Abstract
Background
Airway bacterial carriage might play a role in respiratory disease. We hypothesize that nasal carriage with Staphylococcus aureus or nasopharyngeal carriage with Haemophilus influenzae, Moraxella catarrhalis, and Streptococcus pneumoniae predisposes individuals to adverse respiratory health.
Objective
To examine the association of early‐life airway bacterial carriage with respiratory tract infections and vice versa, and of early‐life airway bacterial carriage with wheezing, lung function, and asthma in later childhood.
Methods
We collected upper airway swabs for bacterial culturing for S aureus, H influenzae, M catarrhalis, and H influenzae at six timepoints between the ages of 6 weeks and 6 years among 945 children participating in a population‐based prospective cohort study. Information on respiratory tract infections and wheezing until age 6 years, and asthma at age 10 years was obtained by questionnaires. Lung function at age 10 years was measured by spirometry. We tested possible bidirectional associations between airway bacterial carriage and respiratory tract infections by cross‐lagged models, and associations of repeatedly measured airway bacterial carriage with wheezing, lung function, and asthma by generalized estimating equations models and regression models.
Results
Cross‐lagged modeling showed that early‐life airway bacterial carriage was not consistently associated with upper and lower respiratory tract infections or vice versa. Nasopharyngeal carriage with any bacteria in infancy was associated with an increased risk of wheezing (OR [95% CI]: 1.66 [1.31, 2.10]). Airway bacterial carriage was not consistently associated with school‐age lung function or asthma.
Conclusion
Nasopharyngeal carriage with any bacteria is associated with wheezing, but not respiratory tract infections, asthma, or lung function.
Keywords: asthma, bacteria, bacterial carriage, child, epidemiology, respiratory function test, respiratory tract infection
Key Message.
Previous studies demonstrated that airway bacterial carriage in infancy is associated with short‐term respiratory health. The persistence of airway bacterial carriage into later childhood and the relation with lung function are not known. In this population‐based prospective cohort study, we demonstrate that nasopharyngeal carriage with any bacteria is associated with wheezing, especially in early life, but not school‐age asthma or lung function. Additionally, airway bacterial carriage is not consistently associated with lower respiratory tract infection or vice versa. These findings suggest that bacterial carriage in infancy is associated with short, but not long‐term respiratory health.
1. INTRODUCTION
Respiratory tract infections in early life have been associated with the development of chronic obstructive respiratory disease in childhood and adulthood. 1 , 2 Previous studies showed that hypopharyngeal bacterial carriage of Haemophilus influenzae, Moraxella catarrhalis, and Staphylococcus pneumoniae in infancy is associated with an increased risk of lower respiratory tract infections in the first 3 years of life. 3 Alternatively, respiratory tract infections might also influence airway bacterial carriage through bacterial superinfection or changed bacterial colonization after a viral infection. 4 , 5 Therefore, it is difficult to disentangle the direction of the association between airway bacterial carriage and respiratory tract infections. Additionally, previous studies have shown that airway bacterial carriage with these pathogens in infancy is associated with increased risks of wheezing and asthma in childhood. 6 , 7 , 8 , 9 Most observational studies have focused on the association of airway bacterial carriage in infancy with wheezing or asthma in early childhood, but its persistence into later childhood and the relation with lung function are not known.
We hypothesized that airway bacterial carriage in early childhood is more prominently associated with an increased risk of respiratory tract infections than vice versa and that bacterial carriage in early childhood is associated with wheezing, lower lung function, and asthma in later childhood. Therefore, we examined in a population‐based prospective cohort study among 945 children the associations of airway bacterial carriage of H influenzae, M catarrhalis, S pneumoniae, or Streptococcus aureus from age 6 weeks to 6 years with respiratory tract infections taking possible bidirectional associations into account. Next, we examined whether airway bacterial carriage with these pathogens was associated with wheezing from age 1 to 6 years, and lung function and asthma at age 10 years.
2. METHODS
2.1. Design
This study was embedded in a subcohort of 1232 Dutch mother and their children of the Generation R Study, a population‐based prospective cohort from early fetal life onwards in Rotterdam, the Netherlands. 10 , 11 The study has been approved by the Medical Ethical Committee of the Erasmus MC, University Medical Center Rotterdam, Rotterdam, the Netherlands (MEC‐2012‐165). Written informed consent was obtained from parents or legal representatives of all children. After exclusion of twins, children without upper airway swabs, and children without any information on respiratory health, 945 children were included in the analysis (Figure S1).
2.2. Bacterial carriage
At the age of 6 weeks; 6 and 14 months; and 2, 3, and 6 years after birth, upper airway swabs of the nose and nasopharyngeal area were taken at the research center, as described earlier. 12 , 13 Swabs were classified as either negative or positive for S aureus, H influenzae, M catarrhalis, or S pneumoniae. Nasopharyngeal carriage of H influenza, M catarrhalis, or S pneumoniae was studied separately, and additionally, nasopharyngeal carriage with any bacteria was classified as positive when either of these three pathogens was positive, and negative if all three were negative.
