Identification (ID) and antimicrobial susceptibility testing (AST) of respiratory pathogens are critical to the management of patients with pneumonia to facilitate optimal antibiotic therapy selection. Few studies have examined the time to results (TTR) for this critical specimen, and such data can be valuable for benchmarking the current paradigm of diagnostic approaches. TTR for bronchoalveolar lavage (BAL) and endotracheal aspirate (ETA) specimens from hospitalized patients was evaluated using the Premier Healthcare Database, a comprehensive database of 194 U.
KEYWORDS: BAL, ETA, pneumonia, bacterial culture, antimicrobial susceptibility testing
ABSTRACT
Identification (ID) and antimicrobial susceptibility testing (AST) of respiratory pathogens are critical to the management of patients with pneumonia to facilitate optimal antibiotic therapy selection. Few studies have examined the time to results (TTR) for this critical specimen, and such data can be valuable for benchmarking the current paradigm of diagnostic approaches. TTR for bronchoalveolar lavage (BAL) and endotracheal aspirate (ETA) specimens from hospitalized patients was evaluated using the Premier Healthcare Database, a comprehensive database of 194 U.S. hospitals. Times from specimen collection to reporting of organism ID/AST were evaluated and compared by specimen types and characteristics. A total of 79,662 (43,129 BAL; 36,533 ETA) specimens were included, of which 19.3% harbored no growth, 47.1% contained normal respiratory flora alone (including yeast), and 0.6% contained mycobacteria/molds. Potential bacterial pathogens (PBP) were recovered from 33.0%. ETA specimens had a higher proportion of specimens with isolation of PBP (39.2% versus 27.7%) and with normal respiratory flora (52.0% versus 43.0%) and were less likely to be negative (8.2% versus 28.6%) than BAL specimens (all P < 0.0001). Staphylococcus aureus and Pseudomonas aeruginosa were isolated in 10.5 and 6.4% of the specimens, respectively, and were the most common organisms identified. Median (interquartile range) TTR were 37.0 h (21.8 to 51.7 h) and 60.5 h (46.6 to 72.4 h) for ID and AST, respectively. Median TTR for major respiratory pathogens by organism ranged from 29.2 to 43.9 h for ID and from 47.9 to 73.9 h for AST. Organism type, specimen collection time, and hospital teaching status influenced TTR. Mechanically vented patients and ETA specimens were more likely to recover PBP.
INTRODUCTION
Identification (ID) and antimicrobial susceptibility testing (AST) of respiratory pathogens are fundamental to the diagnosis and management of patients hospitalized with pneumonia. Guidelines for the treatment of hospital-acquired pneumonia and ventilator-associated pneumonia (HAP/VAP) recommend patients be treated according to the results of microbiologic studies performed on respiratory specimens, primarily because resistant pathogens lead to a significant risk that empirical therapy will be inadequate, which is associated with increased risk of mortality (1). Timely administration of effective antimicrobial therapy requires prompt and accurate determination of the causative pathogen and its antimicrobial susceptibility profile.
Guidelines for the treatment of HAP/VAP recommend broad empirical therapy, followed by tailoring therapy once results of microbiological testing are available. With the growing frequency of multidrug-resistant (MDR) organisms, as well as a better understanding of the harms of unnecessary broad antimicrobial coverage, the microbiology testing turnaround times (TAT) can have significant impact on the management of patients with HAP/VAP (2, 3). However, little data are available regarding contemporary time to results (TTR) for respiratory testing in the microbiology laboratory. Such data can be valuable as a baseline for process improvements aimed at reducing TTR, as a benchmark for TTR across laboratories, to aid in the contextualization of studies aimed at reducing time to therapy optimization for patients with pneumonia and to understand incremental gains possible through novel diagnostic technologies.
To the latter point, new diagnostics tests have been made available to identify potential pathogens in respiratory specimens, include syndromic nucleic acid amplification tests and pathogen identification by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS). These advances have dramatically shortened the time to ID of the causative pathogen, which for some organisms (i.e., Stenotrophomonas maltophilia, Acinetobacter baumannii, etc.) may result in specific adjustments in treatment based on known intrinsic resistance patterns or local antibiogram data. However, for many organisms, phenotypic AST is a rate-limiting step for definitive therapy selection (4). It is not well understood how long U.S. laboratories take to perform pathogen detection, ID, and AST of respiratory pathogens. In the present study, we evaluated timing data for first organism ID and AST from specimen collection for the current bronchoalveolar lavage (BAL) and endotracheal aspirate (ETA) culturing processes in the United States from a large repository of U.S. hospitals. Since current guidelines differ regarding the preferred method of microbiological diagnosis of pneumonia, with “noninvasive” samples (ETA) preferred in some situations and invasive samples (BAL) preferred in others, data were investigated for each method.
MATERIALS AND METHODS
Study design and inclusion criteria.
The Premier Healthcare Database (PHD) was used to identify a retrospective cohort of consecutive, nonduplicate hospitalized patients with BAL or ETA performed between 1 June 2015 and 31 May 2018. PHD is the largest repository of detailed acute care private and academic hospitals in the United States and represents approximately 25% of all U.S. acute-care hospitalizations annually (5). In addition to pharmacy and billing data, the PHD contains microbiology laboratory result data for a subset of hospitals. The number of hospitals contributing microbiology laboratory result data varies by year. Since antibiotic data in the PHD are only provided with the date (no timing available) of administration, therapy changes in relation to timing of ID and AST results was not performed in this analysis. Specimens were excluded from the analysis if they were from patients <18 years old, from patients who had a diagnosis of cystic fibrosis, from patients for whom the time to organism ID was >7 days (∼1% of total specimens), or from patients who were positive for >3 potential pathogens (6, 7). For this analysis, Klebsiella aerogenes was categorized as an Enterobacter spp. Only the first specimen from any individual patient was included across the 3-year period.
The PHD is fully deidentified and compliant with the Health Insurance Portability and Accountability Act of 1996 (HIPAA). Given the deidentified and retrospective nature of the data and the noninterventional study design, written patient consent was neither required nor sought. Administrative permissions were not required to access the raw data.
The primary outcome of the study was to describe the TTR from respiratory specimens in U.S. hospitals. The prevalence of potential bacterial pathogens, normal respiratory flora, and no growth culture results were calculated for the overall population and according to specimen type (BAL versus ETA). Detailed time to result analysis was performed for specimens containing any of the most common agents of pneumonia (MCAP) according to the 2018 Infectious Diseases Society of America (IDSA) and the American Society for Microbiology guide to utilization of the microbiology laboratory for diagnosis of infectious diseases (7).
