Azithromycin in combination with ceftriaxone is recommended as the first-line treatment for uncomplicated gonorrhea in many countries. Therefore, monitoring of azithromycin susceptibility of Neisseria gonorrhoeae isolates is essential. In 2019, the Clinical and Laboratory Standards Institute (CLSI) listed the MIC breakpoint for a susceptible-only category to azithromycin, but breakpoints for disk diffusion are not yet available. In this study, we evaluated the usefulness of disk diffusion for testing the susceptibility of N. gonorrhoeae isolates to azithromycin.
KEYWORDS: azithromycin, disk diffusion, Neisseria gonorrhoeae
ABSTRACT
Azithromycin in combination with ceftriaxone is recommended as the first-line treatment for uncomplicated gonorrhea in many countries. Therefore, monitoring of azithromycin susceptibility of Neisseria gonorrhoeae isolates is essential. In 2019, the Clinical and Laboratory Standards Institute (CLSI) listed the MIC breakpoint for a susceptible-only category to azithromycin, but breakpoints for disk diffusion are not yet available. In this study, we evaluated the usefulness of disk diffusion for testing the susceptibility of N. gonorrhoeae isolates to azithromycin. A total of 189 clinical isolates susceptible and nonsusceptible to azithromycin were used. Agar dilution MICs were correlated with inhibition zone diameters of azithromycin disks (15-μg) manufactured by BBL and Oxoid. In addition, an interlaboratory study involving two clinical microbiology laboratories was conducted. There was a strong correlation between disk diffusion and agar dilution for BBL disks (r = −0.74; P < 0.001) and Oxoid disks (r = −0.75; P < 0.001). Using a zone diameter breakpoint of ≥27 mm (susceptible) and ≤26 mm (nonsusceptible) yielded good separation between susceptible and nonsusceptible isolates and the least number of discrepancies. Compared to agar dilution, disk diffusion showed high agreement and kappa values of 95.2% and 0.899 (P < 0.001) for BBL disks and 96.8% and 0.933 (P < 0.001) for Oxoid disks, respectively. Major and very major discrepancies were observed in isolates with azithromycin MICs (1 and 2 μg/ml, respectively) near to the breakpoint. These data illustrate that disk diffusion could be a reliable method in clinical laboratories to test susceptibility to azithromycin in N. gonorrhoeae isolates.
INTRODUCTION
Antimicrobial resistance in Neisseria gonorrhoeae is considered a public health concern globally (1). Azithromycin in combination with ceftriaxone is recommended as empirical first-line gonorrhea treatment in many countries and also by the World Health Organization (WHO) (2–4). However, the first failure treatment of dual therapy due to isolates with resistance to both antibiotics has been described (5). Recent data from the WHO Global Gonococcal Antimicrobial Surveillance Program (WHO GASP) revealed that 83.0% of 59 countries reporting data on azithromycin susceptibility informed of resistance to this antibiotic in 2016. Moreover, 49.1% of them reported rates of azithromycin resistance higher than the threshold of ≥5% recommended by the WHO (6). Therefore, increasing prevalence of azithromycin resistance, including low- and high-level resistance, threaten the effectiveness of the dual antimicrobial treatment for gonorrhea.
Recently, the Clinical and Laboratory Standard Institute (CLSI) published a susceptible-only interpretive category (MIC ≤1 μg/ml) for azithromycin (7). The MIC determination by agar dilution remains the gold standard method for antimicrobial susceptibility in N. gonorrhoeae, but it is time consuming and labor intensive, and therefore generally used only by reference laboratories and surveillance studies. Antimicrobial gradient strips for MIC determination are simple to set up as well as interpret, and studies have shown good agreement with agar dilution (8, 9). Nevertheless, these may be expensive and not cost effective, especially in low-income settings. The disk diffusion test may be used as an alternative for N. gonorrhoeae antimicrobial susceptibility as it is simple and economical to perform in clinical microbiology laboratories. Studies analyzing simple and fast test procedures that can be applied in routine laboratories and may allow a faster identification of resistant N. gonorrhoeae isolates are greatly needed.
We thus conducted a study to determine whether disk diffusion was a reliable method for assessing in vitro susceptibility of N. gonorrhoeae isolates to azithromycin.
