Abstract
The prevalence and clinical relevance of viremia in patients with coronavirus disease 2019 (COVID-19) have not been well studied. A prospective cohort study was designed to investigate blood viral load and clearance kinetics in 52 patients (median age, 62 years; 31 [59.6%] male) and explore their association with clinical features and outcomes based on a novel one-step RT droplet digital PCR (RT-ddPCR). By using one-step RT-ddPCR, 92.3% (48 of 52) of this cohort was quantitatively detected with viremia. The concordance between the blood and oropharyngeal swab tests was 60.92% (53 of 87). One-step RT-ddPCR was tested with a 3.03% false-positive rate and lower 50% confidence interval of detection at 54.026 copies/mL plasma. There was no reduction in the blood viral load in all critical patients, whereas the general and severe patients exhibited a similar ability to clear the viral load. The viral loads in critical patients were significantly higher than those in their general and severe counterparts. Among the 52 study patients, 30 (58%) were discharged from the hospital. Among half of the 30 discharged patients, blood viral load remained positive, of which 76.9% (10 of 13) completely cleared their blood viral load at follow-up. Meanwhile, none of their close contacts had evidence of infection. Quantitative determination of the blood viral test is of great clinical significance in the management of patients with coronavirus disease 2019.
The end of 2019 witnessed an outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and its associated coronavirus disease 2019 (COVID-19) in Wuhan, China.1 By April 3, 2020, more than 1,000,000 cases and >50,000 deaths were confirmed worldwide. The rapid global spread of COVID-19 has led to subsequent declaration of a pandemic by the World Health Organization.2 Currently, a great number of clinical challenges concerning COVID-19 need to be addressed urgently. Mounting evidence shows that the SARS-CoV-2 test of respiratory specimens turns positive for the virus after discharge,3 which raises concerns about the possible transmission via these patients.
To improve SARS-CoV-2 testing sensitivity, researchers have tried different sampling locations. Peripheral blood should theoretically be an ideal sampling input for a viral test because it contains a variety of biomarkers shed from the whole body, including cell-free DNA and cell-free RNA.4 Despite the critical clinical importance of viremia in the pathogenesis and progression of COVID-19,5 only two studies have thus far examined the viral load in peripheral blood. However, the results were unsatisfactory due to limited sensitivity of real-time PCR.6 The prevalence and clinical significance of viremia in patients with COVID-19 are unknown. Two studies have determined that droplet digital PCR (ddPCR) possesses much higher sensitivity and specificity than conventional traditional real-time PCR in SARS-CoV-2 testing of respiratory specimens,7 which provides the possibility for studying the prevalence and clinical significance of viremia in patients with COVID-19. Furthermore, much evidence has indicated that one-step RT-ddPCR is more precise and fully amenable to the viral RNA absolute quantification, especially when the RNA concentration is low.8
In the current prospective cohort study, we developed a novel test for quantitative and dynamic assessment of the blood viral load of SARS-CoV-2 and validated it in 52 cases of confirmed COVID-19. By using this method, we attempted to study the prevalence and clinical significance of viremia in patients with COVID-19.
Materials and Methods
Study Approval
This study was approved by the medical ethics committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China. Written informed consent was obtained from all patients or the patients’ legal representative if the patient was too unwell to provide the consent.
Patients
Between January 30, 2020, and February 26, 2020, fifty-two patients with COVID-19 were enrolled into this prospective study after screening (Supplemental Figure S1). The diagnosis and the illness severity of COVID-19 were defined according to the Chinese management guideline for COVID-19 version 7.0 (https://www.who.int/docs/default-source/wpro---documents/countries/china/covid-19-briefing-nhc/1-clinical-protocols-for-the-diagnosis-and-treatment-of-covid-19-v7.pdf, last accessed March 18, 2020) and the World Health Organization interim guidance.9 Methods for laboratory confirmation of SARS-CoV-2 infection have been described elsewhere.10 Exclusion criteria included: i) no clinical and chest radiographic findings were available, ii) no written informed consent could be obtained, and iii) patients had received remdesivir or were involved in other clinical trials.
