Abstract
The EFSA Panel on Food Contact Materials, Enzymes and Processing Aids (CEP) was requested by the European Commission to re‐evaluate the safety of styrene (FCM No 193) for use in plastic food contact materials (FCM) following the classification by the International Agency for Research on Cancer (IARC) as ‘probably carcinogenic to humans’. The IARC Monograph pertains to hazard identification, based on studies on high‐dose occupational exposures by inhalation and animal studies, also mainly by inhalation. The Panel considered that the IARC conclusions cannot be directly applied to the evaluation of risks for consumers from the oral exposure to styrene, but also concluded that, based on the data provided in the IARC Monograph and by the industry, a concern for genotoxicity associated with oral exposure to styrene cannot be excluded. The migration of styrene into foods packed in styrenic plastics is below 10 μg/kg for the majority of the foods, but up to 230 μg/kg was reported. Migration tends to be high for contact with fatty foods, and/or with high surface to volume ratios of the FCM. Dietary exposure of the consumers to styrene migrating from styrenic plastics was estimated in the order of 0.1 μg/kg body weight (bw) per day. It is in the same range as exposure from styrene present in foods as such. The dietary exposure (food component plus migration from styrenic plastics) is similar or lower than that by inhalation in the general population. Taking the human exposure data into account, the Panel concluded that a systematic review of genotoxicity and mechanistic data, comparative toxicokinetics and analysis of species differences is required for assessing the safety of styrene for its use in FCM.
Keywords: Styrene, CAS No 100‐42-5, FCM substance No 193, food contact materials, IARC Monograph Vol. 121, safety assessment, evaluation
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
Terms of Reference as provided by the European Commission
“In accordance with Article 12(3) of Regulation (EC) No 1935/20041, the European Commission asks EFSA to evaluate whether the authorisation of styrene (FCM No 193) as provided for in Table 1 of Annex I of Regulation (EU) No 10/2011, is still in accordance with the requirements of Regulation (EC) No 1935/2004.
Styrene is authorised to be used as monomer and/or starting material for the manufacture of plastic food contact materials (FCM) and is currently listed in Annex I of Regulation (EU) No 10/2011, without a Specific Migration Limit (SML).
New scientific evidence on the potential health effects of styrene was recently considered by the International Agency for Research on Cancer (IARC) in the reclassification of its carcinogenicity, where IARC concluded that styrene is ‘probably carcinogenic to humans’.2
The EFSA evaluation should assess whether the evidence examined by IARC leading to its conclusions, would have an impact on the safety of styrene in FCMs. To this end, EFSA should obtain the available information on styrene from IARC.
In case the new evidence indicates that the present authorisation is no longer in accordance with the regulation, then EFSA should, if supported by the data, determine under what conditions the substance could be safely used.
Addendum to the Terms of Reference
On 22 July 2019, EFSA received from the European Commission a cover letter and the Styrene Technical Dossier put together and submitted to the Commission by a consortium of industry operators under the auspices of PlasticsEurope.
To ensure that all available information is utilised in the styrene re‐evaluation, the information submitted by PlasticsEurope needs to be also considered together with the IARC monograph in the styrene re‐evaluation.”
1.2. Interpretation of the Terms of Reference
In 2019, IARC updated the evaluation of styrene (IARC, 2002) that had classified the substance as ‘possibly carcinogenic to humans’ (Group 2B). In the IARC Monograph Volume 121, published on 23 September 2019, IARC evaluated styrene and its metabolite styrene‐7,8‐oxide as ‘probably carcinogenic to humans’ (Group 2A). Furthermore, IARC considered that ‘there is strong evidence that both styrene and styrene‐7,8‐oxide are genotoxic, and that this mechanism can also operate in humans’.
Based on the new IARC classification, the European Commission asked EFSA to evaluate whether the new scientific evidence considered by IARC has an impact on the safety of styrene for use in plastic FCMs.
In addition, the evaluation of the Technical Dossier submitted by Styrenics (a consortium of industry operators under the auspices of PlasticsEurope) on 22 July 2019 was considered as part of this mandate.
The CEP Panel asked for support by the EFSA Scientific Committee cross‐cutting working group on genotoxicity (ccWG Genotoxicity) to address the genotoxicity of styrene, in particular considering oral exposure.
The ccWG Genotoxicity indicated the need to review not only the references considered by IARC and in the Styrene Technical Dossier, but to extend the literature search beyond these datasets. Since this analysis could not be completed in a short time and considering that styrene is in the high priority group of substances needing re‐evaluation among those that are authorised for use in FCMs without an SML (EFSA, 2020), the CEP Panel proposed to address the mandate in two steps:
First (within 1 year following the publication of the IARC Monograph), an opinion is provided based on the information available in the IARC Monograph and in the Styrene Technical Dossier, without appraising all individual papers referenced therein. This opinion also addresses the migration of styrene from styrenic plastics into food and dietary exposure estimates, as well as exposure to styrene from FCMs compared to other sources.
A more comprehensive evaluation of the relevant literature is foreseen in the second phase to conclude on the genotoxic risk of styrene associated with the use of the substance in food contact materials, and, if appropriate, proposing safe limits for consumer exposure. For the evaluation of the exposure to styrene, data on FCMs other than plastics are of interest. Finally, for an assessment of what migrates from polystyrene and styrene copolymers, also oligomers and other related reaction products should be considered.
2. Data and methodologies
2.1. Data
For this first step of the mandate, the following data have been considered:
-
–
the IARC Monograph Volume 121 on styrene, styrene‐7,8-oxide and quinoline, published 23 September 2019 on the IARC website: http://publications.iarc.fr/582;
-
–
the Styrene Technical Dossier submitted in July 2019 by the industry consortium;
-
–
additional information provided by PlasticsEurope in response to requests from EFSA sent on 13 March 2020 (see ‘Documentation provided to EFSA’);
-
–
archive material regarding the earlier assessment performed by the Scientific Committee on Food (SCF) provided by the European Commission on 22 January 2020 (SCF opinions/evaluations 1982, 1991; addenda to initial Technical Dossier 1995–2001).
Some publications referenced in the IARC Monograph and the Styrene Technical Dossier were consulted. No comprehensive literature search was performed.
Data used for the evaluation are:
Non‐toxicological data and information
Chemical identity
Physical and chemical properties
Uses
History of the authorisation
Migration of the substance into simulants and foods
Exposure
Toxicological data
Genotoxicity data as reported by the IARC Monograph and provided by the industry.
2.2. Methodologies
The assessment was conducted in line with the principles laid down in Regulation (EC) No 1935/2004 on materials and articles intended to come into contact with food, the EFSA Guidance on transparency in the scientific aspects of risk assessment (EFSA, 2009) and considering the relevant guidance from the EFSA Scientific Committee. Data sources taken into consideration are listed in Section 2.11.
3. Assessment
3.1. History of the authorisation of styrene in FCMs
In 1982, the Scientific Committee on Food (SCF) evaluated styrene and had no objection accepting the continued use of styrene in FCMs, while recommending to reduce the levels of styrene monomer residues as much as possible. Thus, in the Synoptic Document (European Commission, 2005), the SCF classification for styrene was set as 4B, allocated to ‘substances for which an acceptable daily intake (ADI) or tolerable daily intake (TDI) could not be established, but which could be used if the levels of monomer residues in materials and articles intended to come into contact with foodstuffs are reduced as much as possible’.
In the Directive 90/128/EEC3, the first Directive relating to plastic materials and articles intended to come into contact with foodstuffs, styrene was authorised to be used as monomer and/or starting substance for the manufacture of plastic FCMs without a Specific Migration Limit (SML). No restrictions are defined by the current listing of styrene in Annex I of Regulation (EU) No 10/2011. The substance can be used at up to 100% w/w to make all types of plastics intended for contact with all types of foodstuffs, without restrictions in contact time and temperature. The only limitation in use is linked with the sensory properties of styrene, given that in accordance with Article 3(c) of Regulation 1935/2004, materials and articles should not transfer their constituents to food in quantities which could bring about a deterioration in the organoleptic characteristics thereof.
3.2. Uses of styrene in FCM/conditions of use
The vinyl C=C double bond of styrene can be polymerised to make polystyrene homopolymers. Styrene can also be polymerised with other unsaturated monomers to make a range of copolymers. Typical comonomers and their resulting copolymers are the thermoplastics made by addition polymerisation of styrene with acrylonitrile (styrene/acrylonitrile copolymers, SAN) and butadiene (acrylonitrile/butadiene/styrene copolymers, ABS). Other types of copolymers are the thermoset plastics made by mixed addition/condensation polymerisation of styrene with unsaturated polyesters (styrene/divinylbenzene cross‐linked unsaturated polyester resins) and the copolymers made by reaction with acrylic acid (and its derivatives) to make styrene/acrylate resins.
These polystyrene homopolymers and styrenic copolymers are made into a variety of materials and articles that are intended for either single use (e.g. food packaging) or repeated use (e.g. durable articles). These materials and articles are in turn used in contact with many different categories of food. Examples are:
high impact polystyrene (HIPS) containers for dairy products, such as yoghurt;
sheets and films for dairy products, take away foods, cutlery and tableware made of styrene‐butadiene copolymer (SBC) thermoplastics and blends with general purpose polystyrene (GPPS);
reusable kitchenware and cutlery, made of GPPS, HIPS, ABS or SAN;
disposable plates and containers for hot foods like soups, e.g. for catering, HIPS;
disposable wares for hot solid foods, made of foamed GPPS;
cups for hot beverages, like coffee or tea, made from GPPS or expanded polystyrene (EPS);
trays for packaging meat, poultry, cheese, fruits and vegetables, made of expanded (EPS) or extruded polystyrene (XPS);
cold boxes for fisheries, food or beer, of EPS;
kitchen appliances and machine parts thereof, of ABS;
unsaturated polyester/epoxy vinylester/styrene polymers for food containers (vats, other large vessels) in industrial applications;
can coatings for food and beverages;
sealants, e.g. styrene‐butadiene rubber (SBR) for can ends;
polymer dispersions/lattices (e.g. styrene butadiene or styrene acrylate copolymers) used for coatings of paper and board, adhesives, overprint varnishes etc.;
styrene as a solvent, cross‐linking agent and reactive diluent in the production of glass‐reinforced plastics.
