Abstract
Background
Outbreak of COVID-19 has been recognized as a global health concern since it causes high rates of morbidity and mortality. No specific antiviral drugs are available for the treatment of COVID-19 till date. Drug repurposing strategy helps to find out the drugs for COVID-19 treatment from existing FDA approved antiviral drugs. In this study, FDA approved small molecule antiviral drugs were repurposed against the major viral proteins of SARS-CoV-2.
Methods
The 3D structures of FDA approved small molecule antiviral drugs were retrieved from PubChem. Virtual screening was performed to find out the lead antiviral drug molecules against main protease (Mpro) and RNA-dependent RNA polymerase (RdRp) using COVID-19 Docking Server. Furthermore, lead molecules were individually docked against protein targets using AutoDock 4.0.1 software and their drug-likeness and ADMET properties were evaluated.
Results
Out of 65 FDA approved small molecule antiviral drugs screened, Raltegravir showed highest interaction energy value of -9 kcal/mol against Mpro of SARS-CoV-2 and Indinavir, Tipranavir, and Pibrentasvir exhibited a binding energy value of ≥−8 kcal/mol. Similarly Indinavir showed the highest binding energy of -11.5 kcal/mol against the target protein RdRp and Dolutegravir, Elbasvir, Tipranavir, Taltegravir, Grazoprevir, Daclatasvir, Glecaprevir, Ledipasvir, Pibrentasvir and Velpatasvir showed a binding energy value in range from -8 to -11.2 kcal/mol. The antiviral drugs Raltegravir, Indinavir, Tipranavir, Dolutegravir, and Etravirine also exhibited good bioavailability and drug-likeness properties.
Conclusion
This study suggests that the screened small molecule antiviral drugs Raltegravir, Indinavir, Tipranavir, Dolutegravir, and Etravirine could serve as potential drugs for the treatment of COVID-19 with further validation studies.
Keywords: COVID-19, SARS-CoV-2, Main protease, RNA-dependent RNA polymerase, Antiviral drugs, Docking
Introduction
In December 2019, an unknown virus caused severe pneumonia in Wuhan (Hubei province), China, and it spread rapidly throughout the globe [1]. The unknown etiology was later identified as a new virus and tentatively named as 2019 novel coronavirus (2019-nCoV) [2]. It has a positive-sense single stranded RNA as a genetic material with a genome size of 27−32 kb [3,4]. Furthermore, 2019-nCoV shared 87.5% sequence similarity with two bats derived SARS like CoVs strains (bat-SL-CoVZC45 and bat-SL-CoVZXC21) and SARS-CoV-1. Hence, it was named as SARS-CoV-2 and the disease caused by the virus was called as coronavirus disease (COVID-19) [5,6]. The world Health Organization has declared COVID-19 as a pandemic disease on 12th March 2020 [7]. As on 17th May 2020, a total of 4,525,497 cases were confirmed for COVID-19 infection and 307,395 deaths were occurred worldwide [8]. Human coronaviruses commonly cause mild to severe infections in humans. However, SARS-CoV-2 is a public concern because its disease mechanism and function aspects are still unknown [9]. Disease symptoms of COVID-19 ranged from mild, self-limiting respiratory tract illness to severe progressive pneumonia, multi-organ failure, and sometimes leading to death. SARS-CoV-2 infected patients were clinically managed by symptomatic and supportive therapies [[10], [11], [12]].
Since there is no disease specific vaccine or drug available against COVID-19, there is an immediate need to identify anti-COVID-19 agents to control the outbreak and spread of viral infection [13]. Numerous clinical trials and research efforts are being made by researchers worldwide for identifying potential therapies against COVID-19. However, the current drug discovery program requires high cost and time [14]. Computer-aided drug discovery is the most popular platform for the prediction of potential molecules before synthesis and in-vitro testing [15]. Drug repurposing is the most effective strategy to find out the novel clinical use of already approved drugs to combat COVID-19 in a short period of time. This strategy could reduce the time and cost for a new drug development as the major benefits are that the toxicity and pharmacokinetics of the repurposed drugs already available [16]. Virtual drugs screening even eases the repurposing of old drugs by molecular docking analysis against viral protein targets [17]. The crystal structure of 3-chymotrypsin-like protease (3CLpro), also known as the main protease (Mpro) of SARS-CoV-2 that has been reported as one of the best proven drug discovery target [18]. RNA-dependent RNA polymerase (RdRp) is an important enzyme involves in the life cycle of RNA viruses, and RdRp has been targeted for various viral infections includes hepatitis C virus, Zika virus, and HCoVs [19,20]. In this study, FDA approved small molecule antiviral drugs were screened against protein targets of SARS-CoV-2 using a computational based approach.