Study Design, Specimens, and Data Collection
Specimens and data of the enrolled participants were collected prospectively according to study design (Supplemental Figure S1). The first and the third day after enrollment were designated D1 and D3, respectively. Dx referred to the day when disease progressed according to the Chinese management guideline for COVID-19 (version 7.0). Dend was the day before discharge or death. A patient had to meet the following criteria to be deemed clinically cured and eligible for discharge: i) body temperature remained normal for >3 days, ii) respiratory symptoms resolved, iii) chest CT scan showed substantial improvement of acute exudative lesions, and iv) two consecutive real-time PCR assays, at least 24 hours apart, yielded negative results.
Peripheral blood was taken from patients on D1, D3, Dx, and/or Dend for the determination of viral load according to RT-ddPCR. Blood samples were collected at other time points as necessary. A chest CT scan was performed before enrollment and on Dend. An additional chest CT scan was performed if the patient’s condition deteriorated. Real-time PCR for detecting SARS-CoV-2 RNA in oropharyngeal swab samples was conducted as described previously10 and at an interval as required by routine clinical practice. Anti–SARS-CoV-2 IgG/IgM was serologically detected on D1, D3, Dx, and Dend by using a chemiluminescence immunoassay kit (Shenzhen YHLO Biotech Co., Ltd., Shenzhen, China). All other treatments, laboratory examinations, and clinical evaluation followed routine clinical practice.
Patients’ data were entered into an electronic data capture system. These data included demographic characteristics, medical history, daily clinical findings, oximetric measurements, and laboratory findings, involving complete blood count, serum biochemical parameters, high-sensitivity C-reactive protein, serum IL-6 and IL-8, and ferritin. Also included were the treatments received by the patients.
Follow-Up
Patients were followed up by postdischarge clinic visit. The close contacts of the patients, who were eligible for discharge but were positive for plasma SARS-CoV-2, were also included in clinical and laboratory examination to rule out possible transmission by the patients.
Determination of SARS-CoV-2 Copy Numbers by One-Step RT-ddPCR
For quantitative detection of SARS-CoV-2 copy numbers, a viral RNA purification kit (QIAamp Viral RNA Mini Kit, Qiagen, Hilden, Germany), a one-step RT-ddPCR advanced kit, QX200 droplet generator (Bio-Rad, Hercules, CA), and a QX200 droplet reader (Bio-Rad) were used, following the manufacturers’ instructions. To increase sensitivity, 4 wells were used for each sample. The SARS-CoV-2–specific minor groove binder (MGB) probe-primer set was designed for targeting the ORF1ab region, and the sequences were as follows: forward primer 5′-TGACCCTGTGGGTTTTACACTTAA-3′; reverse primer 5′-CAGCCATAAC-CTTTCCACATACC-3′; probe 5′FAM-AACACAGTCTG-TACCGTCT-3′MGB. Primer blast results showed that 58,667 (99.78%) of 58,799 SARS-CoV-2 genomic sequences collected in the GISAID database (https://db.cngb.org/gisaid; accessed September 2020) were on target templates (Supplemental Table S1). To test the quantitative capacity, SARS-CoV-2 plasmid standards (Sangon Biotech, Shanghai, China) were linearized by using restriction enzyme BamHI, fivefold serially diluted at concentrations ranging from ∼50,000 copies/μL to 16 copies/μL and subjected to ddPCR amplification. To evaluate the lower limit of detection, which was defined as the lower 50% confidence interval of detection (LOD50), plasma samples from patient DF were used as standards.11 Plasma samples from 33 healthy donors served as negative control to estimate the false positivity in healthy samples of the test.
