1. Introduction
South Africa is home to the majority of SARS-CoV-2 infections in Africa and, despite an initial slow rise in infections, it ranked among the top ten highest burden countries globally by September 2020 [1]. In contrast to other high burden countries, the Covid-19 recovery rate remains high (90%) and case fatality rates remain relatively low (2.4%) for reasons yet to be fully elucidated.
Well before the announcement by the Director-General of the World Health Organization (WHO) of the pandemic status of the new coronavirus disease 2019 (Covid-19) on 11 March [2], South Africa had instituted thermal screening measures and Covid-19 symptom screening at all ports of entry into the country. The first case of Covid-19 in South Africa was reported on March 5, 2020 in a traveller who had recently returned from Italy. This was followed by a few more imported cases and individuals who were close work or family contacts of travellers as well as cases of nosocomial transmission in health care workers. The South African government responded swiftly to curtail the community spread of SARS-CoV-2 infections by declaring a national state of disaster on March 15 that restricted international travel, closed schools, limited public gatherings and introduced a mandatory daily curfew between 9pm and 5am. On March 27, a national mandatory lockdown was instituted, restricting the movement of people except for a few categories of essential staff, including health care workers.
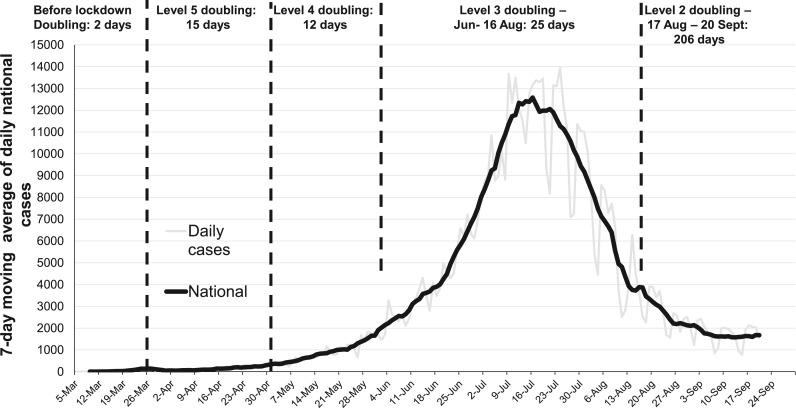
In the absence of a vaccine or immunity, the primary goal of the South African government’s Covid-19 response was to “flatten the curve” by reducing community transmission of SARS-CoV-2. The national lockdown was a mitigation strategy to slow the spread of the virus, and use the time to prepare and strengthen health care facilities particularly in the public health sector, build diagnostic and clinical care capacity to better cope with the anticipated increase in the number of hospital admissions enabling better quality care to be provided with a resultant reduction in the number of deaths. While the lockdown slowed the spread of SARS-CoV-2 infections, it had substantial adverse economic [3] and unintended health effects [4] in the context of multiple existing health burdens and high levels of poverty. As restrictions on movement started to ease incrementally and economic activities have resumed from 1 June, the number of SARS-CoV-2 cases and deaths have increased with the peak infections exceeding 12,000 per day reached in mid-July with a steady decline in cases to about 1400 infections per day in late September (Fig. 1 ).
Fig. 1.
7-day moving average of SARS-CoV-2 cases in South Africa, 5 Mar – September 20, 2020. Daily cases are shown in light grey. The doubling time for cases, corresponding to each lockdown restriction level, is provided above the figure.
The government’s response comprised eight overlapping stages [5] (Table 1 ). In this analysis, we describe how the national testing strategy introduced during Stage 1 of the COVID-19 response changed with the evolving epidemic and reflect on factors that impacted the number of tests undertaken and who had access to diagnostic testing during different stages of the epidemic.
Table 1.