Materials and methods
Ligand preparation
In this study, 3D structures of FDA approved small molecule antiviral drugs were retrieved from PubChem and unavailable 3D structures for antiviral drugs were developed using the chemical tool box Open Babel [21]. All the 3d structures were energy minimized using UFF minimization algorithm. All the minimized structures were converted into PDBQT format before perform docking using Autodock 4.0.1.
Receptor preparation
The 3D structures of Mpro (6LU7) and RdRp (6M71) were downloaded from the Protein Data Bank (PDB). Water molecules, ions, and other ligands present in the protein targets were removed using PyMOL software. The downloaded structures of protein targets were converted into PDBQT format for molecular docking analysis.
Virtual screening
Preliminary virtual screening was performed for screening of all antiviral agents against the two major proteins (Mpro and RdRp) using the virtual screening module of COVID-19 Docking Server. COVID-19 Docking Server was constructed to predict the binding modes between the targets and small molecules, peptides, or antibodies by the implement of Autodock Vina and CoDockPP as docking engines [22].
Molecular docking
The lead drugs screened from virtual screening were individually docked against protein targets using AutoDock 4.0.1 [23]. AutoDock Tools utilized for the preparation of input pdbqt files of Mpro and RdRp. The grid was placed at the center. Kollman charges and polar hydrogen atoms were essential components. Since, two target proteins are used they form a different centre with different x,y, and z coordinates of -25, 12 and 59 for 6LU7 and 120,123, 120 for 6M71, respectively. The docked receptor and ligand interactions were visualized using Pymol.
Lipinski and ADMET analysis
Finally screened antiviral drugs were further checked for their pharmacokinetics, drug-likeness, and medicinal chemistry properties using the SwissADME server. The SMILES format of the ligand molecules were used for input in the tool [24].
Results
Virtual screening of FDA approved drugs against Mpro (6LU7) and RdRp (6M71) of SARS-CoV-2
In this study, a total of 65 FDA approved small molecule antiviral drugs were virtually screened against the Mpro and RdRp protein targets. The highest interaction energy among the 10 poses predicted by the COVID-19 docking server for each drug against the protein target was selected. Antiviral drugs showing a cut off value above -8 kcal/mol were selected for molecular docking. Out of 65 antiviral drugs, 12 were (Dolutegravir, Etravirine, Indinavir, Maraviroc, Raltegravir, Tipranavir, Daclatasvir, Elbasvir, Glecaprevir, Ledipasvir, Pibrentasvir and Velpatasvir) were screened against Mpro based on the binding energy value above the cut off value. Similarly, 16 antiviral drugs (Dolutegravir, Indinavir, Raltegravir, Tipranavir, Daclatasvir, Dihydrochloride, Elbasvir, Glecaprevir, Grazoprevir, Ledipasvir, Lopinavir, Ombitasvir, Paritaprevir, Pibrentasvir, Telaprevir, Velpatasvir and Voxilaprevir) were screened against RdRp target.