As can be seen from the non‐exhaustive list above, the conditions of use of styrene polymers range typically from low temperature (refrigeration) for periods of days to few weeks, e.g. packaged fish, meat and dairy products, to elevated temperatures for short periods of time, e.g. vending cups for hot drinks or fast food take aways.
3.3. Non‐toxicological data
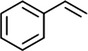
Chemical formula: C8H8
Molecular mass: 104 Da
Chemical structure:
3.3.1. Physical and chemical properties4
The melting point of styrene is ‐30.6°C and the boiling point at standard atmospheric pressure is 145°–146°C. The octanol‐water partition coefficient (log Po/w) is 2.95–3.02. Thermal decomposition of styrene starts above 450°C, which is above the maximum processing temperatures of styrenic plastics (ca. 300°C).
Styrene has a sweet odour.
-
–
Odour thresholds for styrene in water range from 0.004 to 2.6 mg/L, depending on temperature (WHO, 2004); average taste threshold at 40°C: 0.12 mg/L; odour threshold at 60°C: 0.0036 mg/L (Alexander et al., 1982).
-
–
The threshold in milk products is 0.2–6 mg/kg, depending on fat content and the taste of the product itself (Tischner, 1993).
-
–
As provided by the Styrene Technical Dossier,5 generally, concentrations above 1 mg/kg in foods bring about a deterioration of the organoleptic characteristics. The threshold level for organoleptic perception in an oil‐in-water emulsion with 30% oil is about 2 mg/kg. In cream, styrene off‐flavour was reported at 6 mg/kg.
Styrenic plastics contain free styrene monomer at highly variable concentrations. For instance, Genualdi et al. (2014) found styrene in 24 food contact polystyrenes in the range of 9.3–3,100 mg/kg (mean 340 mg/kg). This styrene may consist of unreacted residues from polymerisation, but styrene can also be formed by depolymerisation during thermal processing of styrenic plastics to make materials and articles.
3.3.2. Specific migration6
Migration into foods
In three studies performed by the UK Ministry of Agriculture, Fisheries and Food (MAFF) in 1983, 1994 and 1999, styrene in foods was measured. In the first two studies, the sampling focused on foods packaged in styrenic plastics, but its presence in the foods before contact with the FCM was not checked.
In the 1983 study (MAFF, 1983), retail foods and their styrenic plastic packaging materials were analysed for styrene. Styrene was determined in 133 samples using head space GC‐MS. The samples comprised yogurts, creams, salads, coleslaws, soft cheeses, margarines, hot and cold beverages from dispensing machines, spreads, fresh and cooked meat, candied fruits, fresh strawberries and fast food take aways. Migration ranged from < 1 μg/kg to 200 μg/kg or μg/L, although in the majority of the foods (77%), it was below 10 μg/kg, and in 26% of the foods, it was below 1 μg/kg. Only three foods contained more than 50 μg/kg styrene: a dessert and two fresh meat products.
In the 1994 survey of styrene in food (MAFF 1994), 248 samples of foods of a wide variety (food types, manufacturers, packaging types and pack sizes) were analysed. The sampling focused on foods packaged in styrenic plastics. In the majority of the samples, the styrene concentration ranged from below the detection limit (1–10 μg/kg, depending on the food) to 60 μg/kg. In two out of seven samples of ‘low fat’ table spread (margarine), a mean concentration of 97 μg/kg was reported, but in the other five, the concentrations were 20 μg/kg or less. In 22 samples of milk and cream sold as individual portions (~ 10 g) for tea or coffee, styrene concentrations ranged from 23 to 223 μg/kg, with a mean of 134 μg/kg. For all other food types, mean values were below 30 μg/kg. Within each food type, concentrations were higher in products with a high‐fat content or small pack sizes (high surface to volume ratio, S/V).
In the 1999 total diet study (TDS; MAFF, 1999), 100 composite food samples were analysed. Samples of individual foods in the various categories comprising the 20 UK‐TDS food groups (Peattie et al., 1983) were purchased on different dates during 1997 from retail outlets in different parts of the UK. There was no special focus on samples packaged in styrenic plastics, since the recurring UK‐TDS programme has a ‘general purpose’ character for investigating additives, contaminants, nutrients etc. The foods were prepared as for consumption (including cooking where appropriate) and combined into the 100 composite samples. Styrene was detected at up to 14 μg/kg food group.
In a study performed by the Food and Drug Administration (FDA; Genualdi et al., 2014), migration of styrene into 24 food samples was measured. The polystyrenes contained 9.3–3,100 mg/kg styrene (mean of 340 mg/kg) and the migration into foods ranged from 2.6 μg/kg in raw chicken to 163 μg/kg in sandwich cookies.
El‐Ziney and Tawfik (2016) measured styrene in a range of Egyptian dairy products packed in polystyrene, with results ranging from 11 μg/kg (yogurt) to 102 μg/kg (butter).
Philo et al. (1997) measured styrene‐7,8‐oxide, presumably formed by oxidation of styrene monomer, in polystyrene FCMs. Styrene‐7,8‐oxide was detected in 11 of 16 food packagings at up to 2.9 mg/kg. Assuming a migration pro‐rata to the styrene migration from the plastic and 0.5–3.0 mg styrene oxide/kg packaging, its migration into food was estimated at 0.002–0.150 μg/kg.
It is concluded that styrene migration into foods mostly remains below 10 μg/kg, but the highest concentration measured was 223 μg/kg. Higher migrations were found in high S/V packs and in contact with fatty food.
Migration into simulants
In the Styrene Technical Dossier, two sets of specific migration test reports were provided with different styrene‐based polymers, one from 2001 and the other from 2013.
In the 2001 study,7 rod and sheet test samples of styrene homopolymers and copolymers were tested for migration into the food simulants 3% acetic acid, 10% ethanol, olive oil and dewaxed sunflower oil. The contact conditions were 2 h at 70°C, 2 days at 40°C and 10 days at 5°C, 20°C and 40°C. The rods were tested by total immersion and the sheets by one side contact. For the analysis of styrene, headspace GC‐MS was used. The highest migration of styrene was 590 μg/kg in olive oil after 10 days exposure at 40°C. Migrations into 3% acetic acid and 10% ethanol were three to five times lower. Migrations from rigid and expanded polystyrene (GPPS, HIPS vs. EPS) were in the same range, but those from SAN and ABS five to ten times lower. Taking into account the strongly varying concentrations of styrene in the polymer, presently available data did not enable to classify different polystyrenes and styrene copolymers by styrene migration.
In the study performed in 2013,8 migration tests were performed with styrene homopolymers (GPPS and HIPS) in olive oil for 10 days at 40°C, 50°C and 60°C with one‐sided contact and in 50% ethanol for 10 days at 40°C and 50°C by total immersion. For the analysis of styrene, GC‐FID was used for the olive oil and HPLC‐PDA for the 50% ethanol in water simulant. The highest migration of styrene was 2,270 μg/kg (olive oil after 10 days exposure at 60°C).
■■■■■.9
It is concluded that tests with simulants at standard conditions result in migration of styrene at up to several thousand μg/kg, which is substantially higher than the migration data reported in foods. The Panel noted that such tests refer to foreseeable worst‐case uses and are not suitable for estimating typical oral exposure, including from foods kept under less severe contact conditions and with lower fat content. Furthermore, testing with 50% ethanol at elevated temperatures can swell polystyrene polymers, thus leading to increased (and possibly unrepresentative) migration values. Migration strongly depends on the free styrene content in the plastic. It increases with increasing temperature and is higher for fatty contact than for non‐fatty contact.
3.3.3. Dietary exposure resulting from migration from styrenic plastics into food
Based on migration data in food and the US/FDA consumption factor (CF) assuming that 10% of the food is packed in styrenic plastics, Tang et al. (2000) estimated the average annual exposure to styrene at 1.1–6.5 μg/kg bw or 80–450 μg/adult (70 kg bw). This corresponds to an average daily exposure of 0.003–0.017 μg/kg bw or 0.2–1.2 μg/adult.
Using the migration data in foods from literature and consumption data from the Irish National Children's Food Survey (NCFS), Duffey and Gibney (2007) estimated the mean daily exposure of Irish children to styrene from styrenic plastics at 0.122 μg/kg bw when using the 90th percentile migration values and at 0.169 μg/kg bw when using the maximum value reported.
Lickly et al. (1995, 1997), using a migration model, estimated the styrene exposure from the daily food in the USA packed in styrenic plastics to be 0.15 μg/kg bw per day.
In a study submitted to FDA by the Plastics Foodservice Packaging Group (2015), a concentration of styrene of 2.20 μg/kg in food was attributed to plastic food packaging. Using the default assumption that a person weighing 60 kg may consume daily up to 1 kg of food in contact with the relevant FCM (European Commission, 2001), this concentration in the diet results in an estimated daily intake of approximately 0.04 μg/kg bw.
It is concluded that the daily dietary exposure to styrene migrated from styrenic plastics is in the order of 0.1 μg/kg bw (0.003–0.15 μg/kg bw for adults, and at the 90th percentile 0.169 μg/kg bw for children). Most studies did not include the exposure to styrene from FCMs other than styrenic plastics.
3.3.4. Exposure to styrene present in food
Styrene is present in many foods as such. It is not always clear whether it is a natural component or a biodegradation/fermentation product (e.g. from cinnamic acid). Concentrations vary widely: in fruits, vegetables, milk and meat, they were near the detection limit of 0.1 μg/kg, but much higher concentrations were determined in cinnamon (40,000 μg/kg) as well as in mouldy cheese (up to 5,000 μg/kg) and milled olives after some storage (230 μg/kg) (Tang et al., 2000).
In 27 samples of cinnamon‐flavoured hot cross buns, styrene concentrations ranged from < 10 to 767 μg/kg, with a mean of 26 μg/kg (MAFF, 1983).