MATERIALS AND METHODS
N. gonorrhoeae isolates.
A total of 189 clinical isolates of N. gonorrhoeae were selected from the Gonococcal Antimicrobial Susceptibility Surveillance Program-Argentina (GASSP-AR) culture collection to use in the present study. The selection scheme was designed to provide broad distribution of isolates in the azithromycin MIC range of 0.016 to 16 μg/ml. Seventy-four isolates with a MIC of ≥2 μg/ml collected between 2005 and 2019, and 115 isolates within the susceptible range (MIC of ≤1 μg/ml) collected between 2017 and 2019, were included. Identification of the N. gonorrhoeae isolates was performed by Gram staining, oxidase test, superoxol test (30% hydrogen peroxide), carbohydrate utilization reactions, and matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) (Microflex LT, Bruker Daltonik, Bremen, Germany) (10). Isolates were stored at – 80°C in trypticase soy broth containing 20% glycerol. All isolates were subcultured on Difco GC medium base agar (BD, Franklin Lakes, NJ, USA) supplemented with 1% Britalex enrichment supplement (Britania Lab., Argentina), and incubated from 18 to 24 h at 35°C in a humidified environment and enriched with 5% CO2 prior to testing.
Antimicrobial susceptibility testing.
Agar dilution and disk diffusion methods were performed on the 189 N. gonorrhoeae isolates as described in the CLSI document M07-A11 and M02-A13, respectively (11, 12). For MIC determination, azithromycin was obtained from Sigma-Aldrich (St. Louis, MO, USA; lot 020M4703V). Plates containing 2-fold dilutions of azithromycin at a final concentration ranging from 0.004 to 32 μg/ml were prepared in Difco GC medium base agar (GCMB) (lot 8255978) supplemented with 1% Britalex. The antibiotic-containing plates were prepared the day before testing and stored at 4°C. For each isolate, the inoculum was prepared by suspending bacterial colonies in 0.9% phosphate-buffered saline (pH 7.0) to a 0.5 McFarland standard by using a turbidimeter (DEN-1 McFarland Densitometer, Biosan, Riga, Latvia), and further diluted 1:10 in Mueller-Hinton broth (BD, Franklin Lakes, NJ, USA). The same suspension was used within 15 min to perform a disk diffusion test, as described below. A Steers replicator was used to deliver approximately 104 CFU per spot to the surface of the agar. The plates were incubated from 20 to 24 h at 35°C in a humidified environment and enriched with 5% CO2. The MIC was defined as the lowest concentration of azithromycin that produced complete inhibition of visible growth, disregarding a single colony or a faint haze caused by the inoculum. The CLSI breakpoint (MIC ≤1 μg/ml, susceptible [S]) was used for interpretation of azithromycin MIC results (7). Since intermediate or resistant breakpoints are not yet available, isolates with azithromycin MICs ≥2 μg/ml were categorized in this study as nonsusceptible (NS).
The disk diffusion test was performed on Difco GCMB (lot 8255978) supplemented with 1% Britalex enrichment supplement. Approximately 25 ml of GC medium base was poured into 90-mm-diameter petri dishes to a depth of 4 mm. For each isolate, plates were inoculated by dipping one sterile cotton swab into the inoculum and evenly streaking the entire surface of the plates in three directions. A single lot of azithromycin disks (15-μg) from Oxoid (Basingstoke, UK; lot 2394216) and BBL (Sparks, MD, USA; lot 8177619) was used for all isolates, respectively. The plates were incubated under the same conditions as those indicated for the MIC tests. Using calipers, inhibition zone diameters were measured to the nearest millimeter at the inner zone edge.
N. gonorrhoeae ATCC 49226 and the WHO reference strain P were used throughout the study as quality control (7, 13).
Data analysis.
Data were analyzed using Microsoft Excel 2010 software (Microsoft corporation, Redmond, VA) and GraphPad prism 8.0 software (La Jolla, CA, USA). The correlation between the agar dilution reference method and disk diffusion test was determined by plotting the inhibition zones against their respective MICs, and a linear regression analysis was performed. The correlation coefficient was determined using Pearson’s r at a significance level of 0.05.
