0. Additional assessments included clinical laboratory variables (hematology, blood biochemistry, urinalysis), physical examination, 12‐lead ECG, and vital signs (blood pressure, pulse rate, body temperature, and respiratory rate).
Pharmacokinetic Evaluations
Blood samples (5 mL) were taken for PK analysis on study day 1 (within a 3‐hour period before dosing, and then 4 and 8 hours after dosing) and after dosing on days 2, 3, 4, 5, 6, 8, 11, 15, 22, 29, 43, 57, 71, 85 (end‐of‐study visit for cohorts 1 and 2), and 113 (end‐of‐study visit for cohort 3). Tezepelumab serum concentrations were analyzed by Pharmaceutical Product Development, LLC (Wilmington, North Carolina) using a validated enzyme‐linked immunosorbent assay, with a lower limit of quantification of 10 ng/mL, an intra‐assay variability of 2% to 7%, and an interassay variability of 3% to 7%. Briefly, anti‐tezepelumab–coated microplates were loaded with calibration standards, quality controls (prepared by adding tezepelumab to pooled human serum), and study samples, which were diluted 1:50 in SuperBlock T20 buffer. Microplate wells were washed to remove unbound tezepelumab before addition of horseradish peroxidase (HRP) anti‐tezepelumab to each well. The wells were then washed again to remove unbound HRP anti‐tezepelumab. A 2‐component 3,3’,5,5'‐tetramethylbenzidine substrate solution was added to each well; the reaction of this solution with HRP created a colorimetric signal that was proportional to the amount of tezepelumab present. The optical density of each well was then measured at 450 to 650 nm. Optical density measurements were converted to concentrations using a computer software–mediated comparison to a calibration curve from the same assay, which regressed according to a 4‐parameter logistic equation with a 1/ratio weighting factor.
Immunologic Evaluations
Blood samples (5 mL) for the measurement of anti‐tezepelumab antibodies were collected on study days 1 (within a 3‐hour period before dosing), 29, 57, 85 (end‐of‐study visit for cohorts 1 and 2), and 113 (end‐of‐study visit for cohort 3). Anti‐tezepelumab antibody levels in serum were analyzed by Pharmaceutical Product Development, LLC, using a validated electrochemiluminescence‐based immunoassay. The assay used the Meso Scale Discovery (MSD) platform (Meso Scale Diagnostics, Rockville, Maryland) and followed a 2‐tiered approach consisting of a screening assay and a specificity assay. The screening assay was performed to detect antibodies that bound to tezepelumab; samples with a signal‐to‐noise value greater than the assay cutoff point were then tested to confirm the specificity of the response. Samples were treated with 300 mM acetic acid (to enable antibody complex dissociation before analysis) and incubated with a conjugate/neutralization mixture consisting of biotinylated tezepelumab, ruthenylated tezepelumab, 1 M Tris pH 9.5, and soluble drug (specificity assay only). This mixture was added to a blocked MSD streptavidin‐coated microtiter plate before washing of the plate (to remove any complexes that were not bound to the streptavidin coating) and the addition of tripropylamine MSD read buffer to each well. An MSD Sector Imager 6000 plate reader, which passes a current across the plate‐associated electrodes resulting in a luminescence signal (measured in electrochemiluminescence units), was used to read the plate. The assay sensitivity and lower limit of reliable detection were 20 ng/mL and 40 ng/mL, respectively, of rabbit anti‐tezepelumab polyclonal antibody in neat pooled normal human serum. At the lower limit of reliable detection, the assay tolerated 50 µg/mL of tezepelumab. At 500 ng/mL of rabbit anti‐tezepelumab polyclonal antibody, the assay tolerated ≥300 µg/mL of tezepelumab.
Analysis Sets
The safety analysis set included all volunteers who received study treatment (tezepelumab or placebo). The PK analysis set included all participants in the safety analysis set with at least 1 detectable tezepelumab serum concentration.