In the total diet study performed by MAFF in the UK in 1999 (MAFF, 1999), the dietary exposure to styrene was estimated at 0.03–0.05 μg/kg bw per day, based on the analysis of 100 composite samples. It did not distinguish the sources, i.e. it included styrene exposure from food sources other than packaging and from food packaging materials other than plastics. The Panel noted that at the time the study was performed, styrene was notified as a flavouring substance in use,10 which might have contributed to the dietary exposure values.
In the context of a total diet study performed in Canada, Cao et al. (2018) determined styrene concentrations in 159 foods or food ingredients. This included migration from polystyrene FCMs (not being distinguished from other sources). Styrene was detected in 125 of the 159 samples, with detection limits of 0.023–10.7 μg/kg, depending on the food type. In three samples of bread, the concentration of styrene was 21–34 μg/kg and in white flour 17 μg/kg. Various cereal products contained up to 60 μg/kg styrene (top concentrations in cookies and apple pie), possibly from cinnamon. Pizza contained 33 μg/kg styrene, French fries 24 μg/kg, burgers 7 and 9.8 μg/kg and various other fast foods 6–49 μg/kg, for all of which contact with polystyrene FCMs cannot be excluded. The highest concentration was measured in a composite sample of herbs and spices: 4,934 μg/kg. Overall, the mean daily total dietary exposure to styrene in food (from migration and as a food component) was estimated by the authors as 0.12 μg/kg bw for elderly (71 years and above), 0.15–0.16 μg/kg bw for adults (19–70 years), 0.31 μg/kg bw for children (3–8 years) and 0.38 μg/kg bw for toddlers (2–3 years).
It is concluded that the occurrence of styrene in some foods may contribute to the overall exposure to styrene to a similar extent as the migration from styrenic plastics, but no data were found that would have enabled a clear distinction of the two sources. The total dietary exposure for children is slightly higher than that for adults.
3.3.5. Exposure by inhalation
As summarised by Tang et al. (2000), styrene in air is due to emissions from industrial production of styrene and styrene polymers as well as to combustion or incineration of garbage containing styrene polymers. Further sources are emissions by coal‐fired power stations, vehicle exhaust and cigarette smoke. Concentrations of styrene in outdoor air were often found to be below 1 μg/m3, except near sanitary landfills or in industrial areas. Median concentrations of styrene in indoor air were 0.4–3.6 μg/m3, reaching up to 3.7–54 μg/m3.
Taking into account that ca. 40% of the inhaled styrene is exhaled, Tang et al. (2000) calculated for the majority of the general population a respiratory styrene intake of 18–54 μg/day per person. This was based on a daily respiratory intake of 30 m3. The Panel considered this value as high. Using the mean intake of 20 m3 per day reported for adults in ECHA (2012), the daily intake would amount to 12–36 μg or approximately 0.2–0.6 μg/kg bw. In the smoke of one cigarette, around 1 μg (0.1–10 μg) styrene was measured (Tang et al., 2000).
Cao et al. (2018) cited Canadian data from 1993 on daily exposure by inhalation: 0.107–0.27 μg/kg bw for small children and 0.085–0.27 μg/kg bw for adults. This is less than estimated by Tang et al. (2000), partly because a daily respiratory intake of only 2–23 m3 was considered in the calculations. Cao et al. (2018) concluded that the oral and respiratory exposure levels were similar.
In a detailed review on sources and concentrations of styrene in air, Banton et al. (2019) concluded on a central tendency respiratory daily exposure for non‐smokers of 0.1 μg/kg bw and of 0.12 μg/kg bw for smokers, with upper bound values of 0.31 and 0.37 μg/kg bw, respectively. Highest exposure was derived for children of 1–2 years, due to higher inhalation rates per unit bw, with central tendency respiratory exposure of 0.36 μg/kg bw and an upper bound value of 1.1 μg/kg bw.
Overall, it is concluded that the daily exposure to styrene by inhalation is in the range of 0.1–0.6 μg/kg bw for adults.
3.4. Toxicological data
3.4.1. Genotoxicity of styrene
Genotoxicity is considered the critical endpoint in the safety evaluation of styrene, for the health consequences of somatic and heritable genetic alterations and its implication in cancer. A preliminary evaluation of the genotoxicity of styrene was carried out based on the information presented in the IARC monograph (2019) and in the Styrene Technical Dossier,11 also considering a recently published critical review of genotoxicity data on styrene (Moore et al., 2019), as well as a meta‐analysis of studies of occupationally exposed styrene workers and micronuclei levels (Collins and Moore, 2019), both submitted by PlasticsEurope to EFSA.12
A detailed analysis of these documents, undertaken by the ccWG Genotoxicity, is presented in Annex 1. The main findings are summarised below.
The recent IARC monograph classified styrene as ‘probably carcinogenic to humans’ (Group 2A), on the basis of ‘limited evidence’ in humans and ‘sufficient evidence’ in experimental animals. Increased incidence of, or mortality, from leukaemia and lymphomas were reported in several epidemiological studies in cohorts of workers exposed to styrene by inhalation, mainly in the reinforced plastics industries; there was also a strong signal for sinonasal adenocarcinoma, a rare cancer in humans, based on a few cases observed in a single large study. Overall, IARC concluded that the epidemiological studies provide some credible evidence that exposure to styrene causes lymphohaematopoietic malignancies in humans, but confounding, bias or chance cannot be ruled out.
Increased incidence of lung cancers was detected in several strains of mice exposed to styrene by inhalation and by oral exposure. A single study by inhalation reports increased incidence of malignant mammary tumours in rats.
The IARC Monograph concluded that there is ‘strong evidence’ for a genotoxic mechanism of styrene, mediated by its metabolic activation to the electrophilic styrene‐7,8‐oxide, an epoxide that is genotoxic and directly reactive to DNA. Styrene metabolism and distribution appeared to differ quantitatively among species and toxicokinetic modelling of styrene‐7,8‐oxide concentrations in terminal bronchioles estimated distinct differences among species, with 100‐fold higher tissue concentrations in mice compared with humans, and 10‐fold higher in mice compared with rats from exposure to styrene at 0.01–10.0 ppm by inhalation.
The large majority of in vitro studies on styrene, described in the IARC monograph, showed positive results only in the presence of metabolic activation. Gene mutations in bacterial cells (Ames test) were found in Salmonella Typhimurium strains that detect base‐pair substitutions (TA100, TA1530 and TA1535) but not in strains that detect frameshift mutations (TA98, TA1537 and TA1538) and in Escherichia coli strains. Positive results were reported for gene mutation in mammalian cells. Cytogenetic studies (chromosomal aberration test, micronucleus assay and sister chromatid exchange (SCE)) in mammalian cell lines (V79, CHO) also showed positive results. Positive results without metabolic activation were reported in cytogenetic studies in human whole blood lymphocytes.
The IARC monograph also reports a large number of in vivo genotoxicity studies carried out by inhalation or intraperitoneal injection. These studies showed positive results for markers of DNA damage (DNA‐adducts, single‐strand breaks detected by Comet assay and SCE), while negative or weakly positive results were reported for chromosomal damage (structural chromosomal aberrations and micronuclei). Two in vivo oral studies described in the IARC Monograph reported negative results for chromosomal aberrations in bone marrow of male and female mice exposed up to the maximum tolerated doses, after single or repeated administrations (Loprieno et al., 1978; Sbrana et al., 1983). In one of these oral studies, separate experiments carried out in parallel with styrene oxide at the same range of doses showed a statistically significant dose‐related increase in chromosomal aberrations (Loprieno et al., 1978).
The IARC Monograph supports that the mechanism of genotoxicity of styrene observed in experimental systems is likely to operate also in humans.
The large majority of the human biomonitoring studies were carried out in the reinforced plastics industry, using DNA damage biomarkers, i.e. DNA adducts, oxidative DNA damage, single‐strand breaks by Comet assay, chromosomal aberrations, micronucleus test and SCE. Mixed results were described in studies applying different genotoxicity biomarkers, and a lack of consistency was also shown among the studies using the same genotoxicity biomarker. DNA adducts in peripheral blood cells have been reported to be significantly higher in exposed workers than in unexposed controls in a number of studies. The majority, but not all, of the several available studies showed increased levels of DNA damage as measured by the Comet assay. Studies using the Comet assay to assess oxidative damage to DNA were negative, studies measuring 8‐hydroxy‐2′‐deoxyguanosine in DNA were inconsistent. In the few studies on gene mutation, no clear relationship was found with occupational exposure to styrene. Mixed results were reported in the studies on chromosomal endpoints (chromosomal aberration, micronuclei frequency) in blood cells of exposed workers.
The Panel noted that the conclusion of the IARC monograph and the classification of styrene as ‘probably carcinogenic to humans’ with ‘a strong evidence’ of genotoxicity represent the first step in carcinogen risk assessment (i.e. the hazard identification, with no specific consideration for the route or level of exposure). It involved ‘examination of all relevant information to assess the strength of the available evidence that a substance could alter the incidence of cancer in experimental animals or humans’. In the IARC monograph, genotoxicity and other mechanistic data are considered as supporting evidence; consequently, the genotoxicity studies are not described in detail.
The Panel also evaluated the genotoxicity section of the Styrene Technical Dossier and the recent review by Moore et al. (2019) critically analysing the genotoxicity studies cited in IARC Monographs (IARC, 1985, 1994, 2002). These documents were considered not to provide any further insights into styrene genotoxicity. A critical analysis of their findings is presented in Annex 1.
A meta‐analysis by Collins and Moore (2019) of studies applying the micronucleus assay in peripheral lymphocytes of workers occupationally exposed to styrene by inhalation showed an overall increase of micronuclei in exposed workers when compared to control groups. However, the Panel noted that the large variability and uncertainties in the extent of exposure in the different studies prevent a clear conclusion from being reached. A comprehensive evaluation of all available studies with a critical analysis of the exposure data is needed to reach a conclusion on the genotoxicity of styrene in human subjects exposed by inhalation.
