Abstract
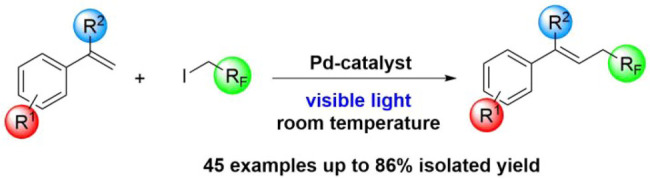
A visible light induced palladium-catalyzed fluoroalkylation method was developed. The Heck-type alkyl coupling reaction enables the introduction of trifluoroethyl, difluoroethyl and other fluoroalkyl fragment into styrenes under mild reaction conditions without the use of additional photosensitizers and ensures access to fluoroalkylated olefins on a broad scale.
The introduction of fluorine-containing functional groups into organic molecules and drug candidates can often improve the enzymatic oxidative and thermal stability as well as increase the lipophilicity and bioavailability of molecules.1 Therefore, in recent years the development of fluoroalkylating methods and reagents, especially trifluoromethylation2 and trifluoroethylation,3 has become an emerging area, including the photocatalytic transformations.4
Besides the fluoroalkylation of aromatic and heteroaromatic rings, the installation of short fluoroalkyl chains into terminal alkene is also important, but is a less explored transformation, which is mostly limited to perfluoroalkylation5 and trifluoroethylation. There are several different strategies to construct the trifluoroethylated styrene structure. It can be synthesized by nucleophilic substitution from the corresponding allyl-bromide derivative,6 oxidative trifluoromethylation,7 classical transition metal coupling with vinyl-boronates,8 or photocatalytic transformation9 using the corresponding fluoroalkyl halides as coupling agents.
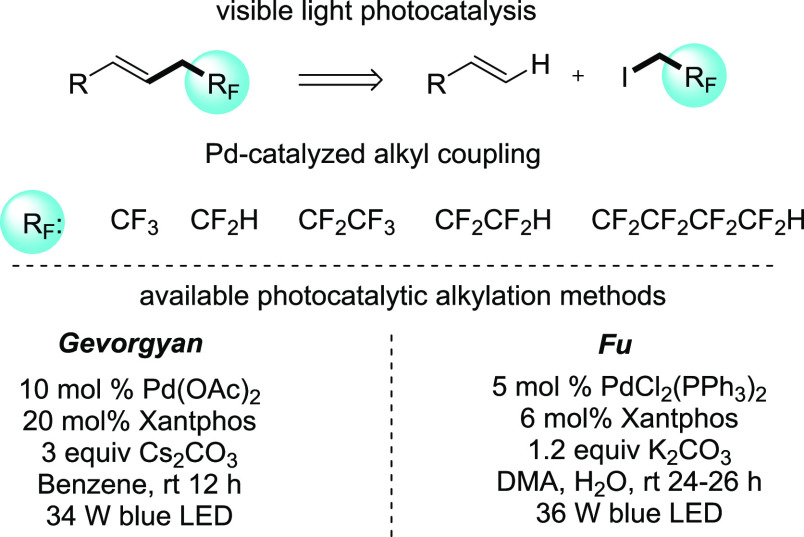
In continuation of our studies in the field of fluoroalkylation reactions3a,3b,10 and photocatalytic transformations,11 we aimed to take advantage of the photo-Heck-type coupling approach to introduce more versatile fluoroalkyl groups into the styrene moiety using palladium catalysis and visible light irradiation (Scheme 1). The classical palladium catalyzed cross-coupling reaction12 involves a well-established two-electron redox mechanism typically between the Pd(0)/Pd(II) oxidation states. However, using the original Heck reaction for the coupling of unactivated alkyl halides with aryl-alkenes is challenging because of two factors:13 the oxidative addition step is relatively slow with low-valent transition metals, and the resulting alkylmetal species can undergo premature β-hydride elimination which leads to side products. These disadvantages can be eliminated by photoexcitation of the applied catalyst.14 The photoexcited Pd-complexes can participate in a single-electron transfer (SET) mechanism to generate a Pd(I)-species, which allows the desired coupling to occur selectively in a photocatalytic manner. Two possible catalytic methods are available to achieve the desired functionalization with fluoroalkyl species (Scheme 1). The Gevorgyan method is based on the Pd(OAc)2/Xantphos catalytic system, and the transformation works efficiently in benzene in the presence of Cs2CO3.14e Similarly, Fu’s procedure uses the PdCl2(PPh3)2/Xantphos catalyst in DMA/H2O solvent in the presence of K2CO3 base.14b Both alkylations require blue light irradiation for appropriate excitation of the photocatalytic system.
Scheme 1. Aim: Direct Fluoroalkylation of Styrene Derivatives.