Statistical Analysis
All data were summarized by treatment group using descriptive statistics. PK parameters were calculated by a noncompartmental approach, using WinNonlin software version 6.3 (Pharsight, Sunnyvale, California). Derived PK parameters included: maximum serum concentration (Cmax); area under the serum concentration–time curve from time 0 to infinity (AUC0–inf); apparent serum clearance (CL/F); apparent volume of distribution (Vz/F); t½,z; and time to Cmax. No formal statistical hypothesis testing of treatment group differences was performed, and there was no imputation of missing data.
Results
Demographics and Baseline Characteristics
In total, 24 healthy Japanese men were enrolled into the study and randomized to receive subcutaneous tezepelumab or placebo within 3 ascending tezepelumab dose cohorts (35 mg, 105 mg, 280 mg). All 24 participants completed the study. The mean (standard deviation) age was 24.8 (5.5) years and mean body mass index was 21.8 kg/m2. There were no significant differences between the treatment groups with regard to demographics or other baseline characteristics (Table 1).
Table 1.
Baseline Demographics and Characteristics
| Placebo (n = 6) | Tezepelumab 35 mg(n = 6) | Tezepelumab 105 mg (n = 6) | Tezepelumab 280 mg (n = 6) | Total(N = 24) | |
|---|---|---|---|---|---|
| Age, y | 22.2 (3.1) | 23.5 (3.0) | 24.5 (5.2) | 28.8 (8.0) | 24.8 (5.5) |
| Weight, kg | 60.4 (7.9) | 60.1 (6.5) | 63.2 (5.8) | 68.7 (12.4) | 63.1 (8.7) |
| Height, cm | 167.3 (2.9) | 165.8 (5.7) | 171.5 (1.6) | 173.9 (5.6) | 169.6 (5.2) |
| BMI, kg/m2 | 21.6 (2.3) | 21.9 (2.2) | 21.5 (1.9) | 22.6 (2.7) | 21.8 (2.2) |
BMI, body mass index.
Data are mean (standard deviation). All participants were healthy Japanese men.
Safety and Tolerability
The safety analysis set comprised all 24 study participants. A summary of TEAEs in the study is presented in Table 2. No TEAEs were reported for the tezepelumab 35 mg or 105 mg cohorts; however, in the tezepelumab 280 mg cohort, 1 participant (16.7%) reported moderate paronychia of the left thumb, and 1 participant (16.7%) reported mild nasopharyngitis (verbatim term: common cold). In the placebo group, 2 participants (33.3%) reported TEAEs of mild nasopharyngitis. None of these TEAEs was judged by the investigator to be treatment related. There were no deaths, other serious AEs, or discontinuations due to AEs. No clinically relevant treatment‐related changes in any of the laboratory variables were identified during the study, and no individual laboratory abnormalities were reported by the investigator as an AE. None of the study participants had serum alanine aminotransferase or aspartate aminotransferase levels more than 3 times the upper limit of normal together with total bilirubin levels more than 2 times the upper limit of normal. There were no clinically relevant treatment‐related changes in blood pressure, pulse rate, body temperature, or respiratory rate. One participant who received placebo experienced an increase in body temperature that was attributed to nasopharyngitis and was reported as a TEAE by the investigator. No clinically important abnormalities in ECG data were reported during the study, and no clinically significant abnormal physical examination findings were reported by the investigator.
Table 2.
Treatment‐Emergent Adverse Events
| Placebo(n = 6) | Tezepelumab 35 mg(n = 6) | Tezepelumab 105 mg(n = 6) | Tezepelumab 280 mg(n = 6) | Total(N = 24) | |
|---|---|---|---|---|---|
| Any TEAE, n (%) | 2 (33.3) | 0 (0.0) | 0 (0.0) | 2 (33.3) | 4 (16.7) |
| Grade 1 (mild) | 2 (33.3) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 3 (12.5) |
| Grade 2 (moderate) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 1 (4.2) |
| Grade ≥3 (severe) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Infections and infestations,a n (%) | 2 (33.3) | 0 (0.0) | 0 (0.0) | 2 (33.3) | 4 (16.7) |
| Nasopharyngitis | 2 (33.3) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 3 (12.5) |
| Paronychia | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 1 (4.2) |
TEAE, treatment‐emergent adverse event.