The Panel considered the data provided by the industry related to the comparison of the daily occupational exposure per person by inhalation, derived from the studies described in the meta‐analysis, and the calculated daily styrene exposure per person via food. These data should be evaluated together with the results of genotoxicity biomarkers in single studies considering the absorption and bioavailability of styrene by inhalation and oral exposure predicted by physiologically based toxicokinetic (PBTK) models.
The Panel concluded that a comprehensive evaluation of the reliability and relevance of all available experimental and human findings on styrene genotoxicity, with consideration of toxicokinetic aspects, would be required for an in‐depth assessment of the genotoxicity of styrene via oral exposure. This task should be undertaken following EFSA methodological criteria for hazard identification and characterisation, weighing the body of the evidence and evaluating uncertainties and risk of bias according to EFSA guidelines (EFSA, 2017, 2018). The Panel also considered that an investigation of the quantitative relationships between DNA adducts and various genotoxicity endpoints (gene mutations, single‐strand breaks and chromosomal damage) would provide useful information on the mode of action of styrene, its genotoxicity profile and human risk characterisation. An extensive evaluation of the human studies through a critical analysis of the exposure data is needed to draw a conclusion on the genotoxicity of styrene in humans.
4. Conclusions
The Panel concluded that:
-
–
For the majority of the foods packed in styrenic plastics, migration is below 10 μg/kg, but in some cases, it ranged up to 230 μg/kg: migration is high when the contact is with fatty foods and/or with high S/V ratios of the FCM.
-
–
In standard testing using food simulants, migration of styrene may reach several thousand μg/kg. Such testing refers to foreseeable worst‐case uses and does not reflect typical exposure.
-
–
Dietary exposure of the consumers to styrene migrating from styrenic plastics was estimated in the order of 0.1 μg/kg bw per day. It is in the same range as exposure from styrene present in foods as such.
-
–
The dietary exposure (food component plus migration from styrenic plastics) for children is slightly higher than that for adults.
-
–
In the general population, the total dietary exposure is similar or lower than that by inhalation.
-
–
Presently, the only limitation of the styrene migration is linked with the sensory properties of styrene (it must not bring about a deterioration in the organoleptic characteristics; Article 3, Regulation (EC) 1935/2004). Reported odour and taste thresholds ranged from 4 to 6,000 μg/kg, depending on the type of the food.
-
–
Free styrene content in the plastic can vary by more than two orders of magnitude, which in turn strongly influences migration levels.
-
–
No classification of polystyrenes and styrene copolymers by styrene migration was possible due to the variability in the levels of styrene within a given type of styrene (co)polymer.
-
–
The IARC conclusion, which is based on studies on high‐dose occupational exposures by inhalation and animal studies, also mainly by inhalation, pertains to hazard identification. Therefore, the Panel considered that the IARC evaluation cannot be directly applied to the evaluation of risks for consumers from the oral exposure to styrene associated with FCM.
-
–
The implications of styrene oral exposure via FCM on the health of consumers should be evaluated based on a comprehensive analysis of the reliability and relevance of all available experimental and human findings on styrene genotoxicity, with consideration of toxicokinetic aspects, ultimately enabling a qualitative and quantitative genotoxic risk estimate associated with the oral exposure to styrene.
The Panel concluded that, based on the data provided in the IARC Monograph and by the industry, a concern for genotoxicity associated with oral exposure to styrene cannot be excluded. Taking the human exposure data into account, and according to the interpretation of the Terms of Reference, a systematic review of genotoxicity and mechanistic data, comparative toxicokinetics and analysis of species differences is required for assessing the safety of styrene for its use in FCM.
Documentation provided to EFSA
Styrene Technical Dossier. July 2019. Submitted by Styrenics, a consortium of industry operators under the auspices of PlasticsEurope.
Addendum to Styrene Technical Dossier: two additional publications (Collins and Moore, 2018; Moore et al., 2019) and a compilation of exposure data in relation to mutagenicity findings. 4 March 2020. Submitted by Styrenics, a consortium of industry operators under the auspices of PlasticsEurope.
Additional information. 20 April 2020. Submitted by Styrenics, a consortium of industry operators under the auspices of PlasticsEurope.
Spontaneous submission of data. May 2020. Submitted by Sherwin‐Williams Company, a packaging coating industry.
Abbreviations
- ABS
acrylonitrile/butadiene/styrene copolymers
- ADI
acceptable daily intake
- Bw
body weight
- CAS
Chemical Abstracts Service
- ccWG Genotoxicity
EFSA Scientific Committee cross‐cutting working group on Genotoxicity
- CEP Panel
EFSA Panel on Food Contact Materials, Enzymes and Processing Aids
- CF
consumption factor
- EPS
expanded polystyrene
- FCM
food contact materials
- FID
flame ionisation detector
- FDA
Food and Drug Administration
- GC
gas chromatography
- GPPS
general purpose polystyrene
- HIPS
high impact polystyrene
- HPLC
high‐performance liquid chromatography
- MAFF
UK Ministry of Agriculture, Fisheries and Food
- MS
mass spectrometry
- PDA
photodiode array detector
- Po/w
octanol/water partition coefficient
- SAN
styrene/acrylonitrile copolymers
- SBC
styrene‐butadiene copolymers
- SBR
styrene‐butadiene rubber
- SCE
sister chromatid exchange
- SCF
Scientific Committee on Food
- SML
specific migration limit
- S/V
surface to volume ratio
- TDI
tolerable daily intake
- TDS
total diet study
- XPS
extruded expanded polystyrene
Annex 1 – Technical report on the genotoxicity evaluation of styrene prepared by the cross‐cutting Working Group Genotoxicity (ccWG Genotoxicity)
Introduction
The cross‐cutting Working Group (ccWG) Genotoxicity was asked by the CEP Panel to address the genotoxicity of styrene, via oral exposure. The assessment to be based on the information from the International Agency for Research on Cancer (IARC) Monograph and the Technical Dossier provided by the industry, without appraising the individual papers referenced therein.
Assessment
Comments on IARC monograph (IARC, 2019 )
The recent IARC monograph (IARC, 2019) classified styrene as ‘probably carcinogenic to humans’ (Group 2A), on the basis of ‘limited evidence’ in humans and ‘sufficient evidence’ in experimental animals. The evidence of cancer risk in humans was mainly based on epidemiological studies in cohorts of workers exposed to styrene by inhalation in the reinforced plastics industries. ‘The stronger and more consistent evidence for cancer was found for leukaemias and, to a lesser extent, lymphomas’, but there was also a strong signal for sinonasal adenocarcinoma, a rare cancer in humans, based on only a few cases in one large study. Overall, IARC concluded that ‘the epidemiological studies provide some credible evidence that exposure to styrene causes lymphohaematopoietic malignancies in humans, but confounding, bias, or chance cannot be ruled out’.
Nine studies of carcinogenicity of styrene in mice were reported (three by gavage, five via inhalation, one intraperitoneal). Increased incidence of bronchioloalveolar adenoma or carcinoma of the lung was described in two studies by inhalation in CD1 mice and in one study of transplacental exposure followed by gavage in O20 mice. In a study in B6C3F1 mice, styrene administered by gavage significantly increased the incidence of bronchioloalveolar adenoma or carcinoma in males, and a significant positive trend in the incidence of hepatocellular adenoma in females.
Nine studies of carcinogenicity of styrene in rats were reported (four by gavage, one in drinking water, two via inhalation, one via intraperitoneal administration and one via subcutaneous injection). One study out of two carcinogenicity studies in rats exposed to styrene by inhalation described a significant increase in the incidences of malignant tumours of the mammary gland. No significant increase in the incidence of any tumour type was observed in the other rat studies.
The IARC monograph concluded, that there is ‘strong evidence’ that styrene is metabolically activated in animals as well as in exposed humans to the electrophilic epoxide styrene‐7,8‐oxide, which reacts directly with DNA to form adducts in different position of DNA bases.
A substantial number of occupational cohort studies or studies with volunteers exposed to styrene in inhalation chambers or by mask are described in IARC monograph. The results of these studies demonstrate that styrene is rapidly absorbed and found in the blood of exposed subjects. The average pulmonary uptake of styrene ranged from 63% to 68%. Multicompartment physiological‐based pharmacokinetic or toxicokinetic models are available for styrene, with which styrene distribution and metabolism in humans, rats and mice can be estimated. Pharmacokinetic modelling of styrene‐7,8‐oxide concentrations shows species differences in the terminal bronchioles predicted tissue concentrations: 100‐fold higher in mouse compared with humans and 10‐fold higher in mouse compared with rats.
The IARC Monograph also concluded that there is ‘strong evidence’ for a genotoxic activity of styrene on the basis of studies in experimental systems.
The large majority of studies on in vitro genotoxicity of styrene described in the IARC monograph showed positive results only in the presence of metabolic activation. Gene mutations in bacterial cells (Ames test) were described in strains that detect base‐pair substitutions (TA100, TA1530, TA1535), but not in strains that detect frameshift mutations (TA98, TA1537 and TA1538) and in E. coli strains. Positive results were reported for gene mutation in mammalian cells. Cytogenetic studies (chromosomal aberration test, micronucleus assay and sister chromatid exchange (SCE)) in cell lines (V79, CHO) showed positive results. Positive results without metabolic activation were reported in cytogenetic studies in human whole blood lymphocytes.
The in vivo studies on genotoxicity of styrene, evaluated in the IARC monograph, were mainly carried out by inhalation or intraperitoneal injection and only a few of them considered the oral route of exposure. Positive results were described for DNA damage (DNA adducts, single‐strand breaks detected by comet assay, SCEs), negative or weakly positive results were reported for chromosomal damage (structural chromosomal aberrations and micronuclei). Two in vivo studies carried out on styrene by oral exposure are described in the IARC monograph, reporting negative results for chromosomal aberrations in bone marrow of male and female mice exposed up to the maximum tolerated doses, after single or repeated administrations (Loprieno et al., 1978; Sbrana et al., 1983). In one of these studies, experiments carried in parallel with styrene‐7,8‐oxide showed a statistically significant increase in chromosomal aberrations (Loprieno et al., 1978).