SARS-CoV-2–Specific IgM and IgG Detection
SARS-CoV-2–specific IgM and IgG were detected by paramagnetic particle chemiluminescent immunoassay using the iFlash-SARS-CoV-2 IgM/IgG assay kit (Shenzhen YHLO Biotech Co., Ltd.) and an iFlash Immunoassay Analyzer (Shenzhen YHLO Biotech Co., Ltd.).
Statistical Analysis
Categorical variables are presented as frequency and proportions. Means ± SD are given for continuous variables; median and ranges are given for variables that were not normally distributed. Means were compared by using t-tests for normally distributed continuous variables; otherwise, a U-test was used. Multiple comparisons were made by using Turkey test. Coefficients of determination (R 2) were computed by using linear regression analysis. Probit analysis for LOD50 was conducted with SPSS Statistics version 25 (IBM SPSS Statistics, IBM Corporation, Armonk, NY).
Results
Patient Characteristics
The study included 52 patients with COVID-19 (Table 1 ). The median age was 62 years (range, 26 to 83 years) with a sex ratio of 31/21 (male/female). The general, severe, and critical status of this cohort comprised 40%, 33%, and 27%, respectively. Of note, the proportion of cases of various severities in our study did not reflect the actual case distribution as Tongji Hospital is a designated medical center caring for severe and critical patients. Comorbidities were present in 54% of the patients, with hypertension and diabetes being the most common. Of patients with comorbidities, 4 (7.7%) and 2 (3.8%) had cancer or immunodeficiency diseases, respectively. The median time from illness onset (always before admission) to enrollment was 17 days (range, 3 to 29 days). Forty-nine (94%) of 52 patients were enrolled into our observational investigation over 7 days following disease onset. About 80% of the patients received corticosteroid treatment. Finally, 30 (58%) patients who met the criteria were discharged with two consecutive real-time PCR assays, 15 (29%) patients died, and seven (13%) patients were still hospitalized for further observation or management.
Table 1.
Patient Characteristics
| Characteristic | Data (N = 52) |
|---|---|
| Age, median (range), years | 62 (26–83) |
| Sex, male/female, n | 31/21 |
| Disease severity status, n (%) | |
| Mild | 0 (0) |
| General | 21 (40) |
| Severe | 17 (33) |
| Critical | 14 (27) |
| Comorbidities, n (%) | 28 (54) |
| Hypertension | 17 (33) |
| Diabetes | 9 (17) |
| Coronary heart disease | 6 (12) |
| Chronic obstructive pulmonary disease | 1 (2) |
| Cancer∗ | 4 (8) |
| Immunodeficiency† | 2 (4) |
| Smoking history, n (%) | 7 (14) |
| Time from illness onset to enrollment, median (range), days | 17 (3–40) |
| >7 days, n (%) | 49 (94) |
| ≤7 days, n (%) | 3 (6) |
| Treatments, n (%) | |
| Antibiotics | 35 (67) |
| Antiviral treatment | 38 (73) |
| Intravenous immunoglobulin | 13 (25) |
| Corticosteroids | 42 (81) |
| High-flow nasal cannula oxygen therapy | 5 (12) |
| Noninvasive mechanical ventilation | 2 (4) |
| Invasive mechanical ventilation | 11 (21) |
| ECMO | 4 (8) |
| Renal replacement therapy | 9 (17) |
| Clinical outcomes at data cutoff, n (%) | |
| Discharge from hospital | 30 (58) |
| Death | 15 (29) |
| Hospitalization | 7 (13) |
ECMO, extracorporeal membrane oxygenation.
Includes: G8, acute B-cell lymphoblastic leukemia (no remission); G21, endometrial cancer (no remission); S8, endometrial cancer (no assessment); and D1, multiple myeloma (complete remission).
Includes: D1: multiple myeloma post anti–B-cell mature antigen chimeric antigen receptor T therapy; and C9, pulmonary sarcoidosis with long-term glucocorticoid administration.
