Cryptococcal epidemiology is shifting toward HIV-negative populations who have diverse presentations. Cryptococcal antigen (CrAg) testing is also changing, with development of the lateral flow assay (LFA) having reported increased sensitivity and specificity, but with minimal knowledge in the HIV-negative population. In this study, we evaluate the real-life performance of CrAg testing in patients with cryptococcal disease. We conducted a retrospective review of patients with cryptococcosis from 2002 to 2019 at Barnes-Jewish Hospital.
KEYWORDS: Cryptococcus neoformans, cryptococcal antigen, diagnosis, test performance
ABSTRACT
Cryptococcal epidemiology is shifting toward HIV-negative populations who have diverse presentations. Cryptococcal antigen (CrAg) testing is also changing, with development of the lateral flow assay (LFA) having reported increased sensitivity and specificity, but with minimal knowledge in the HIV-negative population. In this study, we evaluate the real-life performance of CrAg testing in patients with cryptococcal disease. We conducted a retrospective review of patients with cryptococcosis from 2002 to 2019 at Barnes-Jewish Hospital. Latex agglutination (LA) was used exclusively until April 2016, at which point LFA was used exclusively. Demographics, presentations, and testing outcomes were evaluated. Serum CrAg testing was completed in 227 patients with cryptococcosis. Of 141 HIV-negative patients, 107 had LA testing and 34 had LFA testing. In patients with disseminated disease, serum CrAg sensitivity by LA was 78.1% compared to 82.6% for LFA. In patients with localized pulmonary disease, serum CrAg sensitivity was 23.5% compared to 90.9% for LFA. Of 86 people living with HIV (PLWH), 76 had LA testing, and 10 had LFA testing. Serum CrAg sensitivity for LA was 94.7% compared to 100% for LFA in patients with disseminated disease. We noted a significant improvement in sensitivity from LA testing to LFA testing, predominantly in those with localized pulmonary disease. However, both LFA and LA appear to be less sensitive in HIV-negative patients than previously described in PLWH.
INTRODUCTION
Cryptococcosis remains a significant cause of opportunistic infection in people living with HIV (PLWH) in the developing world (1); however, the proportion occurring among HIV-negative patients has been increasing in high-income countries (2–7). HIV-negative patients have atypical presentations and worse outcomes with cryptococcal infection compared to PLWH (2, 7–9). When clinical suspicion exists for cryptococcosis, cryptococcal antigen (CrAg) tests are the most rapid and widely used diagnostic modality (10–13).
For decades, latex agglutination (LA) has been the mainstay of CrAg testing. However, over the past decade, the new lateral flow assay (LFA) showed improved sensitivity and specificity over the LA (14–18). These studies focused on PLWH, almost exclusively in sub-Saharan Africa, who have higher fungal burdens (14, 16, 19). Combined testing of the LFA and LA suggests that CrAg testing appears to have less favorable characteristics in the HIV-negative population (20). One recent study compared LA to LFA on serum and cerebrospinal fluid (CSF) samples of 31 HIV-negative patients, and found that a significant lower limit of detection was required for the LFA, which improved its sensitivity (15). However, this study has limited generalizability due to the use of a highly select population that does not represent a true sample of cryptococcosis patients (15). To our knowledge, no other systematic studies exist in the HIV-negative population.
In previous work with our cryptococcal cohort, we have shown that our population resembles the national trend of an increasing proportion of transplant recipients and non-HIV nontransplant patients with cryptococcosis (7). In this study, we assess the sensitivity of serum CrAg testing using LA and LFA in a wide population of patients with cryptococcosis.
MATERIALS AND METHODS
Cohort construction.