The genotoxic mechanism in experimental systems was considered by IARC to operate also in humans, although mixed results were described in the reported biomonitoring studies applying different genotoxicity biomarkers in workers occupationally exposed by inhalation. A lack of consistency was also shown among the studies using the same genotoxicity biomarker. The large majority of these studies were carried out in the reinforced plastics industry, using DNA damage biomarkers, i.e. DNA adducts, oxidative DNA damage, single‐strand breaks by comet assay, chromosomal aberrations, micronucleus test, SCE. DNA adducts were found in a number of studies in peripheral blood cells of exposed workers at levels significantly higher than in unexposed controls. The majority, but not all, of the several available studies showed increased levels of DNA damage as measured by the comet assay. Studies using the comet assay to assess oxidative damage to DNA were negative, while studies measuring 8‐hydroxy‐2′‐deoxyguanosine in DNA were inconsistent. In the few studies on gene mutation, no clear relationship was found with occupational exposure to styrene. Mixed results were reported in the available studies on chromosomal endpoints (chromosomal aberration, micronuclei frequency) in blood cells of exposed workers.
The ccWG Genotoxicity notes that the IARC monograph describes the first step in carcinogen risk assessment (i.e. the hazard identification), which involves examination of all relevant information to assess the strength of the available evidence that a substance could alter the incidence of cancer in experimental animals or humans. IARC evaluates cancer hazard using data published in the scientific literature, with no specific consideration of the route of exposure. Genotoxicity and other mechanistic data are also considered as supporting evidence.
Comments on the Styrene Technical Dossier provided by PlasticsEurope
The Styrene Technical Dossier of PlasticsEurope primarily provides an update of the toxicological data on styrene, with an overview of the available literature up to 2018 (including data from 1998). It was finalised prior to the publication of the reassessment in which IARC upgraded the classification of styrene from a Group 2B to a Group 2A carcinogen (probably carcinogenic to humans) (IARC Monograph, 2019).
The present document prepared by the ccWG Genotoxicity comprises comments on the Styrene Technical Dossier. The comments are restricted to the discussion reported in the Genotoxicity section of the dossier.11 It is important to stress that these comments are of a general type (e.g. interpretation of the results obtained in some tests, clarifications of the mechanisms underlying some of the genotoxicity assays) and do not address individual studies on styrene cited in the Dossier.
1. ‘DNA adducts and Single Strand Breaks (SSBs) observed in styrene workers do not necessarily result in heritable changes’.
1A) DNA adducts and gene mutation
The ccWG Genotoxicity notes that styrene‐7,8‐oxide induces gene mutations in human lymphocytes exposed in vitro. In addition, the chemistry of formation, stability and in vitro miscoding properties of nine different primary and two secondary styrene‐induced DNA adducts have been extensively investigated. The findings together with three classes of base substitutions, identified by mutational spectrum analysis, are consistent with a pro‐mutagenic character of several styrene‐induced DNA adducts. Molecular analysis indicates that these gene mutations might derive from direct miscoding or as a result of depurination (spontaneous or DNA repair mediated). Consistent with this second possibility, styrene‐adducted guanine (N7‐SO‐Gua) and adenine (N3‐SO‐Ade) were detected in the urine of styrene‐treated mice and styrene workers indicating that depurination of styrene‐damaged DNA is a likely occurrence in vivo. The possibility that the styrene‐induced DNA damage that induces mutations in vitro also causes heritable mutations in human individuals cannot be excluded.
1B) DNA breaks and gene mutation
The ccWG Genotoxicity notes that SSBs are an indicator of DNA damage and that the in vivo Comet assay (OECD, 2016) is among the standard tests recommended for regulatory purposes to evaluate genotoxicity in vivo as a testing strategy for follow‐up of in vitro positive results (EFSA, 2011). This guideline states that ‘….under alkaline conditions (> pH = 13), the comet assay can detect single and double strand breaks…. These strand breaks may be repaired, resulting in no persistent effect, may be lethal to the cell or may be fixed into a mutation resulting in a permanent viable change. They may also lead to chromosomal damage which is also associated with many human diseases including cancer’.
2. Genotoxicity
In vivo exposure to either styrene or styrene‐7,8‐oxide induced SSBs in several cell types. This induction occurred in a dose‐dependent manner with no evidence of cytotoxicity. According to the current EFSA testing strategy (EFSA, 2011), evidence of a positive in vivo comet assay is considered to be confirmation that the genotoxic effects observed in vitro are also expressed in vivo. The ccWG Genotoxicity cites reliable evidence of styrene‐induced SSBs in some studies in experimental animals and notes that this evidence is supported by studies of increased SSBs levels in styrene‐exposed workers.
3. Oxidative stress
The Technical Dossier reports that the results obtained with the in vivo Fpg‐modified Comet assay may suggest oxidative stress in white blood cells.
The ccWG Genotoxicity notes that an Fpg‐dependent increase of SSBs in the modified comet assay is not definitive proof of the presence of oxidised DNA bases. Styrene‐induced DNA adducts may undergo spontaneous or DNA‐repair mediated depurination (see above) to generate apurinic (AP) sites. Fpg has an AP lyase activity that can incise DNA at AP sites to generate an SSB. Fpg‐mediated induction of SSBs might reflect this processing of secondary DNA lesions rather than the presence of oxidised DNA bases.
4. Chromosomal changes
The Technical Dossier takes issue with the results of studies on sister chromatid exchanges (SCEs) following in vitro and in vivo styrene exposure. The authors criticise the limited knowledge of the molecular mechanisms by which SCEs form and the lack of consistency with the induction of chromosome aberrations.
The ccWG Genotoxicity notes that SCEs generally reflect the presence of persistent DNA damage and its processing during DNA replication. Indeed, persistence of DNA damage in human syndromes with defects in DNA repair (e.g. nucleotide excision repair, mismatch repair, homologous recombination repair) is associated with high levels of SCEs (Wilson III and Thompson, 2007). Even though the mechanism of SCEs formation is not fully clarified, induction of SCEs by styrene deserves some attention. Styrene induces both stable and unstable DNA lesions some of which block DNA replication. SCEs are generally considered to represent homologous recombination events that occur when replication forks collapse. In an alternative pathway, SCEs might also derive from SSBs occurring in S phase during repair of the apurinic sites derived from loss of the alkylated bases. Both these pathways to SCEs may well apply to styrene.
The apparent lack of consistency between styrene‐induced SCEs and chromosome aberrations/micronuclei might simply reflect the different types of DNA damage and/or processing that underlie these endpoints. On the other hand, the ccWG Genotoxicity notes that styrene induction of SCEs is consistent with DNA adducts and SSBs formation.
5. Unscheduled DNA synthesis (UDS)
The Technical Dossier highlights the contradiction between the positive findings in SCEs, SSBs and DNA adducts formation and the negative results obtained by an in vivo unscheduled DNA synthesis (UDS) assay.
The ccWG Genotoxicity notes that UDS detects DNA repair events involving removal and resynthesis of relatively long stretches of DNA (e.g. 24–32 nucleotides by nucleotide excision repair). It is relatively insensitive to DNA repair via base excision repair, which involves the formation of apurinic (AP) sites and the replacement of only one to two bases. Several styrene‐induced DNA lesions are likely to give rise to AP sites. Any repair of styrene‐induced DNA damage may therefore fall below the detection limit of the UDS assay.
6. Tumour induction
The Technical Dossier draws attention to the lack of specificity of styrene‐induced DNA adducts in target tissue for tumour formation in rodents.
The ccWG Genotoxicity points out that DNA adducts are a measure of the ability of the compound to interact with DNA. In the particular case of styrene, the levels of DNA adduction also reflect the capacity of the organs to transform styrene into the DNA‐reactive metabolites. Overall, covalent binding and levels of specific styrene DNA adducts are highest in the lung, followed by liver and spleen. Although the target organ for cancer is among those most reactive to styrene, the formation of DNA adducts may be necessary but insufficient in itself to account for the complex process of carcinogenesis.
Comments on Moore et al., 2019 (submitted by PlasticsEurope in March 2020)
Moore MM, Pottenger LH and House‐Knight T, 2019. Critical review of styrene genotoxicity focused on the mutagenicity/clastogenicity literature and using current organization of economic cooperation and development guidance. Environmental and Molecular Mutagenesis, 60, 624–663.
The industry submitted a critical review of the genotoxicity data set regarding styrene and its metabolite styrene‐7,8‐oxide (Moore et al., 2019).
Summary of the data reviewed and their interpretation by the authors
The authors acknowledged that IARC, NTP and NRC in their recent evaluations (IARC, 2018; NTP, 2008; NRC, 2014) concluded that styrene is a probable human carcinogen and that, in particular, IARC considers styrene and styrene‐7,8‐oxide as genotoxic in humans. This review aimed to evaluate the reliability of the available in vitro and in vivo genotoxicity studies and the IARC's conclusions, verifying the compatibility of the individual studies with the most updated OECD test guidelines. The authors considered that the available in vitro studies show that styrene after its metabolic conversion into styrene‐7,8‐oxide is mutagenic in the Ames test and induces chromosomal aberrations and micronuclei in cultured mammalian cells.
The only two in vivo chromosomal aberration studies considered interpretable by the authors were negative. On this basis, the review's authors concluded that repeated inhalation exposure to styrene does not induce chromosomal aberration. Only one oral study was identified, and it was considered not interpretable because of major shortcomings. The intraperitoneal studies were a priori considered irrelevant, because this route of administration is not recommended by the OECD test guidelines.
Several in vivo micronucleus studies on styrene and styrene‐7,8‐oxide were evaluated. Three of these studies, conducted with inhalation exposure and considered by the authors to be in line with the current experimental standards, reported negative results. In particular, the article published in 2012 by Gaté and co‐workers on styrene and styrene 7,8‐oxide (Gaté et al., 2012) was considered fully reliable.