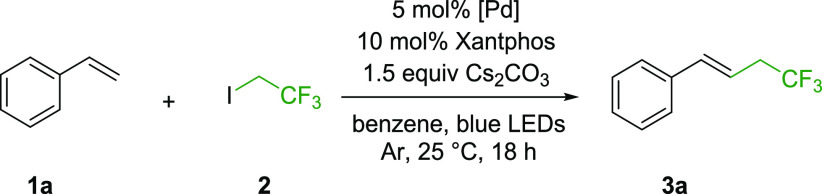
We started our investigation with the optimization of the reaction of styrene (1a) and trifluoroethyl iodide (2) using palladium(II)–Xantphos catalyst systems. The reactions were irradiated by 440–445 nm LEDs for 18 h at 25 °C and analyzed by GC-MS. We found that the Gevorgyan-type Pd(OAc)2–Xantphos photocoupling system14e works efficiently in benzene in the desired trifluoroethylation and provides the expected product 3a in complete conversion. In contrast, Fu’s conditions14b were not suitable for the fluoroalkylation, which shows system sensitivity of the coupling partner and indicates the necessity of fine-tuning of the reaction conditions. After finding the appropriate catalytic system we aimed to fine-tune the reaction conditions to reach more suitable reaction conditions for the fluoroalkylation of styrenes. In these experiments we focused on lowering the catalyst and base loadings, and solvent selection.
We found that the catalyst loading can be lowered in the case of the trifluoroethylation, but the minimum amount of needed Pd(OAc)2 catalyst for the reaction completion was 5 mol %; further reduction of the catalyst loading significantly decreased the conversion (Table 1, entries 2–6). A control experiment without any irradiation indicated that the light is essential for this transformation (Table 1, entry 7). Next, we tested different Pd sources, and we demonstrated that the use of PdCl2 could not result in any product; Pd2(dba)3 and Pd(acac)2 were only moderately active in this reaction, and Pd(TFA)2 performed almost as good as Pd(OAc)2 (Table 1, entries 8–11). Among the tested polar solvents water, acetone and ethyl acetate were relatively ineffective (Table 1, entries 12–14), while in THF the reaction reached 83% conversion. Reactions performed in solvents similar to benzene gave versatile results: chlorobenzene was completely ineffective, while in toluene and benzotrifluoride (BTF) the coupling reaction gave 80% and 90% conversion respectively, which are good results but compared to benzene the transformation did not reach completion. In cyclohexane alongside the desired coupling iodocyclohexane was formed as a byproduct which made this solvent unsuitable (Table 1, entry 19).
Table 1. Fine-tuning of Photocatalytic Conditionsa.
| Entry | Catalyst | Solvent | Conv. |
|---|---|---|---|
| 1 | 10 mol % Pd(OAc)2 | benzene | 100%b |
| 2 | 5 mol %Pd(OAc)2 | benzene | 100% |
| 3 | 4 mol % Pd(OAc)2 | benzene | 99% |
| 4 | 3 mol % Pd(OAc)2 | benzene | 89% |
| 5 | 2 mol % Pd(OAc)2 | benzene | 38% |
| 6 | 1 mol % Pd(OAc)2 | benzene | 12% |
| 7 | 5 mol % Pd(OAc)2 | benzene | 0%c |
| 8 | PdCl2 | benzene | 0% |
| 9 | Pd(acac)2 | benzene | 48% |
| 10 | Pd(TFA)2 | benzene | 83% |
| 11 | Pd2(dba)3 | benzene | 27% |
| 12 | Pd(OAc)2 | water | 7% |
| 13 | Pd(OAc)2 | acetone | 40% |
| 14 | Pd(OAc)2 | ethyl acetate | 46% |
| 15 | Pd(OAc)2 | THF | 83% |
| 16 | Pd(OAc)2 | chlorobenzene | 5% |
| 17 | Pd(OAc)2 | toluene | 80% |
| 18 | Pd(OAc)2 | BTF | 90% |
| 19 | Pd(OAc)2 | cyclohexane | 75% |
Standard reaction conditions: styrene (0.2 mmol), trifluoroethyl-iodide (1.5 equiv), Cs2CO3 (1.5 equiv), catalyst (5 mol %), ligand (10 mol %) in 1 mL degassed benzene under Ar atmosphere, irradiated with single 10 W 440–445 nm LEDs at 25 °C.
3 equiv of Cs2CO3 were used.
In the dark.