Since there is a susceptible-only interpretive category defined for azithromycin, the CLSI M52 guideline was used to evaluate the level of disagreement between the agar dilution and disk diffusion methods (14). Therefore, very major discrepancies (VMDs) and major discrepancies (MDs) were calculated. VMDs occurred with N. gonorrhoeae isolates for which MICs indicated nonsusceptibility by the agar dilution method and susceptibility by the disk diffusion method. MDs occurred with N. gonorrhoeae isolates for which MICs indicated susceptibility by the agar dilution method and nonsusceptibility by the disk diffusion method. Agreement between agar dilution and disk diffusion for each brand of azithromycin disks was estimated using the kappa statistic in a 2-by-2 contingency table at a level of significance of 0.05.
Reproducibility and susceptibility interpretation analysis.
The reproducibility of the disk diffusion method was conducted according to established guidelines, and by using N. gonorrhoeae ATCC 49226 and nine WHO reference strains (F, N, O, P, U, W, X, Y, and Z) (7, 13, 15). Each strain was tested in triplicate each day for three different days. Three separate 0.5 McFarland suspensions of each strain were prepared in 0.9% phosphate-buffered saline (pH 7.0) each day of reproducibility testing. The WHO reference strains were selected according to their MIC values (WHO P and U: 4 μg/ml; WHO Y and Z: 1 μg/ml; WHO W and X: 0.5 μg/ml; WHO N and O: 0.25 μg/ml; WHO F: 0.125 μg/ml) (13). For disk diffusion, the same brands and lots of azithromycin disks were used as described before. Agreement between agar dilution and disk diffusion methods was estimated as previously described.
Susceptibility interpretation analysis was made by using WHONET software ver. 5.6 (16). The National Reference Laboratory of STD has tested using azithromycin disks (15-μg) as the routine for all GASSP-AR isolates since 2016, and inhibition zone data obtained have been stored in the WHONET database. Disk diffusion was performed as described above, using Difco GCMB supplemented with 1% Britalex and 15-μg azithromycin disks obtained from Oxoid and BBL (12). A scatterplot was generated combining data from MIC values and inhibition zone diameters of N. gonorrhoeae isolates collected between 2016 and June 2019 (n = 2,344), and the percentage of discrepancies was calculated as described before.
Interlaboratory study.
An interlaboratory study was conducted at two hospital microbiology laboratories belonging to GASSP-AR. All study materials were provided by the National Reference Laboratory. Each laboratory received, in a blinded fashion, 10 serially numbered N. gonorrhoeae isolates comprising four azithromycin-nonsusceptible isolates (n = 2, MIC 4 μg/ml; n = 2, MIC 2 μg/ml) and six azithromycin-susceptible isolates (n = 2, MIC 1 μg/ml; n = 2, MIC 0.5 μg/ml; n = 2, MIC 0.06 μg/ml). The laboratories were unaware of the azithromycin phenotype.
Disk diffusion tests were performed according to CLSI recommendations (12). Testing of azithromycin disk diffusion was performed using two lots of Difco GCMB (lot-1 8255978; lot-2 6032688), and two lots of azithromycin disks (15-μg) from Oxoid (lot-1 2394216; lot-2 2375531) and BBL (lot-1 8151939; lot-2 8177619). All tests were performed in duplicate on two different days, and a total of 32 zone diameters were determined per each isolate. Significant differences in zone diameter values for the lot number of GCMB and the brand and lot number of azithromycin disks were assessed with a Mann-Whitney U test (17). Moreover, the percentage of discrepancies was calculated as described before.
RESULTS
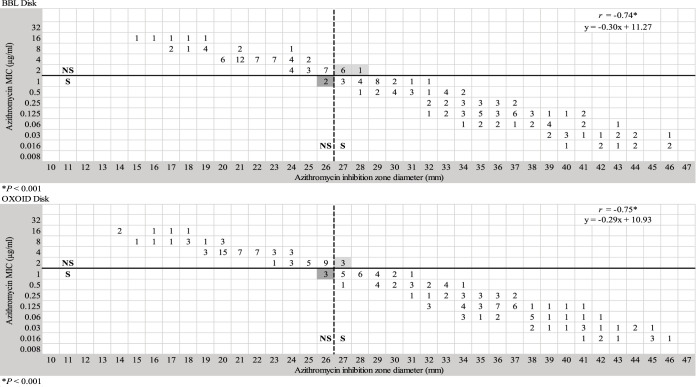
The correlation coefficient, and the scatterplot of the MICs and the zone diameters of azithromycin evaluated in this study, demonstrated a correlation between the reference agar dilution method and disk diffusion test. The Pearson correlation between disk diffusion and agar dilution methods was strong (r = −0.74; P < 0.001) by using BBL disks, and (r = −0.75; P < 0.001) by using Oxoid disks (Fig. 1).
