Abstract
A clearer understanding of the immune-mediated loss of transgene from cutaneous epithelium is necessary for development of effective clinical gene therapy protocols for patients who carry null mutations in the target gene. We have used retrovirus-mediated transfer of lacZ to mouse skin as a model to investigate the mechanism of immune-mediated transgene loss in skin. Transduction of C57Bl/6 mouse skin resulted in elicitation of both humoral and cellular immune responses. Antibody responses did not play a major role in the loss of transgene. Infiltration of the transduced skin with CD4+ and CD8+ cells and induction of transgene-specific cytotoxic T lymphocytes implied a role for T-cell-mediated responses. Transduction of mice deficient in either major histocompatibility complex (MHC) class I or class II molecules resulted in transient transgene expression. Only in MHC−/− mice lacking expression of both class I and class II MHC molecules was persistent transgene expression seen. These data indicate a primary role for T-cell-mediated responses in the immune-mediated loss of transgene expression. Furthermore, CD4 and CD8 T cells have overlapping roles and either population can effectively eliminate transduced cells. Therefore, long-term cutaneous gene therapy may require development of strategies to interfere with activation or function of both T cell populations.
Introduction
Skin is the largest and most accessible organ in the human body and hence an attractive tissue site for development of new gene therapy approaches for treatment of skin and hair disorders as well as systemic genetic disorders [1–6]. In animal models of cutaneous gene therapy, long-term transgene expression has been described following gene transfer to epithelial stem cells with integrating vectors [7,8]. However, the role of immunological response in durability of transgene expression is often ignored, since most of these studies are carried out in immune-deficient mice. The nature of host responses in gene therapy depends on various factors, including the immunogenicity of the transgene product, that of the vector elements, and the type of cell and tissue producing the gene product. Recombinant retroviral vectors (RRVs) are the most suitable vectors for long-term gene therapy in the continuously renewing tissues such as epidermis [7]. RRVs do not encode any viral proteins, leaving the transgene product and the virus envelope as the only source of nonself antigens. Several studies involving retrovirus-mediated gene transfer to liver have shown long-term expression of β-gal transgene by hepatocytes [9,10]. However, autologous grafting of retrovirus-transduced β-gal-expressing keratinocytes onto immunocompetent animals resulted in transgene expression lasting 2–3 weeks [11,12]. While the immunological responses to the transgene product in these studies were not described, the transient expression of β-gal in the grafted skin is suggestive of a role for tissue microenvironment in the host responses to an antigen.
Skin has an important immune-associated function and serves as a primary barrier against foreign antigens [13]. It contains a large number of antigen-presenting cells (APCs), including Langerhans cells and dermal dendritic cells, which are specialized in initiation of immune responses. Furthermore both keratinocytes and dendritic cells are able to secrete inflammatory cytokines that have significant effects on the nature and magnitude of the resulting immune response [13]. While these unique immunological features of the cutaneous microenvironment are ideal for genetic vaccination [14], the ability of skin to mount immune responses to a neoantigen may be a great limitation for therapeutic gene therapy for those patients who carry null mutations in the target gene.
We recently described a method for retrovirus-mediated gene transfer to mouse skin, which resulted in long-term expression of the transgene in immune-deficient mice. In immune-competent mice, however, transgene expression was short-lived. Transduction of mouse skin with a RRV encoding the lacZ reporter gene induced host immune responses against the viral coat protein and the transgene product. A direct correlation between the presence of transgene-specific immunological responses and the duration of transgene expression was demonstrated by persistence of the transgene expression in immune-competent mice that were tolerant to the transgene product or when the transgene product was nonimmunogenic (i.e., neor gene product). Furthermore, these data confirmed the lack of immunogenicity of integrated RRV elements in the transduced cells [15].
In the present study, we have used the in vivo transduction of mouse skin to delineate the type of immune responses involved in the loss of skin-directed transgene expression. The data presented here show that transgene-specific T cell responses play a major role in elimination of transduced cells. Transduction of skin of various knockout mouse models with defined immune-compromised status indicates that suppression of both CD8 and CD4 T cells is required to achieve long-term expression of a neoantigen in skin.
Results
Contribution of Antibody-Mediated Responses in Eliminating Transduced Cells
In vivo transduction of mouse skin with RRVs encoding lacZ has been shown to result in generation of antibodies to the viral envelope protein (neutralizing) and the transgene product [15]. We examined the potential role of antibody-mediated responses in eliminating the transduced cells in mice deficient for immunoglobulin heavy chain (Igh−/−). We transduced C57Bl/6 (B6), Igh−/−, or MTnLZ (β-gal transgenic) mice with β-gal-encoding retrovirus (RRV-LZ, 3 × 107 transducing units/mouse) and assessed transgene expression by X-gal staining of cornified cells removed from the skin surface as described under Material and Methods. The presence of X-gal-positive cornified cells (blue cells) on the skin surface of transduced mice at 1 week posttransduction indicated transgene expression by epidermal keratinocytes (Fig. 1A, left). The transduced area of the skin was identified and the same area was examined by tape stripping at 4 weeks posttransduction. Examination of the cornified cells at this time indicated the loss of transgene expression in both B6 and Igh−/− mice, as no X-gal-positive cells were present on the tapes (Fig. 1A). On the contrary, β-gal expression was observed in MTnLZ mice that were tolerant to β-gal (Fig. 1A). The lower number of transduced cells in the epidermis of these mice at 4 weeks posttransduction reflects the loss of transduced transit amplifying cells shed from the surface due to the normal epidermal turnover [16].
FIG. 1.