No in vivo gene mutation assays for styrene and/or styrene‐7,8‐oxide were identified by the authors.
The authors concluded that, while styrene can induce cytogenetic effects in vitro after metabolic activation, there is no evidence that such effects are produced in vivo.
Several studies in rodents showing DNA adducts in blood, lung, liver and other tissues were reported in the review. In a comet assay after inhalation exposure (Vodicka et al., 2001), slight increases of single‐strand breaks (erroneously reported by the authors of the review as double strand breaks) were observed in lymphocytes after 7 days of continuous exposure, but not after 21 days. In a more recent study (Gaté et al., 2012), only some evidence of oxidative DNA damage was reported in blood cells, but no direct DNA damage, as detected by alkaline comet assay. A positive comet assay after intraperitoneal administration was considered irrelevant by Moore et al. (2019). No data on comet assay after oral exposure are reported in the review.
The ccWG Genotoxicity notes that it was not the aim of the review to consider human studies on DNA adducts and DNA damage (comet assay).
Overall the authors concluded that:
When styrene is metabolised to styrene‐7,8-oxide, positive results are obtained in vitro for gene mutation and clastogenicity.
No evidence that styrene and its oxide are clastogenic in vivo can be found. No in vivo gene mutation study is currently available.
SCEs, DNA adducts and comet assay data, interpreted together as ‘exposure endpoints’, are often positive and demonstrates internal exposure to the metabolite styrene‐7,8-oxide, but not in vivo mutagenicity.
Critical analysis of the authors’ conclusions
The authors of this review concluded that there is no evidence that styrene and styrene‐7,8‐oxide are clastogenic in vivo. However, the ccWG Genotoxicity notes that there are positive experimental results with styrene oxide (IARC, 2019). IARC, taking into account the positive results for DNA damage (comet assay) and induction of DNA adducts obtained in experimental animals and in human studies, concluded that ‘there is strong evidence that both styrene and styrene‐7,8‐oxide are genotoxic, and this mechanism can also operate in humans’.
The authors noted that no in vivo studies for the mutagenicity of styrene or styrene‐7,8‐oxide were identified and, therefore, they acknowledged that no conclusions could be made concerning the ability of in vivo styrene/styrene‐7,8‐oxide exposure to induce gene mutations in the somatic cells of rodents. A key point in the analysis of the available data proposed by the authors is the statement that all the assays detecting DNA damage and/or repair (including also DNA adducts and comet assay) are to be considered only as biomarkers of exposure, as these endpoints do not provide any definitive proof that the test material induces mutations. The ccWG Genotoxicity does not agree with this interpretation. In line with the current testing strategy for the assessment of the genotoxic potential of a substance (EFSA, 2011), when a chemical is found to be positive in an adequate mutagenicity in vitro test, the evidence of in vivo genotoxicity (e.g. obtained with comet assay) is considered sufficient to conclude that this chemical represents a concern for genotoxicity.
Comments on Collins and Moore, 2019 (submitted by PlasticsEurope in March 2020)
Collins JJ and Moore M, 2019. A meta‐analysis of epidemiologic studies of occupationally exposed styrene workers and micronuclei levels. Mutation Research, 837, 15–28.
Short summary of the paper
This study proposes an updated meta‐analysis of the published biomonitoring studies on the application of the micronucleus assay in the peripheral lymphocytes of occupationally exposed styrene workers.
Selection criteria
A PubMed search identified 64 articles; two additional studies were taken from a review. After eliminating reviews, non‐human studies, articles not written in English and publications that did not include primary micronucleus data, 24 studies remained. Then, the studies were further limited to those that used the cytokinesis block method (addition of cytochalasin B), giving 15 studies. Three more publications were excluded from the meta‐analysis, two because they were based on subsets of data later considered in wider studies, and one because it was without an external comparison group (the longitudinal study of Yager et al., 1993). At the end of the selection process, 12 studies were included in the meta‐analysis.
The primary meta‐analysis of the 12 studies (including 516 styrene‐exposed workers and 497 non‐exposed) showed a slight meta‐mean difference between exposed and non‐exposed workers: 1.19 (95% CI 0.20–2.18, random effects model). However, the authors noted some evidence of publication bias, with some small studies with negative findings not being published. Significant heterogeneity among studies was observed.
The authors took into consideration two main quality criteria:
Given the large impact with age and sex for micronuclei frequencies, the studies using matched comparisons were considered more reliable than those that did not. The studies with matched comparisons had a meta‐mean difference of 0.58 (95% CI ‐0.03–0.82, random effects model) compared to those with unmatched comparisons of 1.58 (95% CI 0.03–3.12, random effects model).
The authors also examined the studies with the comparison population mean micronuclei frequencies within and outside the interquartile range, based on the database of the Human Micronucleus Project study (Bonassi et al., 2001). The studies with the mean micronuclei frequencies within the interquartile range were considered of higher quality than those studies outside the range. Following these criteria, the ‘high quality studies’ showed a meta‐mean difference of 0.69 (95% CI ‐1.24–2.61) compared to a difference of 1.56 (95% CI 0.58–2.54) among the ‘low quality studies’.
Based on these two measures of study quality, the authors found that studies with higher quality were consistent with little or no increase in micronucleus frequency among styrene‐exposed workers.
The low styrene‐exposed workers had a meta‐mean difference of 0.44 (95% CI 0.93–1.82) compared to the high styrene‐exposed workers of 1.79 (95% CI 0.38–3.21) in the random effects models.
Authors’ conclusions
This meta‐analysis of the mean standardised differences of micronucleus frequencies found slightly higher levels among styrene‐exposed workers compared to unexposed workers, but there was no consistency across studies. The increase in these frequencies among styrene workers occurred in the studies with lowest quality.
Some evidence of publication bias (one‐sided p = 0.20 for Egger's test) was reported, with small studies of negative findings not being published.
The authors reported that studies with higher styrene exposure had a higher mean standard difference compared with studies with lower styrene exposures. The authors also noted that a longitudinal study did not find any association of styrene exposure and micronucleus frequencies (Yager et al., 1993).
Overall, considering the significant heterogeneity across studies and the equivocal finding concerning any relationship between the level of styrene exposure biomarkers and micronucleus frequencies, the authors concluded that the available data are insufficient to support a conclusion that styrene exposure induces micronuclei in occupationally exposed humans.
Comments
The criteria applied by the authors to evaluate the quality of the studies involve:
the age and gender matching, which is relevant because they are the main confounding factors impacting the micronuclei (MN) frequency. Smoking, which is also an important confounder, was not taken into account;
the inclusion of the mean MN frequencies of control group in the interquartile range, based on the database of the Human Micronucleus Project study (Bonassi et al., 2001). The ccWG Genotoxicity notes that this criterion is questionable because a wide interlaboratory variability in the MN frequencies in healthy unexposed populations has been observed in the large number of available biomonitoring studies. The reasons for this variability include the differences in experimental protocols and scoring criteria applied and the heterogeneity of the populations considered in terms of genetic susceptibility and lifestyle/concurrent exposures. The main criterion to be considered in the analysis of the quality of the studies should be whether or not validated experimental protocols and scoring criteria were applied (Fenech, 2007).
The authors used the mean standardised difference as the summary statistic parameter for the meta‐analysis, which is not incorrect. The currently preferred approach in the meta‐analysis for human MN studies is the use of mean ratio (percent of change of the mean in exposed subjects with respect to the controls) (Ceppi et al., 2010). It makes the effect estimate independent from the absolute values of the means and it results in sufficient comparability across the studies considered. In addition, the mean ratio is less heterogeneous than the mean standardised difference.
In the paper, the description of the statistical methods applied is very limited. The formulae applied in the calculations were not reported. There is some inconsistency in reporting the results in the figure and table.
Some evidence of publication bias (one‐sided p = 0.20 for Egger's test) with small negative studies not being published was reported in the paper, but the funnel plot was not provided. The ccWG Genotoxicity notes that a publication bias should be considered at a significance level of p < 0.05 using a two‐sided test (Egger et al., 1997).
The authors reported that due to a substantial heterogeneity across studies, it is difficult to propose an overall meta‐mean difference of micronuclei increase that represent the results of all studies.
It is important to distinguish between the qualitative and quantitative heterogeneity. In case of qualitative heterogeneity, the meta‐analysis is not recommended. It is not the case for this meta‐analysis. As described in the forest plot, 11 out of 15 studies detected an increase of MN frequency in exposed subjects compare to the control population. The direction of the effect is clear and the heterogeneity is a secondary problem. The quantitative heterogeneity could be due not only to differences in the study quality but also to different exposure conditions in the groups of workers that were analysed in the different studies. The random effects model applied in the meta‐analysis allows that the true effect could vary from study to study and takes into account the variability among the studies.
The ccWG Genotoxicity notes that the meta‐analysis of Collins and Moore (2019), based on the available data from studies applying MN test in humans, does not allow a clear conclusion to be drawn. However, the observation that in studies with higher styrene exposure, the difference of micronucleus frequencies between exposed and unexposed persons was larger than in studies with lower styrene exposures indicates an effect that should not be ignored.
Overall conclusion
The recent IARC monograph classified styrene as ‘probably carcinogenic to humans’ (Group 2A), on the basis of ‘limited evidence’ in humans and ‘sufficient evidence’ in experimental animals.
The IARC working group concluded that there is a ‘strong evidence’ that styrene is metabolically activated in animals, as well as in exposed humans, to the electrophilic epoxide styrene‐7,8‐oxide, which reacts directly with DNA. Styrene metabolism and distribution appeared to differ quantitatively among species. Pharmacokinetic models predicted concentrations of styrene‐7,8‐oxide in the terminal bronchioles 100‐fold higher in mouse compared with humans and 10‐fold higher in mouse compared with rats.
A ‘strong evidence’ for a genotoxic activity of styrene was reported in the IARC Monograph on the basis of the results of studies in experimental systems. The large majority of in vitro genotoxicity studies described in the IARC monograph showed positive results in the presence of metabolic activation. In vivo studies in rodents carried out by inhalation or intraperitoneal injection reported positive results for DNA damage in multiple tissues and negative or equivocal results for chromosomal damage. Only two studies carried out on styrene by oral exposure were described reporting negative results for chromosomal aberrations in mice.