All patients diagnosed with cryptococcosis at Barnes-Jewish Hospital in St. Louis, Missouri from 1 January 2002 to 1 March 2019 were reviewed. Cryptococcal infection was defined as positive serum or CSF CrAg, isolation of Cryptococcus neoformans in culture, or identification by the International Classification of Diseases (ICD) 9th (117.5, 321.0) or 10th (B45.1-B45.9) editions. Resulting date of the diagnostic specimen was used as time of diagnosis. All cases were reviewed by two investigators to confirm accurate diagnosis, and to ensure that all patients had either a proven or probable diagnosis, which served as the gold standard against which the testing was compared. Only patients with available CrAg results were included in our analyses and all CrAg testing was collected within 1 week of diagnosis. Patients were then divided into PLWH and HIV-negative, and further by the type of test performed and by disseminated versus localized disease, to adequately assess test performance.
Disseminated disease was defined as cryptococcosis occurring outside the lungs (i.e., central nervous system, skin, liver, etc). Localized pulmonary cryptococcosis was defined as cryptococcal infection limited to the lungs. Positive CrAg in the serum without any other evidence of dissemination was still classified as localized disease. Central nervous system infection was defined as a positive CSF CrAg or CSF culture growing Cryptococcus neoformans. No isolates of Cryptococcus gattii were identified and other species were excluded. Bloodstream infections were defined as a blood culture positive for Cryptococcus neoformans.
Patient demographics, laboratory testing, treatment data, and outcomes were abstracted from the medical chart and evaluated. All CrAg testing from 1 January 2002 through 31 March 2016 was performed using LA. All CrAg testing from 1 April 2016 through 1 March 2019 was completed exclusively using the LFA (IMMY, Norman, OK, USA).
Statistical analysis.
Categorical variables were analyzed using the Fisher’s exact test and the chi-square test, as appropriate. Continuous variables were analyzed using the t test and the Mann-Whitney U test if assumption of normality was violated. P values of <0.05 were considered statistically significant. Analysis was performed using SPSS [V25] (IBM, Armonk, NY, USA).
RESULTS
Cohort.
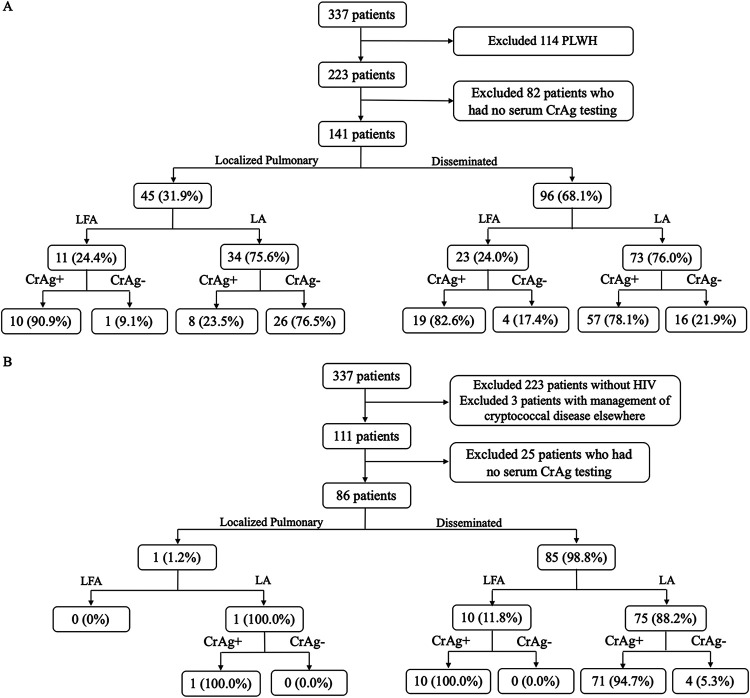
We reviewed 337 patients who met the definition of cryptococcosis from 1 January 2002 until the end of the study period on 1 March 2019, of which 223 were HIV-negative patients and 114 PLWH. One hundred seven patients were excluded who did not have serum CrAg testing done, and an additional three patients were excluded for receiving the majority of their care outside the Barnes-Jewish network. The remaining 227 patients, of which 141 were HIV-negative patients and 86 PLWH, were included in our analysis.