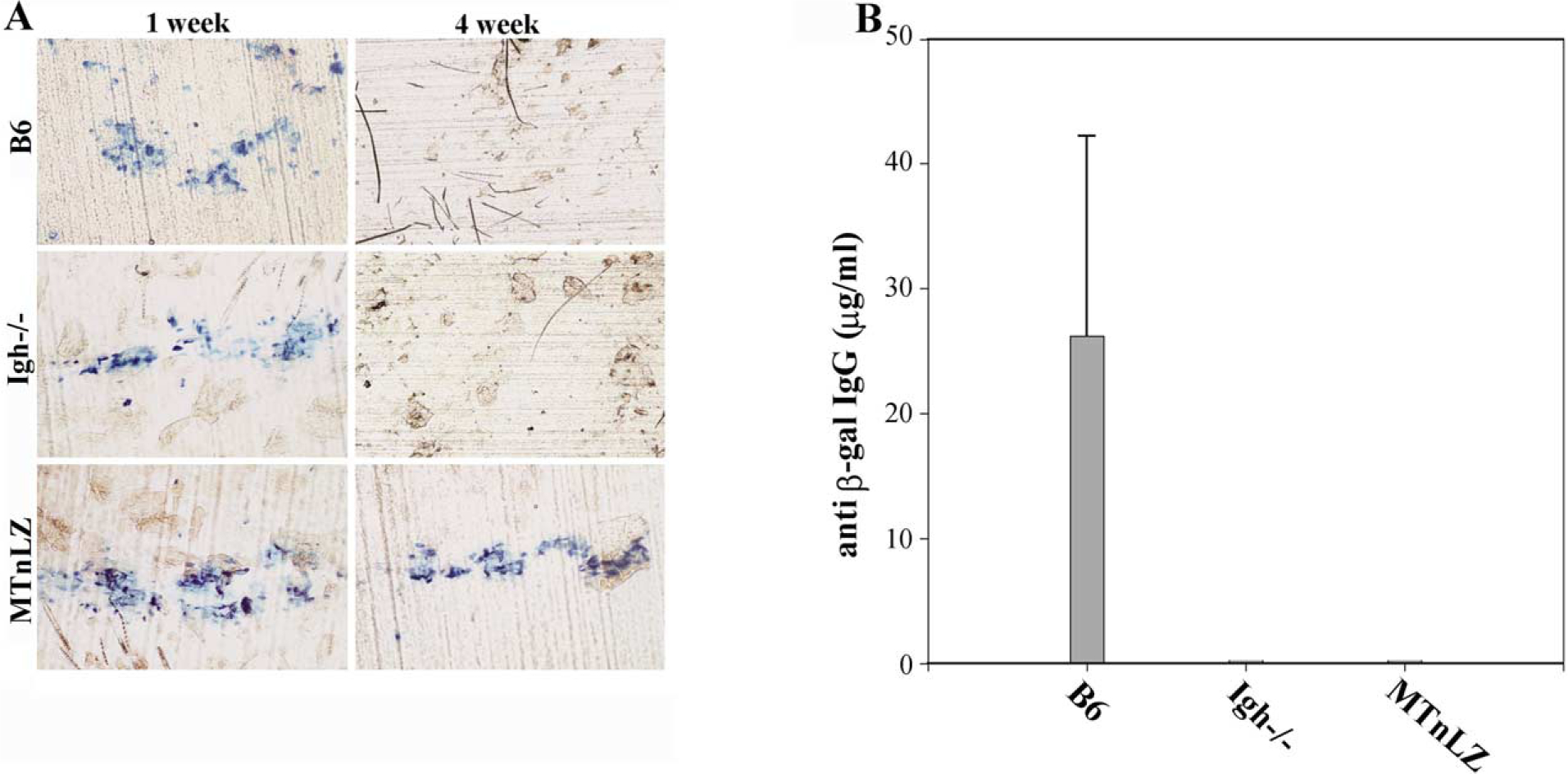
β-Gal expression and induction of β-gal-specific IgG following in vivo transduction of mouse skin. (A) Dorsal skin of B6, Igh−/−, and MTnLZ mice was transduced with MFG-LZ, and at 1 and 4 weeks posttransduction, β-gal expression in the same area of the skin was assessed by tape stripping and staining of adherent cornified cells with X-gal (blue staining). (B) Sera were collected at 4 weeks posttransduction and assayed for the presence of anti-β-gal IgG by ELISA. The concentration of anti-β-gal IgG is expressed based on the concentration of monoclonal anti-β-gal antibody used as a standard in the ELISA. Error bar indicates standard deviation for each group (n = 6).
Examination of sera collected from transduced mice at 4 weeks posttransduction indicated substantial quantities of β-gal-specific antibodies in B6 mice (Fig. 1B). We did not detect such antibodies in the sera collected from Igh−/− or MTnLZ mice (Fig. 1B). However, the lack of antibody response in Igh−/− mice did not alter the transient nature of transgene expression as no X-gal-positive surface cells were detected at 4 weeks posttransduction (Fig. 1A). These data indicate that transgene-specific antibody responses do not play a major role in the clearance of transduced keratinocytes.
Infiltration of CD4- and CD8-Positive Cells and Induction of β-Gal-Specific Cytotoxic T Lymphocytes (CTL) Following In Vivo Transduction of Mouse Skin
To examine the role of cell-mediated responses in the loss of skin-directed transgene expression, we transduced B6 mice (H-2b) or MTnLZ mice (H-2b) at 7 weeks of age with RRV-LZ. The phenotype and relative numbers of infiltrating lymphocytes were assessed by histochemical analysis of the transduced tissue. Both viral infection and derm-abrasion that was used to induce epidermal hyperplasia resulted in an acute inflammatory response in the early phases of in vivo transduction. These responses were di-minished by 7–10 days posttransduction as the epidermis was normalized [15]. To examine cell infiltrates that were specific to the transgene product, we obtained tissue biopsies from transduced skin at 14 days posttransduction. Frozen tissue sections were analyzed by X-gal staining to detect transduced cells, followed by immunostaining to detect CD4+ or CD8+ cells. As shown in Fig. 2, no X-gal-positive cells were observed in the tissue sections obtained from transduced skin of B6 mice, indicating the loss of β-gal expression at 2 weeks posttransduction (Figs. 2A and 2B). Furthermore, a significant number of CD4+ and CD8+ cells infiltrated both dermal and epidermal compartments of the transduced skin (Figs. 2A and 2B). On the contrary, β-gal expression persisted in the transduced skin of MTnLZ mice as indicated by the presence of X-gal-stained epithelial cells in the tissue (Figs. 2C and 2D). CD4+ cells were present at lower numbers and were localized mainly to the dermal compartment of the skin (Fig. 2C) and CD8+ cells were rarely found in the transduced skin of the β-gal-tolerant mice (Fig. 2D). This was similar to the distribution of CD4+ cells (mainly macrophages and dermal dendritic cells) and CD8+ cells in the nontransduced mouse skin (data not shown) [17]. These data indicated the recruitment of CD4+ and CD8+ cells in response to the expression of transgene in the skin.
FIG. 2.