Binding profile of lead antiviral drugs with Mpro (6LU7)
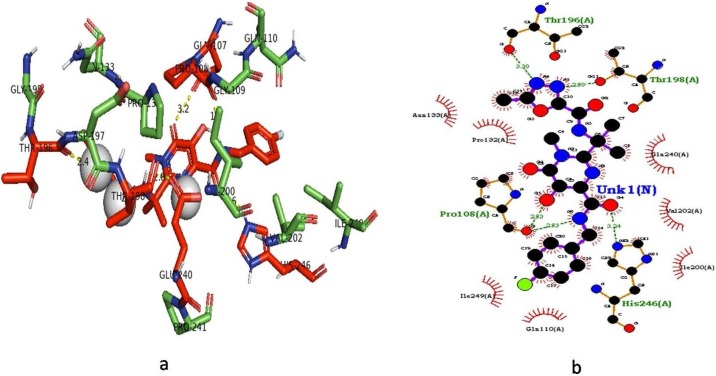
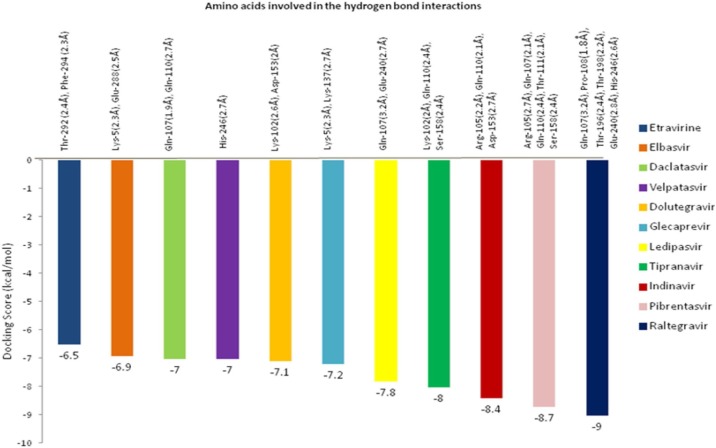
Out of 12 drugs screened against Mpro of SARS-CoV-2, Raltegravir showed the highest binding energy value of -9 kcal/mol (Fig. 1 ). Six HB interactions were observed with amino acid positions Gln-107, Pro-108, Glu-240, Thr-198, Thr-196 and His-246 and there bond length ranged from 1.8 to 3.2 Å. Followed by Raltegravir, the antiviral drug Pibrentasvir showed the binding energy value of -8.7 kcal/mol with HB interactions with amino acids Gln-107, Arg-105, Ser-158, Gln-110, and Thr-111 and bond length was ranged from 2.1 to 2.7 Å. Indinavir drug showed the binding energy value of -8.4 kcal/mol and three amino acids Asp-153, Arg-105 and Gln-110 contributed for HB interactions. Tipranavir showed the binding energy value of -8 kcal/mol and three amino acids Ser-158 (2.4 Å), Lys-102 (2 Å) and Gln-110 (2.4 Å) were involved in the HB interactions followed by Ledipasvir with a binding energy value of -7.8 kcal/mol. Docking score and amino acids involved in the HB interactions and other details of drugs against Mpro were given in Fig. 2 .
Fig. 1.
Docking of Raltegravir with main protease target of SARS-CoV-2.
Fig. 2.
Interaction energy value and hydrogen bond contributing amino acids for main protease target of SARS-CoV-2.
Binding profile of leading antiviral drugs with RdRp (6M71)
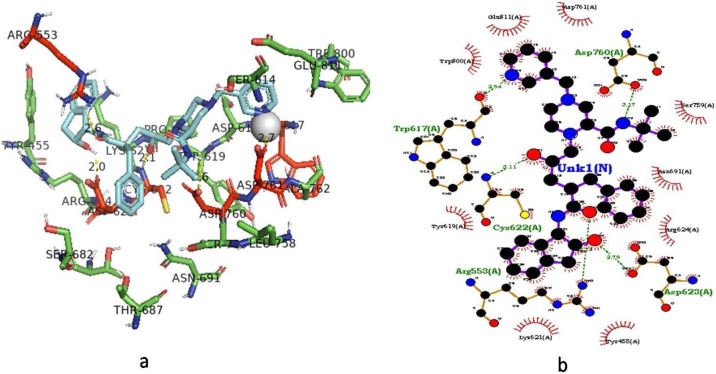
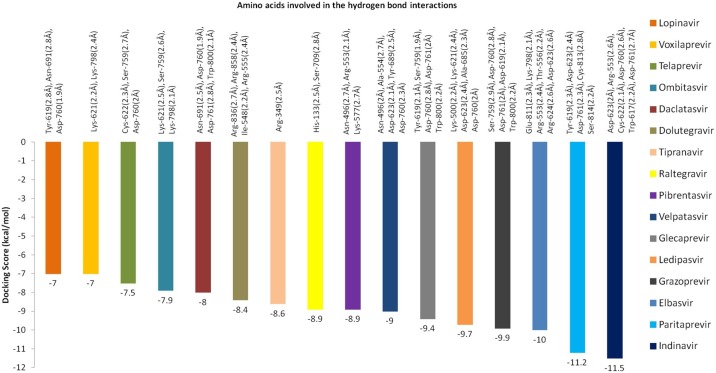
The antiviral drug Indinavir exhibited the highest binding energy of −11.5 kcal/mol against RdRp protein (Fig. 3 ). While analyzing the interaction profile, it was found that six amino acids such as Asp-623 (2 Å), Arg-553 (2.6 Å), Cys-622 (2.1 Å), Asp-760 (2.7 Å), Trp-617 (2.2 Å) and Asp-761 (2.7 Å) were involved in the formation of HB interactions. Paritaprevir drug showed an interaction energy value of -11.2 kcal/mol and five amino acids include Asp-623, Tyr-619, Asp-761, Ser-814 and Cys-813 were contributed for HB interactions and bond length was ranged from 2.2 to 2.8 Å. Grazoprevir drug showed a binding energy value of -9.9 kcal/mol and five amino acids manily Ser-759 (1.9 Å), Asp-760 (2.8 Å), Asp-761 (2 Å), Asp-619 (2.1 Å) and Trp-800 (2.2 Å) contributed for HB interactions. Ledipasvir showed the binding energy value of -9.7 kcal/mol and found HB interactions with amino acids Lys-621 (2.4 Å), Asp-623 (2.4 Å), Asp-760 (2 Å), Lys-500 (2.2 Å) and Ala-685 (3 Å). Velpatasvir drug exhibited the binding energy value of −9 kcal/mol and five amino acids Asn-496 (2.2 Å), Tyr-689 (2.5 Å), Asp-760 (2.3 Å), Asp-623 (2.1 Å) and Ala-554 (2.7 Å) contributed in the HB interactions. The docking score and interaction profile and other features of RdRp were displayed in Fig. 4 .