The 8-stage COVID-19 epidemic response of the South African Government.
| Stage | Date stage initiated | Description of stage |
|---|---|---|
| 1 | Mid-January – 4 March | Preparing for COVID-19 identification of cases, case definition, planning for isolation, contact tracing and care provision, establishing diagnostic testing capacity |
| 2 | 5 March – 26 March | National state of disaster declared, international travel banned, schools closed, large gatherings restricted, mandatory daily curfew introduced and programs to promote social distancing and hand hygiene implemented |
| 3 | 27 March – 31 May | National lockdown, including a mandatory stay at home policy for non-essential workers |
| 4 | 8 April – 8 June | National community-based screening and testing initiated |
| 5 | 30 April – to date | Hotspot identification and mitigation to identify localised outbreaks and implementation of prevention measures |
| 6 | 5 March – to date | Medical care, including construction of field hospitals |
| 7 | 27 March – to date | Preparation for deaths and burials and the mental health challenges of bereavement |
| 8 | 1 August – to date | Ongoing vigilance through case finding and monitoring immunity levels through surveillance and serosurveys |
1.1. Diagnostic testing for SARS-CoV-2
Diagnostic testing is central for the management of the Covid-19 epidemic and has three key and critical purposes; 1) to confirm a diagnosis so that appropriate clinical care can be rendered, 2) to initiate prevention interventions such as contact tracing, isolation and quarantine, and 3) for surveillance purposes wherein temporal trends in the magnitude, demographic and geo-spatial spread of the virus can be monitored and target appropriate resource allocation [6].
Regulatory authorities play a critical role in ensuring that diagnostic assays meet stringent criteria of optimal performance characteristics. The South African Health Products Regulatory Authority has to provide Emergency Use Acceptance (EUA) authorization for a diagnostic test before it can be used.
Key characteristics of a strong diagnostic test is its ability to correctly identify those who are infected (sensitivity) and minimise incorrect diagnosis of those uninfected (specificity). Notwithstanding the relatively recent identification and introduction of SARS-CoV-2, the rapidity of the development of diagnostic tests that establish whether an individual is currently infected by testing for the presence of the virus (molecular diagnostic methods) or previously infected by testing for the presence of antibodies (serological assays) has been unprecedented. The rapid sequencing of the SARS-CoV-2 genome in early January [7] enabled the quick development of a protocol for the detection of SARS-CoV-2 using real time reverse transcription–polymerase chain reaction (RT-PCR) that has been implemented globally [8]. The gold standard to diagnose SARS-CoV-2 infection remains the RT-PCR assay.
Approval of antibody tests has been more challenging. The South African Health Products Regulatory Authority evaluated hundreds of antibody test kits but by September 2020 had only approved one Rapid Lateral Flow test and 3 lab-based Enzyme Linked Immunosorbent assays.
2. SARS-CoV-2 testing in South Africa
2.1. Pre lockdown - building testing capacity
Testing capacity was established in Stage 1, well before the first case of COVID-19 was detected in South Africa. The country had a well-established infrastructure throughout the country for PCR testing, which was developed over almost two decades for HIV viral load testing that facilitated a rapid transition for SARS-CoV-2 detection. Initially, the testing capacity was centralised at the National Institute for Communicable Diseases (NICD) but expanded to include private and research laboratories prior to any cases being identified in South Africa.
The country’s testing strategy was set by the National Department of Health based on advice from the NICD initially and later from the Ministerial Advisory Committee for COVID-19. Initially criteria for testing were restrictive, partially to avoid large numbers of asymptomatic worried-well people seeking tests. In addition to Covid-19 signs and symptoms, the initial criteria included a requirement of recent international travel or having contact with a COVID-19 person who had travelled abroad recently. South Africa declared SARS-CoV-2 infection a notifiable condition in February. Plans were in place for district level teams to initiate, on confirmation of a positive case, contact tracing for further testing and isolation/quarantine as appropriate.
2.2. Early cases – testing of international travellers and contacts
During Stage 2 of the COVID-19 response which followed the identification of the first case in South Africa, testing started to increase. During this stage, the majority of SARS-CoV-2 cases were identified primarily in the private health sector with limited testing in the public sector. Due to concerns regarding the accuracy of tests conducted initially in the private laboratories emanating from a high profile false positive result from a private laboratory very early in the epidemic, for the first few weeks all positive tests from private laboratories had to be confirmed by the NICD.