2.3. Respiratory health
We obtained information on physician‐attended upper (ear infection, throat infection, croup, or whooping cough) and lower (pneumonia, bronchitis, or bronchiolitis) respiratory tract infections by questionnaires at the age of 2, 6, and 12 months, and 2, 3, 4, and 6 years. Information on wheezing was obtained by questionnaire annually from 1 to 4 years, and at age 6 years. Lung function measures were obtained by spirometry during a visit at the research center at the mean age of 9.8 (SD 0.34) years, and converted into sex‐, height‐, age‐, and ethnicity‐adjusted z‐scores according to the Global Lung Initiative reference data. 14 , 15 , 16 Asthma was defined as ever diagnosis of asthma, obtained by questionnaire at the age 10 years, with either wheezing or asthma medication use in the past 12 months. Questions on wheezing and asthma were based on the International Study on Asthma and Allergy in Childhood (ISAAC) Questionnaire, a validated questionnaire for respiratory epidemiological research. 17
2.4. Covariates
Information on maternal history of asthma and atopy, education level, smoking during pregnancy, psychological distress during pregnancy, parity, and pet keeping was obtained by questionnaires during pregnancy. Maternal history of asthma and atopy included diagnosis of asthma, house dust mite allergy, hay fever, or eczema. Maternal psychological distress was measured by the Global Severity Index. 18 Child's gestational age at birth was obtained from midwife and hospital records. Questionnaires in the first year of life provided information on breastfeeding and day care attendance, and questionnaires until age 6 years on antibiotic use.
2.5. Statistical analysis
First, we compared characteristics of mothers and children included in the study to those eligible but not included using t tests, Mann‐Whitney U tests, and chi‐squared tests. Next, we analyzed the associations of airway bacterial carriage with respiratory tract infections using cross‐lagged models. Cross‐lagged models take potential bidirectional associations between the exposure and the outcome into account (Figure S2). With this method, we were able to disentangle the directions of the observed associations of airway bacterial carriage with respiratory tract infections. 2 , 19 Next, we analyzed the associations of airway bacterial carriage at 6 weeks; 6 and 14 months; and 2, 3, and 6 years separately, with childhood wheezing, and school‐age lung function, and asthma. Since we did not expect bidirectional associations between airway bacterial carriage and wheezing until the age of 10 years, we used generalized estimating equation (GEE) models with a toeplitz correlation matrix to examine these associations. Last, we studied the association of airway bacterial carriage with lung function and asthma at school age using linear and logistic regression models, respectively. All models are adjusted for confounders, with missing data imputed by multiple imputation. All measures of association are presented as odds ratios (OR) or Z‐score differences and their corresponding 95% confidence intervals (95% CI). Statistical analyses were performed using Mplus version 7.4 (Muthén & Muthén), SAS version 9.4 (SAS Institute Inc), and SPSS version 25.0 for Windows software (IBM Corp). More detailed information on the methods can be found in Appendix S1.
3. RESULTS
3.1. Subject characteristics
Characteristics of children and their mothers, and of those not included, are presented in Table 1, Figures 1 and 2, and Table S1. Nasal bacterial carriage with S aureus decreased from 54.0% at age 6 weeks to 13.5%‐15.3% at age 2‐3 years and thereafter increased to 27.8% at age 6 years (Figure 1). Nasopharyngeal carriage with any of H influenzae, M catarrhalis, or S pneumoniae increased from 22.9% at age 6 weeks to 66.5% at age 2 years and thereafter decreased to 37.2% at age 6 years. The prevalences of these three pathogens separately showed a similar pattern, but with lower prevalence rates. The prevalences of upper and lower respiratory tract infections increased until age 2 years and decreased thereafter, while the prevalence of wheezing decreased from age 1 to 6 years (Figure 2). The prevalence of asthma was 4.0% at age 10 years (Table 1).
TABLE 1.
Characteristics of children and their mothers
| n = 945 | |
|---|---|
| Maternal characteristics | |
| History of asthma or atopy, yes (%) | 39.6 (374) |
| Educational level, low/middle (%) | 34.0 (321) |
| Smoking during pregnancy, yes (%) | 12.1 (114) |
| Maternal psychiatric symptoms1 | 0.12 (0.00, 0.54) |
| Parity, nulliparous (%) | 63.1 (596) |
| Pet keeping, yes (%) | 43.7 (413) |
| Child's characteristics | |
| Female sex (%) | 49.1 (464) |
| Gestational age at birth (weeks)1 | 40.3 (37.3, 42.1) |
| Ever breastfeeding, yes (%) | 90.8 (858) |
| Day care attendance 1st year, yes (%) | 68.3 (645) |
| Current asthma age 10 y, yes (%) | 4.0 (33) |
| Lung function measures age 10 y | |
| FEV1 (L) | 2.05 (0.29) |
| FVC (L) | 2.38 (0.35) |
| FEV1/FVC (%) | 86.33 (5.58) |
| FEF75 (L/s) | 1.15 (0.34) |
Values are means (SD), valid percentages (absolute numbers), or 1medians (5‐95th percentiles). Forced Expiratory Volume in the first second (FEV1). Forced Vital Capacity (FVC). Forced Expiratory Flow after exhaling 75% of FVC (FEF75). Data were missing and not imputed for asthma (n = 121) and lung function measures (n = 172).
FIGURE 1.
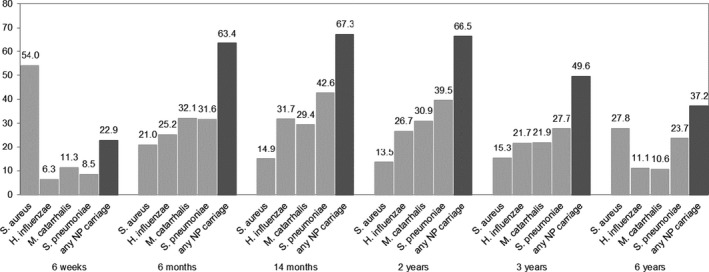
Prevalence (%) of bacterial carriage of the upper airways in childhood. Nasopharyngeal (NP). Nasopharyngeal carriage of any bacteria was classified as positive when either of Haemophilus influenzae, Moraxella catarrhalis, or Streptococcus pneumoniae was positive, and negative if all three were negative
FIGURE 2.