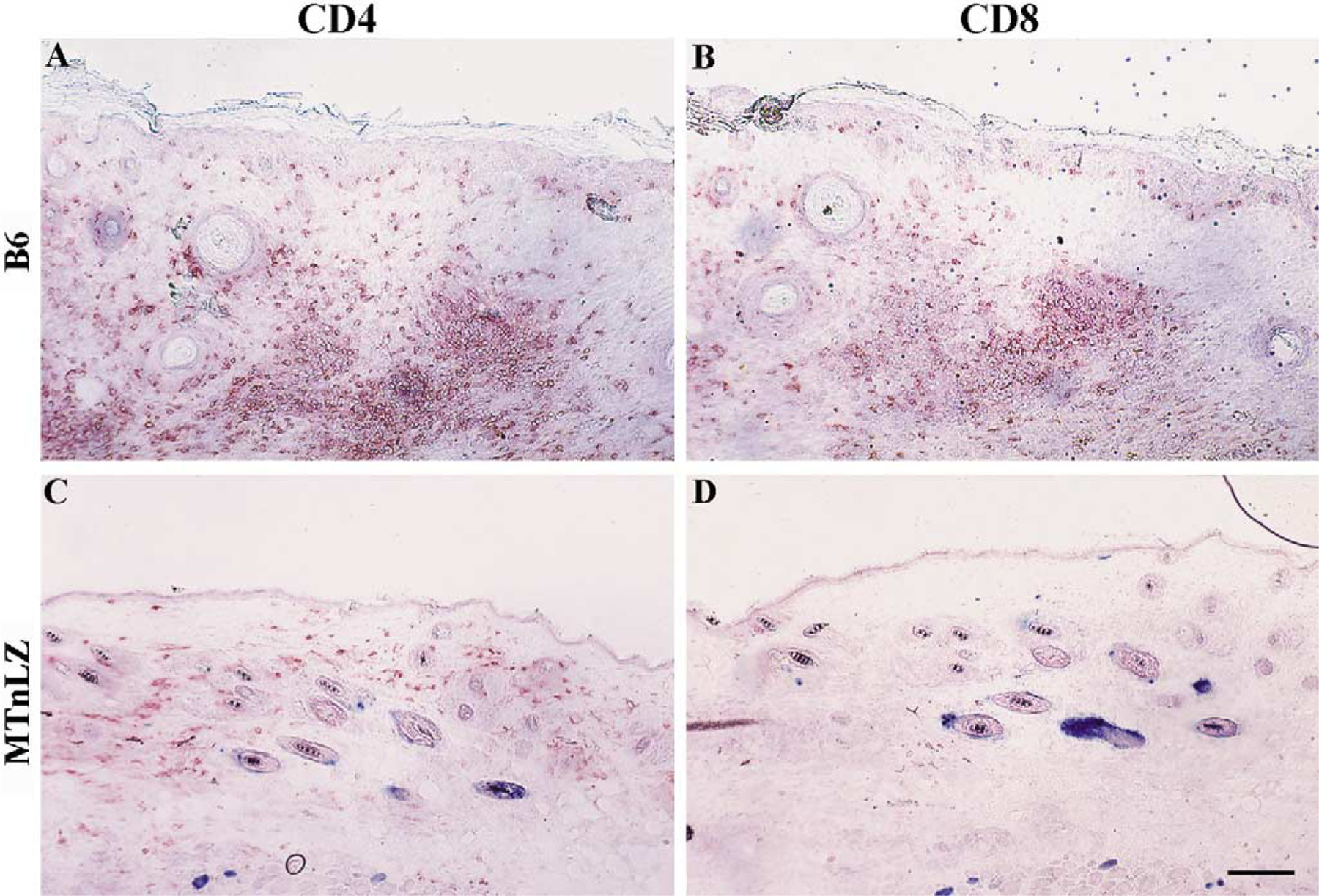
Cellular infiltrates in the RRV-LZ-transduced skin of MTnLZ and B6 mice. B6 mice (A, B) or β-gal transgenic mice (MTnLZ; C, D) were transduced with 3 × 107 infectious viral particles as described under Material and Methods. Two weeks later, transduced skin tissues were harvested from both groups of mice and cryosections were stained with X-gal (blue staining) followed by immunohistochemical staining using anti-CD4 (A, C) or anti-CD8 (B, D) antibodies (red staining). No X-gal-stained cells were present in the transduced skin of B6 mice. Bar, 60 μm.
Since isotype switching is controlled by humoral factors, determination of the transgene-specific IgG subclasses would delineate the type of mediators and the subset of helper T cells that are activated in transduced mice [18]. We analyzed sera collected from lacZ-transduced B6 mice for the presence of anti-β-gal IgG subclasses. The results indicated the prevalence of β-gal-specific IgG2a and IgG2b subtypes with an IgG2a:IgG1 ratio of 8 (Fig. 3). The profile of IgG subtypes suggested a Th-1 polarized response and activation of cell-mediated cytotoxic responses.
FIG. 3.
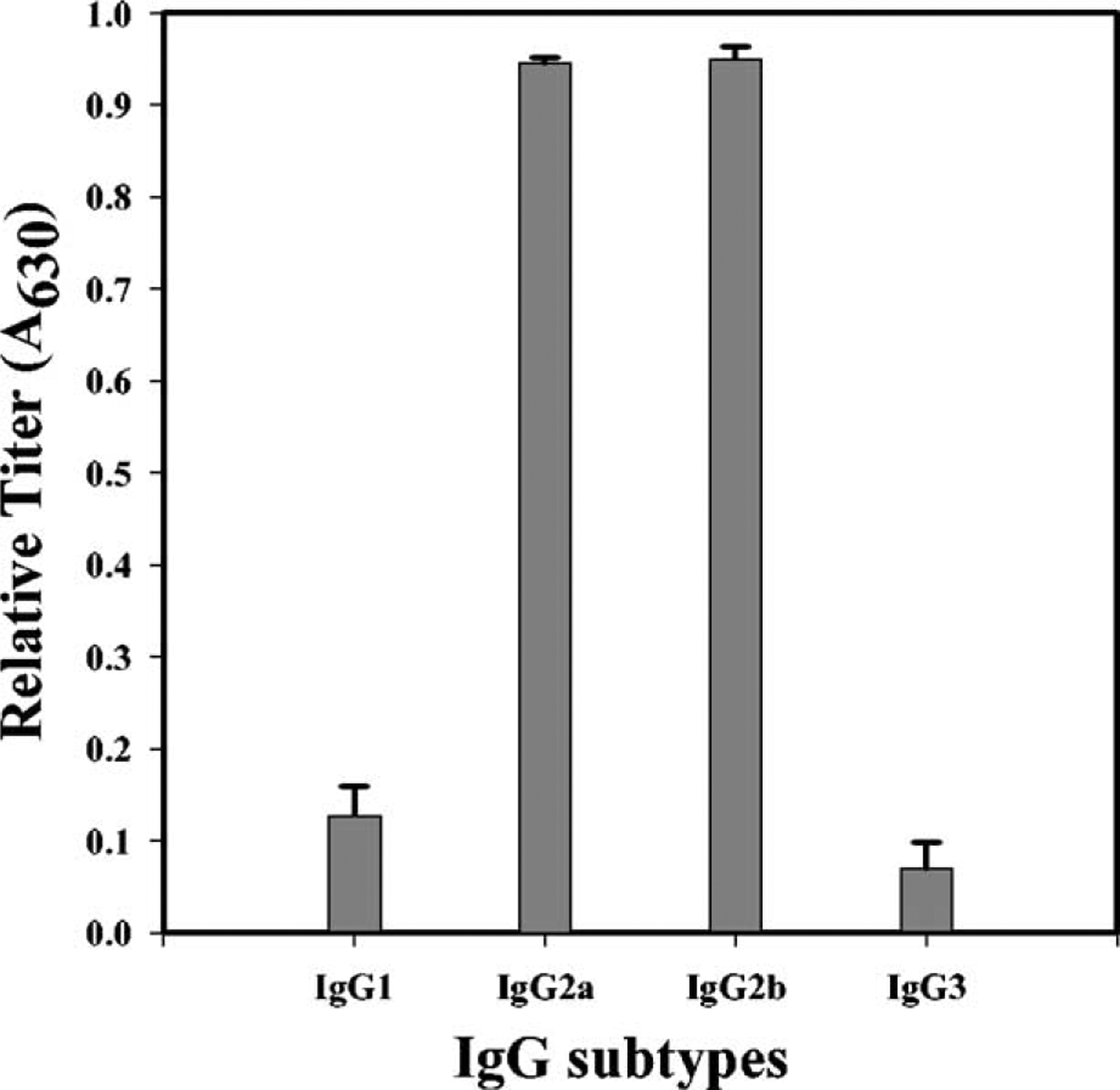
Anti-β-gal IgG subclasses in serum of transduced B6 mice. Serum was collected at 4 weeks posttransduction from RRV-LZ-transduced B6 mice and the isotype of anti-β-gal IgG was determined by ELISA using goat antibodies specific for mouse IgG1, IgG2a, IgG2b, or IgG3. Data are expressed as the means of the optical density at 630 nm (A630) ± SD for 1:50 dilution of the serum samples (n = 6).