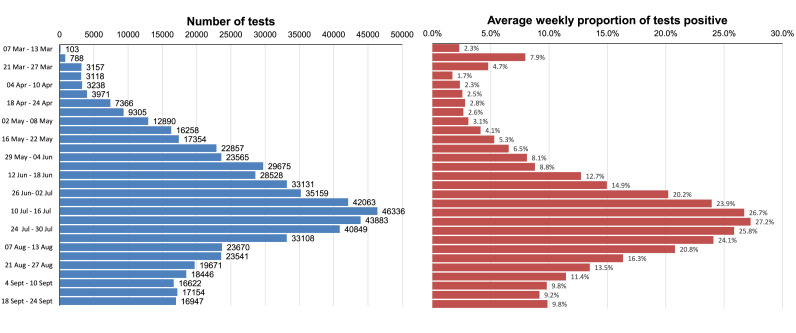
The restrictive criteria for testing relating to travel meant that individuals with Covid-19 signs and symptoms but who had not travelled or had confirmed contact with a traveller were not tested. This limited the extent to which the test-isolate and contact tracing-quarantine strategy could be deployed in Stage 2 to contain community transmission, creating the conditions for SARS-CoV-2 to be seeded in multiple communities. During this stage, testing was predominantly conducted by private laboratories. The public sector capacity through the National Health Laboratory Service started to expand as the number of accredited laboratories increased. The average number of tests per week was below 1000/day with only 6.9% being undertaken in the public sector (Fig. 2 ).
Fig. 2.
Average daily tests and proportion of SARS-CoV-2 tests that are positive, 7 March – 24 September. Total tests completed – 4,102,162.
2.3. Community base strategy for active case finding
About 2 weeks following the start of the national lockdown on 27 March (Stage 3), public sector testing capacity was expanded and the definition of who could be tested was broadened to include symptomatic individuals who had not travelled enabling initiation of active case-finding. This involved identifying 993 socially vulnerable, high density communities throughout the country for a “no-touch” door-to-door screening based on signs and symptoms of Covid-19 by 28,000 trained community health workers who had prior experience from providing tuberculosis and HIV services. As this community-based symptom screening and testing programme gained momentum, the number of community health workers involved increased to over 70,000. The community-based screening programme also set out to increase community awareness of Covid-19, including providing information on the importance of frequent handwashing, social distancing, coughing and sneezing etiquette, and cleaning of household surfaces. Having two or more signs and symptoms was a trigger for referral to a mobile laboratory or primary health care facility for specimen collection for SARS-CoV-2 testing.
The community-based screening, testing and isolation response was a unique approach that aimed to actively find cases rather than passively wait for them to arrive ill at healthcare facilities as was the norm in most countries experiencing epidemics at the time. This approach involved the following key components: 1) house-to-house visits by community healthcare workers screening for symptoms of coronavirus using a standardised set of questions that were recorded in a cellphone app along with geospatial location information, 2) referral of individuals for with symptoms for testing, 3) initiation of self-isolation or assisted isolation at designated centres for people positive for the coronavirus, and 4) self-quarantine of households and other contacts of people with the coronavirus infection.
Between 8 April and 8 June a total of 15,471,559 individuals were screened through this active case-finding strategy and the testing capacity increased dramatically from an average of 3,118 test per week at the end of March to 23,565 test per week by the first week of June. Throughout the community-based screening, the proportion of tests that were positive remained below 10% (Fig. 2) indicating that the community spread of Covid-19 remained low.
2.4. Test kit shortages, overwhelmed testing facilities and growing number of sick patients hospitalized - Shifting testing priorities
While the community-based approach was able to rapidly scale up screening and testing, the global shortage of available test kits, reagents and swabs led to a situation where the demand for SARS-CoV-2 PCR tests outstripped the combined testing capacity of the public and private laboratories in the country. While there was available equipment capacity to undertake about 50,000 tests per day, the massive increase in testing demand without concomitant availability of test kits and increasing hospital admissions created a substantial testing backlog and an increase in turn-around times from 48 hours to several days. These testing challenges defeated the purpose of slowing down transmission through active case finding and isolation of contacts and made differential diagnosis and clinical decision-making difficult. Furthermore, given that the isolation period was 14 days, results that become available after that diminished relevance for the individual or community and compromised population level containment efforts.