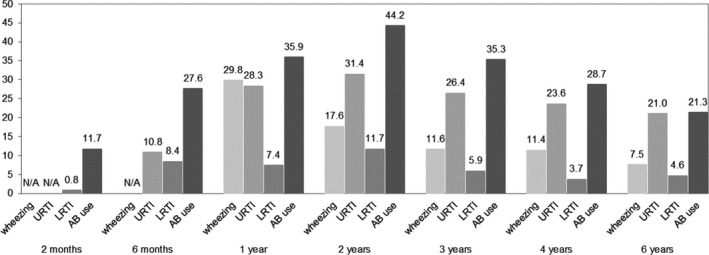
Prevalence (%) of wheezing, upper and lower respiratory tract infections, and antibiotic use in childhood. Upper respiratory tract infections (URTI), lower respiratory tract infections (LRTI), antibiotics (AB)
3.2. Early‐life airway bacterial carriage and respiratory tract infections
For upper respiratory tract infections, cross‐sectional correlations and cross‐lagged associations showed that both nasal and nasopharyngeal bacterial carriages were not associated with upper respiratory tract infections or vice versa (Figure S3). For lower respiratory tract infections, cross‐sectional correlations showed that only M catarrhalis carriage was associated with an increased risk of lower respiratory tract infections at 6 months, but a decreased risk at age 14 months and similarly vice versa (OR [95% CI]: 2.78 [1.30, 5.93] and 0.24 [0.10, 0.59], respectively) (Table 2 and Figure S4). Cross‐lagged associations showed that only H influenza at age 6 weeks was associated with an increased risk of lower respiratory tract infections at age 6 months (OR [95% CI]: 4.79 [1.50, 15.21]), while only lower respiratory tract infections at 6 months were associated with an increased risk of carriage with M catarrhalis at age 14 months (OR [95% CI]: 2.69 [1.21, 5.83]).
TABLE 2.
Direction of associations between airway bacterial carriage and lower respiratory tract infections from age 6 wk until 10 y
| Staphylococcus aureus OR (95% CI) | Haemophilus influenzae OR (95% CI) | Moraxella catarrhalis OR (95% CI) | Streptococcus pneumoniae OR (95% CI) | Any NP carriage OR (95% CI) | |
|---|---|---|---|---|---|
| Cross‐lagged effects | |||||
| Carriage 6 wk → LRTI 6 mo | 0.92 (0.42, 1.98) | 4.79 (1.50, 15.21)* | 0.76 (0.21, 2.71) | 1.47 (0.47, 4.57) | 2.16 (0.88, 5.31) |
| Carriage 6 mo → LRTI 1 y | 1.73 (0.81, 3.70) | 1.32 (0.66, 2.64) | 0.78 (0.36, 1.68) | 0.82 (0.40, 1.70) | 1.14 (0.57, 2.30) |
| Carriage 1 y → LRTI 2 y | 1.39 (0.65, 2.96) | 1.47 (0.83, 2.59) | 1.0.31 (0.72, 2.37) | 0.81 (0.46, 1.41) | 1.44 (0.81, 2.56) |
| Carriage 2 y → LRTI 2 y | 0.18 (0.02, 1.67) | 0.44 (0.16, 1.22) | 1.29 (0.56, 3.01) | 1.07 (0.44, 2.57) | 0.70 (0.28, 1.73) |
| Carriage 3 y → LRTI 4 y | 0.50 (0.13, 1.92) | 1.63 (0.41, 6.40) | 1.35 (0.32, 5.65) | 2.73 (0.84, 8.88) | 1.27 (0.45, 3.62) |
| LRTI 6 wk → carriage 6 mo | 2.64 (0.30, 22.81) | ‐‐ | 0.74 (0.07, 9.93) | ‐‐ | ‐‐ |
| LRTI 6 mo → carriage 1 y | 1.04 (0.37, 2.89) | 1.64 (0.76, 3.47) | 2.69 (1.21, 5.83)** | 1.47 (0.65, 3.26) | 2.26 (0.77, 6.51) |
| LRTI 1 y → carriage 2 y | 0.96 (0.36, 2.60) | 0.47 (0.21, 1.05) | 1.40 (0.74, 2.66) | 0.73 (0.38, 1.41) | 1.07 (0.53, 2.16) |
| LRTI 2 y → carriage 3 y | 1.07 (0.50, 2.29) | 0.53 (0.25, 1.14) | 0.95 (0.48, 1.90) | 0.98 (0.52, 1.83) | 0.75 (0.43, 1.30) |
| LRTI 4 y → carriage 6 y | 0.61 (0.21, 1.78) | 2.11 (0.56, 7.72) | 0.66 (0.08, 5.66) | 2.19 (0.87, 5.46) | 2.11 (0.85, 5.21) |
| Cross‐sectional effects | |||||
| Carriage ↔ LRTI 6 wk | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ |
| Carriage ↔ LRTI 6 mo | 0.53 (0.19, 1.47) | 0.90 (0.38, 2.16) | 2.78 (1.30, 5.93)* | 1.73 (0.75, 4.01) | 1.89 (0.78, 4.50) |
| Carriage ↔ LRTI 1 y | 0.60 (0.24, 1.50) | 0.96 (0.49, 1.90) | 0.24 (0.10, 0.59)** | 0.69 (0.36, 1.33) | 0.50 (0.24, 1.05) |
| Carriage ↔ LRTI 2 y | 1.12 (0.45, 2.82) | 0.97 (0.50, 1.89) | 0.88 (0.47, 1.65) | 1.65 (0.88, 3.10) | 1.10 (0.55, 2.21) |
| Carriage ↔ LRTI 3 y | 1.31 (0.43, 4.03) | 1.10 (0.39, 3.09) | 0.70 (0.24, 2.01) | 1.25 (0.50, 1.22) | 1.14 (0.48, 2.69) |
| Carriage ↔ LRTI 6 y | 1.42 (0.64, 3.13) | 0.32 (0.06, 1.62) | 0.26 (0.03, 2.31) | 0.68 (0.25, 1.85) | 0.47 (0.19, 1.16) |
Associations of S aureus, H influenzae, M catarrhalis, S pneumoniae, and any nasopharyngeal carriage with lower respiratory tract infections and vice versa. Values are odds ratios (95% confidence interval) from cross‐lagged models. Models are adjusted for maternal history of asthma and atopy, education level, smoking during pregnancy, stress during pregnancy, parity and pet keeping, and child's gestational age at birth, breastfeeding, and day care attendance. Bold values indicate significant associations. For some associations, no effect estimate could be given due to limited power, as indicated by ‐‐. Lower respiratory tract infections (LRTI). Nasopharyngeal (NP).