Outcomes, definitions, and statistical analyses.
The study was designed to evaluate the time to result of BAL and ETA specimens, with a focus on the reporting of two results: (i) the time the specimen was collected to the first report of organism ID and (ii) the time the specimen was collected to the first report of AST.
We further compared time to results according to several patient, hospital, and pathogen factors. We also identified the time of day for which ID and AST results were reported. Specimens with organism identification culture results of normal, mixed, or oropharyngeal flora, as well as specimens in which only yeast (i.e., Candida spp.) was isolated, were classified as “normal respiratory flora” for this analysis. Specimen cultures results that were finalized as negative, showing no growth, or specifically described as “no significant pathogens isolated” were classified as “no growth.” Mycobacteria and molds recovered in culture were classified as “mycobacteria/mold.” The time to ID and AST of mycobacteria/mold was not determined due to the low overall prevalence. All remaining specimens which isolated bacteria were classified as “potential bacterial pathogens” in order to capture the range of pathogens that may be associated with a lower respiratory tract infection, since not all clinical features (e.g., symptoms and suggestive radiographic features) required for a confirmed diagnosis were available in the database. Specimens with only a single potential bacterial pathogen isolated from the culture were monomicrobial, regardless of whether normal respiratory flora were present. Specimens in which ≥2 potential bacterial pathogens were isolated were classified as polymicrobial.
Statistical analysis.
Baseline patient and hospital characteristics were analyzed via the chi-square test for categorical variables and by the Mann-Whitney U or a Student t test for continuous variables, as appropriate. Prevalence of culture results were calculated using the total number of specimens as the denominator or the number of specimens by type (BAL versus ETA), as appropriate. Time to result comparisons between BAL and ETA were performed using the Mann-Whitney U test, since these data were not normally distributed. All analyses were performed in JMP 13.0.0 (SAS Institute, Inc., Cary, NC).
Data availability. The data that support the findings of this study are available from Premier Inc., but restrictions apply to the availability of these data, which were used under license for the present study and so are not publicly available. However, data are available from the authors upon reasonable request and with the permission of Premier Inc.
RESULTS
Between 1 June 2015 and 31 May 2018, 79,662 (43,129 BAL; 36,533 ETA) specimens from 194 U.S. hospitals met all of the study inclusion criteria. The patient and hospital characteristics are listed in Table 1. Approximately 70% of specimens were from institutions with >300 beds, with 35% being from hospitals with >500 beds. South (46.7%) and Midwest (33.7%) U.S. census regions contributed the most specimens to the analysis. More than two-thirds of specimens were from patients with a non-health care facility admission source, including from home.
TABLE 1.
Patient and hospital baseline characteristics
| Characteristic | No. (range or %)a
|
||
|---|---|---|---|
| Total (n = 79,662) | BAL (n = 43,129) | ETA (n = 36,533) | |
| Median age (yr), IQR | 65 (54–74) | 65 (54–74) | 64 (53–74) |
| Male | 43,546 (54.7) | 22,857 (53.0) | 20,689 (56.6) |
| Yr | |||
| 2015 | 21,090 (26.5) | 10,711 (24.8) | 10,379 (28.4) |
| 2016 | 32,302 (40.6) | 16,980 (39.4) | 15,322 (41.9) |
| 2017 | 20,534 (25.8) | 11,953 (27.7) | 8,581 (23.5) |
| 2018 | 5,732 (7.2) | 3,481 (8.1) | 2,251 (6.2) |
| Admission source | |||
| Nonhealthcare facility (including from home) | 56,867 (71.4) | 30,312 (70.3) | 26,555 (72.7) |
| Transfer from SNF/ICF | 1,284 (1.6) | 522 (1.2) | 762 (2.1) |
| Transfer from another acute care facility | 10,190 (12.8) | 4,912 (11.4) | 5,278 (14.5) |
| Transfer from another nonacute care facility | 2,199 (2.8) | 745 (1.7) | 1,454 (4.0) |
| Clinic | 7,809 (9.8) | 5,435 (12.6) | 2,374 (6.5) |
| Others | 1,313 (1.6) | 1,203 (2.8) | 110 (0.3) |
| Region | |||
| Midwest | 26,830 (33.7) | 16,053 (37.2) | 10,777 (29.5) |
| Northeast | 7,925 (9.9) | 5,191 (12.0) | 2,734 (7.5) |
| South | 37,187 (46.7) | 18,959 (44.0) | 18,228 (49.9) |
| West | 7,720 (9.7) | 2,926 (6.8) | 4,794 (13.1) |
| No. of hospital beds | |||
| 0–99 | 1,583 (2.0) | 621 (1.4) | 962 (2.6) |
| 100–199 | 9,686 (12.2) | 5,761 (13.4) | 3,925 (10.7) |
| 200–299 | 12,374 (15.5) | 6,977 (16.2) | 5,397 (14.8) |
| 300–399 | 12,963 (16.3) | 6,194 (14.4) | 6,769 (18.5) |
| 400–499 | 15,119 (19.0) | 7,620 (17.7) | 7,499 (20.5) |
| 500+ | 27,937 (35.1) | 15,956 (37.0) | 11,981 (32.8) |
| Teaching facility | 37,674 (47.3) | 22,487 (52.1) | 15,187 (41.6) |
| Specimen collection | |||
| Within 2 days of admission | 41,245 (51.8) | 20,913 (48.5) | 20,332 (55.7) |
| Days 3 to 4 of admission | 18,941 (23.8) | 11,052 (25.6) | 7,889 (21.6) |
| Submitted ≥day 5 of admission | 19,476 (24.4) | 11,164 (25.9) | 8,312 (22.8) |
| ICU admission (any) | 48,720 (61.2) | 17,699 (41.0) | 31,021 (84.9) |
| ICU at collection | 34,847 (43.7) | 11,653 (27.0) | 23,194 (63.5) |
| Mechanical ventilation | |||
| Any | 41,651 (52.3) | 14,514 (33.7) | 27,137 (74.3) |
| Within 48 h of collection | 29,722 (37.3) | 8,918 (20.7) | 20,804 (57.0) |
| Principal diagnosis | |||
| Pneumonia | 4,923 (6.2) | 3,869 (9.0) | 1,054 (2.9) |
| Sepsis | 17,621 (22.1) | 7,645 (17.7) | 9,976 (27.3) |
| Respiratory failure/arrest | 94 (0.1) | 58 (0.1) | 36 (0.1) |
| Secondary diagnosis of pneumonia | 28,604 (35.9) | 14,177 (32.9) | 14,427 (39.5) |
Except as noted otherwise in column 1.