We assessed the presence of β-gal-specific CTLs in transduced mice in a standard 6-h 51Cr-release assay. Lymphocytes were obtained from the spleen and the lymph nodes of transduced mice and stimulated for 5 days in vitro with RRV-LZ-transduced cells. The cytolytic responses of these effector cells were measured by their ability to kill syngeneic fibroblasts (BLKSV cells) expressing β-gal or neomycin phosphotransferase (neor gene product used as a control). As shown in Fig. 4, substantial cytolysis of β-gal-expressing target cells was observed when lymphocytes from transduced B6 mice were used as the effector population (top). This lysis was specific for β-gal since control neor-expressing target cells were not lysed. No cytolysis of lacZ or neor transduced target cells was observed with lymphocytes obtained from β-gal-transduced MTnLZ mice (bottom). These results indicated that the loss of β-gal expression in the transduced skin is likely due to the elimination of transduced cells by transgene-specific CTLs.
FIG. 4.
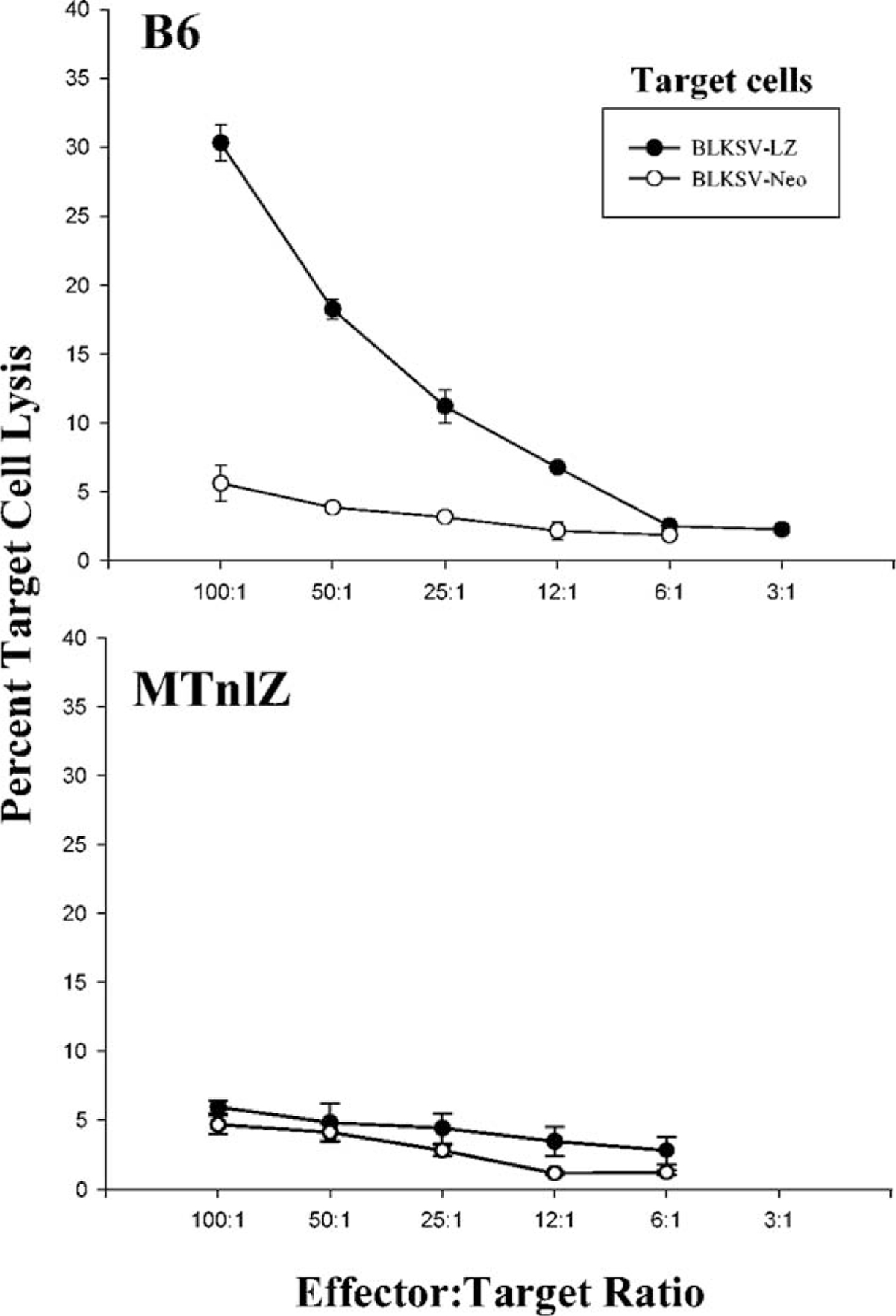
CTL responses in RRV-LZ-transduced B6 or MTnLZ transgenic mice. Lymphocytes harvested from the spleen and lymph nodes of RRV-LZ-transduced B6 mice (top) or MTnLZ transgenic mice (bottom) at 21 days posttransduction were restimulated in vitro for 5 days and tested for specific lysis of LZ-infected (closed circle) or neo-infected (open circle) BLKSV target cells in a 6-h 51Cr-release assay. Percentage specific lysis is expressed as a function of different effector-to-target ratios as shown.
Contribution of CD4+ and CD8+ T Cells to the Loss of Skin Cells Expressing a Foreign Antigen
Presentation of foreign antigens by MHC molecules to either cytotoxic CD8 T cells or helper CD4 T cells is a key component of adaptive host immune responses [19,20]. We analyzed the relative importance of CD4 and CD8 T cells in the loss of transgene expression from transduced skin in mice deficient in expression of either MHC class I (β2m−/−) or class II molecules (Aβb−/−). Since the maturation of T cells in the thymus is dependent on the expression of MHC molecules, the mature CD8+ T cells are virtually absent in class I-deficient mice and class II-deficient mice are depleted of mature CD4+ T cells [21,22]. The dorsal skin of mice was transduced with RRV-LZ and after a week, the initial β-gal expression in epidermis was examined by X-gal staining of the cornified cells to identify the transduced area. Tissue sections were obtained from each group at 2 weeks posttransduction and analyzed for the presence X-gal+, CD4+, or CD8+ cells in the transduced tissue.