3.3. Early‐life bacterial carriage and wheezing
Staphylococcus aureus was not associated with overall wheezing (Figure 3A). Nasopharyngeal carriage of any bacteria at 6 months was associated with an increased risk of overall wheezing (1.66 [1.31, 2.10]) (Figure 3B). Per year, nasopharyngeal carriage of any bacteria at the age of 6 months was associated with an increased risk of wheezing at the age of 1 year (1.81 [1.23, 2.66]), and at age 1 year with an increased risk of wheezing at 2 years (1.72 [1.02, 2.90]). Associations of nasopharyngeal carriage of H influenzae, M catarrhalis, and S pneumoniae individually with wheezing showed similar tendencies (Figure S5). M catarrhalis at age 6 months was associated with an increased risk of overall wheezing (1.33 [1.07, 1.65]), while M catarrhalis at age 6 weeks and 2 years was associated with a decreased risk of overall wheezing (0.65 [0.42, 1.00] and 0.66 [0.47, 0.91]s, respectively).
FIGURE 3.
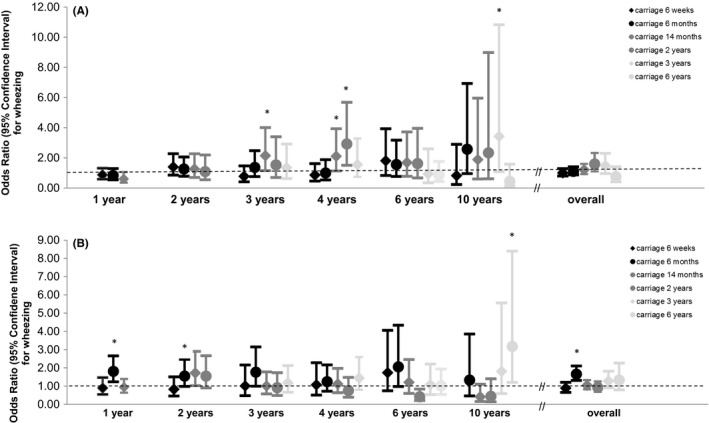
Associations of S aureus (A) and any nasopharyngeal carriage (B) with wheezing from age 1‐10 y. Values are odds ratios with 95% confidence interval from generalized estimating equation models. *P‐value < .05. Models are adjusted for maternal history of asthma and atopy, education level, smoking during pregnancy, stress during pregnancy, parity and pet keeping, and child's gestational age at birth, breastfeeding, day care attendance, and antibiotic use in the past 2 mo (2 mo), 6 mo (6 mo), or year (1‐6 y). Only airway bacterial carriage before or at the age of assessment of wheezing was included in the models, and models were additionally adjusted for any wheezing in the preceding period if the first age at which wheezing was assessed was later than 1 y of age. For nasopharyngeal carriage with any bacteria at age 6 wk and wheezing at age 10 y, no effect estimate could be given due to limited power
3.4. Early‐life bacterial carriage, lung function, and asthma
Overall, no consistent associations of early‐life airway bacterial carriage with lung function or asthma in later childhood were observed (Table 3). Only H influenza and any nasopharyngeal carriage at age 14 months were most prominently associated with a decreased risk of current asthma at age 10 years (OR [95% CI]: 0.28 [0.08, 0.99] and 0.38 [0.15, 0.94], respectively). For all analyses, effect estimates did not materially change when excluding antibiotic use as a confounder (data not shown).
TABLE 3.