Epidemiology.
Overall, 19.3% of specimens were reported as harboring no growth, 47.1% contained normal respiratory flora alone (including yeast), and 0.6% contained mycobacteria/molds. Potential bacterial pathogens were recovered from 26,302 (33.0%) specimens, with at least one MCAP recovered in 28.2% (n = 22,429) of specimens. ETA yielded a higher proportion of specimens isolated with a potential bacterial pathogen (39.2% versus 27.7%) and normal respiratory flora (52.0% versus 43.0%) and were less likely to be negative (8.2% versus 28.6%) than did BAL (all P < 0.0001). At least one MCAP was recovered in 35.1% (n = 12,840) of ETA specimens and in 22.2% of BAL specimens (n = 9,589), respectively (P < 0.0001).
Staphylococcus aureus and Pseudomonas aeruginosa were recovered in 8,366 (10.5%) and 5,138 (6.4%) of all specimens, respectively, and were the most common bacterial pathogens reported by laboratories (Table 2). Klebsiella spp. (3.1%), Haemophilus spp. (2.9%), Streptococcus pneumoniae (2.1%), Escherichia coli (2.1%), Enterobacter spp. (1.7%), Stenotrophomonas maltophilia (1.4%), and Serratia marcescens (1.2%) were also common. All other potential bacterial pathogens were recovered in <1% of the total specimens. Isolation of all the MCAP was higher in ETA than in BAL specimens (Table 2; all P < 0.0001). There was a higher proportion of ETA specimens, with >1 potential bacterial pathogen isolated compared to BAL specimens, but such polymicrobial specimens were <10% in both cases (8.7% versus 6.2%; P < 0.0001). Laboratory reports indicated 30.6% of ETA specimens had one potential pathogen recovered, 7.5% had two, and 1.2% had three. In contrast, 21.6% of BAL specimens had one, 5.4% had two, and 0.8% had three potential pathogens.
TABLE 2.
Rank order of pathogens isolated from ≥1% of BAL and ETA specimens in the United States
| Rank | Pathogen (%) |
||
|---|---|---|---|
| Overall | BAL | ETA | |
| 1 | S. aureus (10.5) | S. aureus (7.9) | S. aureus (13.6) |
| 2 | P. aeruginosa (6.4) | P. aeruginosa (5.1) | P. aeruginosa (8.1) |
| 3 | Klebsiella spp. (3.1) | Haemophilus spp. (2.6) | Klebsiella spp. (4.1) |
| 4 | Haemophilus spp. (2.9) | Klebsiella spp. (2.1) | Haemophilus spp. (3.2) |
| 5 | S. pneumoniae (2.1) | S. pneumoniae (1.8) | E. coli (2.7) |
| 6 | E. coli (2.1) | E. coli (1.6) | S. pneumoniae (2.4) |
| 7 | Enterobacter spp. (1.7) | Enterobacter spp. (1.3) | Enterobacter spp. (2.3) |
| 8 | S. maltophilia (1.4) | S. maltophilia (1.1) | S. maltophilia (1.7) |
| 9 | S. marcescens (1.2) | S. marcescens (0.9) | S. marcescens (1.6) |
Forty-seven percent of specimens were from teaching facilities. Teaching hospitals reported a higher prevalence of E. coli (2.2% versus 2.0%; P = 0.040), Enterobacter spp. (1.9% versus 1.6%; P < 0.0001), Haemophilus spp. (3.2% versus 2.6%; P < 0.0001), Klebsiella spp. (3.4% versus 2.8%; P < 0.0001), S. marcescens (1.3% versus 1.1%; P = 0.0047), and S. aureus (11.2% versus 9.9%; P < 0.0001) than non-teaching hospitals. The rates of Acinetobacter baumannii (0.8% versus 0.8%; P = 0.84), P. aeruginosa (6.4% versus 6.5%; P = 0.69), S. maltophilia (1.4% versus 1.4%; P = 0.71), and S. pneumoniae (2.2% versus 2.0%; P = 0.13) were similar between teaching and non-teaching facilities.
Approximately 52% of specimens were submitted to the lab within the first 2 days of hospitalization. S. pneumoniae (3.2% versus 0.9%; P < 0.0001). Haemophilus spp. (3.8% versus 1.9%; P < 0.0001), and P. aeruginosa (6.7% versus 6.2%; P = 0.007) were more likely to be reported in specimens submitted within the first 2 days of hospitalization, suggestive of community onset infection compared to specimens submitted >2 days after admission. A. baumannii (0.9% versus 0.7%; P = 0.0009), Enterobacter spp. (2.5% versus 1.1%; P < 0.0001), E. coli (2.5% versus 1.8%; P < 0.0001), Klebsiella spp. (3.6% versus 2.6%; P < 0.0001), S. marcescens (1.4% versus 1.0%; P < 0.0001), S. aureus (11.1% versus 9.9%; P < 0.0001), and S. maltophilia (1.9% versus 0.9%; P < 0.0001) were more commonly reported in specimens submitted >2 days after admission.
All MCAP samples (S. aureus [11.0% versus 10.4%; P = 0.020], A. baumannii [1.2% versus 0.6%; P < 0.0001], Enterobacter spp. [3.0% versus 1.3%; P < 0.0001], E. coli [2.7% versus 1.9%; P < 0.0001], Klebsiella spp. [4.3% versus 2.7%; P = 0.0055], P. aeruginosa (7.6% versus 6.1%; P < 0.0001), S. marcescens [1.6% versus 1.1%; P < 0.0001], and S. maltophilia [2.7% versus 1.0%; P < 0.0001]) were recovered at higher frequency from specimens submitted >5 days after admission to hospital, with the exception of S. pneumoniae (0.5% versus 2.6%; P < 0.0001) and Haemophilus spp. (1.4% versus 3.4%; P < 0.0001), which was less commonly reported later in the hospitalization.