In β2m−/− mice, a significant number of CD4+ cells had infiltrated both dermal and epidermal compartments of the transduced skin (Fig. 5A) and as expected, CD8+ cells were almost absent from the skin (Fig. 5B). We observed no X-gal-stained keratinocytes in all the sections examined from the transduced area (n = 150); however, a few X-gal-positive fibroblasts were present (Fig. 5C). The loss of transgene expression in these mice suggests that CD4 T cells can effectively eliminate transduced cells in the absence of CD8 CTLs. Conversely, examination of Aβb−/− mouse skin demonstrated a significant CD8+ cell infiltration in response to β-gal expression in the epidermis (Fig. 5E). We found β-gal-expressing cells only in the upper layers of the epidermis about to be shed from the surface (Fig. 5F), demonstrating that CD8 CTLs can effectively eliminate the transduced cells in the absence of class II-restricted T cell help. We detected a small number of CD4+ cells in the skin of Aβb−/− mice (Fig. 5D). This was not surprising, since some CD4+ cells have been shown to remain in the periphery of Aβb−/− mice; however, the T helper activity is completely absent. A small number of CD4+ cells (3–7%) detected in the periphery of the class II-deficient mice represented CD4+ γδ T cells, macrophages, and a subset of dendritic cells [22,23]. Examination of sera collected from transduced Aβb−/− mice at 4 weeks posttransduction confirmed the lack of CD4 T cell activity, as no anti-β-gal IgG was present (data no shown).
FIG. 5.
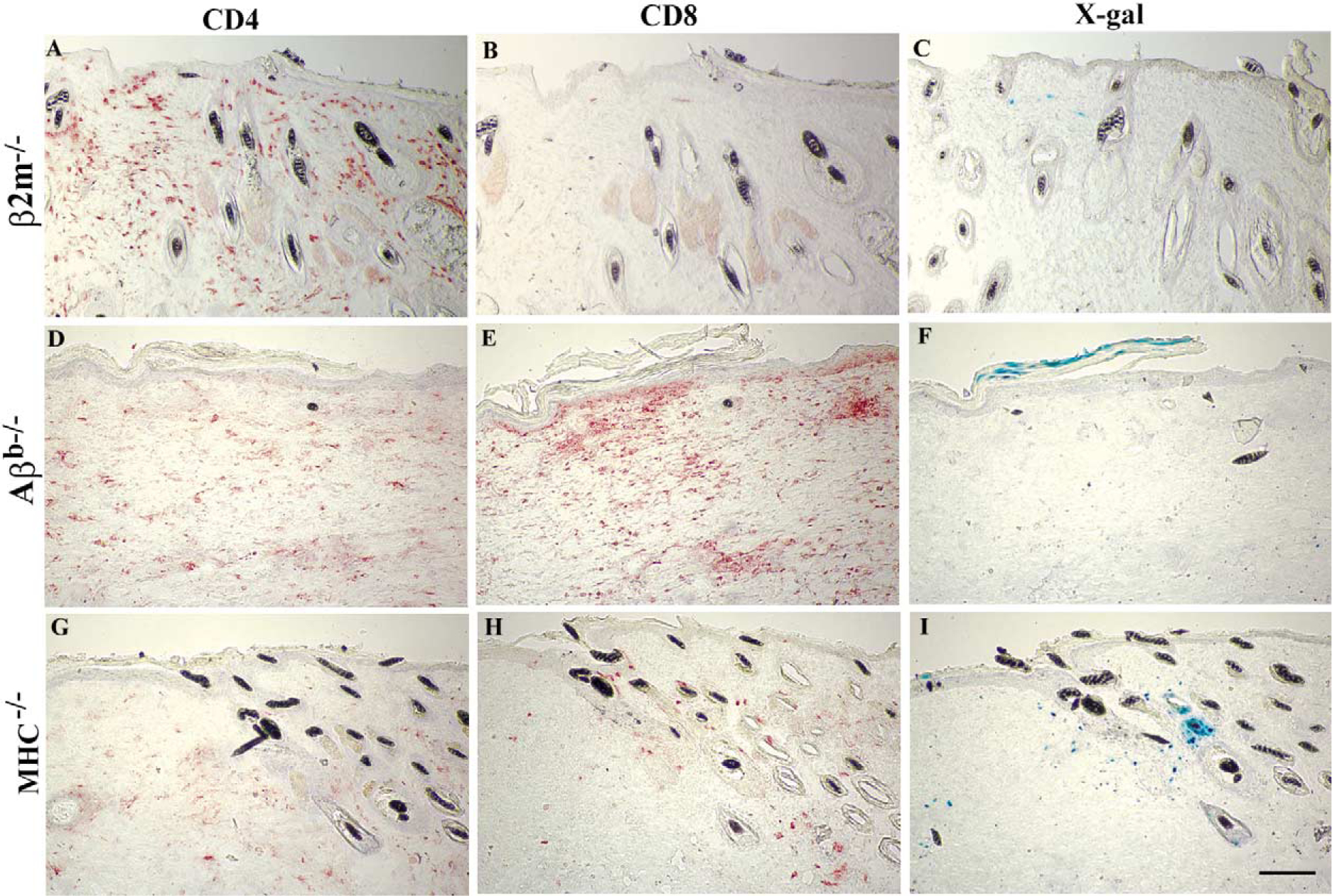
Analysis of β-gal expression and cellular infiltrates in mice with defined immune deficiencies. The skin of mice deficient in expression of class I MHC (β2m−/−; A–C), class II MHC (Aβb−/−; D–F), or both molecules (MHC−/−; G–I) was transduced with 3 × 107 infectious viral particles as described under Material and Methods. Transduced skin tissue from a representative mouse in each group was harvested at 2 weeks posttransduction and serial cryosections were immunostained for detection of CD4+ or CD8+ cells (red staining) or stained with X-gal for detection of transduced cells (blue staining) as indicated. No X-gal-stained cell was present in the transduced skin of β2m−/− mice at 2 weeks posttransduction. Bar, 50 μm.