Detection and Validation of Plasma SARS-CoV-2 by One-Step RT-ddPCR
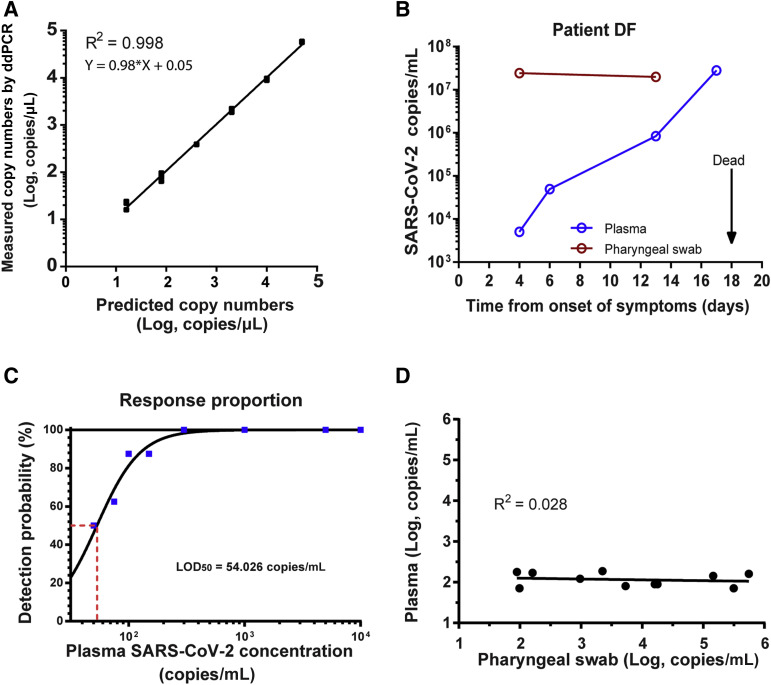
We developed a SARS-CoV-2 detection system based on RT-ddPCR. Linearized plasmids with ORF1ab inserted were serially diluted and used as standards to test the quantitative capacity of the system. Each concentration, measured in triplicate, was well in line with expected concentration (R 2 = 0.998, P < 0.001), indicating that ddPCR using the probe-primer set for SARS-CoV-2 could accurately quantify SARS-CoV-2 copies (Figure 1 A). Next, COVID-19 patient DF, with a severe defect in humoral immunity, was continuously measured for SARS-CoV-2 load in blood and oropharyngeal swabs by using one-step RT-ddPCR. Patient DF had a complete depletion of plasma cells due to anti–B-cell mature antigen chimeric antigen receptor T-cell treatment for relapsed multiple myeloma 3 months earlier. B-cell mature antigen chimeric antigen receptor T cells could be persistently detected in the patient and caused the humoral immune deficiency to persist. Although the patient’s viral load in oropharyngeal swabs stayed at a constant level, the plasma SARS-CoV-2 load showed a continuous rise, with his disease progressing to death (Figure 1B).
Figure 1.
The sensitivity and accuracy of plasma severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) cell-free RNA quantification. A: Dilution curve of plasmid standards quantification by RT droplet digital PCR (ddPCR). Correlation between expected and observed copy number are shown. Each black square represents a single replicate well of the dilution experiment, whereas the regression line is based on the average concentration at each dilution. B: SARS-CoV-2 concentration–time curve of plasma (blue) and oropharyngeal swab (red) samples from patient DF. C: Probit analysis sigmoid curve reporting the lower 50% confidence interval of detection (LOD50) of one-step RT-ddPCR. The red dashed line represents the plasma SARS-CoV-2 concentration when the detection probability is 50%. D: Comparison of plasma and oropharyngeal swab SARS-CoV-2 concentration of time-matched samples.