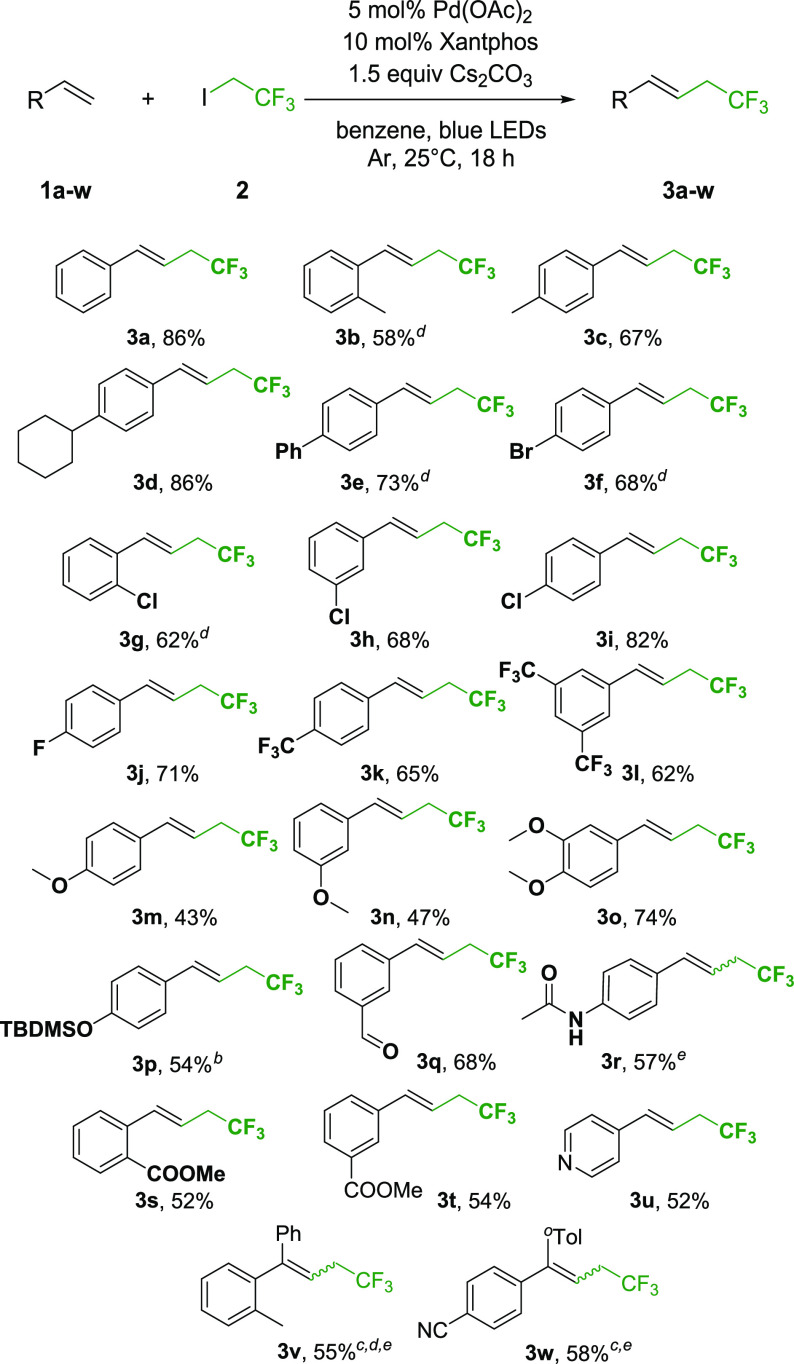
With the optimized conditions in hand first we investigated the generality of the trifluoroethylation reaction (Scheme 2).
Scheme 2. Scope of Trifluorethylation.
Standard reaction conditions: substrate (0.5 mmol), trifluoroethyl iodide (1.5 equiv), Cs2CO3 (1.5 equiv), Pd(OAc)2 (5 mol %), and Xantphos (10 mol %) was used in 2.5 mL degassed benzene under an Ar atmosphere, irradiated with single 10 W 440–445 nm LEDs at 25 °C.
The reaction time was 24 h.
10 mol % catalyst, 20 mol % Xantphos and 3 equiv. base were used.
Average of two reactions.
E/Z ratio 3r 13:1; 3v 3:1; 3w: 33:1.
The transformation proceeded smoothly with styrene derivatives with electron-withdrawing and electron-donating groups as well, regardless of their position on the aromatic ring. We synthesized different alkyl (3b, 3c, 3d) and aryl (3e) derivatives in good yields, and the reaction worked efficiently with halogenated styrenes (3f–j) and methoxy derivatives (3m, 3n, 3o) as well. The silyl protected phenol functional group is also tolerated under the reaction conditions, and product 3p was isolated in 54% yield after a 24 h reaction time. Aldehyde (3q), amide (3r) and ester (3s, 3t) derivatives were also prepared successfully from the corresponding styrene. In this series the pyridine derivative (3u) was also obtained in 52% yield. On the other hand, 1,1-diphenylethylene derivatives proved to be less reactive in this transformation. Therefore, increased catalyst and base loadings were necessary to accomplish the desired coupling. We managed to synthesize the 3v and 3w derivatives in 55% and 58% yields, respectively.