Although alternative rapid testing technologies, such as the Xpert® Xpress SARS-CoV-2 test, which can detect SARS-CoV-2 within 30 minutes on the GeneXpert point-of-care testing platform, became available, global demand and US stockpiling constrained the widespread use of this technology for SARS-CoV-2 diagnosis in South Africa. In some provinces with low test positivity rates, the RT-PCR assays were adapted for testing pooled samples to increase throughput and reduce the demand for test kits and reagents, and associated costs. However, as lockdown restrictions gradually eased, cases started increasing in most provinces and the proportion of tests that were positive gradually increased and by mid-June exceeded 10% and pooling of samples was limited (Fig. 2).
2.5. Testing for hotspot identification
During Stage 5, the focus of testing shifted from active case finding to hotspot identification and mitigation. This involved the investigation of clusters of cases as they emerged to prevent the outbreaks from expanding and to mitigate their spread or recurrence. Those involved in the community screening, were redirected to work with the hotspot identification and mitigation teams.
2.6. Prioritising testing when demand exceeded availability of test kits
As demand for testing increased beyond available supply, it became necessary to re-prioritise target populations for SARS-CoV-2 testing, especially in public laboratories. Several priority testing populations were identified (Table 2 ) by the Ministerial Advisory Committee for Covid-19 and samples from these populations were prioritised for testing to reduce the turn-around times for these groups and to ensure that the tests remained clinically relevant. Three priority levels – high, medium, and low – were identified [9]. The rationale for the “high priority” level was to maximise clinical benefit and preserve healthcare capacity and the rationale for the “medium priority” level was to reduce the risk of localised outbreaks and preserve essential services. Testing in the “low priority” level populations was only recommended if testing capacity had not been exhausted. If testing capacity was exhausted, people were advised to isolate if symptomatic regardless of test results and to quarantine if they had direct contacts, regardless of Covid-19 status.
Table 2.
Testing populations according to priority level.
| High priority populations |
|---|
|
|
Medium priority populations |
|
|
Low priority populations |
Populations prioritised for point point-of-care testing.
pooled testing could be considered for these populations given likely low prevalence.
In the context of diagnostic kit shortages, the prioritization as outlined in Table 2 provided an evidenced-based approach to rationing available test kits. Diagnosis of patients being admitted to hospitals and mitigating risk in health care workers were highest priority. While the rationale for this prioritization is focused on saving lives of hospitalized patients and reducing nosocomial transmission, it may have paradoxically exacerbated the magnitude and severity of the epidemic by not containing spread at a community level and identifying sick individuals earlier in disease progression that a public health approach to testing would do. The challenge with rationing diagnostic kits became a balancing act between the care and diagnosis of hospitalized patients versus containing the spread of the virus.
As the peak of the epidemic passed, the demand for PCR testing declined rapidly. Hence, the focus for testing has shifted to ongoing vigilance through case finding, testing of contacts, monitoring emergence of new infections and clusters. The priority populations for testing now include: symptomatic patients in hospitals and any level of the health service, patients admitted electively to hospital for high-risk hospital procedures and emergencies, asymptomatic individuals that are close contacts of confirmed cases and natural deaths where Covid-19 could be a differential diagnosis.
2.7. Introduction of serological testing
Monitoring of immunity levels through serosurveys in preparation for subsequent waves of the epidemic started as the number of cases declined after the epidemic peak. Although results from these antibody (serological) studies are being interpreted with caution because of possible cross-reaction with other coronavirus proteins, which could lead to false positive results, the studies will provide clues about the true SARS-CoV-2 infection rate and the proportion of asymptomatic infections in South Africa. It also enables the estimation of an infection mortality rate over and above the available case fatality rates.
Expansion of PCR testing and identification of SARS-CoV-2 cases remains important even as cases start to decline because the threat of a second wave is ever present. The number of validated and regulatory authority approved, rapid, easy-to-use, point-of-care serological test kits are making serology more accessible. Newer antigen based tests utilising naso-pharyngeal swabs are in the process of being licensed in South Africa. Some of these antigen tests are rapid point-of-case tests. These antigen tests are expected to reduce reliance on PCR tests in a variety of populations and settings. Ultimately, a low cost, easy-to-use, home-based self-testing antigen kit would be ideal to enable rapid identification of infection and immediate isolation to prevent viral spread.