We then determined the LOD50 of one-step RT-ddPCR by using serially diluted blood samples of patient DF. Eight replicates of each concentration were tested. The LOD50 of plasma SARS-CoV-2 load was 54.026 copies/mL plasma (Figure 1C). Furthermore, the false-positive rate was estimated from 33 healthy plasma samples, and only one of them exhibited one positive droplet. Thus, the false-positive rate was 3.03% (1 of 33).
To investigate the correlation in the SARS-CoV-2 load between oropharyngeal swabs and the corresponding plasma samples, 11 time-matched SARS-CoV-2–positive oropharyngeal swab samples were re-detected by using one-step RT-ddPCR. Although the SARS-CoV-2 load in oropharyngeal swab samples was highly variable, the plasma viral load was relatively low, ranging from 80.4 to 187.5 copies/mL (excluding samples of patient DF) and bore no correlation with the load of their paired swab samples (P = 0.488) (Figure 1D).
Clinical Concordance between Blood Tests and Oropharyngeal Swab Tests
We compared 87 pairs of time-matched samples, which were the plasma samples tested by using one-step RT-ddPCR and oropharyngeal swab samples by real-time PCR. While 16 (18.39%) of 87 pairs of plasma and oropharyngeal swab samples were positive with both tests, 37 of 87 (42.53%) were negative with the two tests. Thus, the concordance between these two testing methods was 60.92% (53 of 87). Of the 17 positive oropharyngeal swab samples, 16 (94.12%) were also positive for plasma SARS-CoV-2. Conversely, there were 33 paired tests with plasma viral positivity but negative oropharyngeal swabs.
Clearance of Blood SARS-CoV-2 in the Course of Disease
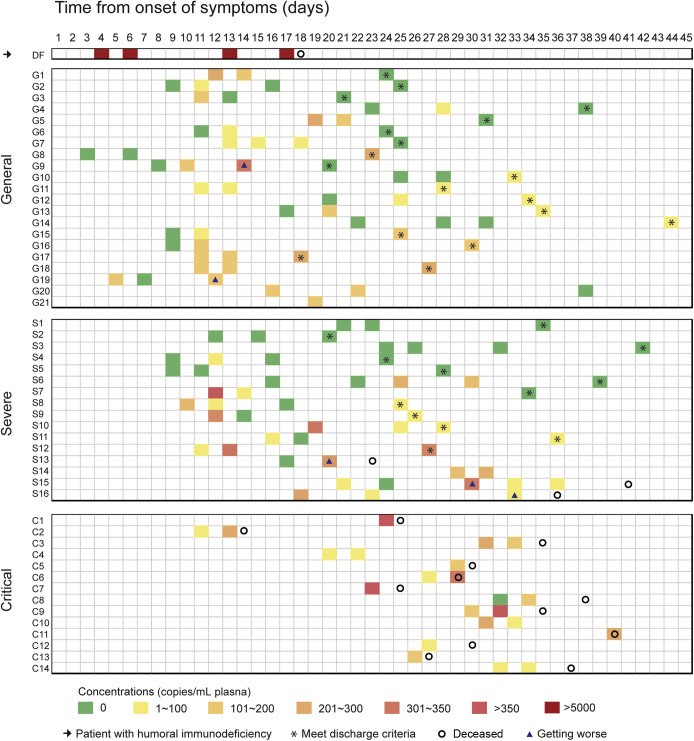
By applying one-step RT-ddPCR, 92.3% (48 of 52) of our cohort could be quantitatively detected with viremia. Clearance of blood SARS-CoV-2 in the course of the disease was tracked according to the classification of clinical severity (Figure 2 ). A continuous increase of viremia was observed only in patient DF, who had no appreciable level of SARS-CoV-2 antibody. At the cutoff day of the study, clearance of blood viral load was observed in 42.86% (9 of 21) of the general patients, with a median clearance time of 24 days (13 to 38 days). 43.75% (7 of 16) of the severe patients cleared the virus in their blood, with a median clearance time of 21 days (9 to 39 days). There was no statistical difference in the median clearance time between mild and severe patients (P = 0.484). Remarkably, all critical patients were unable to clear blood SARS-CoV-2. Critical patients C4 and C10 were followed up on day 45 and day 56, respectively. Their plasma viral loads remained positive.