In HIV-negative patients, 96 (68.1%) had disseminated disease and 45 (31.9%) had localized pulmonary disease. Of those with disseminated disease, 20 (20.8%) had negative serum CrAgs, while of those with localized pulmonary disease, 27 (60%) had negative serum CrAgs. In disseminated disease, LA was positive in 78.1% of cases and LFA was positive in 82.6%. For localized pulmonary disease, LA was positive in 23.5% of cases and 90.9% positive for LFA testing (Fig. 1A).
FIG 1.
CONSORT diagram of 337 patients by their HIV status, dissemination and type of cryptococcal antigen test performed (2002 to 2019). Among the 223 HIV-negative (A) and 111 PLWH (B) diagnosed with cryptococcal disease by their dissemination status, the lateral flow assay appears to have a higher sensitivity, especially among HIV-negative patients and localized disease. LFA, lateral flow assay; LA, latex agglutination; CrAg, serum cryptococcal antigen; HIV, human immunodeficiency virus; PLWH, people living with HIV.
In PLWH, 85 (98.8%) had disseminated disease and 1 (1.2%) had localized pulmonary disease. Of the 85 patients with disseminated disease, 4 (4.7%) were negative by CrAg testing, while the one patient with localized disease had a positive CrAg by LA. In disseminated disease, LA was positive in 94.7% of cases (71 of 75 patients) compared to LFA, which was positive in 100% of cases (10 of 10) (Fig. 1B).
False negatives were seen in a variety of clinical presentations, including meningitis, bloodstream infections, and localized pulmonary disease (data not shown), and there were no specific differences in comorbidities or immune function for this group. Of the four false negatives with disseminated disease and LFA testing, two had end-stage liver disease, and of the three bloodstream infection patients with false-negative results, one had a history of end-stage liver disease and another had a history of liver transplant.
There were no significant differences in the site of cryptococcal infection, nor differences in patient comorbidities associated with antigen negativity in PLWH or HIV-negative patients (Table 1; see also Tables S1 and S2 in the supplemental material). No differences were seen in 90-day mortality when comparing CrAg positivity, nor when comparing time to diagnosis or time to initiation of antifungals. In HIV-negative patients with disseminated disease, patients with negative serum CrAgs (n = 5, 25%) were more likely to have no treatment compared to those with positive serum CrAgs (n = 1, 1.3%) (P < 0.001).
TABLE 1.
Baseline characteristics of HIV-negative individuals with disseminated cryptococcosis by positivity of serum cryptococcal antigen testing 2002 to 2019 (n = 96)
| Variablea | Positive serum CrAg (n = 76) | Negative serum CrAg (n = 20) | P value |
|---|---|---|---|
| No. male (%) | 52 (68) | 12 (60) | 0.477 |
| Age in years (median, IQR) | 58 (49, 68) | 54 (42, 63) | 0.144 |
| No. African American (%) | 8 (11) | 3 (15) | 0.758 |
| No. transplant recipients (%) | 20 (26) | 7 (35) | 0.442 |
| No. by site of infection (%) | |||
| Pulmonary | 14 (18) | 5 (25) | 0.511 |
| CNS disease | 46 (61) | 12 (60) | 0.966 |
| Bloodstream | 45 (59) | 8 (40) | 0.124 |
| Other site of infection | 12 (16) | 3 (15) | 0.931 |
| No. immunocompetent (%) | 15 (20) | 7 (35) | 0.148 |
| No. with 90-day mortality (%) | 28 (37) | 5 (25) | 0.286 |
| Median days to diagnosis from symptom onset (IQR) | 20.5 (7, 34) | 18 (5, 24) | 0.511 |
| Median days to initiation of antifungals from symptom onset (IQR) | 20 (7, 35) | 18 (5, 24) | 0.561 |
| Median days to initiation of antifungals from admission date (IQR) | 3 (1,7) | 1 (0, 7) | 0.309 |
IQR, interquartile range; CNS, central nervous system; CrAg, cryptococcal antigen.