Associations of bacterial carriage of the upper airways with lung function and asthma at age 10 y
|
FEV1 Z‐score difference (95% Confidence Interval) n = 719 |
FVC Z‐score difference (95% Confidence Interval) n = 719 |
FEV1/FVC Z‐score difference (95% Confidence Interval) n = 719 |
FEF75 Z‐score difference (95% Confidence Interval) n = 719 |
Current asthma Odds ratio (95% Confidence Interval) n = 762 |
|
|---|---|---|---|---|---|
| 2 mo | |||||
| Staphylococcus aureus | 0.05 (−0.12, 0.21) | 0.03 (−0.13, 0.19) | 0.02 (−0.16, 0.21) | 0.01 (−0.16, 0.17) | 0.75 (0.27, 2.03) |
| Haemophilus influenzae | 0.01 (−0.37, 0.40) | 0.15 (−0.23, 0.52) | −0.28 (−0.71, 0.15) | −0.30 (−0.70, 0.07) | −− |
| Moraxella catarrhalis | 0.01 (−0.25, 0.28) | −0.00 (−0.26, 0.26) | 0.01 (−0.29, 0.31) | 0.04 (−0.23, 0.30) | 0.55 (0.07, 4.62) |
| Streptococcus pneumoniae | 0.09 (−0.23, 0.41) | −0.01 (−0.32, 0.30) | 0.19 (−0.16, 0.54) | 0.10 (−0.21, 0.41) | 0.70 (0.08, 6.14) |
| Any nasopharyngeal carriage | 0.03 (−0.20, 0.25) | 0.00 (−0.21, 0.22) | 0.03 (0.21, 0.28) | −0.02 (−0.24, 0.20) | 0.45 (0.09, 2.20) |
| 6 mo | |||||
| Staphylococcus aureus | −0.01 (−0.18, 0.16) | 0.01 (−0.15, 0.18) | −0.04 (−0.23, 0.15) | −0.06 (−0.23, 0.11) | 0.84 (0.30, 2.39) |
| Haemophilus influenzae | 0.03 (−0.15, 0.20) | 0.02 (−0.14, 0.19) | −0.02 (−0.21, 0.17) | −0.06 (−0.23, 0.11) | 0.59 (0.19, 1.86) |
| Moraxella catarrhalis | −0.02 (−0.18, 0.13) | −0.03 (−0.17, 0.12) | −0.02 (−0.19, 0.15) | −0.00 (−0.16, 0.16) | 1.53 (0.65, 3.61) |
| Streptococcus pneumoniae | 0.02 (−0.14, 0.17) | −0.01 (−0.16, 0.14) | 0.04 (−0.13, 0.21) | 0.04 (−0.12, 0.20) | 0.42 (0.14, 1.32) |
| Any nasopharyngeal carriage | −0.09 (−0.24, 0.07) | −0.07 (−0.22, 0.08) | −0.02 (−0.19, 0.15) | −0.05 (−0.21, 0.10) | 0.81 (0.33, 1.98) |
| 14 mo | |||||
| Staphylococcus aureus | −0.13 (−0.34, 0.08) | −0.05 (−0.25, 0.15) | −0.13 (−0.35, 0.10) | −0.17 (−0.38, 0.04) | 1.65 (0.58, 4.71) |
| Haemophilus influenzae | −0.19 (−0.35, −0.02)* | −0.14 (−0.30, 0.02) | −0.05 (−0.23, 0.13) | −0.13 (−0.30, 0.03) | 0.28 (0.08, 0.99)* |
| Moraxella catarrhalis | 0.12 (−0.04, 0.29) | 0.10 (−0.06, 0.26) | 0.01 (−0.17, 0.19) | 0.06 (−0.10, 0.22) | 0.91 (0.34, 2.43) |
| Streptococcus pneumoniae | 0.07 (−0.09, 0.24) | 0.07 (−0.09, 0.22) | −0.03 (−0.20, 0.15) | 0.07 (−0.09, 0.23) | 0.47 (0.18, 1.22) |
| Any nasopharyngeal carriage | 0.03 (−0.14, 0.21) | 0.05 (−0.12, 0.21) | −0.04 (−0.23, 0.15) | −0.02 (−0.20, 0.15) | 0.38 (0.15, 0.94)* |
| 2 y | |||||
| Staphylococcus aureus | 0.13 (−0.11, 0.37) | −0.02 (−0.25, 0.21) | 0.26 (0.01, 0.51)* | 0.21 (−0.02, 0.44) | 1.46 (0.46, 4.63) |
| Haemophilus influenzae | 0.18 (−0.01, 0.37) | 0.13 (−0.06, 0.32) | 0.05 (−0.15, 0.26) | 0.18 (−0.01, 0.36) | 0.63 (0.20, 1.97) |
| Moraxella catarrhalis | 0.06 (−0.12, 0.24) | 0.00 (−0.17, 0.18) | 0.08 (−0.11, 0.27) | 0.15 (−0.02, 0.33) | 0.45 (0.15, 1.39) |
| Streptococcus pneumoniae | 0.16 (−0.02, 0.33) | 0.12 (−0.05, 0.28) | 0.06 (−0.13, 0.24) | 0.14 (−0.03, 0.31) | 0.86 (0.34, 2.19) |
| Any nasopharyngeal carriage | 0.17 (−0.01, 0.35) | 0.10 (−0.07, 0.27) | 0.10 (−0.09, 0.29) | 0.21 (0.04, 0.39)* | 0.68 (0.27, 1.71) |
| 3 y | |||||
| Staphylococcus aureus | 0.10 (−0.12, 0.31) | 0.07 (−0.15, 0.25) | 0.13 (−0.09, 0.35) | 0.11 (−0.10, 0.32) | 1.79 (0.62, 5.17) |
| Haemophilus influenzae | 0.01 (−0.18, 0.20) | 0.00 (−0.18, 0.19) | 0.03 (−0.17, 0.22) | 0.00 (−0.18, 0.18) | 1.61 (0.57, 4.54) |
| Moraxella catarrhalis | 0.10 (−0.09, 0.28) | 0.14 (−0.05, 0.32) | −0.04 (−0.23, 0.15) | 0.05 (−0.13, 0.23) | 1.38 (0.51, 3.70) |
| Streptococcus pneumoniae | 0.09 (−0.09, 0.27) | 0.10 (−0.07, 0.28) | −0.00 (−0.18, 0.18) | −0.00 (−0.17, 0.17) | 1.66 (0.65, 4.25) |
| Any nasopharyngeal carriage | 0.15 (−0.01, 0.30) | 0.15 (0.01, 0.31)* | −0.00 (−0.16, 0.16) | 0.07 (−0.09, 0.22) | 1.65 (0.65, 4.14) |
| 6 y | |||||
| Staphylococcus aureus | −0.01 (−0.16, 0.14) | 0.02 (−0.12, 0.16) | −0.01 (−0.17, 0.15) | 0.05 (−0.10, 0.19) | 0.95 (0.39, 2.32) |
| Haemophilus influenzae | 0.03 (−0.17, 0.23) | 0.05 (−0.15, 0.24) | −0.04 (−0.26, 0.19) | −0.03 (−0.22, 0.17) | 0.96 (0.28, 3.30) |
| Moraxella catarrhalis | 0.10 (−0.10, 0.31) | 0.13 (−0.07, 0.33) | −0.08 (−0.30, 0.15) | −0.08 (−0.28, 0.12) | 1.08 (0.31, 3.73) |
| Streptococcus pneumoniae | −0.06 (−0.22, 0.09) | −0.12 (−0.26, 0.03) | 0.10 (−0.06, 0.27) | 0.08 (−0.07, 0.23) | 1.10 (0.45, 2.69) |
| Any nasopharyngeal carriage | 0.01 (−0.13, 0.14) | −0.00 (−0.13, 0.13) | 0.00 (−0.14, 0.15) | 0.01 (−0.13, 0.14) | 1.38 (0.63, 3.03) |