Fifty-two percent of specimens (n = 41,651) were from patients who were mechanically ventilated during hospitalization. Nearly all of the MCAP specimens (S. aureus [13.6% versus 7.1%; P < 0.0001], S. pneumoniae [2.3% versus 1.8%; P < 0.0001], A. baumannii [1.0% versus 0.5%; P < 0.0001], Enterobacter spp. [2.3% versus 1.2%; P < 0.0001], E. coli [2.6% versus 1.6%; P < 0.0001], Haemophilus spp. [3.1% versus 2.7%; P = 0.0008], Klebsiella spp. [4.0% versus 2.1%; P < 0.0001], S. marcescens [1.5% versus 0.9%; P < 0.0001], and S. maltophilia [1.6% versus 1.1%; P < 0.0001]) were more frequently isolated from mechanically ventilated patients than from patients that were did not receive mechanical ventilation during hospitalization. Only P. aeruginosa was isolated as frequently in both specimens from mechanically ventilated (6.3%) and nonventilated patients (6.6%; P = 0.66).
AST.
AST was performed for 86.1% (14,858/17,253) of MCAP from monomicrobial specimens. The proportion of bacterial isolates tested for AST ranged from 28.1% for Haemophilus spp. (β-lactamase testing was performed for 61.5%) to 96.0% for Enterobacter spp. (Table 3). AST was performed for more than 90% of lactose-fermenting (E. coli, Enterobacter spp., and Klebsiella spp.) Gram-negative bacteria (95.2%), P. aeruginosa (91.6%), and S. aureus (91.8%) isolated from respiratory specimens. AST was more frequently performed for lactose-fermenting Gram-negative bacteria (95.2%) than non-lactose-fermenting Gram-negative bacteria (91.6%; P < 0.0001). Tables S1 and S2 in the supplemental material provide additional details on AST of MCAP according to the hospital day of admission and the mechanical ventilation status, respectively.
TABLE 3.
Antimicrobial susceptibility test distribution by specimen type for the most common etiologic agents of HAP/VAP
| Etiologic agent | BAL |
ETA |
Overall |
||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of isolates | AST |
No. of isolates | AST |
No. of isolates | AST |
||||
| n | % | n | % | n | % | ||||
| Gram positive, monomicrobial | |||||||||
| Staphylococcus aureus | 2,544 | 2,252 | 88.5 | 3,660 | 3,440 | 94.0 | 6,204 | 5,692 | 91.7 |
| Streptococcus pneumoniae | 542 | 469 | 86.5 | 588 | 500 | 85.0 | 1,130 | 969 | 85.8 |
| Gram negative, monomicrobial | |||||||||
| Acinetobacter spp. | 122 | 108 | 88.5 | 206 | 195 | 94.7 | 328 | 303 | 92.4 |
| Escherichia coli | 464 | 427 | 92.0 | 618 | 595 | 96.3 | 1,082 | 1,022 | 94.5 |
| Enterobacter spp. | 338 | 317 | 93.8 | 512 | 499 | 97.5 | 850 | 816 | 96.0 |
| Haemophilus spp. (full AST) | 791 | 201 | 25.3 | 798 | 246 | 30.8 | 1,589 | 447 | 28.1 |
| Haemophilus spp. (β-lactamase test) | 791 | 439 | 55.5 | 798 | 538 | 67.4 | 1,589 | 977 | 61.5 |
| Klebsiella spp. | 523 | 483 | 92.4 | 861 | 834 | 96.9 | 1,384 | 1,317 | 95.2 |
| Pseudomonas aeruginosa | 1,617 | 1,424 | 88.0 | 1,892 | 1,790 | 94.6 | 3,509 | 3,214 | 91.6 |
| Serratia marcescens | 218 | 190 | 87.1 | 312 | 304 | 97.4 | 530 | 494 | 93.2 |
| Stenotrophomonas maltophilia | 317 | 283 | 89.3 | 330 | 301 | 91.2 | 647 | 584 | 90.3 |
| Total | 7,476 | 6,154 | 82.3 | 9,777 | 8,704 | 89.0 | 17,253 | 14,858 | 86.1 |
Time to results.
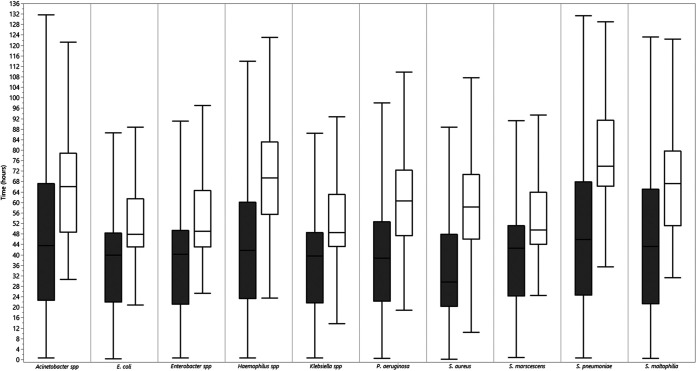
Overall, the median (interquartile range) TTR values from specimen collection for potential bacterial pathogens were 37.0 h (21.8 to 51.7 h) and 60.5 h (46.6 to 72.4 h) for ID and AST, respectively. The median TTR varied by the type of organism for ID and AST. The median TTR for monomicrobial specimens with MCAP ranged from 29.2 h (S. aureus) to 44.9 h (S. pneumoniae) for ID and from 47.9 h (E. coli) to 73.9 h (S. pneumoniae) for AST (Fig. 1).
FIG 1.
TTR for first ID and AST of the most common etiologic agents of HAP/VAP: combined BAL and ETA data. Bars show medians (and interquartile ranges) for time to first organism ID (gray) and AST (white) of monomicrobial specimens that isolated the 10 most common etiologic agents of HAP/VAP.