Figure 2.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) dynamics of 52 patients. One humoral immunodeficient, 21 general, 16 severe, and 14 critical patients are presented. Squares in different colors represent different virus loads. Asterisks indicate patients met discharge standards at the specific days; circles, patients were deceased at the specific days; triangles, patients were getting worse at the specific days.
Correlation of Blood Viral Load, Antibodies, and Inflammatory Factors to Illness Severity of COVID-19
The average plasma viral load in general, severe, and critical patients was 81.68 (0 to 312.5) copies/mL, 73.62 (0 to 317) copies/mL, and 176 (0 to 392) copies/mL, respectively (Figure 3 A). Although the plasma viral load was significantly higher in critical patients than in general and severe patients (P < 0.001), no significant difference was found between the general and severe patients (P = 0.885). In our cohort, disease progression occurred in 2 general (G19 and G9) and 3 severe (S13, S15, and S16) patients, and 4 of the 5 patients concomitantly experienced a substantial increase in the blood viral load (Figure 3B). The peak level of anti-IgG of SARS-CoV-2 in critical patients was lower than in general (P = 0.044) and severe (P = 0.011) patients (Figure 3D); no significant difference was found in peak IgM levels among the three groups (Figure 3C). All inflammatory factors, including high-sensitivity C-reactive protein, ferritin, soluble IL-6, and soluble IL-8, were profoundly higher in critically ill patients compared with all other patients (Figure 3, E–H). Interestingly, although high-sensitivity C-reactive protein was significantly higher in severe patients than in general patients, no significant difference was observed in the levels of ferritin, soluble IL-6, and soluble IL-8, suggesting that other cytokines might be responsible for the increased inflammatory reaction in severe patients.
Figure 3.
Analysis of severity-associated clinical factors. A: Plasma severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) loads in general, severe, and critical patients were compared. B: Levels of plasma SARS-CoV-2 cell-free RNA before and after disease progression were analyzed. C–H: The levels of peak SARS-CoV-2–specific IgM (C), IgG (D), high-sensitivity C-reactive protein (hsCRP) (E), peak serum ferritin (F), peak soluble IL-6 (sIL-6) (G), and peak soluble IL-8 (sIL-8) (H) were compared among general, severe, and critical patients. Error bars indicate means ± SD. ∗∗∗P < 0.001 (paired t-test); †P < 0.05, ††P < 0.01, and †††P < 0.001 (Tukey multiple comparisons test). AU, arbitrary units.
Outcomes of Discharged Patients with Positive Blood Test Results and Their Close Contacts
Among 30 discharged patients, 18 were general and 12 were severe (Figure 2). At the time of discharge, the blood viral test was positive in 50% (15 of 30) of the patients. Important indicators, including disease severity, serum antibody level, viral cell-free RNA copies, time from illness onset to discharge, absolute lymphocyte count, and corticosteroid exposure, were compared between the positive and negative patients (Supplemental Table S2). There were no significant differences in these indicators between the two subgroups (Supplemental Table S2). To address the concerns of the final clinical outcome as well as contagion in these blood-positive patients, we followed up 13 of 15 patients and their close contacts. Physical examination, oropharyngeal and rectal swabs, and blood viral tests of SARS-CoV-2 were performed (Table 2 ). The median from the illness onset to the follow-up was 49 (45 to 61) days. The median from close family contact to follow-up was 7 (4 to 14) days. Interestingly, 10 of 13 patients eventually cleared their blood viral load without any further treatment. All seven close contacts exhibited no evidence of SARS-CoV-2 infection.
Table 2.