Values are odds ratios (OR) or changes in Z‐score with 95% confidence interval, derived from logistic and linear regression models, respectively. *P‐value < .05, **P‐value < .01. Models are adjusted for maternal history of asthma and atopy, education level, smoking during pregnancy, stress during pregnancy, parity and pet keeping, and child's gestational age at birth, breastfeeding, day care attendance, and antibiotic use in the past 2 mo (2 mo), 6 mo (6 mo), or year (1‐6 y). For the association of H influenza at the age of 6 wk with current asthma, not effect estimate could be given due to limited power, as indicated by ‐‐‐. Forced Expiratory Volume in 1 s (FEV1). Forced Vital Capacity (FVC), Forced Expiratory Flow after exhaling 75% of FVC (FEF75). Information on lung function and asthma was obtained at age 10 y.
4. DISCUSSION
4.1. Principal findings
Within this population‐based prospective cohort study, studying the effect of airway bacterial carriage with S aureus, H influenzae, M catarrhalis, and S pneumoniae, we observed no consistent directions of associations between airway bacterial carriage and respiratory tract infections during childhood. Nasopharyngeal carriage of any bacteria at the age of 6 months was associated with an increased risk of wheezing in childhood, especially until age 2 years. We did not find any consistent associations of airway bacterial carriage with lung function and asthma in later childhood. This suggests that airway bacterial carriage might have a short‐term effect only.
4.2. Comparison with previous studies
Studies examining longitudinal associations of bacterial carriage and respiratory tract infections on a population‐based level are scarce. A prospective cohort study among 411 children in Denmark studied the associations of hypopharyngeal carriage with H influenzae, M catarrhalis, or S pneumoniae at the age of 4 weeks with respiratory health up to age 5 years. 3 , 6 , 8 This study demonstrated that hypopharyngeal carriage with any of these bacteria was associated with an increased risk of pneumonia or bronchiolitis in the first 3 years of life. 3 We only found few associations between airway bacterial carriage and lower respiratory tract infections. This is most probably due to insufficient power, since the prevalence of lower respiratory tract infections in our study was maximum 11.7%, while in this study, this was 58%. However, we did observe that H influenzae at age 6 weeks was associated with an increased risk of lower respiratory tract infections at age 6 months and that M catarrhalis at the ages of 6 months is associated with an increased risk, and at 14 months with a decreased risk of lower respiratory tract infections at those ages and vice versa. A case‐control study assessing the airway microbiome also showed that children with lower respiratory tract infections had a low abundance of M catarrhalis compared to age‐, sex‐, and time‐matched healthy controls. 20 These findings, combined with ours, suggest that mostly M catarrhalis and lower respiratory tract infections in early life seem to influence each other. Why specifically M catarrhalis seems to have an effect remains open for discussion, given the different findings in other studies. 20 , 21 The Danish study also demonstrated that hypopharyngeal carriage is associated with an increased risk of wheezing especially in the first 3 years of life, and an increased risk of asthma at age 5 years. 6 , 8 In line with these findings, we observed that nasopharyngeal carriage with any bacteria at age 6 months was associated with an increased risk of overall wheezing until age 10 years, mostly with wheezing in the first 2 years. We, however, did not observe an association of airway bacterial carriage with asthma at school age. Prospective cohort studies that used microbiome analyses showed an association of a high abundance of Streptococcus, but not Moraxella with wheeze at age 5 or 10 years. 21 , 22 Differences in findings, especially with regard to the association of hypopharyngeal bacterial carriage with lower respiratory tract infections and asthma between our study and the Danish study, might be explained by the higher prevalence of lower respiratory tract infections in their cohort and assessment of asthma at an earlier age. Additionally, effects might differ between the high‐risk and our general population, although we did not observe interaction of maternal asthma or atopy. Lastly, results may differ when focusing on individual pathogens, as compared to more recent studies that focus on the entire microbiome. To date, findings suggest that bacterial carriage in infancy is associated with short‐term, but not long‐term respiratory health.