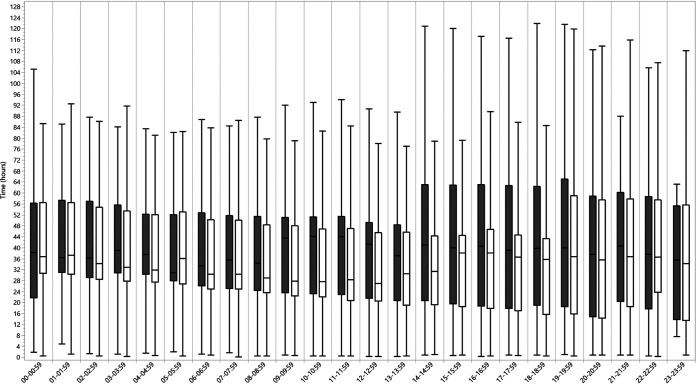
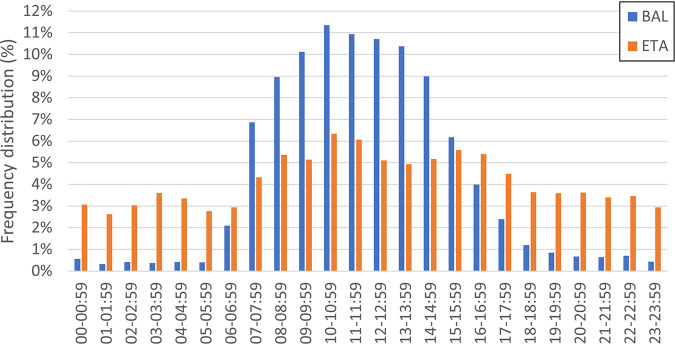
Overall, median TTR for ETA specimens were shorter than BAL specimens for ID (34.8 h versus 41.0 h; P < 0.0001) and AST (58.8 versus 63.8 h; P < 0.0001) for potential bacterial pathogens collectively and for the majority of MCAP (Table 4). The TTR for ID varied according to hour of specimen collection (Fig. 2), with longer TTR values for specimens collected in the late evening and overnight hours. Most BAL specimens were collected between 7:00 a.m. and 3:00 p.m., whereas the collection of ETA specimens was distributed more evenly throughout the day (Fig. 3). BAL specimens collected after 2:00 p.m. were observed to have a wider interquartile range for TTR for ID, compared to BAL specimens collected prior to 2:00 p.m. (Fig. 2).
TABLE 4.
Time to result for most common etiologic agents of HAP/VAP isolated from BAL and ETA specimensa
| Etiologic agent | Time to first ID |
Time to first AST |
||||
|---|---|---|---|---|---|---|
| Median time (h) |
P | Median time (h) |
P |
|||
| BAL | ETA | BAL | ETA | |||
| Gram positive, monomicrobial | ||||||
| Staphylococcus aureus | 32.0 (21.5–49.2) | 28.5 (19.3–45.9) | <0.0001 | 60.5 (45.7–70.2) | 57.7 (46.5–71.3) | 0.54 |
| Streptococcus pneumoniae | 47.3 (25.7–70.1) | 39.7 (22.9–57.4) | <0.0001 | 72.9 (67.5–91.0) | 75.5 (63.8–92.1) | 0.82 |
| Gram negative, monomicrobial | ||||||
| Acinetobacter spp. | 46.6 (23.7–69.0) | 42.7 (22.6–63.9) | .10 | 68.3 (49.8–77.3) | 63.6 (47.5–79.4) | 0.11 |
| Escherichia coli | 43.2 (23.8–49.1) | 38.2 (20.8–47.8) | 0.0005 | 48.2 (44.1–66.1) | 47.5 (41.9–58.8) | 0.0024 |
| Enterobacter spp. | 42.2 (23.1–50.5) | 39.5 (20.9–49.7) | 0.031 | 48.0 (43.7–66.4) | 49.9 (42.7–63.2) | 0.93 |
| Haemophilus spp. | 43.3 (24.4–54.9) | 34.1 (21.1–50.1) | <0.0001 | 68.4 (50.9–78.8) | 69.9 (58.2–85.8) | 0.071 |
| Klebsiella spp. | 41.9 (22.9–50.1) | 38.4 (21.3–48.1) | 0.021 | 47.9 (43.8–66.3) | 48.8 (42.9–60.6) | 0.15 |
| Pseudomonas aeruginosa | 43.4 (23.7–62.3) | 34.3 (21.2–50.3) | <0.0001 | 64.4 (46.8–72.6) | 58.8 (47.8–72.4) | 0.048 |
| Serratia marcescens | 44.7 (24.0–50.6) | 41.6 (26.3–51.1) | 0.55 | 49.2 (45.0–64.1) | 50.0 (43.1–63.6) | 0.71 |
| Stenotrophomonas maltophilia | 46.2 (23.9–69.0) | 38.4 (21.3–58.4) | 0.0046 | 68.3 (51.2–85.3) | 64.3 (51.2–78.1) | 0.039 |
| Polymicrobial | ||||||
| Mixed Gram positive (n = 611) | 38.3 (23.4–51.2) | 35.9 (22.0–53.1) | 0.41 | 67.3 (48.6–81.1) | 67.2 (54.2–77.9) | 0.65 |
| Mixed Gram negative (n = 1,627) | 44.3 (23.9–67.5) | 42.3 (23.3–63.6) | 0.35 | 66.7 (48.0–73.5) | 64.5 (50.0–75.6) | 0.80 |
| Mixed Gram positive and negative (n = 3,589) | 36.4 (22.2–52.7) | 34.3 (20.3–53.3) | 0.11 | 66.1 (47.4–73.5) | 62.3 (48.6–74.8) | 0.18 |
IQR values are indicated in parentheses.
FIG 2.
Median TTR to ID for potential bacterial pathogens by specimen collection hour of the day. Bars show medians (and interquartile ranges) for time to first organism ID for BAL (gray) and ETA (white) specimens.
FIG 3.
Frequency distribution of respiratory specimen collection.
The median TTR values for first organism ID and AST were shorter (P < 0.0001) for monomicrobial specimens (35.7 h [21.2 to 50.6 h] and 58.8 h [46.2 to 71.9 h]) than specimens multiple potential bacterial pathogens (38.1 h [22.3 to 56.0 h] and 64.7 h [48.6 to 74.8 h]).
Compared to non-teaching facilities, teaching facilities had TTR for organism ID (median, 32.9 h [20.8 to 49.6 h] versus 40.3 h [22.9 to 54.4 h]; P < 0.0001) and AST (56.9 h [46.1 to 70.9 h] versus 63.4 h [47.5 to 74.8 h]; P < 0.0001) for bacterial pathogens (Table 5).
TABLE 5.