Follow-Up Outcome of Discharged Patients with Positive Blood Test Result and Their Close Contacts
| Participants |
Relationship to patients | COVID-19– associated clinical manifestations | Follow-up to illness onset, days | Follow-up to discharge, days | Close contact time, days | Viral load, copies/mL plasma |
Swabs on follow-up |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Discharge point | Follow-up point |
Nasal | Throat | Rectal | ||||||||
| Patients | Contacts | Patients | Contacts | |||||||||
| G10 | No | 55 | 22 | 71.4 | 0 | N | N | N | ||||
| G11 | No | 49 | 21 | 71.4 | 98 | N | N | N | ||||
| C1 of G11 | Wife | No | 7 | 0 | N | N | N | |||||
| G13 | No | 52 | 17 | 89.3 | 0 | N | N | N | ||||
| G14 | No | 61 | 17 | 89.3 | 0 | N | N | N | ||||
| G15 | No | 47 | 22 | 142.9 | 0 | N | N | N | ||||
| G18 | No | 49 | 22 | 258.9 | 0 | N | N | N | ||||
| C1 of G18 | Husband | No | 8 | 0 | N | N | N | |||||
| C2 of G18 | Father-in-law | No | 8 | 0 | N | N | N | |||||
| G16 | No | 46 | 16 | 169.6 | 107 | N | N | N | ||||
| G17 | No | 46 | 28 | 250.0 | 0 | N | N | N | ||||
| C1 of G17 | Husband | No | 14 | 0 | N | N | N | |||||
| S8 | No | 45 | 20 | 62.5 | 0 | N | N | N | ||||
| C1 of S8 | Daughter | No | 6 | 0 | N | N | N | |||||
| C2 of S8 | Son-in-law | No | 6 | 0 | N | N | N | |||||
| S9 | No | 47 | 21 | 71.4 | 0 | N | N | N | ||||
| S10 | No | 58 | 30 | 80.4 | 0 | N | N | N | ||||
| S11 | No | 54 | 18 | 98.2 | 0 | N | N | NA | ||||
| C1 of S11 | Wife | No | 4 | 0 | N | N | N | |||||
| S12 | No | 46 | 20 | 232.1 | 250 | N | N | N | ||||
| Total | 0/20 | 49 (45–61) | 21 (16–30) | 7 (4–14) | 89.3 (62.5–258.9) | 10/13 | 7/7 | 20/20 | 20/20 | 19/19 | ||
COVID-19, coronavirus disease 2019; N, negative; NA, not available.
Discussion
In this prospective cohort study, we developed and validated a novel detection method to quantitatively measure the viral load of SARS-CoV-2 in the blood of patients with COVID-19. Quantitative capacity was evaluated by linearized DNA, within which the reverse-transcriptase step was not included, and hence the results were not completely applicable to RNA.
By using this technique, we were able to address the prevalence and clinical significance of viremia in patients with COVID-19. To our knowledge, this report is the first to depict the clearance kinetics of blood SARS-CoV-2 over the entire course of COVID-19. Remarkably, this study, including a protracted follow-up of survivors, suggests that all critical patients failed to eliminate blood viral load. In contrast, the general and severe patients were equally capable of completely clearing the blood viral load. Second, blood viral load was found to be associated with the severity of COVID-19. The viral load was significantly higher in critical patients than in their general/severe counterparts, which could be, at least partially, ascribed to the impaired capability to generate SARS-CoV-2–specific antibodies on the part of critical patients. Among the patients whose condition deteriorated, a corresponding rise in blood viral load was detected. Interestingly, although there was no significant difference in the level of blood viral load between the general and severe patients, the severe patients exhibited a stronger inflammatory response, as defined by high-sensitivity C-reactive protein level. This finding provides a rationale for a prospective clinical trial to reduce the overactivated host inflammatory response in severe patients. Moreover, no significant difference was found in the levels of cytokines between the general and severe patients, suggesting that other cytokines or chemokines might be more crucial for the immunopathologic changes.