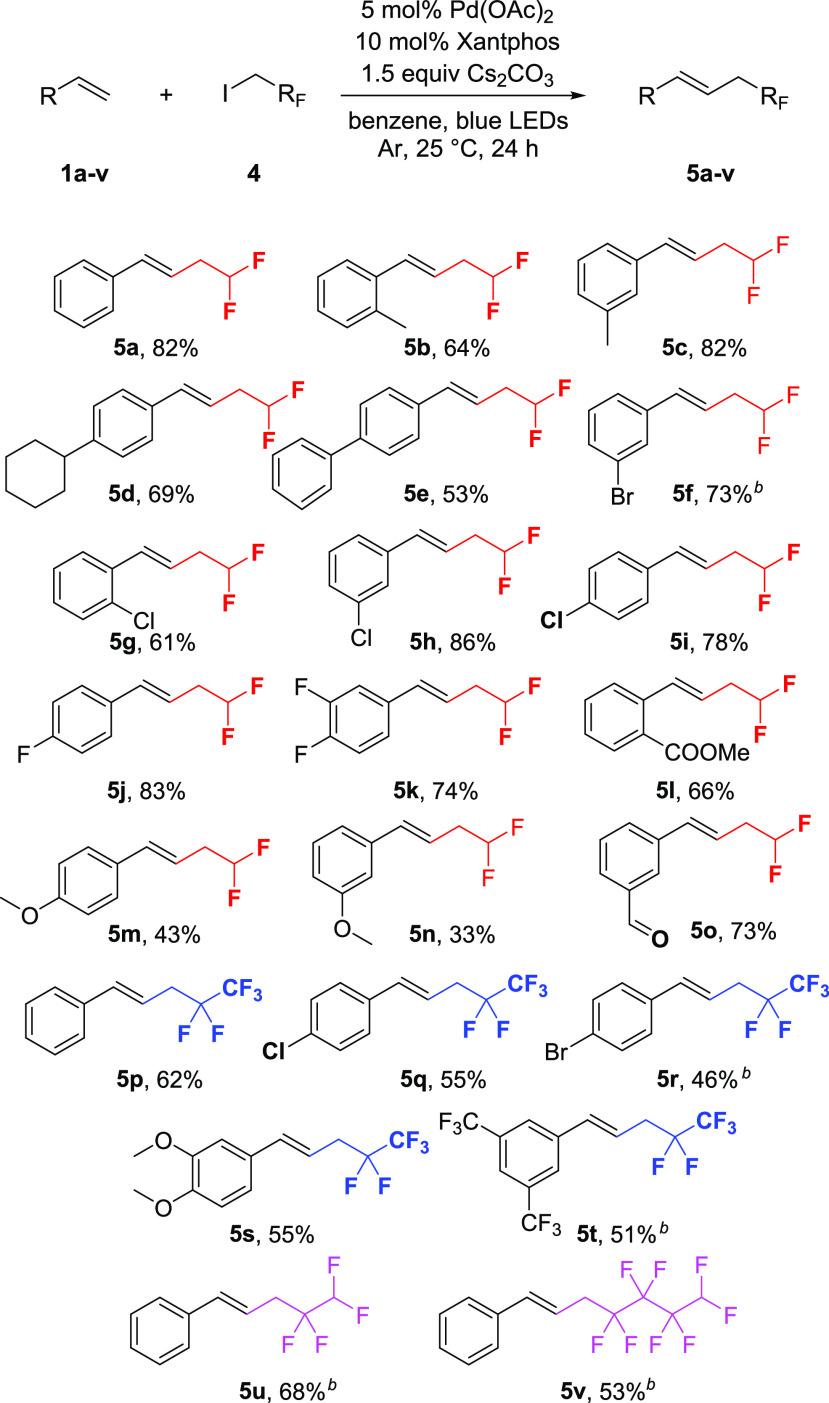
Next, we broadened the scope of the photocatalytic fluoroalkylation method with versatile fluoroalkyl iodides (Scheme 3). We found that 1,1-difluoro-2-iodoethane is also capable of participating in the coupling reaction.
Scheme 3. Scope of Different Fluoroalkyl Iodides.
Standard reaction conditions: substrate (0.5 mmol), fluoroalkyl iodide (1.5 equiv), Cs2CO3 (1.5 equiv), Pd(OAc)2 (5 mol %), Xantphos (10 mol %) was used in 2.5 mL degassed benzene under Ar atmosphere, irradiated with single 10 W 440–445 nm LEDs at 25 °C.
Average of two reactions.
This attracted our interest because the difluoromethyl moiety is a well-studied motif in medicinal chemistry. The difluoromethyl group is isosteric and isopolar with the −OH and −SH groups and can behave as a H-donor as well.15 The preparation of difluoroethylated styrene derivatives is performed only through the functionalization of allylic halides with difluorocarbene sources,16 and direct difluoroethylation of terminal alkene is unprecedented.
Applying the previously used reaction conditions, we found that the coupling of 1,1-difluoro-2-iodoethane with styrene requires a 24 h reaction time to reach completion, and we could isolate the corresponding difluoroethylated styrene product (5a) in 82% yield. With the slightly modified procedure, 12 additional derivatives were prepared to explore the scope of the photocatalyzed coupling reaction. This transformation also possesses good functional group tolerance, and alkyl (5b, 5c, 5d), aryl (5e), halogen (5f–k), ester (5l), methoxy (5m, 5n), and aldehyde (5o) derivatives were successfully synthesized.
Increasing further the versatility of the fluoroalkyl part we utilized 1H,1H-pentafluoropropyl iodide as a coupling partner, and we obtained the corresponding coupling products under the standard reaction conditions. In this case the pentafluoropropylated derivatives (5p–t) were isolated in 46–62% yield. Additionally, two other fluoralkyl iodides were also tested in this transformation, 1,1,2,2-tetrafluoro-3-iodopropane and 1H,1H,5H-octafluoropentyl iodide, and successfully gave the desired coupled products (5u, 5v) in 68% and 53% yield, respectively, which demonstrates that longer fluorous chains are also applicable in this palladium-catalyzed photochemical transformation.