Time to result for most common etiologic agents of HAP/VAP according to the teaching status of the hospitala
| Etiologic agent | Time to first ID |
Time to first AST |
||||
|---|---|---|---|---|---|---|
| Median time (h) |
P | Median time (h) |
P | |||
| Teaching | Nonteaching | Teaching | Nonteaching | |||
| Gram positive, monomicrobial | ||||||
| Staphylococcus aureus | 26.5 (18.8–46.0) | 31.9 (21.4–49.1) | <0.0001 | 54.8 (45.6–69.2) | 62.0 (46.7–72.3) | <0.0001 |
| Streptococcus pneumoniae | 36.8 (22.2–60.0) | 47.3 (29.7–68.5) | <0.0001 | 72.5 (64.4–86.6) | 75.2 (67.2–93.2) | 0.0006 |
| Gram negative, monomicrobial | ||||||
| Acinetobacter spp. | 36.0 (18.9–52.0) | 49.0 (28.7–69.0) | 0.0001 | 57.4 (46.2–70.2) | 69.1 (56.7–86.1) | 0.0001 |
| Escherichia coli | 36.6 (20.5–47.2) | 42.0 (23.4–50.2) | 0.0003 | 47.6 (43.3–59.8) | 48.5 (43.1–62.9) | 0.24 |
| Enterobacter spp. | 38.8 (19.1–48.6) | 41.2 (23.6–50.1) | 0.030 | 49.4 (43.3–65.0) | 48.9 (42.9–64.4) | 0.88 |
| Haemophilus spp. | 33.0 (20.2–49.9) | 41.1 (25.1–54.2) | <0.0001 | 55.5 (45.6–71.1) | 61.2 (47.4–76.6) | 0.0072 |
| Klebsiella spp. | 35.8 (19.7–46.8) | 41.3 (23.6–50.1) | 0.0001 | 47.9 (43.1–61.1) | 49.3 (43.4–64.4) | 0.19 |
| Pseudomonas aeruginosa | 34.1 (21.3–49.9) | 41.2 (23.2–57.8) | <0.0001 | 57.1 (46.6–70.9) | 63.5 (48.5–74.5) | <0.0001 |
| Serratia marcescens | 42.4 (25.0–56.1) | 42.5 (24.3–49.1) | 0.54 | 51.6 (45.2–65.9) | 47.6 (42.5–50.6) | 0.0007 |
| Stenotrophomonas maltophilia | 40.9 (21.2–63.1) | 44.6 (22.9–66.7) | 0.08 | 65.1 (49.8–73.6) | 68.3 (53.0–87.1) | 0.0011 |
| Polymicrobial | ||||||
| Mixed Gram positive (n = 611) | 32.1 (22.6–49.1) | 43.0 (25.3–59.2) | 0.0002 | 64.0 (48.3–74.5) | 70.4 (60.8–82.1) | <0.0001 |
| Mixed Gram negative (n = 1,627) | 40.3 (21.9–63.2) | 46.1 (25.7–67.2) | <0.0001 | 62.4 (47.6–72.3) | 67.4 (52.8–77.7) | <0.0001 |
| Mixed Gram positive and negative (n = 3,589) | 31.3 (21.2–51.1) | 38.7 (22.2–54.5) | 0.0010 | 62.0 (47.3–72.5) | 65.2 (49.7–76.3) | <0.0001 |
IQR values are indicated in parentheses.
Report timing.
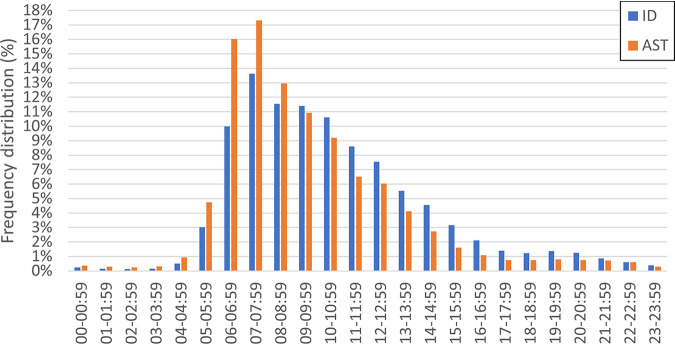
The distribution of respiratory culture result reporting times by hour are shown in Fig. 4. Approximately two-thirds (65.8%) of ID and three-quarters (72.9%) of AST results were reported between the hours of 6:00 a.m. and 12:00 p.m. When analyzed by shift work, both ID and AST results for respiratory specimens with potential bacterial pathogens was reported during the day shift (7:00 a.m. to 2:59 p.m.) for 70% of specimens. Only 12% of the ID and 7% of the AST results are reported on the second shift (3:00 p.m. to 10:59 p.m.).
FIG 4.
Frequency distribution of respiratory culture results.
DISCUSSION
Pneumonia is the eighth most common cause of death in the United States overall, accounting for more than 55,000 deaths in 2017 alone (8). Many studies demonstrate that delayed effective therapy or failing to receive an antimicrobial with activity against the causative pathogen(s) is associated with higher morbidity and mortality in patients with HAP and VAP (9–11). Since clinical features of pneumonia do not distinguish them from other diseases, chest radiography and bacterial culture are often necessary for confirmation of a pneumonia diagnosis. Therefore, a diagnosis of pneumonia may take several days due to the time required for bacteria growth from the respiratory culture. Among 194 U.S. hospitals, the median time to results from respiratory specimen collection to first reporting of organism identification was approximately 37 h, and nearly another 24 h was required for AST results. In general, clinical microbiology laboratories take approximately 1 to 2 days from specimen collection to ID, and 2 to 3 days to report AST for most specimens. However, the median time to result varies significantly between different types of organisms, with AST for Enterobacterales taking ∼48 h and S. aureus and P. aeruginosa each taking closer to ∼60 h. An analysis of 1,288 BAL cultures from Barnes Jewish Hospital, a 1,400-bed nonprofit teaching hospital in Missouri, reported a slightly shorter median TTR for ID and a comparable time to AST at 30.5 and 59 h for Enterobacterales, 21 and 67 h for S. aureus, and 27 and 48 h for P. aeruginosa, respectively, which is consistent with our observation that teaching hospitals have a shorter TTR (12). Of note, the organisms with the most challenging and unpredictable antimicrobial resistance profiles (A. baumannii, P. aeruginosa, and S. maltophilia) often take up to 72 h for AST in each of these studies. Like Jean and Burnham, we also found that polymicrobial cultures have longer TAT for ID and AST.