FIG 1.
Scattergram of MICs versus zone diameters (n = 189) for 15-μg azithromycin disks from BBL and Oxoid. The horizontal solid line represents the CLSI breakpoint for azithromycin. The vertical dashed line indicates the zone diameter breakpoint proposed in this study. Very major and major discrepancies are highlighted in light gray and dark gray, respectively.
A scattergram of MICs versus zone diameters was analyzed to determine a tentative zone diameter breakpoint for azithromycin. Using a zone diameter breakpoint of ≥27 mm (susceptible) and ≤26 mm (nonsusceptible) provided the best separation between susceptible and nonsusceptible isolates and the lowest number of discrepancies (Fig. 1). Compared to agar dilution, disk diffusion showed an agreement and kappa index of 95.2% and 0.899 (P < 0.001) for BBL disks, and 96.8% and 0.933 (P < 0.001) for Oxoid Disks, respectively. The rates of VMDs and MDs were 4.0% (3/74) and 2.6% (3/115) with Oxoid disks, and 9.4% (7/74) and 1.7% (2/115) with BBL disks, respectively. MDs and VMDs were observed in isolates with azithromycin MICs of 1 and 2 μg/ml, respectively (Fig. 1).
Reproducibility and susceptibility interpretation analysis.
To test for reproducibility, the zone breakpoint proposed in this study was used. The agreement of disk diffusion with agar dilution was 100% across all strains tested, with no VMDs or MDs detected. Table 1 summarizes the results of the reproducibility study. Azithromycin MICs obtained for WHO reference strains were equal or did not differ more than one dilution steps of those previously reported (13). Moreover, based on disk diffusion data for nine replicates per isolate and combining the BBL and Oxoid results obtained, tentative quality control ranges for the nine N. gonorrhoeae WHO reference strains were determined.
TABLE 1.
Azithromycin MIC and inhibition zone diameter results of N. gonorrhoeae ATCC 49226 and nine WHO reference strains
| Reference strain | Azithromycin MIC (μg/ml) |
Zone diam (mm) |
||
|---|---|---|---|---|
| Reference MICa | MIC in present study | Range | Median | |
| WHO F | 0.125 | 0.125–0.25 | 32–37 | 34 |
| WHO N | 0.25 | 0.125–0.25 | 36–38 | 37 |
| WHO O | 0.25 | 0.25–0.5 | 32–37 | 34 |
| WHO P | 4 | 4 | 22–25 | 24 |
| WHO U | 4 | 4 | 22–25 | 23 |
| WHO W | 0.5 | 0.25–0.5 | 35–38 | 36 |
| WHO X | 0.5 | 0.25–0.5 | 36–38 | 38 |
| WHO Y | 1 | 0.5–1 | 32–37 | 36 |
| WHO Z | 1 | 1 | 30–33 | 31 |
| ATCC 49226 | 0.25–1 | 0.25–0.5 | 35–37 | 36 |
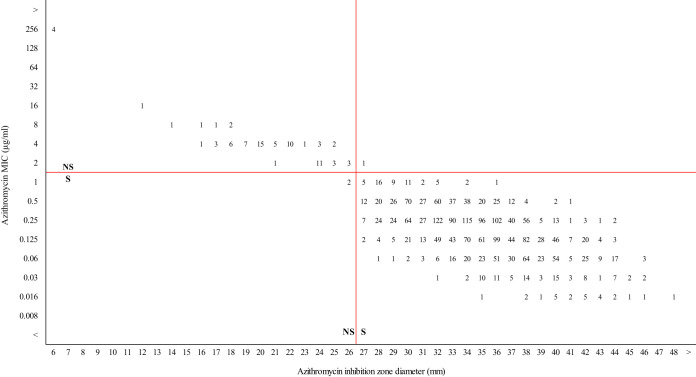