As expected, examination of the cornified cells removed from the surface of transduced areas of the skin at 4 weeks posttransduction by X-gal staining indicated the loss of transgene expression in both groups of mice (Table 1). These data suggested that depletion of both T cell populations might be necessary for long-term transgene expression in the skin. To test this hypothesis, we transduced the dorsal skin of MHC−/− mice, which are deficient in both class I and class II MHC molecules and demonstrate a significant depletion of CD4 and CD8 T cells [24], with RRV-LZ and identified the transduced area of the skin by X-gal staining of cornified cells at 1 week posttransduction. A week later, examination of tissue sections obtained from MHC−/− mice indicated the presence of a significant number of X-gal-positive keratinocytes and fibroblasts in the tissue (Fig. 5I). A small but significant number of CD4+ and CD8+ cells infiltrated the transduced area of the skin (Figs. 5G and 5H). The recruitment of these cells was specific to the transduced cells since no such infiltrate was present in the adjacent areas of the skin (Figs. 5G and 5H and data not shown). Immunostaining of serial sections for TCRαβ confirmed the presence of T lymphocytes in the area (data not shown). It is known that a small number of CD4 and CD8 T cells are present in the peripheral lymphoid organs of MHC−/− mice; however, their functional importance has not been defined [24]. Analysis of sera collected from transduced mice at 4 weeks posttransduction for the presence of anti-β-gal IgG confirmed the lack of CD4 T cell activity in MHC−/− mice [24]. On the contrary, significant levels of anti-β-gal IgG were present in the sera collected from β2m−/− mice, indicating the functional integrity of T helper cells in mice deficient in class I MHC molecules (data not shown).
TABLE 1:
Summary of transgene expression in the epidermis of mice with defined immune deficiencies
| β-Cal expression | C57Bl/6 | IgGh−/− | β2m−/− | Aβb−/− | MHC−/− | MTnLZ |
| 1-week post-Trd | + (n = 8) | + (n = 6) | + (n = 6) | + (n = 6) | + (n = 4) | + (n = 8) |
| 4-week post-Trd | − (0/8) | − (0/6) | − (0/6) | − (0/6) | + (4/4) | + (8/8) |
β-Gal expression was monitored at 1 or 4 weeks posttransduction by X-gal staining of cornified cells that were removed from the surface of epidermis by tape stripping. β-Gal-positive area of skin was mapped at 1 week posttransduction and the same area was examined for continued expression at 4 weeks posttransduction. A positive sign indicates continued β-gal expression in the cornified cells and a negative sign when the expression was lost. The number of animals in each group is presented in parentheses.
As indicated in Table 1, examination of the cornified cells removed from the transduced area of epidermis at 4 weeks posttransduction indicated the persistent expression of β-gal in the epidermis of doubly deficient mice despite the recruitment of a small number of CD4 and CD8 T cells (Fig. 5). Overall, these results indicate a compensatory role of CD8 and CD4 T cells in the loss of skin-directed transgene expression and that the inhibition of both CD4 and CD8 T cells is required to achieve long-term transgene expression in the skin.
Discussion
Cutaneous gene-based therapies may provide a way to treat inherited skin and systemic disorders. Long-term therapy requires durable transgene expression. Significant progress has been made in using integrating vectors to target epithelial stem cells and achieving long-term transgene expression in the absence of immunological responses [4,7]. Transgene-specific immune responses, however, may have profound effects on the outcome of therapy for patients with null mutations in the target gene. In such patients transgene products are presented as neoantigens. Expression of a foreign gene in skin has been shown to induce potent cellular and humoral immune responses against the encoded protein [25–27]. A direct correlation between the transient nature of transgene expression in mouse skin and the induction of transgene-specific immune responses has been shown previously [15]. In the present study, we have used in vivo transduction of mouse skin as a model to study the mechanisms of immune-mediated loss of transgene from cutaneous epithelium. A role for transgene-specific T-cell-mediated elimination of transduced skin cells was demonstrated by (1) the prevalence of β-gal-specific IgG2a and IgG2b subclasses in the serum of transduced animals, suggesting activation of T helper 1-like responses; (2) infiltration of a significant number of CD4 and CD8 cells into the transduced area of the skin at day 14 when the transgene expression is lost; (3) the presence of β-gal-specific CTLs; and (4) the persistent expression of the transgene in mice deficient in both CD4 and CD8 T cells or in mice that were tolerant to the transgene products. Furthermore, the transient transgene expression in the skin of mice deficient in either CD4 or CD8 T cells suggested a compensatory cytolytic role for CD4 T cells in the absence of CD8 CTLs.
Skin is enriched in resident macrophages and dendritic cells including the most powerful antigen-presenting cells, the Langerhans cells, which are specialized for the initiation of immune response [28]. Subsequent to the inoculation of RRV-LZ into skin, APCs could acquire antigens either through direct transduction or through the uptake of viral particles by endocytosis or both. The RRV-encoded proteins (i.e., β-gal) are expressed in the producer cells and included in the viral particles as the viruses exit the producer cell by budding through the plasma membrane [29]. These antigen-filled viral particles could provide an exogenous source of antigen for presentation to MHC class II-restricted helper T cells [30]. An endogenous source of antigens for presentation to MHC class I-restricted CTLs is provided either by direct transduction of APCs or via cross-presentation of the transgene products synthesized in keratinocytes or fibroblasts by APCs [31,32].
MHC class I-restricted CD8 CTLs are thought to be the primary effector cells for elimination of virally infected or tumor cells. Nevertheless, it is generally assumed that CTLs require MHC class II-restricted CD4 helper T cells for their differentiation and expression of effector function [18]. For example, in the liver-directed adenovirus-mediated model of gene therapy, CD4 T cells were necessary for a fully competent CD8 CTL response to mediate elimination of β-gal-expressing hepatocytes, and therefore, the depletion of either CD8 or CD4 T cells was sufficient to abrogate the immune-mediated destruction of transduced hepatocytes [33]. Our data indicate that in skin-directed gene therapy; however, CD8 CTLs are able to eliminate transduced cells in the absence of class II-restricted T cell help. The loss of transgene expression in mice deficient in CD8 T cells accompanied by a significant CD4+ cell infiltrate in the transduced area of the skin suggests a cytolytic role for CD4 T cells in these mice. Lymphocytes expressing either CD4 or CD8 have been shown to express comparable cytolytic activity [34]. CD4 T cells can emerge as primary CTL effectors, especially when CD8 CTLs are depleted [35,36]. CD4 killer cells, however, are presumably restricted to recognize mainly cells expressing MHC class II molecules that are targets of helper T cell recognition. While the molecular mechanism(s) responsible for elimination of transduced cells in β2m−/− mice that are deficient in CD8 CTLs is not clear, expression of class II molecules on keratinocytes is induced by IFN-γ and has been shown in many skin disorders with prominent lymphocytic infiltration [37,38]. Expression of class II MHC molecules by keratinocytes in the transduced skin of either normal or β2m−/− mice was confirmed by immunofluorescent staining of the tissue sections with anti-I-A/I-E antibody (data not shown).
The persistence of transgene expression in MHC double-knockout mice that are deficient in expression of class I and class II MHC molecules, and show a significant depletion of both CD4 and CD8 T cell populations, confirms the role of T-cell-mediated responses in the loss of transgene expression. Interestingly in these mice, β-gal expression persisted despite infiltration of a small but significant number of CD8 and CD4 T cells in the transduced area. This suggests that the expression of class I or class II MHC molecules by transduced skin cells may be required for T-cell-mediated loss of the transgene from the skin.