System Organ Class/Preferred Term (Medical Dictionary for Regulatory Activities, Version 16.1).
Pharmacokinetics
All 18 participants who received tezepelumab had at least 1 detectable tezepelumab serum concentration and were included in the PK analysis set. One participant in the tezepelumab 280 mg dose group exhibited an atypical 5‐fold lower PK exposure. This was unexpected and considered an outlier based on low interindividual variability in exposures at the other dose levels and from previous PK studies with tezepelumab.22, 23 PK data from this participant have been excluded from the PK analyses presented herein. The tezepelumab concentration‐time profiles (Figure 2) showed a slow absorption and prolonged exposure following subcutaneous administration, characteristic of a human immunoglobulin G monoclonal antibody.27 The median time to Cmax of tezepelumab was 7.0 to 10.0 days, and the mean t½,z was 23.9 to 26.3 days (Table 3). Cmax (mean, 5.2‐39.7 µg/mL) and AUC0–inf (mean, 207.2–1612.0 μg · day/mL) were approximately dose‐proportional over the 8‐fold tezepelumab dose range administered (35–280 mg). The Vz/F of tezepelumab (mean, 5568‐6050 mL) was equivalent to approximately 100 mL/kg (assuming a 60‐kg body weight), and the mean CL/F of tezepelumab was low (147.8‐175.0 mL/day) (Table 3).
Figure 2.

Tezepelumab serum concentration–time profiles. After administration of tezepelumab, none of the serum concentrations measured in any participant at any time point were below the LLOQ (10 ng/mL). One participant in the tezepelumab 280‐mg dose group exhibited an atypical 5‐fold lower PK exposure. This was unexpected and considered an outlier based on low interindividual variability in exposures at the other dose levels and from previous PK studies with tezepelumab. PK data from this participant have been excluded from the PK analyses presented herein. LLOQ, lower limit of quantification; PK, pharmacokinetic; SD, standard deviation.
Table 3.
Tezepelumab Pharmacokinetic Parameters
| Tezepelumab 35 mg | Tezepelumab 105 mg | Tezepelumab 280 mg | |
|---|---|---|---|
| (n = 6) | (n = 6) | (n = 5)a | |
| tmax, days | 7.0 (3.0‐10.0) | 8.5 (5.0‐14.0) | 10.0 (7.0‐14.0) |
| Cmax, µg/mL | 5.2 (0.8) | 15.7 (1.7) | 39.7 (7.8) |
| t½,z, days | 23.9 (2.8) | 26.3 (3.4) | 24.0 (2.8) |
| AUC0–inf, µg · day/mL | 207.2 (32.9) | 718.0 (78.0) | 1612.0 (157.7) |
| Vz/F, mL | 5907 (872.9) | 5568 (584.8) | 6050 (764.4) |
| CL/F, mL/day | 172.3 (26.5) | 147.8 (17.3) | 175.0 (16.7) |
AUC0–inf, area under the serum concentration–time curve from time 0 to infinity; CL/F, apparent serum clearance; Cmax, maximum serum concentration; t½,z, terminal serum half‐life; tmax, time to Cmax; PK, pharmacokinetic; Vz/F, apparent volume of distribution.
Data are mean (standard deviation) except for tmax (median [range]).
One participant in the tezepelumab 280 mg dose group exhibited an atypical 5‐fold lower PK exposure. This was unexpected and considered an outlier based on low interindividual variability in exposures at the other dose levels and from previous PK studies with tezepelumab. PK data from this participant have been excluded from the PK analyses presented herein.