Susceptibility interpretation analysis was performed using a collection of N. gonorrhoeae clinical isolates from the National Reference Laboratory. The tentative zone diameter breakpoint for azithromycin proposed in this study was used to generate a scatterplot of MIC values versus inhibition zone diameters for 2,344 clinical isolates of N. gonorrhoeae. Based on MIC testing, 85.9% (2,013/2,344) of the strains were resistant to ≥1 antimicrobial tested, and 24.3% (569/2,344) were resistant to ≥3 antimicrobial. A summary of the resistance profile of the isolates is shown in Table S1 in the supplemental material. Disk diffusion had an agreement of 99.9% and a kappa index of 0.981 (P < 0.001) compared to agar dilution, and the overall rates of VMDs and MDs were 0.04% (1/2,344) and 0.08% (2/2,344), respectively. Isolates with azithromycin MICs of ≤1 μg/ml (n = 2,262) showed zone diameters between 26 and 48 mm, and MDs were observed in 0.09% (2/2,262) of the isolates, which showed an azithromycin MIC of 1 μg/ml (Fig. 2). Meanwhile, isolates with azithromycin MICs of ≥2 μg/ml (n = 82) showed zone diameters between 6 and 27 mm, and VMDs were observed in 1.2% (1/82) of the isolates, which showed an azithromycin MIC of 2 μg/ml (Fig. 2).
FIG 2.
Retrospective analysis of azithromycin MICs versus inhibition zone diameters for 2,344 N. gonorrhoeae isolates cultured across Argentina from 2016 to June 2019. The horizontal red solid line represents the CLSI breakpoint for azithromycin. The vertical red solid line indicates the zone diameter breakpoint proposed in this study.
Interlaboratory study.
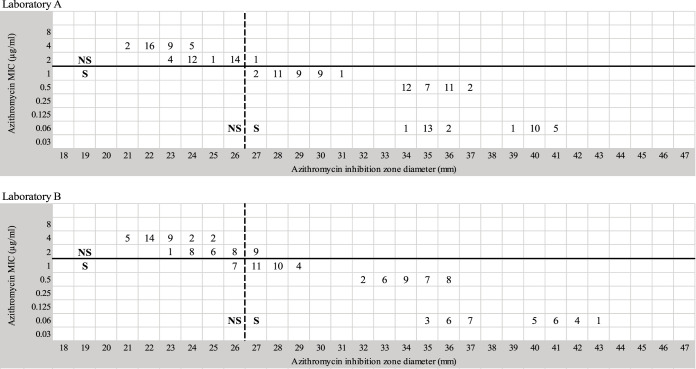
Testing of the 10 N. gonorrhoeae isolates at the two laboratories (identified as A and B) resulted in similar zone diameter determinations by each laboratory. For laboratories A and B, there were no significant differences in zone diameters based on the lot number of GCMB (U = 3096.5; P = 0.723 and U = 2992.0; P = 0.477, respectively) or the brand or lot number of azithromycin disks used (U = 3134.0; P = 0.821 and U = 2928.5; P = 0.353, respectively). Therefore, results were pooled for analysis. Figure 3 illustrates the similarity in MIC and zone diameter distributions by using a 15-μg azithromycin disk in the two laboratories. Compared to agar dilution, disk diffusion showed an agreement and kappa index of 99.4% and 0.987 (P < 0.001) for laboratory A, and 90% and 0.791 (P < 0.001) for laboratory B, respectively. Comparing laboratories results, the Oxoid disks performed slightly better at identifying isolates correctly than the BBL disks. A summary of MIC values and inhibition zone diameters for BBL and Oxoid disks is shown in Fig. S1. The rates of VMDs and MDs were 3.1% (2/64) and 4.2% (4/96) with Oxoid disks, and 12.5% (8/64) and 3.1% (3/96) with BBL disks, respectively. These results are related to the correlation analysis where MDs and VMDs were observed in isolates with azithromycin MICs of 1 and 2 μg/ml, respectively.
FIG 3.