Results of the radical quenching and light on–off experiments17 support that the fluoroalkylation reaction follows the general mechanism of the palladium-catalyzed alkylations.14 The Xantphos–Pd(0) complex can be excited with blue light (440–460 nm), and then this Pd species reacts with fluoroalkyl iodide in the SET reaction, supposedly generating a Xantphos–Pd(I)–I and fluoroalkyl radical pair (the latter was trapped with TEMPO to prove its presence),17 which are in close proximity. Alkene reacts with this palladium(I) intermediate through insertion or radical addition, and then a β-hydrogen radical elimination could form the coupled styrene product and the common H–Pd(II)–I. This latter species undergoes base-assisted reductive elimination to produce Xantphos–Pd(0), ready for the next catalytic cycle.
In conclusion we developed a visible light driven palladium-catalyzed Heck-type coupling between styrenes and fluoroalkyl iodides at room temperature, which enables the introduction of versatile fluoroalkyl chains into terminal alkene functionality. A series of styrene derivatives were subjected to the present reaction conditions and formed the corresponding fluoroalkyl derivatives in good yields. In our synthetic studies five different fluoroalkyl iodides were successfully utilized. This method offers an efficient disconnection to incorporate fluorine-containing functional groups into styrene derivatives, which could serve as a useful derivatization method to obtain fluoroalkenylated compounds.
Acknowledgments
This research was funded by the National Research, Development and Innovation Office (K132077 and KH125230). This work was completed in the ELTE Institutional Excellence Program (1783-3/2018/FEKUTSRAT), Cooperation Excellence Program with Research Centre for Natural Sciences and the ÚNKP-19-3 New National Excellence program supported by the Hungarian Ministry of Human Capacities (A.R.). The authors are thankful for the analytical measurements by László Burai, Soma Szabó, Márton Zwillinger, and Tamás Gáti at Servier Research Institute of Medicinal Chemistry and the donation of some styrene substrates. The authors thank Dr. Tibor Soós and Mr. Máté Berta (Research Centre for Natural Sciences, Budapest, Hungary) for the NMR measurements and Attila Kiss (University of Debrecen) for HRMS measurements.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.0c03043.
General information, synthesis procedures, characterizations of products (1H, 13C, 19F NMR, IR, HRMS), and spectra (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Shimizu M.; Hiyama T. Modern Synthetic Methods for Fluorine-Substituted Target Molecules. Angew. Chem., Int. Ed. 2005, 44, 214–231. 10.1002/anie.200460441. [DOI] [PubMed] [Google Scholar]; b Muller K.; Faeh C.; Diederich F. Fluorine in Pharmaceuticals: Looking Beyond Intuition. Science 2007, 317, 1881–1886. 10.1126/science.1131943. [DOI] [PubMed] [Google Scholar]; c Johnson B. M.; Shu Y.-Z.; Zhuo X.; Meanwell N. A. Metabolic and Pharmaceutical Aspects of Fluorinated Compounds. J. Med. Chem. 2020, 63, 6315–6386. 10.1021/acs.jmedchem.9b01877. [DOI] [PubMed] [Google Scholar]; d Ojima I.Fluorine in Medicinal Chemistry and Chemical Biology; Wiley-Blackwell: 2009. [Google Scholar]; e Wang J.; Sánchez-Roselló M.; Aceña J. L.; del Pozo C.; Sorochinsky A. E.; Fustero S.; Soloshonok V. A.; Liu H. Fluorine in pharmaceutical industry: fluorine-containing drugs introduced to the market in the last decade (2001–2011). Chem. Rev. 2014, 114, 2432–2506. 10.1021/cr4002879. [DOI] [PubMed] [Google Scholar]; f Zhou Y.; Wang J.; Gu Z.; Wang S.; Zhu W.; Aceña J. L.; Soloshonok V. A.; Izawa K.; Liu H. Next Generation of Fluorine-Containing Pharmaceuticals, Compounds Currently in Phase II–III Clinical Trials of Major Pharmaceutical Companies: New Structural Trends and Therapeutic Areas. Chem. Rev. 2016, 116, 422–518. 10.1021/acs.chemrev.5b00392. [DOI] [PubMed] [Google Scholar]