Immunologic Evaluations
All 24 participants tested negative for anti‐tezepelumab antibodies at all time points.
Discussion
The results of this phase 1, single‐ascending dose study indicate that tezepelumab, administered as subcutaneous doses of 35, 105, and 280 mg, has a good safety and tolerability profile in the study population of healthy Japanese men. No serious AEs were reported during the study, and there were no discontinuations due to AEs. The incidences and severities of AEs were similar in the tezepelumab and placebo groups, consistent with safety findings from a phase 2b study of tezepelumab in adults with moderate to severe uncontrolled asthma, in which at least 1 TEAE was reported in 65.9% and 66.0% of patients in the placebo and tezepelumab groups, respectively, and in which the majority of TEAEs (71%) were mild or moderate in severity.21
Tezepelumab demonstrated linear PK in healthy Japanese men and a systemic exposure that was dose proportional over the dose range investigated. The PK profile indicated a slow rate of absorption and a small Vz/F, suggesting limited distribution into peripheral tissues. These characteristics are consistent with those of other therapeutic monoclonal antibodies.28, 29, 30 When administered via subcutaneous injection, the primary route of absorption of monoclonal antibodies is the lymphatic system.30 Subsequent distribution into peripheral tissues is limited because it is mediated primarily by convective extravasation.31 The mean CL/F of tezepelumab was low (147.8‐175.0 mL/day), consistent with the mean CL/F value reported in a previous study of tezepelumab PK in 21 adolescents (aged 12‐17 years) with mild to moderate asthma (159 mL/day). Another pronounced PK characteristic of tezepelumab that it shares with other monoclonal antibodies28, 29, 30 was its long t½,z (mean, 23.9‐26.3 days). This is consistent with work demonstrating that monoclonal antibodies are protected from lysosomal degradation by the binding of immunoglobulin G to the neonatal Fc receptor.28, 29, 30 The mean t½,z reported in this study was consistent with that reported in a study of tezepelumab PK in 21 adolescents with mild to moderate asthma (25.3 days)23 and that reported when a population PK model was developed for tezepelumab using pooled PK data from clinical studies (22.0 days).24
None of the participants who received tezepelumab developed anti‐tezepelumab antibodies, suggesting that the immunogenicity of tezepelumab is low. Consistent with this finding, a low incidence of anti‐tezepelumab antibodies was also reported in the phase 2b study: 3.7% (5/136), 0.8% (1/131), and 2.3% (3/131) of patients in the 70 mg (every 4 weeks), 210 mg (every 4 weeks), and 280 mg (every 2 weeks) dose groups developed antidrug antibodies, respectively.21 The immunogenicity of tezepelumab was also found to be lower than that of other monoclonal antibodies indicated for the treatment of asthma; for example, in a study of the PK and pharmacodynamics of a single dose of mepolizumab in healthy Japanese male adults, 37.5% (3/8), 12.5% (1/8), and 12.5% (1/8) of patients in the 10 mg, 75 mg, and 250 mg single‐dose groups, respectively, developed antidrug antibodies.32 Moreover, in a study of the long‐term safety and efficacy of reslizumab in patients with eosinophilic asthma, 5.0% (24/481) of patients who had not previously been treated with reslizumab and 5.1% (29/571) of those who had previously received reslizumab developed antidrug antibodies.33
Conclusions
Here, we report the first clinical evaluation of the safety, tolerability, and PK of tezepelumab in a Japanese population. In this phase 1, single‐ascending‐dose study, tezepelumab 35 to 280 mg had an acceptable safety profile and linear PK in healthy Japanese men. No clear differences in tezepelumab safety and PK between Japanese and non‐Japanese populations were identified.
Conflicts of Interest
K.S., N.H., and M.H. are employees of AstraZeneca K.K., Japan. S.M. and S.I. are employees of SOUSEIKAI Fukuoka Mirai Hospital Clinical Research Center, Fukuoka, Japan, which received funding for this study from AstraZeneca. N.U. is an employee of the Showa University School of Medicine, Tokyo, Japan, which received funding for this study from AstraZeneca. S.R. is an employee of AstraZeneca, USA.