The genotoxic mechanism was proposed by IARC to operate also in humans. DNA adducts were reported in peripheral blood cells of exposed workers. Mixed results were described in the reported biomonitoring studies applying different genotoxicity biomarkers in workers occupationally exposed by inhalation.
The ccWG Genotoxicity notes that the IARC monograph describes the first step in carcinogen risk assessment (i.e. the hazard identification), which involves examination of all relevant information to assess the strength of the available evidence that a substance could alter the incidence of cancer in experimental animals or humans. IARC evaluates cancer hazard using data published in the scientific literature, with no specific consideration of the route of exposure. Genotoxicity and other mechanistic data are also considered as supporting evidence.
The ccWG Genotoxicity notes that the evidence for styrene carcinogenicity in animals and its relevance to humans has been critically evaluated by other bodies, including the European authorities (ECHA RAC, 2012; SCHER, 2012). These reports were not considered by the ccWG Genotoxicity in the current assessment since it was out of the mandate and they will be analysed in the second phase.
In addition to the IARC monograph, the ccWG Genotoxicity also considered the genotoxicity part of the Technical Dossier provided by the industry PlasticsEurope and a critical analysis of the genotoxicity and carcinogenicity data (Moore et al., 2019) and a meta‐analysis (Collins and Moore, 2019), which were submitted to EFSA.
The meta‐analysis of Collins and Moore (2019) concerns studies applying the micronucleus assay in peripheral blood lymphocytes in workers occupationally exposed to styrene by inhalation. An overall increase of micronuclei frequencies was reported in exposed workers when compared to control groups. However, the ccWG Genotoxicity notes that the large variability and uncertainties in the extent of exposure in the different evaluated studies prevent a clear conclusion to be reached on the genotoxicity of styrene in humans exposed by inhalation.
The ccWG Genotoxicity concludes that a comprehensive evaluation of the reliability and relevance of all available experimental and human findings on styrene genotoxicity, with consideration of metabolic and toxicokinetic aspects, would be required for a sound evaluation of the genotoxicity of styrene, via oral exposure. This task should be undertaken following EFSA methodological criteria for hazard identification and characterisation, weighing the body of evidence and evaluating uncertainties and risk of bias according to EFSA guidelines (EFSA, 2017, 2018). The ccWG Genotoxicity also considers that an investigation of the quantitative relationships between DNA adducts and various genotoxicity endpoints (gene mutations, single‐strand breaks and chromosomal damage) would provide useful information on the mode of action of styrene, its genotoxicity profile, and human risk characterisation.
This task could not be completed solely based on the IARC Monograph because the experimental details provided in the Monographs are not always sufficient for an in‐depth evaluation.
References
Bonassi S, Fenech M, Lando C, Lin YP, Ceppi M, Chang WP, Holland N, Kirsch‐Volders M, Zeiger E, Ban S, Barale R, Bigatti MP, Bolognesi C, Jia C, Di Giorgio M, Ferguson LR, Fucic A, Lima OG, Hrelia P, Krishnaja AP, Lee TK, Migliore L, Mikhalevich L, Mirkova E, Mosesso P, Müller WU, Odagiri Y, Scarffi MR, Szabova E, Vorobtsova I, Vral A and Zijno A, 2001. Human MicroNucleus project: international data base comparison for results with the cytokinesis‐block micronucleus assay in human lymphocytes: I. Effect of laboratory protocol, scoring criteria, and host factors on the frequency of micronuclei, Environmental Molecular Mutagensis, 37, 31–45.
Ceppi M, Biasotti B, Fenech M and Bonassi S, 2010. Human population studies with the exfoliated buccal micronucleus assay: statistical and epidemiological issues, Mutation Research, 705, 11–19.
Collins JJ and Moore M, 2019. A meta‐analysis of epidemiologic studies of occupationally exposed styrene workers and micronuclei levels. Mutation Research, 837, 15–28.
Egger M, Smith GD, Schneider M and Minder C, 1997. Bias in meta‐analysis detected by a simple, graphical test. British Medical Journal, 315, 629–634.
ECHA RAC (European Chemical Agency ‐ Committee for Risk Assessment), 2012. Annex 1: Background document to the Opinion proposing harmonised classification and labelling at EU level of styrene. CAS no. 100‐42‐5. Available online: https://echa.europa.eu/documents/10162/744d52f2-26b6-a41d-da81-6e8415a45557
EFSA Scientific Committee, 2011. Scientific Opinion on genotoxicity testing strategies applicable to food and feed safety assessment. EFSA Journal 2011;9:2379. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/2379
EFSA Scientific Committee, 2017. Guidance on the use of the weight of evidence approach in scientific assessments. EFSA Journal 2017;15(8):4971. https://doi.org/10.2903/j.efsa.2017.4971
EFSA Scientific Committee, 2018. Guidance on Uncertainty Analysis in Scientific Assessments, EFSA Journal 2018;16(1):5123. https://doi.org/10.2903/j.efsa.2018.5123
Fenech M, 2007. Cytokinesis‐block micronucleus cytome assay. Nature Protocol, 2, 1084–1104.
Gaté L, Micillino JC, Sebillaud S, Langlais C, Cosnier F, Nunge H, Darne C, Guichard Y and Binet S, 2012. Genotoxicity of styrene‐7,8‐oxide and styrene in fisher 344 rats: A 4‐week inhalation study. Toxicological Letter, 211, 211–219.
IARC (International Agency for Research on Cancer), 2019. Styrene, styrene‐7,8‐oxide, and quinoline. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, 121, 83–341, Lyon, France.
Loprieno N, Presciuttini S, Sbrana I, Stretti G, Zaccaro L, Abbondandolo A, Bonatti S, Fiorio R, and Mazzaccaro A, 1978. Mutagenicity of industrial compounds. VII. Styrene and styrene oxide: II. Point mutations, chromosome aberrations and DNA repair induction analyses. Scandian Journal of Work Environmental Health, 4(Suppl 2), 169–178.
Moore MM, Pottenger LH and House‐Knight T, 2019. Critical review of styrene genotoxicity focused on the mutagenicity/clastogenicity literature and using current organization of economic cooperation and development guidance. Environmental and Molecular Mutagenesis, 60, 624–663.
NRC (National Research Council), 2014. Review of the Styrene Assessment in the National Toxicology Program 12th Report on Carcinogens. Washington, DC: The National Academies Press. https://doi.org/10.17226/18725
NTP (National Toxicology Program), 2008. Report on Carcinogens Background Document for Styrene. United States Department of Health and Human Services, Public Health Services, National Toxicology Program, Research Triangle Park, N.C., (8‐5978):i‐462.
OECD (Organisation for Economic Co‐operation and Development), 2016, Test No. 489: In Vivo Mammalian Alkaline Comet Assay, OECD Guidelines for the Testing of Chemicals, Section 4, OECD Publishing, Paris. https://doi.org/10.1787/9789264264885-en
SCHER (Scientific Committee on Health and Environmental Risks), 2008. SCHER scientific opinion on the risk assessment report on styrene human health part, CAS 100‐42‐6, 6 May 2008. Available online: http://ec.europa.eu/health/ph_risk/committees/04_scher/docs/scher_o_095.pdf
Sbrana I, Lascialfari D, Rossi AM, Loprieno N, Bianchi M, Tortoreto M and Pantarroto C, 1983. Bone marrow cell chromosomal aberrations and styrene biotransformation in mice given styrene on a repeated oral schedule. Chemical Biology Interactions, 45, 349–357.
Vodicka P, Koskinen M, Vodickova L, Stetina R, Smerak P, Barta I and Hemminki K, 2001. DNA adducts, strand breaks and micronuclei in mice exposed to styrene by inhalation. Chemical Biology Interactions, 137, 213–227.
Wilson III DM and Thompson LH, 2007. Molecular mechanisms of sister‐chromatid exchange. Mutation Research, 616, 11–23.
Yager JW, Paradisin WM and Rappaport SM, 1993. Sister‐chromatid exchanges in lymphocytes are increased in relation to longitudinally measured occupational exposure to low concentrations of styrene, Mutation Research, 319, 155–165.
Suggested citation: EFSA Panel on Food Contact Materials, Enzymes and Processing Aids (CEP) , Silano V, Barat Baviera JM, Bolognesi C, Chesson A, Cocconcelli PS, Crebelli R, Gott DM, Grob K, Lambré C, Lampi E, Mengelers M, Mortensen A, Steffensen I‐L, Tlustos C, Van Loveren H, Vernis L, Zorn H, Castle L, Di Consiglio E, Franz R, Hellwig N, Milana MR, Pfaff K, Carfi M, Van Haver E and Rivière G, 2020. Scientific Opinion on the assessment of the impact of the IARC Monograph Vol. 121 on the safety of the substance styrene (FCM No 193) for its use in plastic food contact materials. EFSA Journal 2020;18(10):6247, 23 pp. 10.2903/j.efsa.2020.6247
Requestor: European Commission
Question number: EFSA‐Q‐2019‐00686
Panel members: José Manuel Barat Baviera, Claudia Bolognesi, Andrew Chesson, Pier Sandro Cocconcelli, Riccardo Crebelli, David Michael Gott, Konrad Grob, Claude Lambré, Evgenia Lampi, Marcel Mengelers, Alicja Mortensen, Gilles Rivière, Vittorio Silano, Inger‐Lise Steffensen, Christina Tlustos, Henk Van Loveren, Laurence Vernis and Holger Zorn.