4.3. Underlying mechanisms
The hygiene hypothesis postulates that early‐life exposure to certain microorganisms might be protective for the risk of later‐life atopic diseases. 23 , 24 Microorganisms might influence the immune system, and specifically the type 1 and 2 helper T‐cell balance, and regulatory T cells, which could subsequently influence the risk of atopic diseases such as asthma. 25 Additionally, the immune system might also affect the risk of bacterial airway colonization with microorganisms. 26 Airway bacterial carriage in early life has been associated with a low‐grade systemic inflammation at age 6 months, including C‐reactive protein and interleukin‐6, which could also affect the risk of wheezing or asthma. 27 Bacterial carriage might influence the risk of lower respiratory tract infections and vice versa. 21 However, we observed limited evidence for this. Bacterial carriage in infancy seems most prominently associated with adverse respiratory health, which might be explained by the high dynamics of bacterial carriage between 6 weeks and 6 months. Future large‐scale prospective studies are needed to explore whether assessment of the microbiome as opposed to specific bacterial culturing is more informative in the association with respiratory health, and whether airway bacterial carriage and the microbiome only affect short‐term or also long‐term respiratory health.
4.4. Strengths and limitations
The main strength of our study is that it is embedded in a population‐based prospective cohort study with repeated assessment of airway bacterial carriage and respiratory health. Limitations include the low prevalences of a positive culture and lower respiratory tract infections in the first 6 months of life, leading to limited power. Second, only a small group of children had airway swabs taken at every visit. Therefore, we were not able to study the effect of cumulative or persistent positive swabs on respiratory health. Third, questionnaire data were used to obtain information on respiratory tract infections, wheezing, and asthma. We only used information on doctor‐diagnosed respiratory tract infections and validated questionnaires for wheezing and asthma. However, variability in agreement between parents' and clinicians' report on respiratory health cannot be fully excluded, which might have led to non‐differential misclassification and therefore dilution of the effect estimates. 28 Fourth, although we used standard material and commonly used methods, sensitivity of culturing might be affected. This will, however, most likely be operator dependent and lead to random misclassification. 29 Lastly, we should consider the possibility that any found associations are due to chance. Although this might be the case for the associations with lung function and asthma, we believe this to be less likely for the associations with lower respiratory tract infections and wheezing, given the pattern of effects, with mostly low P‐values.
In conclusion, we observed no consistent associations in either direction between airway bacterial carriage and respiratory tract infections during childhood, or direction between airway bacterial carriage with lung function and asthma in later childhood. Only nasopharyngeal carriage of any bacteria at the age of 6 months was associated with an increased risk of wheezing in childhood, especially with wheezing in the first 2 years of life. This implies that airway bacterial carriage seems to have an effect on short‐term effect on respiratory health only. Future studies should focus on the effect of the airway microbiome, which comprises more than individual pathogens, on short‐ and long‐term respiratory health.
CONFLICT OF INTEREST
The authors report no conflict of interest.
AUTHOR CONTRIBUTIONS
Evelien R. van Meel: Conceptualization (equal); formal analysis (lead); investigation (equal); methodology (equal); visualization (lead); writing‐original draft (lead); writing‐review and editing (equal). Vincent W. V. Jaddoe: Conceptualization (equal); funding acquisition (equal); supervision (supporting); writing‐review and editing (equal). Kirsten I. M. Looman: Conceptualization (supporting); writing‐review and editing (supporting). Johan C. de Jongste: Conceptualization (supporting); supervision (supporting); writing‐review and editing (supporting). Henriëtte A. Moll: Conceptualization (equal); funding acquisition (equal); writing‐review and editing (supporting). Liesbeth Duijts: Conceptualization (equal); formal analysis (supporting); funding acquisition (equal); methodology (supporting); supervision (lead); visualization (supporting); writing‐original draft (supporting); writing‐review and editing (lead).
Peer Review
The peer review history for this article is available at https://publons.com/publon/10.1111/pai.13310.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
The Generation R Study is conducted by the Erasmus Medical Centre in close collaboration with the School of Law and the Faculty of Social Sciences at the Erasmus University, Rotterdam, the Municipal Health Service, Rotterdam area and the Stichting Trombosedienst and Artsen laboratorium Rijnmond (Star‐MDC), Rotterdam. We gratefully acknowledge the contribution of children and their parents, general practitioners, hospitals, midwives, and pharmacies in Rotterdam.
van Meel ER, Jaddoe VWV, Looman KIM, de Jongste JC, Moll HA, Duijts L. Airway bacterial carriage and childhood respiratory health: A population‐based prospective cohort study. Pediatr Allergy Immunol. 2020;31:774–782. 10.1111/pai.13310
Funding information
The Generation R Study is made possible by financial support from the Erasmus Medical Centre, Rotterdam, the Erasmus University Rotterdam and the Netherlands Organization for Health Research and Development. Dr Vincent Jaddoe received an additional grant from the European Research Council (ERC‐2014‐CoG‐648916). Dr Liesbeth Duijts received funding from the European Union's Horizon 2020 co‐funded program ERA‐Net on Biomarkers for Nutrition and Health (ERA HDHL) (ALPHABET project (no 696295; 2017), ZonMW The Netherlands (no 529051014; 2017). The researchers are independent from the funders. The study sponsors had no role in the study design, data collection and analysis, interpretation of data, writing of this report, or the decision to submit the article for publication.