In HIV-negative patients with localized pulmonary disease (n = 45), patients positive for CrAg also had similar sites of infection and comorbidities as those that were negative. Time to initiation of antifungals from admission date was significantly longer in those with negative serum CrAgs (median 13 days, interquartile range [IQR] 4 to 26) compared to those with positive serum CrAgs (median 3 days, IQR 2 to 7) (P = 0.042) (Table S2). Rates of treatment were similar based on CrAg positivity, although those with negative serum CrAgs were numerically less likely to receive liposomal amphotericin B for induction therapy (n = 2, 7.4%) compared to those with positive CrAgs (n = 7, 38.9%) (P = 0.598), although the numbers are small.
Latex agglutination.
We included 183 patients who had LA testing. Of these, 148 had disseminated disease and 35 had localized pulmonary disease. The overall sensitivity of LA was 74.9% (137 of 183). Within disseminated disease, 128 of 148 patients receiving LA testing were CrAg positive (86.5%). Within localized pulmonary disease, only 9 of 35 patients receiving LA testing were CrAg positive (25.7%).
Comparing HIV-negative patients to PLWH, 107 of 141 HIV-negative patients had LA testing versus 76 of 86 PLWH. The overall sensitivity of LA in HIV-negative patients was 60.7% (65 of 107 patients). In HIV-negative patients, sensitivity of LA in disseminated disease was 78.1% (57 of 73 patients) and the sensitivity of LA in localized pulmonary patients was 23.5% (8 of 34 patients) (Tables 2 and 3). In PLWH, the sensitivity of LA in disseminated disease was 94.7% (71 of 75 patients) and the sensitivity of LA in localized pulmonary disease was 100% (1 of 1 patient) (Table 3).
TABLE 2.
Baseline characteristics of HIV-negative individuals with disseminated cryptococcosis by type of test performed (n = 96)
| Variablea | Latex agglutination (n = 73) | Lateral flow assay (n = 23) | P value |
|---|---|---|---|
| No. male (%) | 49 (67) | 15 (65) | 0.866 |
| Age in years (median, IQR) | 56 (45, 67) | 61 (53, 69) | 0.234 |
| No. African American (%) | 8 (11) | 3 (13) | 0.324 |
| No. transplant recipients (%) | 19 (26) | 8 (35) | 0.415 |
| No. by site of infection (%) | |||
| Pulmonary | 15 (21) | 4 (17) | 0.740 |
| CNS disease | 42 (58) | 16 (70) | 0.304 |
| Bloodstream | 43 (59) | 10 (43) | 0.195 |
| Other site of infection | 11 (15) | 4 (17) | 0.789 |
| No. immunocompetent (%) | 19 (26) | 3 (13) | 0.196 |
| No. with 90-day mortality (%) | 21 (29) | 12 (52) | 0.048 |
| Median days to diagnosis from symptom onset (IQR) | 19 (7, 34) | 20 (7, 34) | 0.664 |
| Median days to initiation of antifungals from symptom onset (IQR) | 19 (7, 32) | 21 (7, 38) | 0.753 |
| Median days to initiation of antifungals from admission date (IQR) | 3 (0, 7) | 1 (0, 5) | 0.058 |
IQR, interquartile range; CNS, central nervous system; CrAg, cryptococcal antigen.
TABLE 3.
Cryptococcal antigen test sensitivity by disease presentation and HIV statusa
| Infection type | Population | % LA sensitivity (95% CI) | % LFA sensitivity (95% CI) | P value |
|---|---|---|---|---|
| Localized pulmonary | PLWH | 100 (0–100) | — | — |
| HIV-negative | 23.5 (10.8–41.2) | 90.9 (58.7–99.8) | <0.001 | |
| Disseminated | PLWH | 94.7 (86.9–98.5) | 100 (65.1–100) | 0.517 |
| HIV-negative | 78.1 (66.9–86.9) | 82.6 (61.2–95.1) | 0.641 |
PLWH, people living with HIV; LA, latex agglutination; LFA, lateral flow assay; CI, confidence interval; —, not applicable.