Fig. 3.
Docking of Indinavir with RNA-dependent RNA polymerase target of SARS-CoV-2.
Fig. 4.
Interaction energy value and hydrogen bond contributing amino acids for RdRp target of SARS-CoV-2.
Physiochemical, Pharmacokinetic, and drug likeness properties of lead antiviral drugs
Screened drugs in this study possessed molecular weight ranged from 444 to 1113 kDa. While checking the pharmacokinetic profile, it was noted that all the screened drugs showed poor gastrointestinal adsorption except Indinavir but almost all the drugs are a substrate of P-glycoprotein (Table 1 ). While analyzing drug likeness properties, it was noted that all the drugs have few violations in these rules, but still it seems to fit into the FDA approval drugs. The bioavailability value seems to be 0.55 for Indinavir, Raltegravir, Tipranavir, Dolutegravir and Etravirine while other drugs had shown bioavailability value of 0.17.
Table 1.
Physiological, pharmacokinetics and drug-likeness properties of screened small molecule antiviral drugs.
| Antiviral drugs | Physiological properties |
Pharmacokinetics properties |
Drug-likeness filters |
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Formula | MW | Heavy atoms | Aromatic heavy atoms | Rotatable bonds | H-bond acceptors | H-bond donors | MR | TPSA | GI absorption | BBB penetration | Pgp substrate | CYP1A2 inhibitor | CYP2C19 inhibitor | CYP2C9 inhibitor | CYP2D6 inhibitor | CYP3A4 inhibitor | log Kp (cm/s) | Lipinski #violations | Ghose #violations | Veber #violations | Egan #violations | Muegge #violations | Bioavailability Score | |
| Dolutegravir | C20H19F2N3O5 | 419.38 | 30 | 12 | 4 | 7 | 2 | 104.48 | 100.87 | High | No | Yes | No | No | No | No | No | −7.13 | 0 | 0 | 0 | 0 | 0 | 0.55 |
| Indinavir | C36H47N5O4 | 613.79 | 45 | 18 | 14 | 7 | 4 | 182.62 | 118.03 | High | No | Yes | No | No | No | No | No | −7.97 | 1 | 3 | 1 | 0 | 1 | 0.55 |
| Raltegravir | C20H21FN6O5 | 444.42 | 32 | 17 | 8 | 9 | 3 | 109.03 | 152.24 | Low | No | Yes | No | No | No | No | Yes | −8.23 | 1 | 0 | 1 | 1 | 1 | 0.55 |
| Tipranavir | C31H33F3N2O5S | 602.66 | 42 | 18 | 12 | 9 | 2 | 153.8 | 113.97 | Low | No | Yes | No | Yes | No | Yes | Yes | −5.03 | 1 | 4 | 1 | 1 | 2 | 0.56 |
| Daclatasvir | C40H52Cl2N8O6 | 811.8 | 56 | 22 | 17 | 8 | 4 | 225.79 | 174.64 | Low | No | Yes | No | No | No | No | Yes | −6.5 | 2 | 4 | 2 | 2 | 4 | 0.17 |
| Elbasvir | C49H55N9O7 | 882.02 | 65 | 31 | 17 | 9 | 4 | 252.67 | 188.8 | Low | No | Yes | No | Yes | No | No | Yes | −6.94 | 2 | 4 | 2 | 2 | 5 | 0.17 |
| Glecaprevir | C38H46F4N6O9S | 838.87 | 58 | 10 | 9 | 15 | 3 | 205.91 | 203.6 | Low | No | Yes | No | No | No | No | No | −8.19 | 2 | 4 | 1 | 1 | 3 | 0.17 |
| Grazoprevir | C38H50N6O9S | 766.9 | 54 | 10 | 10 | 11 | 3 | 206.38 | 203.6 | Low | No | Yes | No | No | No | No | No | −7.61 | 2 | 3 | 1 | 1 | 3 | 0.17 |
| Ledipasvir | C49H54F2N8O6 | 889 | 65 | 26 | 16 | 10 | 4 | 247.1 | 174.64 | Low | No | Yes | No | No | No | No | Yes | −6.5 | 2 | 4 | 2 | 2 | 5 | 0.17 |