In summary, the purpose of this study was to define the type of immune responses mediating the loss of transgene expression in cutaneous epithelium to design vectors that can evade or modulate these responses. The basic finding is that inhibition of both CD4 or CD8 T-cell-mediated responses may be necessary for long-term skin-directed transgene expression.
Material and Methods
Animals and in vivo transduction.
Igh-6−/− (immunoglobulin deficient), MTnLZ (a transgenic line with lacZ expression targeted to the liver), and C57Bl/6 mice were from The Jackson Laboratory (Bar Harbor, ME). MHC class I-deficient (β2m−/−), MHC class II-deficient (Aβb−/−), and MHC-deficient (Aβb−/− β2m−/−) mice were purchased from Taconic. All strains of mice used in this study had C57Bl/6 genetic background (H-2b) and 7- to 8-week-old female mice were used in all experiments. At this age, the dorsal skin of mice is in the resting phase of the hair growth cycle. For in vivo transduction of cutaneous epithelium, the clipped, depilated dorsal skin of the mice was dermabraded using a felt wheel, and on day 3 postabrasion, 30 μl (containing 3 × 107 transducing units) of vesicular stomatitis virus G-pseudotyped MFG-LZ retroviral vector was used to transduce the healing skin as described previously [15]. The MFG-LZ vector encodes the lacZ reporter gene under the control of the 5′ long terminal repeat. Animal studies were performed in accordance with the institutional guidelines set forth by the State University of New York.
Detection of transgene expression in transduced skin.
To assess β-gal expression in transduced skin of live animals, cornified cells on the surface of epidermis were removed by tape stripping using adhesive tape (Scotch, 3M; St. Paul, MN). The tape and adherent cells were washed in PBS; fixed briefly in 0.2% glutaraldehyde; rinsed in PBS; incubated for 1 h in 1 mg/ml X-gal in 0.1 M sodium phosphate buffer (pH 7.5) containing 5 mM K3Fe(CN)6, 5 mM K4Fe(CN)6, 1 mM MgCl2, 0.02% NP-40, and 0.01% sodium deoxycholate for 1 h at 37°C; and examined by low-power light microscopy. For detection of β-gal expression in tissue sections, frozen sections were dried and stained en face with X-gal solution for 1 h at 37°C as described previously [15].
Immunohistochemical analysis.
Sections were rinsed with PBS, dried, and fixed with cold acetone for 2 min. Fixed tissue sections were blocked in 5% nonfat milk in PBS and stained with 1 μg/ml rat anti-mouse CD4 or anti-mouse CD8a monoclonal antibodies (BD-Pharmingen, San Diego, CA) for 60 min. Antibodies were detected using the BioGenex ready-to-use Links and Labels as described by the manufacturer (BioGenex, San Ramon, CA). In some experiments, sections were incubated in X-gal solution for 1 h at room temperature prior to acetone fixation and immunostaining.
Analysis of humoral responses in transduced mice.
Antibody responses to β-gal were determined by ELISA using purified Escherichia coli β-gal (Sigma, St. Louis, MO). Microtiter plates (Costar, Corning, NY) were coated with 1 μg of β-gal in 100 μl carbonate buffer for 16 h at 4°C. The wells were washed and blocked with 5% nonfat dry milk in Tris-buffered saline for 1 h at room temperature. Diluted serum samples were added to the blocking solution and incubated for 2 h. As a standard control, serial dilutions of monoclonal anti-β-gal antibody (Sigma) were used. The wells were washed extensively and refilled with 100 μl of 1:10,000 diluted horseradish peroxidase-conjugated goat anti-mouse IgG (Sigma), and bound antibodies were detected using tetramethylbenzidine as substrate (Sigma).
For antibody isotyping, after the 2-h incubation of serum samples, the identical sample wells were incubated with 1:1000 dilution of goat anti-mouse IgG1, IgG2a, IgG2b, or IgG3 (Sigma) for 60 min followed by incubation with horseradish peroxidase-conjugated rabbit anti-goat antibodies before detection of the bound antibodies.
CTL assay.
Spleen and draining lymph nodes from transduced C57Bl/6 or MTnLZ mice were collected at 3 weeks posttransduction. Single-cell sus-pensions were stimulated for 5 days with BLKSV fibroblast line (H-2b haplotype, ATCC TIB-88) transduced previously with LZRN vector encoding both LZ and neor [15]. The latter was used for selection and enrichment of LZ-expressing cells. The stimulation ratio was 50 lymphocytes:1 stimulator cell. Cytolytic activity was determined in a standard 6-h 51Cr-release assay. BLKSV transduced with LZRN or LXSN (as a control) were used as target cells to analyze cytotoxic responses specific to β-gal. Target cells (1 × 107 cells/ml) were incubated with 100 μCi of 51Cr (Amersham Pharmacia Biotech, Inc., Piscataway, NJ) for 1 h at 37°C. Effector lymphocytes were plated at different effector-to-target ratios (100:1 to 3:1) in 96-well plates containing 4 × 104 target cells per well. After incubation for 6 h at 37°C, plates were centrifuged and aliquots of 100 μl supernatant were collected and counted in a gamma counter. Spontaneous release was determined by culturing target cells in medium. Maximum release was determined by lysing target cells with 1% NP-40. Results of triplicate culture are expressed as percentage specific 51Cr release = 100 × (experimental release − spontaneous release)/(maximum release − spontaneous release).
ACKNOWLEDGMENTS
We are grateful to Ning Lin for technical assistance. This research was supported by grants from the NIH to S.G. (K01-AR02100) and to L.T. (R01-DE04511).