- a Merino E.; Nevado C. Addition of CF3 across Unsaturated Moieties: A Powerful Functionalization Tool. Chem. Soc. Rev. 2014, 43, 6598–6608. 10.1039/C4CS00025K. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Li G.; Zhang C.; Song C.; Ma Y. Progress in Copper-Catalyzed Trifluoromethylation. Beilstein J. Org. Chem. 2018, 14, 155–181. 10.3762/bjoc.14.11. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Tomashenko O. A.; Grushin V. V. Aromatic trifluoromethylation with metal complexes. Chem. Rev. 2011, 111, 4475–4521. 10.1021/cr1004293. [DOI] [PubMed] [Google Scholar]; d Studer A. A “renaissance” in radical trifluoromethylation. Angew. Chem., Int. Ed. 2012, 51, 8950–8958. 10.1002/anie.201202624. [DOI] [PubMed] [Google Scholar]; e Besset T.; Schneider C.; Cahard D. Tamed arene and heteroarene trifluoromethylation. Angew. Chem., Int. Ed. 2012, 51, 5048–5050. 10.1002/anie.201201012. [DOI] [PubMed] [Google Scholar]; f Bizet V.; Besset T.; Ma J.-A.; Cahard D. Recent progress in asymmetric fluorination and trifluoromethylation reactions. Curr. Top. Med. Chem. 2014, 14, 901–940. 10.2174/1568026614666140202205531. [DOI] [PubMed] [Google Scholar]; g Liu X.; Xu C.; Wang M.; Liu Q. Trifluoromethyltrimethylsilane: nucleophilic trifluoromethylation and beyond. Chem. Rev. 2015, 115, 683–730. 10.1021/cr400473a. [DOI] [PubMed] [Google Scholar]; h Alonso C.; de Marigorta E. M.; Rubiales G.; Palacios F. Carbon Trifluoromethylation Reactions of Hydrocarbon Derivatives and Heteroarenes. Chem. Rev. 2015, 115, 1847–1935. 10.1021/cr500368h. [DOI] [PubMed] [Google Scholar]; i Egami H.; Sodeoka M. Trifluoromethylation of alkenes with concomitant introduction of additional functional groups. Angew. Chem., Int. Ed. 2014, 53, 8294–8308. 10.1002/anie.201309260. [DOI] [PubMed] [Google Scholar]; j Besset T.; Poisson T.; Pannecoucke X. Direct vicinal difunctionalization of alkynes: an efficient approach towards the synthesis of highly functionalized fluorinated alkenes. Eur. J. Org. Chem. 2015, 2015, 2765–2789. 10.1002/ejoc.201403507. [DOI] [Google Scholar]
- a Tóth B. L.; Kovács S.; Sályi G.; Novák Z. Mild and Efficient Palladium-Catalyzed Direct Trifluoroethylation of Aromatic Systems by C-H Activation. Angew. Chem., Int. Ed. 2016, 55, 1988–1992. 10.1002/anie.201510555. [DOI] [PubMed] [Google Scholar]; b Tolnai G. L.; Székely A.; Makó Z.; Gáti T.; Daru J.; Bihari T.; Stirling A.; Novák Z. Efficient Direct 2,2,2-Trifluoroethylation of Indoles via C-H Functionalization. Chem. Commun. 2015, 51, 4488–4491. 10.1039/C5CC00519A. [DOI] [PubMed] [Google Scholar]; c Maraswami M.; Pankajakshan S.; Chen G.; Loh T.-P. Palladium-Catalyzed Direct C–H Trifluoroethylation of Aromatic Amides. Org. Lett. 2017, 19, 4223–4226. 10.1021/acs.orglett.7b01859. [DOI] [PubMed] [Google Scholar]
- a Barata-Vallejo S.; Bonesi S. M.; Postigo A. Photocatalytic fluoroalkylation reactions of organic compounds. Org. Biomol. Chem. 2015, 13, 11153–11183. 10.1039/C5OB01486G. [DOI] [PubMed] [Google Scholar]; b Koike T.; Akita M. Fine Design of Photoredox Systems for Catalytic Fluoromethylation of Carbon-Carbon Multiple Bonds. Acc. Chem. Res. 2016, 49, 1937–1945. 10.1021/acs.accounts.6b00268. [DOI] [PubMed] [Google Scholar]
- Feng Z.; Min Q.-Q.; Zhao H.-Y.; Gu J.-W.; Zhang X. A General Synthesis of Fluoroalkylated Alkenes by Palladium-Catalyzed Heck-Type Reaction of Fluoroalkyl Bromides. Angew. Chem., Int. Ed. 2015, 54, 1270–1274. 10.1002/anie.201409617. [DOI] [PubMed] [Google Scholar]
- Miyake Y.; Ota S.; Nishibayashi Y. Copper-Catalyzed Nucleophilic Trifluoromethylation of Allylic Halides: A Simple Approach to Allylic Trifluoromethylation. Chem. - Eur. J. 2012, 18, 13255–13258. 10.1002/chem.201202853. [DOI] [PubMed] [Google Scholar]