Funding
AstraZeneca and Amgen Inc. funded the study and contributed to study design, data collection, analysis, and interpretation.
Author Contributions
All authors had full access to the study data. The corresponding author had final responsibility for the decision to submit the manuscript for publication.
Data Sharing
All data requests should be submitted to the corresponding author for consideration.
Acknowledgments
The authors thank the participants in this study. Medical writing support was provided by Laura Knapp, PhD, of PharmaGenesis London, London, UK.
References
- 1. Papi A, Brightling C, Pedersen SE, Reddel HK. Asthma. Lancet. 2018;391(10122):783‐800. [DOI] [PubMed] [Google Scholar]
- 2. Global Burden of Disease 2015 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990‐2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1545‐1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ichinose M, Sugiura H, Nagase H, et al. Japanese guidelines for adult asthma 2017. Allergol Int. 2017;66(2):163‐189. [DOI] [PubMed] [Google Scholar]
- 4. Peters SP, Ferguson G, Deniz Y, Reisner C. Uncontrolled asthma: a review of the prevalence, disease burden and options for treatment. Respir Med. 2006;100(7):1139‐1151. [DOI] [PubMed] [Google Scholar]
- 5. Dixon AE, Kaminsky DA, Holbrook JT, Wise RA, Shade DM, Irvin CG. Allergic rhinitis and sinusitis in asthma: differential effects on symptoms and pulmonary function. Chest. 2006;130(2):429‐435. [DOI] [PubMed] [Google Scholar]
- 6. Bateman ED, Buhl R, O'Byrne PM, et al. Development and validation of a novel risk score for asthma exacerbations: the risk score for exacerbations. J Allergy Clin Immunol. 2015;135(6):1457‐1464 e1454. [DOI] [PubMed] [Google Scholar]
- 7. Bateman ED, Bousquet J, Busse WW, et al. Stability of asthma control with regular treatment: an analysis of the Gaining Optimal Asthma controL (GOAL) study. Allergy. 2008;63(7):932‐938. [DOI] [PubMed] [Google Scholar]
- 8. Anderson GP. Endotyping asthma: new insights into key pathogenic mechanisms in a complex, heterogeneous disease. Lancet. 2008;372(9643):1107‐1119. [DOI] [PubMed] [Google Scholar]
- 9. Corren J. Asthma phenotypes and endotypes: an evolving paradigm for classification. Discov Med. 2013;15(83):243‐249. [PubMed] [Google Scholar]
- 10. Skloot GS. Asthma phenotypes and endotypes: a personalized approach to treatment. Curr Opin Pulm Med. 2016;22(1):3‐9. [DOI] [PubMed] [Google Scholar]
- 11. Agache IO. From phenotypes to endotypes to asthma treatment. Curr Opin Allergy Clin Immunol. 2013;13(3):249‐256. [DOI] [PubMed] [Google Scholar]
- 12. Robinson D, Humbert M, Buhl R, et al. Revisiting type 2‐high and type 2‐low airway inflammation in asthma: current knowledge and therapeutic implications. Clin Exp Allergy. 2017;47(2):161‐175. [DOI] [PubMed] [Google Scholar]
- 13. Allakhverdi Z, Comeau MR, Jessup HK, et al. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J Exp Med. 2007;204(2):253‐258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kitajima M, Lee HC, Nakayama T, Ziegler SF. TSLP enhances the function of helper type 2 cells. Eur J Immunol. 2011;41(7):1862‐1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Soumelis V, Reche PA, Kanzler H, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3(7):673‐680. [DOI] [PubMed] [Google Scholar]