| Lopinavir | C37H48N4O5 | 628.8 | 46 | 18 | 17 | 5 | 4 | 187.92 | 120 | High | No | Yes | No | Yes | No | No | Yes | −5.93 | 1 | 3 | 1 | 0 | 3 | 0.55 |
| Ombitasvir | C50H67N7O8 | 894.11 | 65 | 18 | 22 | 8 | 4 | 262.84 | 178.72 | Low | No | Yes | No | No | No | No | Yes | −6.11 | 2 | 3 | 2 | 1 | 4 | 0.17 |
| Paritaprevir | C40H43N7O7S | 765.88 | 55 | 20 | 9 | 10 | 3 | 211.96 | 198.03 | Low | No | Yes | No | No | No | No | Yes | −7.67 | 2 | 3 | 1 | 1 | 3 | 0.17 |
| Pibrentasvir | C57H65F5N10O8 | 1113.18 | 80 | 30 | 21 | 15 | 4 | 300.07 | 199.58 | Low | No | Yes | No | No | No | No | No | −7.82 | 2 | 4 | 2 | 2 | 6 | 0.17 |
| Etravirine | C20H15BrN6O | 435.28 | 28 | 18 | 4 | 5 | 2 | 109.56 | 120.64 | Low | No | No | Yes | Yes | Yes | No | Yes | −5.8 | 0 | 0 | 0 | 0 | 0 | 0.55 |
| Velpatasvir | C49H54N8O8 | 883 | 65 | 30 | 17 | 10 | 4 | 251.54 | 193.1 | Low | No | Yes | No | Yes | No | No | Yes | −7.31 | 2 | 4 | 2 | 1 | 5 | 0.17 |
| Voxilaprevir | C40H52F4N6O9S | 868.93 | 60 | 10 | 11 | 15 | 3 | 216.59 | 203.6 | Low | No | Yes | No | No | No | No | No | −7.39 | 2 | 4 | 2 | 2 | 4 | 0.17 |
| Telaprevir | C36H53N7O6 | 679.85 | 49 | 6 | 19 | 8 | 4 | 186.74 | 179.56 | Low | No | Yes | No | No | No | No | Yes | 7.47 | 2 | 3 | 2 | 1 | 3 | 0.17 |
Discussion
COVID-19 causes huge health crisis worldwide. Since there are no approved drugs available, new treatment options are urgently required for treating COVID-19 [9]. Computer based drug discovery methods are easing the identification of drugs and used to be aware of molecular aspects of protein targets and target-ligand interactions [25]. Repurposing of FDA approved drugs will be the right choice at this time for discovering drugs against COVID-19 since, they have already evaluated the toxicity and safety in human against the management of different diseases [17].
In this study, FDA approved small molecule antiviral drugs were virtually screened against Mpro and RdRp of SARS-CoV-2. The Mpro controls viral replication and is essential for the life cycle of virus. Discovery of the Mpro structure in SARS-CoV-2 has provided an opportunity in identifying potential drug candidates for COVID-19 [26]. While performing virtual screening against Mpro target, 11 antiviral drugs were screened based on the high interaction energy and highest number of amino acids involved in the HB interactions. It was found that Raltegravir showed the highest interaction energy (−9 kcal/mol) and other drugs such as Indinavir, Tipranavir, and Ledipasvir showed interaction energy value above −8 kcal/mol. Raltegravir, Indinavir, and Tipranavir were originally approved as HIV-1 drugs and Ledipasvir was approved as anti-HCV drug [27]. Kandeel and Al-Nizawi [17] reported on the repurpose of antiviral drugs against Mpro of SARS-CoV-2 and found antivirals (Ribavirin and Telbivudine), antituberculous drugs (Aminosalicylate sodium and Pyrazinamide), vitamins (Vitamin B12 and Nicotinamide) and anticancer drug (Temozolomide) exhibited the highest binding energy.