Lateral flow assay.
We included 44 patients who had LFA testing. Of these, 33 (75%) had disseminated disease and 11 (25%) had localized pulmonary disease. The overall sensitivity of LFA testing was 88.6% (39 of 44 patients) compared to the overall sensitivity of LA of 74.9% (P = 0.055). Among those with disseminated disease, 29 of 33 patients (87.9%) had a positive LFA test, comparable to 86.5% of those having a positive LA test (P = 0.901). In patients with localized pulmonary disease, 90.9% (10 of 11 patients) had a positive LFA test compared to 25.7% positivity with LA (P < 0.001).
Comparing HIV-negative patients to PLWH, the overall sensitivity for LFA in HIV-negative patients was 85.3% (29 of 34 patients) compared to 100% in PLWH (10 of 10 patients). In HIV-negative patients, sensitivity of LFA in disseminated disease was 82.6% (19 of 23 patients) and the sensitivity in localized pulmonary disease was 90.9% (10 of 11 patients) (Table 3).
In our cohort, there appeared to be a trend toward a decrease in time to antifungal initiation in patients with cryptococcal disease when LFA testing was used (1 day versus 3 days, P = 0.058) (Table 2).
DISCUSSION
With the changing epidemiology of cryptococcosis (7, 20, 21) and with HIV-negative patients facing higher mortality, possibly associated with delays in diagnosis due to low clinical suspicion, it is important that these delays are minimized to ensure prompt treatment initiation. False-negative CrAg tests have been linked to delays in diagnosis, and HIV-negative patients are more likely to have false-negative serum CrAg testing given lower fungal burdens compared to PLWH (20). Our study is unique from previous studies in that we included all cryptococcosis cases, consistent with cohorts likely to be seen in other academic centers (22). Therefore, it likely represents a cohort that is more representative of cryptococcosis in the United States. Additionally, disseminated and localized disease were analyzed separately, and we intentionally separated LA from LFA testing to compared their performance (20).
Previous work has reported serum CrAg by LFA test sensitivity to be between 97.0 and 100% and specificity between 99.0 and 100%, but this literature does not focus on HIV-negative populations (14, 17, 19, 23). Our overall LFA sensitivity of 88.6% (95% confidence interval [CI] 74.6 to 95.7%) was lower than what has been previously reported, but this is the first real-life analysis of the performance of the assay in this patient population. In our HIV-negative group with disseminated disease, serum CrAg sensitivity with LFA was 82.6%, a slight improvement over the LA sensitivity of 78.1% (Fig. 1A and Table 3). However, the most substantial finding of our study was the improvement in serum CrAg sensitivity within localized pulmonary disease, from 23.5% with LA to 90.9% with LFA (Fig. 1A and Table 3).
LA performance in serum samples of PLWH has been reported to have a sensitivity of between 83 and 100% and a specificity of between 93 and 100% (24, 25). The new LFA CrAg test has shown promising results, especially in PLWH (14). In a review by Nalintya, Kiggundu, and Meya, median serum LFA sensitivity was 100% (IQR 95.6%, 100%) and median specificity was 99.5% (IQR 95.7%, 100%) (14). There appeared to be a modest improvement in PLWH with disseminated disease in our cohort, 94.7% to 100%; however, the numbers for the LFA group are too small to confidently estimate that this was not due to chance alone.
Overall, CrAg positivity was higher in our cohort’s PLWH compared to HIV-negative patients. This is consistent with previous studies and possibly due to increased fungal burden in PLWH (18). This could also be due to the prozone or the postzone effect (26, 27), but this should have also then occurred in the PLWH (27). However, the improvements in the HIV-negative population were predominantly in the diagnosis of localized disease. This suggests there may be opportunities to further optimize performance of this assay for HIV-negative patients with disseminated disease.