- a Xu J.; Fu Y.; Luo D.-F.; Jiang Y.-Y.; Xiao B.; Liu Z.-J.; Gong T.-J.; Liu L. Copper-Catalyzed Trifluoromethylation of Terminal Alkenes through Allylic C-H Bond Activation. J. Am. Chem. Soc. 2011, 133, 15300–15303. 10.1021/ja206330m. [DOI] [PubMed] [Google Scholar]; b Parsons A. T.; Buchwald S. L. Copper-Catalyzed Trifluoromethylation of Unactivated Olefins. Angew. Chem., Int. Ed. 2011, 50, 9120–9123. 10.1002/anie.201104053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Zhao Y.; Hu J. Palladium-Catalyzed 2,2,2-Trifluoroethylation of Organoboronic Acids and Esters. Angew. Chem., Int. Ed. 2012, 51, 1033–1036. 10.1002/anie.201106742. [DOI] [PubMed] [Google Scholar]; b Zhang Y.; Du H.; Zhu M.; Li J.; Zou D.; Wu Y.; Wu Y. Copper-Catalyzed Decarboxylative Trifluoroethylation of Cinnamic Acids. Tetrahedron Lett. 2017, 58, 880–883. 10.1016/j.tetlet.2017.01.060. [DOI] [Google Scholar]
- a Kreis L. M.; Krautwald S.; Pfeiffer N.; Martin R. E.; Carreira E. M. Photocatalytic Synthesis of Allylic Trifluoromethyl Substituted Styrene Derivatives in Batch and Flow. Org. Lett. 2013, 15, 1634–1637. 10.1021/ol400410m. [DOI] [PubMed] [Google Scholar]; b Li L.; Huang M.; Liu C.; Xiao J.-C.; Chen Q.-Y.; Guo Y.; Zhao Z.-G. 2,2,2-Trifluoroethylation of Styrenes with Concomitant Introduction of a Hydroxyl Group from Molecular Oxygen by Photoredox Catalysis Activated by Visible Light. Org. Lett. 2015, 17, 4714–4717. 10.1021/acs.orglett.5b02177. [DOI] [PubMed] [Google Scholar]; c Roh G.; Iqbal N.; Cho E. J. Trifluoroethylation of Alkynes: Synthesis of Allylic-CF3 Compounds by Visible-Light Photocatalysis. Chin. J. Chem. 2016, 34, 459–464. 10.1002/cjoc.201500919. [DOI] [Google Scholar]; d Straathof N. J. W.; Cramer S. E.; Hessel V.; Noël T. Practical Photocatalytic Trifluoromethylation and Hydrotrifluoromethylation of Styrenes in Batch and Flow. Angew. Chem., Int. Ed. 2016, 55, 15549–15553. 10.1002/anie.201608297. [DOI] [PubMed] [Google Scholar]
- Mészáros Á.; Székely A.; Stirling A.; Novák Z. Design of Trifluoroalkenyl Iodonium Salts for a Hypervalency-Aided Alkenylation–Cyclization Strategy: Metal-Free Construction of Aziridine Rings. Angew. Chem., Int. Ed. 2018, 57, 6643–6647. 10.1002/anie.201802347. [DOI] [PubMed] [Google Scholar]
- a Varga B.; Gonda Zs.; Tóth B.; Kotschy A.; Novák Z. Development of Ni-Ir dual photocatalytic Liebeskind coupling of sulfonium salts for the synthesis of 2-benzylpyrrolidines. Eur. J. Org. Chem. 2020, 2020, 1466–1471. 10.1002/ejoc.201900957. [DOI] [Google Scholar]; b Földesi T.; Adamik R.; Sipos G.; Nagy B.; Tóth B. L.; Bényei A.; Szekeseres K.; Láng Gy.; Demeter A.; Peelen T. J.; Novák Z. Design and application of diimine-based copper(I) complexes in photoredox catalysis. Org. Biomol. Chem. 2019, 17, 8343–8347. 10.1039/C9OB01331H. [DOI] [PubMed] [Google Scholar]
- a Handbook of Organopalladium Chemistry for Organic Synthesis; Negishi E., de Meijere A., Eds.; John Wiley & Sons: New York, NY, 2002. [Google Scholar]; (b)Metal-Catalyzed Cross-Coupling Reactions; de Meijere A., Diederich F., Eds.; Wiley-VCH: Weinheim, 2004. [Google Scholar]
- a Bloome K. S.; McMahen R. L.; Alexanian E. J. Palladium-Catalyzed Heck-Type Reactions of Alkyl Iodides. J. Am. Chem. Soc. 2011, 133, 20146–20148. 10.1021/ja2091883. [DOI] [PubMed] [Google Scholar]; b McMahon C. M.; Alexanian E. J. Palladium-Catalyzed Heck-Type Cross-Couplings of Unactivated Alkyl Iodides. Angew. Chem., Int. Ed. 2014, 53, 5974–5977. 10.1002/anie.201311323. [DOI] [PubMed] [Google Scholar]