Competing interests: R. Franz declared that Fraunhofer institute at which he was employed provides advisory services to private business operators active in the sector on food contact materials. In line with EFSA's Policy on Independence (http://www.efsa.europa.eu/sites/default/files/corporate_publications/files/policy_independence.pdf) and the Decision of the Executive Director on Competing Interest Management (http://www.efsa.europa.eu/sites/default/files/corporate_publications/files/competing_interest_management_17.pdf), a waiver was granted to R. Franz regarding his participation to the EFSA's Working Group on Food Contact Materials (FCM WG) in accordance with Article 21 of the Decision of the Executive Director on Competing Interest Management. Pursuant to Article 21(6) of the above‐mentioned Decision, the involvement of R. Franz is authorised as member in the FCM WG, allowing him to take part in the discussions and in the drafting phase of the scientific output, but he is not allowed to be, or act as, a chairman, a vice‐chairman or rapporteur of the working group.
Acknowledgements: The Panel wishes to thank the following members of the EFSA Scientific Committee cross‐cutting working group on genotoxicity (ccWG Genotoxicity) and EFSA staff members for their support provided to this scientific output: Diane Benford, Gabriele Aquilina, Margherita Bignami, Rainer Guertler, Francesca Marcon, Elsa Nielsen, Josef Rudolf Schlatter, Christiane Vleminckx, Daniela Maurici, and Rositsa Serafimova.
Note: The full opinion will be published in accordance with Article 10(6) of Regulation (EC) No 1935/2004 once the decision on confidentiality, in line with Article 20(3) of the Regulation, will be received from the European Commission. Some data on styrene migrating from copolymers into food simulants have been provided under confidentiality and it is redacted awaiting the decision of the Commission.
Adopted: 9 September 2020
Notes
OJ L 338, 13.11.2004, p. 4.
IARC Monograph volume 121: styrene, styrene‐7,8‐oxide and quinoline.
OJ L 75, 21.3.1990, p. 19–40.
Technical dossier/sections 1.1 and 2.1.
Technical dossier/section 5.1.
Technical dossier/section 5.1 and Annexes 6–10.
Technical dossier/section 5.1 and Annexes 7, 10.
Technical dossier/section 5.1 and Annexes 6, 8, 9.
Technical dossier/additional data April 2020.
OJ L 84, 27.3.1999, p. 1–137.
Technical dossier/section 8.1.
Technical dossier/additional data March 2020.
References
- Alexander HC, McCarty WM and Syverud AN, 1982. Aqueous odor and taste threshold values of industrial chemicals. Journal of the American Water Works Association, 74, 595–599. [Google Scholar]
- Banton MI, Bus JS, Collins JJ, Delzell E, Gelbke HP, Kester JE, Moore MM, Waites R and Sarangi SS, 2019. Evaluation of the potential health effects associated with occupational and environmental exposure to styrene – an update. Journal of Toxicology and Environmental Health, Part B: Critical Reviews. https://www.tandfonline.com/doi/full/10.1080/10937404.2019.1633718 [DOI] [PubMed] [Google Scholar]
- Cao XL, Sparling M, Pelletier L and Dabeka R, 2018. Styrene in foods and dietary exposure estimates. Food Additives & Contaminants: Part A, 35, 2045–2051. [DOI] [PubMed] [Google Scholar]
- Collins JJ and Moore M, 2019. A meta‐analysis of epidemiologic studies of occupationally exposed styrene workers and micronuclei levels. Mutation Research, 837, 15–28. [DOI] [PubMed] [Google Scholar]
- Duffey E and Gibney MF, 2007. Use of a food‐consumption database with packaging information to estimate exposure to food‐packaging migrants; epoxidized soybean oil and styrene monomer. Food Additives & Contaminants, 24, 216–225. [DOI] [PubMed] [Google Scholar]
- ECHA , 2012. Guidance on information requirements and chemical safety assessment Chapter R.8: Characterisation of dose [concentration]‐response for human health. Available online: https://echa.europa.eu/documents/10162/13632/information_requirements_r8_en.pdf
- EFSA (European Food Safety Authority), 2009. Guidance of the Scientific Committee on transparency in the scientific aspects of risk assessments carried out by EFSA. Part 2: general principles. EFSA Journal 2009;7(5):1051, 22 pp. 10.2903/j.efsa.2009.1051 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2017. Scientific Opinion on the guidance on the use of the weight of evidence approach in scientific assessments. EFSA Journal 2017;15(8):4971, 69 pp. 10.2903/j.efsa.2017.4971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2018. Guidance on Uncertainty Analysis in Scientific Assessments. EFSA Journal 2018;16(1):5123, 39 pp. 10.2903/j.efsa.2018.5123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2020. Scientific Opinion on the review and priority setting for substances that are listed without a specific migration limit in Table 1 of Annex 1 of Regulation 10/2011 on plastic materials and articles intended to come into contact with food. EFSA Journal 2020;18(6):6124, 104 pp. 10.2903/j.efsa.2020.6124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El‐Ziney MG and Tawfik MS, 2016. Migration levels of monostyrene from polystyrene containers to dairy products. Food Processing Technology, 3, 00066. [Google Scholar]
- European Commission , 2001. Guidelines of the Scientific Committee on Food for the presentation of an application for safety assessment of a substance to be used in food contact materials prior to its authorisation. Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/sci-com_scf_out82_en.pdf
- European Commission , 2005. Provisional list of monomers and additives notified to European Commission as substances which may be used in the manufacture of plastics or coatings intended to come into contact with foodstuffs (updated to June 2005) ‐ “Synoptic document”. SANCO D3/AS D(2005).
- Genualdi S, Nyman P and Begley T, 2014. Updated evaluation of the migration of styrene monomer and oligomers from polystyrene food contact materials to foods and food simulants. Food Additives & Contaminants. Part A, Chemistry, Analysis, Control, Exposure & Risk Assessment, 31, 723–733. 10.1080/19440049.2013.878040 [DOI] [PubMed] [Google Scholar]
- IARC , 1985. Allyl compounds, aldehydes, epoxides and peroxides>. IARC Monogr Eval Carcinog Risk Chem Hum, 36, 1–369. Available online: http://publications.iarc.fr/54
- IARC , 1994. Some industrial chemicals. IARC Monogr Eval Carcinog Risks Hum, 60, 1–560. Available online: http://publications.iarc.fr/78; PMID:7869568 [PMC free article] [PubMed]
- IARC , 2002. Some traditional herbal medicines, some mycotoxins, naphthalene and styrene. IARC Monogr Eval Carcinog Risks Hum, 82, 1‐556. Available online: http://publications.iarc.fr/100; PMID:12687954 [PMC free article] [PubMed]
- IARC , 2019. Styrene, styrene‐7,8‐oxide, and quinoline. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, 121, 83–341, Lyon, France. [PMC free article] [PubMed] [Google Scholar]
- Lickly TD, Breder CV and Rainey ML, 1995. A Model for estimating the daily dietary‐intake of a substance from food‐contact articles ‐ styrene from polystyrene food‐ contact polymers. Regulatory Toxicology and Pharmacology, 21, 406–417. [DOI] [PubMed] [Google Scholar]
- Lickly TD, Rainey ML, Burgert LC, Breder CV and Borodinsky L, 1997. Using a simple diffusion model to predict residual monomer migration ‐ Considerations and limitations. Food Additives and Contaminants, 14, 65–74. [DOI] [PubMed] [Google Scholar]
- Loprieno N, Presciuttini S, Sbrana I, Stretti G, Zaccaro L, Abbondandolo A, Bonatti R, Fiorio R and Mazzaccaro A, 1978. Mutagenicity of industrial compounds. VII. Styrene and styrene oxide: II. Point mutations, chromosome aberrations and DNA repair induction analyses. Scandinavian Journal of Work, Environment & Health, 4(Suppl 2), 169–178. [PubMed] [Google Scholar]
- MAFF , 1983. Survey of styrene levels in food contact materials and in foods. Food Surveillance Paper Number 11, HMSO.
- MAFF , 1994. Survey of styrene in food. Food Surveillance Information Sheet, Number 38, HMSO.
- MAFF , 1999. Total Diet study: styrene. Food Surveillance Paper Number 189, HMSO.
- Moore MM, Pottenger LH and House‐Knight T, 2019. Critical review of styrene genotoxicity focused on the mutagenicity/clastogenicity literature and using current organization of economic cooperation and development guidance. Environmental and Molecular Mutagenesis, 60, 624–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peattie ME, Buss DH, Lindsay DG and Smart GQ, 1983. Reorganisation of the British Total Diet Study for Monitoring Food Constituents from 1981. Food and Chemical Toxicology, 21, 503–507. [DOI] [PubMed] [Google Scholar]
- Philo MR, Fordham PJ, Damant AP and Castle L, 1997. Measurement of styrene oxide in polystyrenes, estimation of migration to foods, and reaction kinetics and products in food simulants. Food and Chemical Toxicology, 35, 821–826. 10.1016/S0278-6915(97)00054-9 PMID:9350227. [DOI] [PubMed] [Google Scholar]
- Plastics Foodservice Packaging Group , 2015. The safety of styrene‐based polymers for food contact use 2013. Available online: https://www.plasticfoodservicefacts.com/wp-content/uploads/2017/10/Polystyrene-Report.pdf [Accessed 19 June 2019]. [Google Scholar]
- Sbrana I, Lascialfari D, Rossi AM, Loprieno N, Bianchi M, Tortoreto M and Pantarotto C, 1983. Bone marrow cell chromosomal aberrations and styrene biotransformation in mice given styrene on a repeated oral schedule. Chemico‐Biological Interactions, 45, 349–357. [DOI] [PubMed] [Google Scholar]
- Tang W, Hemm I and Eisenbrand G, 2000. Estimation of human exposure to styrene and ethylbenzene. Toxicology, 144, 39–50. 10.1016/S0300-483X(99)00188-2 PMID:10781869. [DOI] [PubMed] [Google Scholar]
- Tischner T, 1993. Vergleich der Migrationskinetik von Monostyrol aus Polystyrolbechern in Milchprodukte und Simulanzlebensmittel. Thesis Fachhochschule München.
- WHO (World Health Organisation), 2004. Guidelines for Drinking‐water Quality. Vol. 1. 3rd Edition. Vol. 1 Recommendations. World Health Organisation, Geneva, 2004. https://www.who.int/water_sanitation_health/dwq/GDWQ2004web.pdf