More than 1 in 3 ETA cultures were positive for one of the organisms that are most associated with HAP/VAP, whereas just under 1 of 4 BAL cultures were positive for one of these organisms. The rates of positivity were highest for patients receiving mechanical ventilation. ETA specimens were more likely to be positive for potential bacterial pathogens than BAL specimens. The observed positivity rate of BAL specimens is consistent with the work of Jean and Burnham, who reported that 20% of BAL cultures were positive for bacterial pathogens, of which P. aeruginosa, S. aureus, Enterobacterales, and S. maltophilia were predominantly recovered (12). Overall, S. aureus and P. aeruginosa were the most prevalent organisms recovered, and this observation was consistent across BAL and ETA specimens when analyzed separately. Klebsiella spp., Haemophilus spp., S. pneumoniae, and E. coli were also common, being recovered in >2% of all specimens. The frequency of organisms observed in the present study is comparable to a study of >6,000 patients hospitalized with pneumonia in intensive care units (ICUs) of 75 U.S. medical centers from 2015 to 2017, as well as a 20-year review (from 1997 to 2016) of 102,995 bacterial respiratory isolates from North America, Europe, and the Asia-Pacific region, and Latin America collected for the SENTRY Antimicrobial Surveillance Program (13). Interestingly, several important respiratory pathogens were isolated more frequently at teaching hospitals versus non-teaching hospitals. The reasons for this observation are not clear from the available data elements, but this may be a result of more sophisticated laboratory methods/reporting and higher-acuity patients at teaching hospitals. Future studies could help understand these disparities in reporting between academic and community hospitals.
Our finding that ETA specimens are less likely to be negative than BAL specimens is to be expected, as noninvasive diagnostic methods such as ETA collection are known to yield higher rates of clinical false positives due to oral and tracheal contamination from colonization or during the sampling procedure itself. Moreover, it may be difficult to differentiate pathogenic specimens from asymptomatic carriage for certain bacteria (14). Another interesting finding of this study was that the median TTR values ID for ETA specimens were ca. 5 to 6 h shorter than for BAL specimens for ID and AST. This finding likely reflects the fact that BAL specimens were predominantly collected between 6 a.m. and 4 pm (i.e., the day shift), whereas ETA specimens were collected across all hours. Based on reporting times, it is likely these are both batched to be read and reported on the day shift. As such, an opportunity for quality improvement in the clinical microbiology laboratory could be to review BAL cultures on the night shifts, providing a more rapid time to results. Unfortunately, we were not able to assess whether the additional detections and difference in TTR for ID/AST between ETA and BAL specimens had an impact on antimicrobial selection and decision making due to the lack of antibiotic administration data, but this should be an area of future investigation.
Therapeutic adjustment following the availability of culture results is encouraged in HAP/VAP guidelines issued both by The European Respiratory Society (ERS)/European Society of Intensive Care Medicine (ESCIM)/European Society of Clinical Microbiology/Infectious Diseases (ESCMID)/Asociación Latinoamericana del Tórax (ALAT) and by the IDSA/American Thoracic Society (ATS) (14, 15). Selecting appropriate empirical therapy for suspected HAP/VAP has become increasingly difficult with the increasing prevalence of MDR bacteria because clinicians must weigh the importance of providing early effective antimicrobials with the risk of broad-spectrum antimicrobials. Most consensus guidelines state that de-escalation is beneficial because it likely reduces antimicrobial resistance, side effects, and costs (16–18). Having diagnostic respiratory specimens with minimal turnaround time means reevaluation of patient management can occur earlier in the course of disease progression, prompting additional diagnostic evaluation or streamlining of pharmacologic interventions. Antimicrobial therapy can be modified based on these culture results, whether that be for a failure of initial therapy coverage identified by the culture results, or a change of antimicrobial coverage to a pathogen-targeted regimen to minimize unnecessary antimicrobial exposure. As a result, the turnaround time for culture results of respiratory specimens may have important implications on the care of patients with HAP/VAP. In fact, there are now molecular panels approved by the FDA for ETA and BAL specimens, which have shown increased pathogen detection and shortened time to result for identification and the presence of resistance markers from common HAP/VAP pathogens compared to traditional culture. Rapid phenotypic testing of these specimens would provide additional actionable information much sooner than the current standard of care and has the potential to significantly impact patient outcomes. On the other hand, the time to obtain ID and AST results from respiratory specimens by conventional culture could be reduced through real-time ID and AST processing during multiple shifts versus bulk testing during selected hours of the day. Although it was not possible to determine the working hours of the labs in this study, the assessment of time to ID according to specimen collection hour (Fig. 2) suggests that a meaningful portion of labs are not performing ID and AST in real time, but rather batching according to first shift, which unavoidably lengthens the overall turnaround time.
One limitation of this study was our inability to make an assessment on the quality of initial Gram stain and semiquantitative cultures. The database does not label culture results as Gram stain findings or quantitation, and <5% of specimens had data that resembled these data points. Without an indicator of specimen quality, it is not possible to know the conditions under which the specimen was obtained, which may have implications on differentiating colonization from a potential infectious episode. In particular, some of the organisms reported can be isolated from respiratory specimens of both healthy individuals and those with bronchopulmonary infections (e.g., S. pneumoniae). Moreover, the present study database does not provide information on individual hospital policies for methods utilized for organism ID/AST or guidance on the classification of organism pathogenicity in respiratory specimens, such as which organisms are considered pathogenic and individually reported in the patient medical record versus organisms that are nonpathogenic and constitute normal microbiota of the respiratory tract and are collectively referred to as normal respiratory flora. Therefore, it is possible that organisms of uncertain pathogenicity in respiratory specimens (i.e., certain Corynebacterium species and coagulase-negative Staphylococcus spp.) may not be accurately represented in the prevalence estimates of the current analysis. Lastly, it is important to note that we evaluated the first identification result reported. Although it is our hope clinicians respond to all data coming from the microbiology laboratory, some may wait until the culture is finalized before adjusting therapy, since such values may underestimate the time to response for these clinical specimens.
Conclusions.
The median respiratory specimen TAT from specimen collection to ID and AST were approximately 1.5 and 2.5 days, respectively. The type of organism, the time of specimen collection, and the hospital teaching status influenced the TAT. Mechanically vented patients and ETA specimens were more likely to recover common etiologic agents of HAP/VAP.