Azithromycin MIC values and disk diffusion zone diameters (using BBL and Oxoid disks) recorded by two laboratories (A and B) when testing ten N. gonorrhoeae strains in duplicate on two different days. The horizontal solid line represents the CLSI breakpoint for azithromycin. The vertical dashed line indicates the zone diameter breakpoint proposed in this study.
DISCUSSION
Accurate laboratory susceptibility testing is essential for proper usage of an antibiotic. Moreover, early detection of antimicrobial resistance to first-line drugs represents an important task for public health agencies. Many clinical laboratories, particularly in developed countries, use gradient strips for susceptibility determination in clinical and research setting. Disk diffusion testing is one of the oldest approaches to antimicrobial susceptibility testing. However, this method is mostly used in developing countries due to its affordability and accessibility, and in some areas, it may be the only method available. Dual therapy of azithromycin plus ceftriaxone is currently recommended as first-line treatment for gonorrhea in many countries. Therefore, testing the feasibility of alternative methods such as the disk diffusion for monitoring azithromycin and ceftriaxone susceptibility is essential. Our aim was to compare the level of reliability and performance of disk diffusion against agar dilution for susceptibility testing of N. gonorrhoeae isolates to azithromycin. Linear regression analysis showed a strong degree of correlation between disk diffusion and agar dilution method. Based on the MIC breakpoint for azithromycin recently recommended by the CLSI, the disk diffusion breakpoint of ≤26 mm (nonsusceptible) and ≥27 mm (susceptible) showed the best separation between susceptible and nonsusceptible isolates and the fewest number of discrepancies. These results are in line with a previous study using 15-μg azithromycin disks, in which a good correlation was observed between zone diameters of ≤27 mm and isolates with azithromycin MICs of >1 μg/ml (18). Two sources of azithromycin 15-μg disks were evaluated, which showed a high agreement of 95.2% (BBL disks) and 96.8% (Oxoid disks), respectively. Moreover, in terms of the kappa, an almost perfect agreement (kappa >0.8) was also observed between agar dilution and disk diffusion methods (19). However, an unacceptable rate of VMDs for BBL disks (9.4%) and Oxoid disks (4.0%) was observed (14). The BBL disk provided larger zone diameters (≥1 mm) than the Oxoid disk. Therefore, a higher rate of VMDs was observed. These results were also reflected in the interlaboratory study. Two clinical laboratories achieved a high agreement when testing a collection of 10 N. gonorrhoeae strains, which included susceptible and nonsusceptible strains. However, the rates of VMD and MD were 3.1% and 4.2% for Oxoid disks, and 12.5% and 3.1% for BBL disks, respectively, each one above acceptable limits (<3%) (14). Discrepancies were observed in isolates with azithromycin MICs of 1 and 2 μg/ml, which are near the upper limit of the susceptible breakpoint. The percentages of VMDs and MDs may be attributed, in part, to the absence of an intermediate range for azithromycin, and/or the fact that the population of strains with an azithromycin MIC between 1 and 2 μg/ml studied (n = 42) was small. The CLSI recently established a “susceptible-only” MIC breakpoint for azithromycin, as there are few clinical data available to support the efficacy of azithromycin in infections caused by isolates with MICs between 2 and 16 μg/ml (20). Therefore, additional surveillance and clinical data with special emphasis on isolates with MICs in the range of 2 to 16 μg/ml are necessary for the establishment of optimal susceptibility interpretive criteria for azithromycin. These criteria will also help determine significant performance differences between MIC determination and disk diffusion methods according to the error rate-bounded method recommended by the CLSI M23 document (21). For now, the lack of an intermediate and resistant category means that category errors between a test method and the reference method cannot be categorized as major, minor, or very major errors.
We found that the agreement between disk diffusion and agar dilution method was >95% (15). Whether disk diffusion testing reliably detected both categories of susceptibility, we used a challenge data set that included azithromycin zone diameters of 2,344 clinical isolates with a high diversity of antibiotic resistance profiles. The disk diffusion showed a high equivalent categorization of the isolates to agar dilution, and an acceptable percentage of discrepancies. One limitation of this data set was the low number of isolates with azithromycin MICs of 1 μg/ml, 2 μg/ml, and so forth. However, in Argentina, these azithromycin MICs are represented in <5% of the isolates (6).