REFERENCES
- 1.Taichman LB (1999). Systemic replacement therapy from genetically modified epidermal keratinocytes. Proc. Assoc. Am. Physicians 111: 206–210. [DOI] [PubMed] [Google Scholar]
- 2.Cao T, Wang XJ, and Roop DR (2000). Regulated cutaneous gene delivery: the skin as a bioreactor. Hum. Gene Ther 11: 2297–2300. [DOI] [PubMed] [Google Scholar]
- 3.Uitto J, and Pulkkinen L (2000). The genodermatoses: candidate diseases for gene therapy. Hum. Gene Ther 11: 2267–2275. [DOI] [PubMed] [Google Scholar]
- 4.Khavari PA, Rollman O, and Vahlquist A (2002). Cutaneous gene transfer for skin and systemic diseases. J. Intern. Med 252: 1–10. [DOI] [PubMed] [Google Scholar]
- 5.Hoffman RM (2000). The hair follicle as a gene therapy target. Nat. Biotechnol 18: 20–21. [DOI] [PubMed] [Google Scholar]
- 6.Gupta S, Domashenko A, and Cotsarelis G (2001). The hair follicle as a target for gene therapy. Eur. J. Dermatol 11: 353–356. [PubMed] [Google Scholar]
- 7.Ghazizadeh S, and Taichman LB (2000). Virus-mediated gene transfer for cutaneous gene therapy. Hum. Gene Ther 11: 2247–2251. [DOI] [PubMed] [Google Scholar]
- 8.Ortiz-Urda S, et al. (2002). Stable nonviral genetic correction of inherited human skin disease. Nat. Med 8: 1166–1170. [DOI] [PubMed] [Google Scholar]
- 9.Kay MA, et al. (1993). In vivo gene therapy of hemophilia B: sustained partial correction in factor IX-deficient dogs. Science 262: 117–119. [DOI] [PubMed] [Google Scholar]
- 10.Ferry N, Duplessis O, Houssin D, Danos O, and Heard JM (1991). Retroviral-mediated gene transfer into hepatocytes in vivo. Proc. Natl. Acad. Sci. USA 88: 8377–8381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stockschlader MA, Schuening FG, Graham TC, and Storb R (1994). Transplantation of retrovirus-transduced canine keratinocytes expressing the β-galactosidase gene. Gene Ther. 1: 317–322. [PubMed] [Google Scholar]
- 12.Ng RLH, Woodward B, Bevan S, Green C, and Martin R (1997). Retroviral marking identifies grafted autologous keratinocytes in porcine wounds receiving cultured epithelium. J. Invest. Dermatol 108: 457–462. [DOI] [PubMed] [Google Scholar]
- 13.Streilein JW (1990). Skin-associated lymphoid tissues (SALT): the next generation In Skin Immune System (SIS) (Bos JD, Ed.), pp. 25–48. CRC Press, Boca Raton, FL. [Google Scholar]
- 14.Larregina AT, and Falo LDJ (2000). Generating and regulating immune responses through cutaneous gene delivery. Hum. Gene Ther 11: 2301–2305. [DOI] [PubMed] [Google Scholar]
- 15.Ghazizadeh S, Harrington R, and Taichman LB (1999). In vivo transduction of mouse epidermis with recombinant retroviral vectors: implications for cutaneous gene therapy. Gene Ther. 6: 1267–1275. [DOI] [PubMed] [Google Scholar]
- 16.Ghazizadeh S, and Taichman LB (2001). Multiple classes of stem cells in cutaneous epithelium: a lineage analysis of adult mouse skin. EMBO J. 20: 1215–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paul WE, and Seder RA (1994). Lymphocyte responses and cytokines. Cell 76: 241–251. [DOI] [PubMed] [Google Scholar]
- 18.Paus R, Christoph T, and Muller-Rover S (1999). Immunology of the hair follicle: a short journey into terra incognita. J. Invest. Dermatol. Symp. Proc 4: 226–234. [DOI] [PubMed] [Google Scholar]
- 19.Janeway CAJ, and Bottomly K (1994). Signals and signs for lymphocyte responses. Cell 76: 275–285. [DOI] [PubMed] [Google Scholar]
- 20.Watts C (1997). Capture and processing of exogenous antigens for presentation on MHC molecules. Annu. Rev. Immunol 15: 821–850. [DOI] [PubMed] [Google Scholar]
- 21.Zijlstra M, et al. (1990). Beta 2-microglobulin deficient mice lack CD4−8+ cytolytic T cells. Nature 344: 742–746. [DOI] [PubMed] [Google Scholar]
- 22.Grusby MJ, Johnson RS, Papaioannou VE, and Glimcher LH (1991). Depletion of CD4+ T cells in major histocompatibility complex class II-deficient mice. Science 253: 1417–1420. [DOI] [PubMed] [Google Scholar]
- 23.Cosgrove D, et al. (1991). Mice lacking MHC class II molecules. Cell 66: 1051–1066. [DOI] [PubMed] [Google Scholar]
- 24.Grusby MJ, et al. (1993). Mice lacking major histocompatibility complex class I and class II molecules. Proc. Natl. Acad. Sci. USA 90: 3913–3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raz E, et al. (1994). Intradermal gene immunization: the possible role of DNA uptake in the induction of cellular immunity to viruses. Proc. Natl. Acad. Sci. USA 91: 9519–9523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fan H, Lin Q, Morrissey GR, and Khavari PA (1999). Immunization via hair follicles by topical application of naked DNA to normal skin. Nat. Biotechnol 17: 870–872. [DOI] [PubMed] [Google Scholar]
- 27.Tang D, Shi Z, and Curiel DT (1997). Vaccination onto bare skin. Nature 388: 729–730. [DOI] [PubMed] [Google Scholar]
- 28.Steinman RM (1991). The dendritic cell system and its role in immunogenicity. Annu. Rev. Immunol 9: 271–296. [DOI] [PubMed] [Google Scholar]
- 29.Varmus H (1988). Retroviruses. Science 240: 1427–1435. [DOI] [PubMed] [Google Scholar]
- 30.Germain RN (1994). MHC-dependent antigen processing and peptide presentation: providing ligands for T lymphocyte activation. Cell 76: 287–299. [DOI] [PubMed] [Google Scholar]
- 31.Song ES, et al. (1997). Antigen presentation in retroviral vector-mediated gene transfer in vivo. Proc. Natl. Acad. Sci. USA 94: 1943–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heath WR, and Carbone FR (2001). Cross-presentation in viral immunity and self-tolerance. Nat. Rev. Immunol 1: 126–134. [DOI] [PubMed] [Google Scholar]
- 33.Yang Y, Xiang Z, Ertl HCJ, and Wilson JM (1995). Upregulation of class I major histocompatibility complex antigens by interferon gamma is necessary for T-cell-mediated elimination of recombinant adenovirus-infected hepatocytes in vivo. Proc. Natl. Acad. Sci. USA 92: 7257–7261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lancki DW, Hsieh CS, and Fitch FW (1991). Mechanisms of lysis by cytotoxic T lymphocyte clones. Lytic activity and gene expression in cloned antigen-specific CD4+ and CD8+ T lymphocytes. J. Immunol 146: 3242–3249. [PubMed] [Google Scholar]
- 35.Muller D, et al. (1992). LCMV-specific, class II-restricted cytotoxic T cells in beta 2-microglobulin-deficient mice. Science 255: 1576–1578. [DOI] [PubMed] [Google Scholar]
- 36.Eichelberger M, Allan W, Zijlstra M, Jaenisch R, and Doherty PC (1991). Clearance of influenza virus respiratory infection in mice lacking class I major histocompatibility complex-restricted CD8+ T cells. J. Exp. Med 174: 875–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Albanesi C, Cavani A, and Girolomoni G (1998). Interferon-gamma-stimulated human keratinocytes express the genes necessary for the production of peptide-loaded MHC class II molecules. J. Invest. Dermatol 110: 138–142. [DOI] [PubMed] [Google Scholar]
- 38.Aubock J, Romani N, Grubauer G, and Fritsch P (1986). HLA-DR expression on keratinocytes is a common feature of diseased skin. Br. J. Dermatol 114: 465–472. [DOI] [PubMed] [Google Scholar]