- a Kurandina D.; Parasram M.; Gevorgyan V. Visible Light-Induced Room-Temperature Heck Reaction of Functionalized Alkyl Halides with Vinyl Arenes/Heteroarenes. Angew. Chem., Int. Ed. 2017, 56, 14212–14216. 10.1002/anie.201706554. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Wang G.-Z.; Shang R.; Cheng W.-M.; Fu Y. Irradiation-Induced Heck Reaction of Unactivated Alkyl Halides at Room Temperature. J. Am. Chem. Soc. 2017, 139, 18307–18312. 10.1021/jacs.7b10009. [DOI] [PubMed] [Google Scholar]; c Wang G.-Z.; Shang R.; Fu Y. Irradiation-Induced Palladium-Catalyzed Decarboxylative Heck Reaction of Aliphatic N-(Acyloxy)phthalimides at Room Temperature. Org. Lett. 2018, 20, 888–891. 10.1021/acs.orglett.8b00023. [DOI] [PubMed] [Google Scholar]; d Koy M.; Sandfort F.; Tlahuext-Aca A.; Quach L.; Daniliuc C. G.; Glorius F. Palladium-Catalyzed Decarboxylative Heck-Type Coupling of Activated Aliphatic Carboxylic Acids Enabled by Visible Light. Chem. - Eur. J. 2018, 24, 4552–4555. 10.1002/chem.201800813. [DOI] [PubMed] [Google Scholar]; e Kurandina D.; Rivas M.; Radzhabov M.; Gevorgyan V. Heck Reaction of Electronically Diverse Tertiary Alkyl Halides. Org. Lett. 2018, 20, 357–360. 10.1021/acs.orglett.7b03591. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Zhao B.; Shang R.; Wang G.-Z.; Wang S.; Chen H.; Fu Y. Palladium-Catalyzed Dual Ligand-Enabled Alkylation of Silyl Enol Ether and Enamide under Irradiation: Scope, Mechanism, and Theoretical Elucidation of Hybrid Alkyl Pd(I)-Radical Species. ACS Catal. 2020, 10, 1334–1343. 10.1021/acscatal.9b04699. [DOI] [Google Scholar]; g Chuentragool P.; Kurandina D.; Gevorgyan V. Catalysis with Palladium Complexes Photoexcited by Visible Light. Angew. Chem., Int. Ed. 2019, 58, 11586–11598. 10.1002/anie.201813523. [DOI] [PMC free article] [PubMed] [Google Scholar]; h Xing W.-L.; Shang R.; Wang G.-Z.; Fu Y. Visible Light-Induced Palladium-Catalyzed Ring Opening β-H Elimination and Addition of Cyclobutanone Oxime Esters. Chem. Commun. 2019, 55, 14291–14294. 10.1039/C9CC08077E. [DOI] [PubMed] [Google Scholar]; i Feng L.; Guo L.; Yang C.; Zhou J.; Xia W. Visible-Light-Induced Palladium-Catalyzed Intermolecular Narasaka-Heck Reaction at Room Temperature. Org. Lett. 2020, 22, 3964–3968. 10.1021/acs.orglett.0c01267. [DOI] [PubMed] [Google Scholar]
- a Erickson J. A.; McLoughlin J. I. Hydrogen Bond Donor Properties of the Difluoromethyl Group. J. Org. Chem. 1995, 60, 1626–1631. 10.1021/jo00111a021. [DOI] [Google Scholar]; b Narjes F.; Koehler K. F.; Koch U.; Gerlach B.; Colarusso S.; Steinkühler C.; Brunetti M.; Altamura S.; Francesco R. D.; Matassa V. G. A Designed P1 Cysteine Mimetic for Covalent and Non-Covalent Inhibitors of HCV NS3 Protease. Bioorg. Med. Chem. Lett. 2002, 12, 701–704. 10.1016/S0960-894X(01)00842-3. [DOI] [PubMed] [Google Scholar]; c Prakash G. K. S.; Mandal M.; Schweizer S.; Petasis N. A.; Olah G. A. Stereoselective Synthesis anti-α-(Difluoromethyl)-β-Amino Alcohols by Boronic Acid Based Three-Component Condensation. Stereoselective Preparation of (2S,3R)-Difluorothreonine. J. Org. Chem. 2002, 67, 3718–3723. 10.1021/jo011116w. [DOI] [PubMed] [Google Scholar]; d Chowdhury M. A.; Abdellatif K. R. A.; Dong Y.; Das D.; Suresh M. R.; Knaus E. E. Synthesis of Celecoxib Analogues Possessing a N-Difluoromethyl-1,2-Dihydropyrid-2-One 5-Lipoxygenase Pharmacophore: Biological Evaluation as Dual Inhibitors of Cyclooxygenases and 5-Lipoxygenase with Anti-Inflammatory Activity. J. Med. Chem. 2009, 52, 1525–1529. 10.1021/jm8015188. [DOI] [PubMed] [Google Scholar]
- a Burton D. J.; Hartgraves G. A. The Preparation of HCF2CdX and HCF2ZnX via Direct Insertion into the Carbon Halogen Bond of CF2HY (Y = Br, I). J. Fluorine Chem. 2007, 128, 1198–1215. 10.1016/j.jfluchem.2007.05.015. [DOI] [Google Scholar]; b Aikawa K.; Ishii K.; Endo Y.; Mikami K. Copper-Catalyzed Allylic Difluoromethylation of Allyl Carbonates with (Difluoromethyl)zinc Reagent. J. Fluorine Chem. 2017, 203, 122–129. 10.1016/j.jfluchem.2017.07.018. [DOI] [Google Scholar]; c Gu Y.; Lu C.; Gu Y.; Shen Q. Ligand-Controlled Copper-Catalyzed Highly Regioselective Difluoromethylation of Allylic Chlorides/Bromides and Propargyl Bromides. Chin. J. Chem. 2018, 36, 55–58. 10.1002/cjoc.201700594. [DOI] [Google Scholar]
- For details, see Supporting Information.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.