In a study, six antiviral compounds include Nelfinavir, Rhein, Withanolide D, Withaferin A, Enoxacin, and Aloe-emodin were identified against Mpro of SARS-CoV-2 and found that following amino acid positions Phe-3, Arg-4, Lys-5, Phe-8, Thr-29, Lys-102, Phe-103, Val-104, Arg-105, Ile-106, Gln-107, Val-108, Gly-109, Gln-110, Thr-111, Phe-112, Tyr-126, Gln-127, Val-135, Lys-137, Gly-138, Ser-139, Asn-151, Ile-152, Asp-153, Tyr-154, Ser-158, Glu-178, Trp-207, Ile-281, Leu-282, Gly-283, Glu-288, Phe-291, Thr-292, Phe-294, and Asp-295 were involved in the interactions [28]. In this study, most of the amino acid positions involved in the HB interactions between lead antiviral drugs screened against Mpro were similar to the amino acid interactions reported in the study of Chandel et al. [28]. Qamar et al. [29] found five potential protease inhibitors, Nelfinavir, Remdesivir, Lopinavir, Ritonavir, and Ketoamide against protease on COVID-19 with a negative energy value. Nelfinavir recorded the best binding energy of −7.54 kcal/mol and Thr75, Arg141, Gln175, and His176 were involved in the HB interactions and suggested Nelfinavir as a drug of choice for treating COVID-19. They also suggested that other drugs screened in their study showed negative dock energy against the target proteins; equal importance can be given to them as protease inhibitor ligands. In our study, other screened antiviral drugs such as Indinavir, Tipranavir, and Pibrentasvir showed dock energy value more than -8 kcal/mol and these drugs might also serve as an inhibitors of Mpro target of SARS-CoV-2. Studies revealed that the repurposed drugs Remdesivir [30,31] and a combination of Lopinavir and Ritonavir significantly improved the clinical condition of SARS-CoV patients [32]. In a study, Lopinavir drug has been reported to have the highest interaction energy against Mpro, followed by Atazanavir, Saquinavir, Ritonavir, Nelfinavir, Darunavir, Tipranavir, Amprenavir and Fosamprenavir in antiviral drugs screening. Finally, they recommended Indinavir and Remdesivir could be the potential therapeutic agents for COVID-19. These both drugs have been used in the clinical practices with limited toxicity [33].
RdRp is another important protein involved in the replication and transmission of coronavirus [34]. In PDB, two structures for SARS-CoV-2 RdRp were released (PDB ID: 6M71 and 7BTF) [35]. These protein models have the Root Mean Square Deviation (RMSD) value less than 2 Å [36]. While performing virtual screening of antiviral drugs against the protein target RdRp in this study, Indinavir (HIV drug) drug showed the highest interaction energy (-11.5 kcal/mol) and formed 5 hydrogen bond interactions with Asp-623, Arg-553, Cys-622, Asp-760 and Trp-617. Among other drugs, majority of drugs were anti-HCV (Elbasvir, Grazoprevir, Daclatasvir, Glecaprevir, Ledipasvir, Pibrentasvir, Velpatasvir and Paritaprevir and few were anti-HIV drugs (Dolutegravir, Tipranavir, and Raltegravir) that showed interaction energy of higher than −8 kcal/mol.
In a study, the solved structure 7BTF was docked with 31 compounds and they reported that Hydroxychloroquine had showed an interaction energy value of −6.13 kcal/mol and Sofosbuvir showed −7.46 kcal/ mol. They suggested that these drugs can bind with the active site of RdRp and induce RdRp inhibition. Since the safety profile for these drugs has already been studied and approved by FDA, these can be successful candidates for COVID-19 [20]. In this study, the PDB ID 6M71 has been targeted and it was observed that the antiviral drugs Elbasvir, Indinavir, Grazoprevir, Gelcaprevir, Ledispavir, Velpatasvir and Paritaprevir could serve as potential inhibitors of RdRp. In a similar study, Chang et al. [33] reported that the antiviral drug Remdesivir showed highest interaction energy value (−7.803 kcal/mol) followed by Galidesivir, Ribavirin and Fipiravir.