Previous studies have shown higher sensitivities for LFA compared to LA in cryptococcosis; however, they were limited by a lower heterogeneity in the presentation of cryptococcosis (15). We noted that the most significant improvement in sensitivity from LA to LFA was in those with localized pulmonary disease. This finding is clinically important because localized disease is shown to be more common among patients without HIV (5), and given the evolving epidemiology of cryptococcosis in HIV-negative populations, we can expect that the proportion of atypical presentations will continue to increase.
In our study, patients with positive CrAg testing had shorter times to administration of antifungals. We also noted significant differences in treatment regimens depending on CrAg positivity in HIV-negative patients. For those with disseminated disease, patients with a negative LA test were significantly more likely to receive no treatment for their cryptococcosis. Patients with localized pulmonary disease were also significantly more likely to receive liposomal amphotericin B induction if they had a positive serum CrAg. In a previous study, we noted delays in diagnosis in non-HIV, nontransplant patients compared to PLWH (7). While there did not appear to be delays in diagnosis, in HIV-negative patients with localized pulmonary disease, those with negative serum CrAgs had a significantly longer median time to the initiation of antifungals compared to those with positive serum CrAgs.
In our HIV-negative group, there were false-negative CrAgs in cryptococcal meningitis and bloodstream infections, as well as localized pulmonary infections. There did not appear to be any specific difference in their comorbidities or immune function. However, of the four disseminated-disease patients with negative LFA tests, two had end-stage liver disease, and of the three bloodstream patients with negative CrAgs, one had a history of end-stage liver disease and the other had a history of liver transplant. Patients with cirrhosis are already known to have more severe cryptococcal disease and poorer outcomes (9). A possible explanation for the false-negative CrAgs in the setting of bloodstream infection is the postzone phenomenon. This effect has been noted in other infections conditions, such as syphilis. This effect has been described for both LA and LFA serum CrAgs (26, 28).
Limitations of this study include being performed at a single, Midwest academic tertiary care center, which may limit generalizability. This study is also retrospective and data can be limited by missing records and documentation. We are also limited by a relatively small number of patients who had LFA tests given the recent change to this test at our institution. Specificity for the CrAg tests could not be calculated as only patients with cryptococcosis were included with no control population; however, specificity of these tests appears to be excellent (17, 18). We are also limited in that some cases of cryptococcosis were diagnosed solely by positive CrAg test and compatible syndrome, therefore there are likely missed diagnoses in patients with negative cultures who had false-negative CrAgs.
In conclusion, we saw an improvement in sensitivity with serum LFA compared to LA in both HIV-negative patients and PLWH with cryptococcosis, especially in those with localized pulmonary disease. While we did not reach sensitivities previously cited, this may be due to the relatively small size of our LFA cohort, but the previously cited works have predominantly studied only PLWH and other select populations that may not be representative of cryptococcosis in the United States. LA tests should not be used to rule out cryptococcosis, especially in isolated pulmonary and even in those with disseminated disease. While LFA has higher a higher sensitivity, there were still cases of false-negatives in some of our HIV-negative patients.
Supplementary Material
ACKNOWLEDGMENTS
A.S. has grant support from Astellas and consults for Mayne, Scynexis, Viamet, Astellas, and Minnetronix. All other authors have no conflicts of interest.