Quality assurance is essential to ensure the quality of antimicrobial susceptibility testing by disk diffusion (12). The results from this study provide initial ranges for 9 WHO reference strains, which may be used in conjunction with the strain ATCC 49226 to support accurate disk diffusion testing for monitoring the in vitro activity of azithromycin during routine clinical antimicrobial susceptibility testing.
In conclusion, these data extend the possible usefulness of disk diffusion to determine in vitro susceptibility of N. gonorrhoeae isolates to azithromycin. Moreover, these results appear to be consistent, derived from comparative and statistical analysis, and testing a high number of clinical isolates with different susceptibility profiles. Until azithromycin zone breakpoints become standardized, the inclusion of a 15-μg azithromycin disk in the routine panel of antibiotics for susceptibility testing provides a feasible method that may be used in resource-limited settings in which MIC determinations methods are not readily available. Additional multicenter studies are required to evaluate the suitability of the tentative breakpoint proposed in this study.
Supplementary Material
ACKNOWLEDGMENTS
The GASSP-AR Working Group is composed of the following people: Ciudad Autónoma de Buenos Aires: S. Bergese, J. Smayevski, M. Turco, C. Garbaz, M. Morales, C. Alfonso, M. Montoto, M. Marcato, M. Cervetto, M. Giovanakis, L. Scocozza, L. Cardozo, N. Prieto, A. Tarzia, V. Cames; Buenos Aires: M. Machaín, M. Garrone, V. Vilches, M. Sparo, A. Tognieri, M. Rizzo, N. Casanova, G. Sly, O. Mariñasqui, P. Simone, L. Moreno, S. Odriz, L. Spadachini; Córdoba: T. Lopez; Santa Fe: J. Valles, V. Manias; Chaco: A. Brihuela, H. Solís, A. Burzla; Salta: V. Silva, N. Sponton, A. Berejnoi; Neuquén: V. Guillermel; Rio Negro: G. Rivollier, M. Roncallo, M. Alvarez; Chubut: M. Flores; La Pampa: A. Pereyra, N. Scarone; Mendoza: F. Ampuero, C. Bandoni, A. C. Lopez, A. Lopez; Santa Cruz: W. Krause; Formosa: N. Pereyra; Entre Ríos: N. Yoya, A. Prestifilippo; Tucumán: L. Basco, N. Cudmani; Jujuy: M. Mernes; San Juan: P. Ranea; Tierra del Fuego: M. Vargas; Corrientes: R. Pato; Misiones: G. Bello Velázquez, S. Roginski.
We declare we have no competing interests.
This study was supported by the National Administration of Laboratories and Health Institutes (ANLIS) Dr. Carlos G. Malbrán-Ministry of Health, Argentina.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Wi T, Lahra MM, Ndowa F, Bala M, Dillon JR, Ramon-Pardo P, Eremin SR, Bolan G, Unemo M. 2017. Antimicrobial resistance in Neisseria gonorrhoeae: global surveillance and a call for international collaborative action. PLoS Med 14:e1002344. doi: 10.1371/journal.pmed.1002344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bignell C, Unemo M, European STI Guidelines Editorial Board. 2013. 2012 European guideline on the diagnosis and treatment of gonorrhoea in adults. Int J STD AIDS 24:85–92. doi: 10.1177/0956462412472837. [DOI] [PubMed] [Google Scholar]
- 3.Workowski KA, Bolan GA, Centers for Disease Control and Prevention. 2015. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 64:1–137. [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. 2016. WHO guidelines for the treatment of Neisseria gonorrhoeae. World Health Organization, Geneva, Switzerland: http://www.who.int/reproductivehealth/publications/rtis/gonorrhoea-treatment-guidelines/en/. [PubMed] [Google Scholar]
- 5.Fifer H, Natarajan U, Jones L, Alexander S, Hughes G, Golparian D, Unemo M. 2016. Failure of dual antimicrobial therapy in treatment of gonorrhoea. N Engl J Med 374:2504–2506. doi: 10.1056/NEJMc1512757. [DOI] [PubMed] [Google Scholar]