The Lipinski’s rule of 5 was also checked for the screened antiviral drugs in this study by observing the five criteria such as molecular weight <500 Da, <5, hydrogen bonds, <10 H-bond acceptors and LogP <5 for considering as a drug-like molecule [37]. Drug-likeness analysis showed that all the screened drugs have few violations despite they fit into the FDA approval drugs. Early estimation of ADME in the drug discovery phase reduces the pharmacokinetics-related failure in the clinical phase [38]. In this study, the pharmacokinetic parameters such as absorption, distribution, metabolism, and excretion (ADME) were analyzed for screened antiviral drugs. Pharmacokinetic analysis revealed that all the screened drugs had a poor gastrointestinal adsorption except Indinavir but all served as a substrate of P-glycoprotein. It is believed that Pgp plays an important role in the oral bioavailability, CNS distribution, and biliary and renal elimination of drugs, which are substrates of this transporter. The drugs Raltegravir, Indinavir, Tipranavir, Dolutegravir and Etravirine have high bioavailability value.
Conclusions
The repurposing of FDA approved antiviral drugs for COVID-19 could be the appropriate drug discovery option in this crisis time. This study finding revealed that Raltegravir, Indinavir, Tipranavir, Dolutegravir, and Etravirine had shown high binding energy against both Mpro and RdRp. These antiviral drugs have good bioavailability and drug-likeness properties. This study suggests that the small molecule antiviral drugs Raltegravir, Indinavir, Tipranavir, Dolutegravir, and Etravirine could be the potential drug of choice for the treatment of COVID-19 with further in vitro and in vivo evaluations.
Author statement
We agree the submission of the revised manuscript entitled “Raltegravir, Indinavir, Tipranavir, Dolutegravir, and Etravirine against main protease and RNA-dependent RNA polymerase of SARS-CoV-2: A molecular docking and drug repurposing approach” to this journal.
Funding
No funding. Sources: The authors extend their appreciation to The Researchers supporting project number (RSP 2020/20) King Saud University, Riyadh, Saudi Arabia.
Competing interests
None declared.
Ethical approval
Not required.
Acknowledgement
The authors extend their appreciation to The Researchers supporting project number (RSP 2020/20) King Saud University, Riyadh, Saudi Arabia.
References
- 1.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China, 2019. New Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cui J., Li F., Shi Z.L. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17:181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monchatre-Leroy E., Boué F., Boucher J.M., Renault C., Moutou F., Ar Gouilh M. Identification of alpha and beta coronavirus in wildlife species in France: bats, rodents, rabbits, and hedgehogs. Viruses. 2017;9:364. doi: 10.3390/v9120364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coronaviridae Study Group of the International Committee on Taxonomy of Viruses The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang L., Lin D., Sun X., Curth U., Drosten C., Sauerhering L. Crystal structure of SARSCoV-2 main protease provides a basis for design of improved alpha-ketoamide inhibitors. Science. 2020;368:409–412. doi: 10.1126/science.abb3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization . WHO; 2020. Coronavirus disease (COVID-19) situation report — 118. Available at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200517-covid-19-sitrep-118.pdf?sfvrsn=21c0dafe_6. [Accessed 18 May 2020] [Google Scholar]
- 9.Kumar D., Chandel V., Raj S., Rathi B. In silico identification of potent FDA approved drugs against Coronavirus COVID-19 main protease: a drug repurposing approach. Chem Biol Lett. 2020;7:166–175. [Google Scholar]
- 10.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu K., Fang Y.Y., Deng Y., Liu W., Wang M.F., Ma J.P. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J. 2020;133:1025–1031. doi: 10.1097/CM9.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.EU Clinical Trial Register. 2020. https://www.clinicaltrialsregister.eu/ctr-search/search?query=covid-19.