Research reported in this publication was supported by the Washington University Institute of Clinical and Translational Sciences grant KL2TR002346 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Rajasingham R, Smith RM, Park BJ, Jarvis JN, Govender NP, Chiller TM, Denning DW, Loyse A, Boulware DR. 2017. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis 17:873–881. doi: 10.1016/S1473-3099(17)30243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brizendine KD, Baddley JW, Pappas PG. 2013. Predictors of mortality and differences in clinical features among patients with Cryptococcosis according to immune status. PLoS One 8:e60431. doi: 10.1371/journal.pone.0060431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bratton EW, El Husseini N, Chastain CA, Lee MS, Poole C, Sturmer T, Juliano JJ, Weber DJ, Perfect JR. 2012. Comparison and temporal trends of three groups with cryptococcosis: HIV-infected, solid organ transplant, and HIV-negative/non-transplant. PLoS One 7:e43582. doi: 10.1371/journal.pone.0043582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pyrgos V, Seitz AE, Steiner CA, Prevots DR, Williamson PR. 2013. Epidemiology of cryptococcal meningitis in the US: 1997–2009. PLoS One 8:e56269. doi: 10.1371/journal.pone.0056269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.George IA, Spec A, Powderly WG, Santos CAQ. 2018. Comparative epidemiology and outcomes of human immunodeficiency virus (HIV), non-HIV non-transplant, and solid organ transplant Aassociated cryptococcosis: a population-based study. Clin Infect Dis 66:608–611. doi: 10.1093/cid/cix867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samudralwar RD, Spec A, Cross AH. 2019. Case report: fingolimod and cryptococcosis: collision of immunomodulation with infectious disease. Int J Mol Sci Care 21:275–280. doi: 10.7224/1537-2073.2018-080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hevey MA, George IA, Raval K, Powderly WG, Spec A. 2019. Presentation and mortality of cryptococcal infection varies by predisposing illness: a retrospective cohort study. Am J Med 132:977–983. doi: 10.1016/j.amjmed.2019.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hevey MA, Presti RM, O'Halloran JA, Larson L, Raval K, Powderly WG, Spec A. 2019. Mortality after cryptococcal infection in the modern antiretroviral therapy era. J Acquir Immune Defic Syndr 82:81–87. doi: 10.1097/QAI.0000000000002095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spec A, Raval K, Powderly WG. 2016. End-stage liver disease is a strong predictor of early mortality in cryptococcosis. Open Forum Infect Dis 3:ofv197. doi: 10.1093/ofid/ofv197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Halloran JA, Powderly WG, Spec A. 2017. Cryptococcosis today: it is not all about HIV infection. Curr Clin Microbiol Rep 4:88–95. doi: 10.1007/s40588-017-0064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maziarz EK, Perfect JR. 2016. Cryptococcosis. Infect Dis Clin North Am 30:179–206. doi: 10.1016/j.idc.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spec A, Mejia-Chew C, Powderly WG, Cornely OA. 2018. EQUAL Cryptococcus Score 2018: a European Confederation of Medical Mycology score derived from current guidelines to measure QUALity of clinical cryptococcosis management. Open Forum Infect Dis 5:ofy299. doi: 10.1093/ofid/ofy299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spec A, Powderly WG. 2018. Cryptococcal meningitis in AIDS. Handb Clin Neurol 152:139–150. doi: 10.1016/B978-0-444-63849-6.00011-6. [DOI] [PubMed] [Google Scholar]