Supplementary Material
ACKNOWLEDGMENT
The authors are employees of Accelerate Diagnostics, Inc.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Chung DR, Song J-H, Kim SH, Thamlikitkul V, Huang S-G, Wang H, So TM-K, Yasin RMD, Hsueh P-R, Carlos CC, Hsu LY, Buntaran L, Lalitha MK, Kim MJ, Choi JY, Kim SI, Ko KS, Kang C-I, Peck KR, Asian Network for Surveillance of Resistant Pathogens Study Group. 2011. High prevalence of multidrug-resistant nonfermenters in hospital-acquired pneumonia in Asia. Am J Respir Crit Care Med 184:1409–1417. doi: 10.1164/rccm.201102-0349OC. [DOI] [PubMed] [Google Scholar]
- 2.Seddon MM, Bookstaver PB, Justo JA, Kohn J, Rac H, Haggard E, Mediwala KN, Dash S, Al-Hasan MN. 2019. Role of early de-escalation of antimicrobial therapy on risk of Clostridioides difficile infection following Enterobacteriaceae bloodstream infections. Clin Infect Dis 69:414–420. doi: 10.1093/cid/ciy863. [DOI] [PubMed] [Google Scholar]
- 3.Schuts EC, Hulscher MEJL, Mouton JW, Verduin CM, Stuart JWTC, Overdiek HWPM, van der Linden PD, Natsch S, Hertogh CMPM, Wolfs TFW, Schouten JA, Kullberg BJ, Prins JM. 2016. Current evidence on hospital antimicrobial stewardship objectives: a systematic review and meta-analysis. Lancet Infectious Diseases 16:847–856. doi: 10.1016/S1473-3099(16)00065-7. [DOI] [PubMed] [Google Scholar]
- 4.Doern CD. 2018. The slow march toward rapid phenotypic antimicrobial susceptibility testing: are we there yet? J Clin Microbiol 56:e01999-17. doi: 10.1128/JCM.01999-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Premier Healthcare Database. 2019. Premier Healthcare Database White Paper: data that informs and performs Premier Applied Sciences, Boyertown, PA. [Google Scholar]
- 6.Murray PR, Rosenthal KS, Pfaller MA. 2020. Role of bacteria in disease, p 152–160. In Medical microbiology, 9th ed Elsevier, Philadelphia, PA. [Google Scholar]
- 7.Miller JM, Binnicker MJ, Campbell S, Carroll KC, Chapin KC, Gilligan PH, Gonzalez MD, Jerris RC, Kehl SC, Patel R, Pritt BS, Richter SS, Robinson-Dunn B, Schwartzman JD, Snyder JW, Telford S, Theel ES, Thomson RB, Weinstein MP, Yao JD. 2018. A Guide to Utilization of the Microbiology Laboratory for Diagnosis of Infectious Diseases: 2018 Update by the Infectious Diseases Society of America and the American Society for Microbiology. Clin Infect Dis 67:e1–e94. doi: 10.1093/cid/ciy381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heron M. 2019. Deaths: leading causes for 2017—national vital statistics reports from the Centers for Disease Control and Prevention, National Center for Health Statistics. National Vital Statistics System 68:77. [PubMed] [Google Scholar]
- 9.Guillamet CV, Vazquez R, Noe J, Micek ST, Kollef MH. 2016. A cohort study of bacteremic pneumonia: the importance of antibiotic resistance and appropriate initial therapy? Medicine 95:e4708. doi: 10.1097/MD.0000000000004708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuti EL, Patel AA, Coleman CI. 2008. Impact of inappropriate antibiotic therapy on mortality in patients with ventilator-associated pneumonia and blood stream infection: a meta-analysis. J Crit Care 23:91–100. doi: 10.1016/j.jcrc.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 11.Muscedere JG, Shorr AF, Jiang X, Day A, Heyland DK, Canadian Critical Care Trials Group. 2012. The adequacy of timely empiric antibiotic therapy for ventilator-associated pneumonia: an important determinant of outcome. J Crit Care 27:322.e7–14–322.e14. doi: 10.1016/j.jcrc.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 12.Jean S, Burnham C-AD. 2019. Turnaround time for pathogen identification and antimicrobial susceptibility testing for culture-based analysis of bronchoalveolar lavage specimens. ASM Microbe 2019, San Francisco, CA, 20 to 24 June 2019. [Google Scholar]
- 13.Sader HS, Castanheira M, Mendes RE, Flamm RK. 2018. Frequency and antimicrobial susceptibility of Gram-negative bacteria isolated from patients with pneumonia hospitalized in ICUs of U.S. medical centres (2015–17). J Antimicrob Chemother 73:3053–3059. doi: 10.1093/jac/dky279. [DOI] [PubMed] [Google Scholar]
- 14.Torres A, Niederman MS, Chastre J, Ewig S, Fernandez-Vandellos P, Hanberger H, Kollef M, Li Bassi G, Luna CM, Martin-Loeches I, Paiva JA, Read RC, Rigau D, Timsit JF, Welte T, Wunderink R. 2017. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociación Latinoamericana del Tórax (ALAT). Eur Respir J 50:1700582. doi: 10.1183/13993003.00582-2017. [DOI] [PubMed] [Google Scholar]
- 15.Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, Napolitano LM, O’Grady NP, Bartlett JG, Carratalà J, El Solh AA, Ewig S, Fey PD, File TM, Restrepo MI, Roberts JA, Waterer GW, Cruse P, Knight SL, Brozek JL. 2016. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 63:e61–e111. doi: 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kollef MH, Morrow LE, Niederman MS, Leeper KV, Anzueto A, Benz-Scott L, Rodino FJ. 2006. Clinical characteristics and treatment patterns among patients with ventilator-associated pneumonia. Chest 129:1210–1218. doi: 10.1378/chest.129.5.1210. [DOI] [PubMed] [Google Scholar]
- 17.Lisboa T, Rello J. 2006. De-escalation in lower respiratory tract infections. Curr Opin Pulm Med 12:364–368. doi: 10.1097/01.mcp.0000239555.01068.dd. [DOI] [PubMed] [Google Scholar]
- 18.Höffken G, Niederman MS. 2002. Nosocomial pneumonia: the importance of a de-escalating strategy for antibiotic treatment of pneumonia in the ICU. Chest 122:2183–2196. doi: 10.1378/chest.122.6.2183. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.