- 14.Romano J.D., Tatonetti N.P. Informatics and computational methods in natural product drug discovery: a review and perspectives. Front Genet. 2019;10:368. doi: 10.3389/fgene.2019.00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gahtori J., Pant S., Srivastava H.K. Modeling antimalarial and antihuman African trypanosomiasis compounds: a ligand- and structure-based approaches. Mol Divers. 2019;23:1–8. doi: 10.1007/s11030-019-10015-y. [DOI] [PubMed] [Google Scholar]
- 16.Khan R.J., Jha R.K., Amera G.M., Jain M., Singh E., Pathak A. Targeting SARS-CoV-2: a systematic drug repurposing approach to identify promising inhibitors against 3C-like proteinase and 2′-O-ribose methyltransferase. J Biomol Struct Dyn. 2020;2:1–40. doi: 10.1080/07391102.2020.1753577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kandeel M., Al-Nazawi M. Virtual screening and repurposing of FDA approved drugs against COVID-19 main protease. Life Sci. 2020;251 doi: 10.1016/j.lfs.2020.117627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mothay D., Ramesh K.V. Binding site analysis of potential protease inhibitors of COVID-19 using AutoDock. Virus Disease. 2020;2:1. doi: 10.1007/s13337-020-00585-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elfiky A.A. Novel guanosine derivatives as anti-HCV NS5b polymerase: a QSAR and molecular docking study. Med Chem. 2019;15:130–137. doi: 10.2174/1573406414666181015152511. [DOI] [PubMed] [Google Scholar]
- 20.Elfiky A.A. Ribavirin, Remdesivir, Sofosbuvir, Galidesivir, and Tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): a molecular docking study. Life Sci. 2020;253 doi: 10.1016/j.lfs.2020.117592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Boyle N.M., Banck M., James C.A., Morley C., Vandermeersch T., Hutchison G.R. Open babel: an open chemical toolbox. J Cheminform. 2011;3:33. doi: 10.1186/1758-2946-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morris G.M., Huey R., Olson A.J. Using AutoDock for ligand-receptor docking. Curr Protoc Bioinformatics. 2008;8:14. doi: 10.1002/0471250953.bi0814s24. [DOI] [PubMed] [Google Scholar]
- 23.Kong R., Yang G., Xue R., Liu M., Wang F., Hu J. COVID-19 Docking Server: A meta server for docking small molecules, peptides and antibodies against potential targets of COVID-19. Bioinformatics. 2020 doi: 10.1093/bioinformatics/btaa645. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daina A., Michielin O., Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep. 2017;7:2717. doi: 10.1038/srep42717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheikh A., Al-Taher A., Al-Nazawi M., Al-Mubarak A.I., Kandeel M. Analysis of preferred codon usage in the coronavirus N genes and their implications for genome evolution and vaccine design. J Virol Methods. 2020;277 doi: 10.1016/j.jviromet.2019.113806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khaerunnisa S., Kurniawan H., Awaluddin R., Suhartati S., Soetjipto S. Pre Prints; 2020. Potential inhibitor of COVID19 main protease (Mpro) from several medicinal plant compounds by molecular docking study; pp. 1–14. [Google Scholar]
- 27.Chaudhuri S., Symons J.A., Deval J. Innovation and trends in the development and approval of antiviral medicines: 1987–2017 and beyond. Antivir Res. 2018;155:76–88. doi: 10.1016/j.antiviral.2018.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chandel V., Raj S., Rathi B., Kumar D. In silico identification of potent COVID-19 main protease inhibitors from FDA approved antiviral compounds and active phytochemicals through molecular docking: a drug repurposing approach. Chem Biol Lett. 2020;7:166–175. [Google Scholar]
- 29.Qamar M.T., Alqahtani S.M., Alamri M.A., Chen L.L. Structural basis of SARS-CoV-2 3CLpro and anti-COVID-19 drug discovery from medicinal plants. J Pharm Anal. 2020;10:313–319. doi: 10.1016/j.jpha.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;4:1–3. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chu C.M., Cheng V.C., Hung I.F., Wong M.M., Chan K.H., Chan K.S. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang Y.C., Tung Y.A., Lee K.H., Chen T.F., Hsiao Y.C., Chang H.C. Potential therapeutic agents for COVID-19 based on the analysis of protease and RNA polymerase docking. Preprints. 2020 [Google Scholar]
- 34.Calligari P., Bobone S., Ricci G., Bocedi A. Molecular investigation of SARS–CoV-2 proteins and their interactions with antiviral drugs. Viruses. 2020;12:445. doi: 10.3390/v12040445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown ap-parent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14:72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 36.Elfiky A.A. SARS-CoV-2 RNA dependent RNA polymerase (RdRp) targeting: an in silico perspective. J Biomol Struct Dyn. 2020;26:1–5. doi: 10.1080/07391102.2020.1761882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lipinski C.A. Lead- and drug-like compounds: the rule-of-five revolution. Drug Discov Today Technol. 2004;1:337–341. doi: 10.1016/j.ddtec.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 38.Hay M., Thomas D.W., Craighead J.L., Economides C., Rosenthal J. Clinical development success rates for investigational drugs. Nature Biotechnol. 2014;32:40–51. doi: 10.1038/nbt.2786. [DOI] [PubMed] [Google Scholar]