- 14.Nalintya E, Kiggundu R, Meya D. 2016. Evolution of cryptococcal antigen testing: what is new? Curr Fungal Infect Rep 10:62–67. doi: 10.1007/s12281-016-0256-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jitmuang A, Panackal AA, Williamson PR, Bennett JE, Dekker JP, Zelazny AM. 2016. Performance of the cryptococcal antigen lateral flow assay in non-HIV-related cryptococcosis. J Clin Microbiol 54:460–463. doi: 10.1128/JCM.02223-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kabanda T, Siedner MJ, Klausner JD, Muzoora C, Boulware DR. 2014. Point-of-care diagnosis and prognostication of cryptococcal meningitis with the cryptococcal antigen lateral flow assay on cerebrospinal fluid. Clin Infect Dis 58:113–116. doi: 10.1093/cid/cit641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Binnicker MJ, Jespersen DJ, Bestrom JE, Rollins LO. 2012. Comparison of four assays for the detection of cryptococcal antigen. Clin Vaccine Immunol 19:1988–1990. doi: 10.1128/CVI.00446-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boulware DR, Rolfes MA, Rajasingham R, von Hohenberg M, Qin Z, Taseera K, Schutz C, Kwizera R, Butler EK, Meintjes G, Muzoora C, Bischof JC, Meya DB. 2014. Multisite validation of cryptococcal antigen lateral flow assay and quantification by laser thermal contrast. Emerg Infect Dis 20:45–53. doi: 10.3201/eid2001.130906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vidal JE, Boulware DR. 2015. Lateral flow assay for cryptococcal antigen: an important advance to improve the continuum of HIV care and reduce cryptococcal meningitis-related mortality. Rev Inst Med Trop S Paulo 57(Suppl 19):38–45. doi: 10.1590/S0036-46652015000700008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marr KA, Sun Y, Spec A, Lu N, Panackal A, Bennett J, Pappas P, Ostrander D, Datta K, Zhang SX, Williamson PR, Lyons J, Bhimraj A, Trotman R, Health C, Perfect J, Lyon GM, Vazquez J, Piwoz J, Marr K, Hopkins J, Spindel S, Wray D, Bennett J, Garcia-Diaz J, Nolt D, Subramanian A, Pappas P, Schaenman J, Taplitz R, Diego S, Miceli M, Lee SA, Nguyen H, Pannaraj P, Hasbun R, Limaye A, Powderly W, Spec A, Cryptococcus Infection Network Cohort Study Working Group. 2020. A multicenter, longitudinal cohort study of cryptococcosis in human immunodeficiency virus-negative people in the United States. Clin Infect Dis 70:252–261. doi: 10.1093/cid/ciz193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Messina JA, Maziarz EK, Spec A, Kontoyiannis DP, Perfect JR. 2017. Disseminated cryptococcosis with brain involvement in patients with chronic lymphoid malignancies on ibrutinib. Open Forum Infect Dis 4:ofw261. doi: 10.1093/ofid/ofw261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williamson PR, Jarvis JN, Panackal AA, Fisher MC, Molloy SF, Loyse A, Harrison TS. 2017. Cryptococcal meningitis: epidemiology, immunology, diagnosis and therapy. Nat Rev Neurol 13:13–24. doi: 10.1038/nrneurol.2016.167. [DOI] [PubMed] [Google Scholar]
- 23.Lelievre L, Garcia-Hermoso D, Abdoul H, Hivelin M, Chouaki T, Toubas D, Mamez AC, Lantieri L, Lortholary O, Lanternier F, French Mycosis Study G. 2014. Posttraumatic mucormycosis: a nationwide study in France and review of the literature. Medicine (Baltimore) 93:395–404. doi: 10.1097/MD.0000000000000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Panackal AA, Dekker JP, Proschan M, Beri A, Williamson PR. 2014. Enzyme immunoassay versus latex agglutination cryptococcal antigen assays in adults with non-HIV-related cryptococcosis. J Clin Microbiol 52:4356–4358. doi: 10.1128/JCM.02017-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanner DC, Weinstein MP, Fedorciw B, Joho KL, Thorpe JJ, Reller L. 1994. Comparison of commercial kits for detection of cryptococcal antigen. J Clin Microbiol 32:1680–1684. doi: 10.1128/JCM.32.7.1680-1684.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee G-H, Arthur I, Leung M. 2018. False-negative serum cryptococcal lateral flow assay result due to the prozone phenomenon. J Clin Microbiol 56:e01878-17. doi: 10.1128/JCM.01878-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kojima N, Chimombo M, Kahn DG. 2018. False-negative cryptococcal antigen test due to the postzone phenomenon. AIDS 32:1201–1202. doi: 10.1097/QAD.0000000000001805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Navabi N, Montebatsi M, Scott M, Gluckman SJ, Reid MJ. 2015. Case report: false negative serum cryptococcal latex agglutination test in a patient with disseminated cryptococcal disease. J Int Assoc Provid AIDS Care 14:123–126. doi: 10.1177/2325957414555233. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.