- 6.Unemo M, Lahra MM, Cole M, Galarza P, Ndowa F, Martin I, Dillon JR, Ramon-Pardo P, Bolan G, Wi T. 2019. World Health Organization Global Gonococcal Antimicrobial Surveillance Program (WHO GASP): review of new data and evidence to inform international collaborative actions and research efforts. Sex Health 16:412–425. doi: 10.1071/SH19023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clinical and Laboratory Standards Institute. 2019. Performance standards for antimicrobial susceptibility testing: twenty-ninth informational supplement M100-S29. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 8.Papp JR, Rowlinson MC, O'Connor NP, Wholehan J, Razeq JH, Glennen A, Ware D, Iwen PC, Lee LV, Hagan C. 2018. Accuracy and reproducibility of the Etest to detect drug-resistant Neisseria gonorrhoeae to contemporary treatment. J Med Microbiol 67:68–73. doi: 10.1099/jmm.0.000651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desjardins M, Lefebvre B, Labbé AC, Martin I, Mauffrey F, Longtin J, Fortin C. 2017. Agar gradient diffusion susceptibility testing for Neisseria gonorrhoeae: a reliable alternative to agar dilution? Open Forum Infect Dis 4:S107. doi: 10.1093/ofid/ofx163.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. 2013. Laboratory diagnosis of sexually transmitted infections, including human immunodeficiency virus. World Health Organization, Geneva, Switzerland: http://apps.who.int/iris/bitstream/10665/85343/1/9789241505840_eng.pdf. [Google Scholar]
- 11.Clinical and Laboratory Standards Institute. 2018. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard-eleventh edition, CLSI document M07-A11. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 12.Clinical and Laboratory Standards Institute. 2018. Performance standards for antimicrobial disk susceptibility tests; approved standard-thirteenth edition, CLSI document M02-A13. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 13.Unemo M, Golparian D, Sánchez-Busó L, Grad Y, Jacobsson S, Ohnishi M, Lahra MM, Limnios A, Sikora AE, Wi T, Harris SR. 2016. The novel 2016 WHO Neisseria gonorrhoeae reference strains for global quality assurance of laboratory investigations: phenotypic, genetic and reference genome characterization. J Antimicrob Chemother 71:3096–3108. doi: 10.1093/jac/dkw288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clinical and Laboratory Standards Institute. 2015. Verification of commercial microbial identification and antimicrobial susceptibility testing systems; first edition CLSI guideline M52. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 15.Clark RB, Lewinski MA, Loeffelholz MJ, Tibbetts RJ. 2009. Cumitech 31A, verification and validation of procedures in the clinical microbiology laboratory. In Sharp SE. (ed), ASM Press, Washington, DC. [Google Scholar]
- 16.O’Brien TF, Stelling JM. 1995. WHONET: an information system for monitoring antimicrobial resistance. Emerg Infect Dis 1:66. doi: 10.3201/eid0102.950209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mann HB, Whitney DR. 1947. On a test of whether one of two random variables is stochastically larger than the other. Ann Math Statist 18:50–60. doi: 10.1214/aoms/1177730491. [DOI] [Google Scholar]
- 18.Chisholm SA, Neal TJ, Alawattegama AB, Birley HD, Howe RA, Ison CA. 2009. Emergence of high-level azithromycin resistance in Neisseria gonorrhoeae in England and Wales. J Antimicrob Chemother 64:353–358. doi: 10.1093/jac/dkp188. [DOI] [PubMed] [Google Scholar]
- 19.Landis JR, Koch GG. 1977. The measurement of observer agreement for categorical data. Biometrics 33:159–174. doi: 10.2307/2529310. [DOI] [PubMed] [Google Scholar]
- 20.Kersh EN, Allen V, Ransom E, Schmerer M, Cyr S, Workowski K, Weinstock H, Patel J, Ferraro MJ. 2020. Rationale for a Neisseria gonorrhoeae susceptible-only interpretive breakpoint for azithromycin. Clin Infect Dis 70:798–804. doi: 10.1093/cid/ciz292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clinical and Laboratory Standards Institute. 2018. Development of in vitro susceptibility testing criteria and quality control parameters; approved guideline-fifth edition. CLSI document M23-A5. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.