- 16. Ziegler SF, Roan F, Bell BD, Stoklasek TA, Kitajima M, Han H. The biology of thymic stromal lymphopoietin (TSLP). Adv Pharmacol. 2013;66:129‐155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ying S, O'Connor B, Ratoff J, et al. Expression and cellular provenance of thymic stromal lymphopoietin and chemokines in patients with severe asthma and chronic obstructive pulmonary disease. J Immunol. 2008;181(4):2790‐2798. [DOI] [PubMed] [Google Scholar]
- 18. Ying S, O'Connor B, Ratoff J, et al. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2‐attracting chemokines and disease severity. J Immunol. 2005;174(12):8183‐8190. [DOI] [PubMed] [Google Scholar]
- 19. Watson B, Gauvreau GM. Thymic stromal lymphopoietin: a central regulator of allergic asthma. Expert Opin Ther Targets. 2014;18(7):771‐785. [DOI] [PubMed] [Google Scholar]
- 20. Gauvreau GM, O'Byrne PM, Boulet LP, et al. Effects of an anti‐TSLP antibody on allergen‐induced asthmatic responses. N Engl J Med. 2014;370(22):2102‐2110. [DOI] [PubMed] [Google Scholar]
- 21. Corren J, Parnes JR, Wang L, et al. Tezepelumab in adults with uncontrolled asthma. N Engl J Med. 2017;377(10):936‐946. [DOI] [PubMed] [Google Scholar]
- 22. Parnes JR, Sullivan JT, Chen L, Dias C. Pharmacokinetics, safety, and tolerability of tezepelumab (AMG 157) in healthy and atopic dermatitis adult subjects. Clin Pharmacol Ther. 2019;106(2):441‐449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Roseti SL, Howell MD, Stelmach I, et al. Pharmacokinetic and safety evaluation of tezepelumab in adolescents with mild to moderate asthma. Am J Resp Crit Care Med. 2017;195:A5103. [Google Scholar]
- 24. Ly N, Zheng Y, Griffiths JM, et al. Exposure‐response analysis of tezepelumab in patients with severe asthma to guide phase 3 dose selection. Eur Respir J. 2018;52:PA1688. [Google Scholar]
- 25. Wang JY, Chou CH, Lee LN, et al. Diagnosis of tuberculosis by an enzyme‐linked immunospot assay for interferon‐gamma. Emerg Infect Dis. 2007;13(4):553‐558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim SH, Choi SJ, Kim HB, Kim NJ, Oh MD, Choe KW. Diagnostic usefulness of a T‐cell based assay for extrapulmonary tuberculosis. Arch Intern Med. 2007;167(20):2255‐2259. [DOI] [PubMed] [Google Scholar]
- 27. Wang W, Wang EQ, Balthasar JP. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2008;84(5):548‐558. [DOI] [PubMed] [Google Scholar]
- 28. Keizer RJ, Huitema AD, Schellens JH, Beijnen JH. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49(8):493‐507. [DOI] [PubMed] [Google Scholar]
- 29. Vugmeyster Y, Xu X, Theil FP, Khawli LA, Leach MW. Pharmacokinetics and toxicology of therapeutic proteins: advances and challenges. World J Biol Chem. 2012;3(4):73‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dirks NL, Meibohm B. Population pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49(10):633‐659. [DOI] [PubMed] [Google Scholar]
- 31. Ryman JT, Meibohm B. Pharmacokinetics of monoclonal antibodies. Pharmacometrics Sys Pharmacol. 2017;5(2):102‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tsukamoto N, Takahashi N, Itoh H, Pouliquen I. Pharmacokinetics and pharmacodynamics of mepolizumab, an anti‐interleukin 5 monoclonal antibody, in healthy Japanese male subjects. Clin Pharmacol Drug Dev. 2016;5(2):102‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Murphy K, Jacobs J, Bjermer L, et al. Long‐term safety and efficacy of reslizumab in patients with eosinophilic asthma. J Allergy Clin Immunol Pract. 2017;5(6):1572‐1581. [DOI] [PubMed] [Google Scholar]