Notwithstanding the many challenges in the evolving epidemic, dynamic changes in knowledge about Covid-19, procuring test kits and undertaking the diagnostic tests, as of September 24, 2020 a total of 667,049 Covid-19 cases were identified through the conduct of 4,102,162 SARS-CoV-2 PCR tests in South Africa [10].
In summary, diagnostic testing for SARS-CoV-2 was a key component of the Covid-19 response in South Africa. As the epidemic evolved, the country’s testing strategy changed to meet changing demands and changing availability of test kits and reagents. South Africa has therefore needed to continually adapt its SARS-CoV-2 testing strategy as the epidemic shifted from imported cases to community spread as the severity of the epidemic increased with increasing hospitalizations and as it competed with better resourced countries in procuring diagnostic kits and reagents in the midst of global demand for diagnostic kits in a pandemic context. The increasing availability of reliable, low cost, rapid SARS-CoV-2 molecular and serological assays will assist with the control of future SARS-CoV-2 epidemics. Investments in local diagnostic assay and reagent production and innovation needs more emphasis to enable middle-income countries like South Africa to be better prepared and more responsive to current and future epidemics.
Declaration of competing interest
Salim S. Abdool Karim is the Chair of the South African Ministerial Advisory Committee on COVID-19 and is a Member of the Africa Task Force for Coronavirus as well as the Lancet Commission on COVID-19. Quarraisha Abdool Karim is a member of the South African Covid-19 Ministerial Advisory Committee and is an Executive Group member of the WHO Solidarity Therapeutics and Vaccine Trials. Cheryl Baxter has no conflicts to declare.
Acknowledgements
The authors receive funding from the South African Department of Science and Innovation and South African Medical Research Council for research on COVID-19.
References
- 1.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020;20(5):533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization, editor. Timeline: WHO’s Covid-19 Response. World Health Organization; Geneva, Switzerland: 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/interactive-timeline#event-5 Available from: Accessed: 4 September 2020. [Google Scholar]
- 3.Arndt C, Davies R, Gabriel S, Harris L, Makrelov K, Modise B, Robinson S, Simbanegavi W, van Seventer D, Anderson L. Impact of Covid-19 on the South African economy: an initial analysis. SA-TIED Working Paper 111. Available from: https://sa-tied.wider.unu.edu/sites/default/files/pdf/SA-TIED-WP-111.pdf. Accessed: 4 September 2020. South Africa: International Food Policy Research Institute 2020.
- 4.Abdool Karim Q., Abdool Karim S.S. COVID-19 affects HIV and tuberculosis care. Science. 2020 Jul 24;369(6502):366–368. doi: 10.1126/science.abd1072. [DOI] [PubMed] [Google Scholar]
- 5.Abdool Karim S.S. The South African response to the pandemic. N. Engl. J. Med. 2020 Jun 11;382(24):e95. doi: 10.1056/NEJMc2014960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hellewell J., Abbott S., Gimma A., Bosse N.I., Jarvis C.I., Russell T.W., Munday J.D., Kucharski A.J., Edmunds W.J., Centre for the Mathematical Modelling of infectious diseases C-WG, Funk S., Eggo R.M. Feasibility of controlling COVID-19 outbreaks by isolation of cases and contacts. Lancet Glob. Health. 2020 Apr;8(4):e488–e496. doi: 10.1016/S2214-109X(20)30074-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Jiang R.D., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020 Mar;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corman V, Bleicker T, Brünink S, Drosten C. Diagnostic detection of 2019-nCoV by real-time RT-PCR. Available from: https://www.who.int/docs/default-source/coronaviruse/protocol-v2-1.pdf?sfvrsn=a9ef618c_2. Accessed: 4 September 2020. Geneva, Switzerland: World Health Organisation 2020.
- 9.Ministerial Advisory Committee for Covid-19. Advisory on the urgent need to address the current challenges in testing through prioritisation for the SARS-CoV-2 daily testing targets. Availabe from: https://sacoronavirus.co.za/wp-content/uploads/2020/08/Memo_Advisory-Testing-Prioritisation_-02-June-2020_final.pdf. Accessed: 8 September. Pretoria: National Department of Health 2020.
- 10.Department of Health. COVID-19 corona virus south african resource portal. Available from: https://sacoronavirus.co.za/. Accessed: 2 September 2020.