Edited by: Ömer Kalaycı
REFERENCES
- 1. Barker DJ, Godfrey KM, Fall C, Osmond C, Winter PD, Shaheen SO. Relation of birth weight and childhood respiratory infection to adult lung function and death from chronic obstructive airways disease. BMJ. 1991;303:671‐675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van Meel ER, den Dekker HT, Elbert NJ, et al. A population‐based prospective cohort study examining the influence of early‐life respiratory tract infections on school‐age lung function and asthma. Thorax. 2018;73:167‐173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vissing NH, Chawes BL, Bisgaard H. Increased risk of pneumonia and bronchiolitis after bacterial colonization of the airways as neonates. Am J Respir Crit Care Med. 2013;188:1246‐1252. [DOI] [PubMed] [Google Scholar]
- 4. Korten I, Mika M, Klenja S, et al. Interactions of respiratory viruses and the nasal microbiota during the first year of life in healthy infants. mSphere. 2016;1(6):e00312‐16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bosch AA, Biesbroek G, Trzcinski K, Sanders EA, Bogaert D. Viral and bacterial interactions in the upper respiratory tract. PLoS Pathog. 2013;9:e1003057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bisgaard H, Hermansen MN, Buchvald F, et al. Childhood asthma after bacterial colonization of the airway in neonates. N Engl J Med. 2007;357:1487‐1495. [DOI] [PubMed] [Google Scholar]
- 7. Davis MF, Peng RD, McCormack MC, Matsui EC. Staphylococcus aureus colonization is associated with wheeze and asthma among US children and young adults. J Allergy Clin Immunol. 2015;135(811–3):e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. von Linstow M‐L, Schønning K, Hoegh AM, Sevelsted A, Vissing NH, Bisgaard H. Neonatal airway colonization is associated with troublesome lung symptoms in infants. Am J Respir Crit Care Med. 2013;188:1041‐1042. [DOI] [PubMed] [Google Scholar]
- 9. Tsai M‐H, Huang S‐H, Chen C‐L, et al. Pathogenic bacterial nasopharyngeal colonization and its impact on respiratory diseases in the first year of life: the PATCH Birth Cohort Study. Pediatr Infect Dis J. 2015;34:652‐658. [DOI] [PubMed] [Google Scholar]
- 10. Kooijman MN, Kruithof CJ, van Duijn CM, et al. The Generation R Study: design and cohort update 2017. Eur J Epidemiol. 2016;31:1243‐1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jaddoe VWV, Mackenbach JP, Moll HA, et al. The Generation R Study: design and cohort profile. Eur J Epidemiol. 2006;21:475‐484. [DOI] [PubMed] [Google Scholar]
- 12. Lebon A, Labout JAM, Verbrugh HA, et al. Dynamics and determinants of Staphylococcus aureus carriage in infancy: the Generation R Study. J Clin Microbiol. 2008;46:3517‐3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Labout JAM, Duijts L, Lebon A, et al. Risk factors for otitis media in children with special emphasis on the role of colonization with bacterial airway pathogens: the Generation R study. Eur J Epidemiol. 2011;26:61‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. den Dekker HT, Jaddoe VWV, Reiss IK, de Jongste JC, Duijts L Fetal and infant growth patterns and risk of lower lung function and asthma. The generation R study. Am J Respir Crit Care Med. 2018;197:183‐192. [DOI] [PubMed] [Google Scholar]
- 15. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319‐338. [DOI] [PubMed] [Google Scholar]
- 16. Quanjer PH, Stanojevic S, Cole TJ, et al. Multi‐ethnic reference values for spirometry for the 3–95‐yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40:1324‐1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Asher MI, Keil U, Anderson HR, et al. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J. 1995;8:483‐491. [DOI] [PubMed] [Google Scholar]
- 18. LR. D . BSI Brief Symptom Inventory, Administration, Scoring, and Procedures Manual. (4th ed.). Minneapolis: MN: National Computer Systems; 1993. [Google Scholar]
- 19. Kenny DA. Correlation and Causality. New York, NY: Wiley; 1979;282‐308. [Google Scholar]
- 20. Man WH, van Houten MA, Mérelle ME, et al. Bacterial and viral respiratory tract microbiota and host characteristics in children with lower respiratory tract infections: a matched case‐control study. Lancet Respir Med. 2019;7:417‐426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Teo S, Mok D, Pham K, et al. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe. 2015;17:704‐715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Powell EA, Fontanella S, Boakes E, et al. Temporal association of the development of oropharyngeal microbiota with early life wheeze in a population‐based birth cohort. EBioMedicine. 2019;46:486‐498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lambrecht BN, Hammad H. The immunology of the allergy epidemic and the hygiene hypothesis. Nat Immunol. 2017;18:1076‐1083. [DOI] [PubMed] [Google Scholar]
- 24. Okada H, Kuhn C, Feillet H, Bach JF. The 'hygiene hypothesis' for autoimmune and allergic diseases: an update. Clin Exp Immunol. 2010;160:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Man WH, de Steenhuijsen Piters WA, Bogaert D. The microbiota of the respiratory tract: gatekeeper to respiratory health. Nat Rev Microbiol. 2017;15:259‐270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ramos‐Sevillano E, Ercoli G, Brown JS. Mechanisms of Naturally Acquired Immunity to Streptococcus pneumoniae . Front Immunol. 2019;10:358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rahman Fink N, Chawes BL, Thorsen J, et al. Neonates colonized with pathogenic bacteria in the airways have a low‐grade systemic inflammation. Allergy. 2018;73:2150‐2159. [DOI] [PubMed] [Google Scholar]
- 28. Cane RS, Ranganathan SC, McKenzie SA. What do parents of wheezy children understand by "wheeze"? Arch Dis Child. 2000;82:327‐332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bogaert D, van Belkum A, Sluijter M, et al. Colonisation by Streptococcus pneumoniae and Staphylococcus aureus in healthy children. Lancet. 2004;363:1871‐1872. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