Finally, 30 patients met the current discharge criteria, and blood viral load remained positive in 15 of these patients. Consistent with our data, other independent studies have reported frequent detection of nucleic acid of SARS-CoV-2 in urine and stool.12 These findings raise serious concerns over the long-term outcome and infectivity of these patients, which present future public health issues. Ten of the 13 discharged patients completely cleared their blood viral load, as indicated by our follow-up (spanning 45 to 61 days from the illness onset to the follow-up). Moreover, none of their close contacts exhibited evidence of infection, as indicated by ddPCR. Our results suggest that the clearance of blood SARS-CoV-2 might take longer than expected and occurs in a gradual manner. Nevertheless, there was no evidence suggesting that the patients in the course of viral clearance were contagious. The most recent publication from the Wendtner group has confirmed our findings.13 Although further investigation in a large cohort is warranted to draw definitive conclusions, the current findings are very encouraging.
This study has important clinical implications. By using this method, were able to detect viremia in 92.3% of our cohort, which can serve as a useful tool for diagnosis, monitoring therapeutic response in terms of viral load, confirming the viral clearance, and following up with patients after discharge. Our study further supports the notion that COVID-19 is a self-limited disease, with most of the general and severe cases capable of completely clearing their viral load. Conversely, critical patients seem to be a distinct subgroup, characterized by defective viral clearance,14 lower levels of SARS-CoV-2–specific antibodies, and a strong inflammatory response. The management of COVID-19 should therefore be tailored because of the heterogeneous nature of these patients. For the general/severe cases, supportive therapies could be essential, and, with some severe cases, immunomodulatory therapies might be explored to suppress aberrant host inflammatory response. For the critical patients, antiviral therapy, in combination with anti-inflammatory and supportive treatments, should be considered and tested in a clinical trial setting to improve clinical outcomes. In this regard, dynamic monitoring of blood viral load by RT-ddPCR will assist physicians in identifying and monitoring the early signs of aggravation in critical cases. Finally, our study showed that the viremia in patient DF and other critical patients did not suffice to produce SARS-CoV-2–specific antibodies, suggesting that the host humoral immunity plays a critical part in the removal of SARS-CoV-2, and the vaccines and therapeutic antibodies promise to be effective for the prevention and cure of COVID-19.
Acknowledgments
We thank eStart Medical Technology Co., Ltd., Professor Jennifer Hou, and Professor Guangliang Shan for discussion and building the electronic data capture system.
Footnotes
Supported by the Emergency Research Project of Tongji Hospital (J.Zho.), Emergency Research Project of Tongji Hospital of Huazhong University of Science and Technology grant 2020kfyXGYJ045 (J.Zho.), and the Emergency Research Project of Hubei Province grant 2020FCA006 (W.W.).
L.C., G.W., X.L., and H.H. contributed equally to this work.
Disclosures: None declared.
Supplemental material for this article can be found at http://doi.org/10.1016/j.jmoldx.2020.10.007.
Author Contributions
W.W., J.Zho. concieved and designed the study; L.C., G.W., X.L., H.H., J.T., J.W., Y.C., W.L. acquired and analyzed data; J.Zha., Y.C., Lia.H., F.M., Lif.H., N.W. provided care to patients and provided clinical information; J.Zho., J.W., L.C., G.W., Y.C. wrote the manuscript; J.Zho., W.W., Z.S., G.H., J.Zha. revised the manuscript; L.C., X.L., Y.C. performed statistical analysis; W.W., J.Zho. obtained funding; W.W., J.Zho. supervised the study.
Supplemental Data
Flowchart of study design. COVID-19, coronavirus disease 2019; D1 and D3, first and the third day after the enrollment, respectively; Dend, the day before discharge or death; Dx, the day when disease progressed.
References
- 1.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W., China Novel Coronavirus Investigating and Research Team A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahase E. Covid-19: WHO declares pandemic because of “alarming levels” of spread, severity, and inaction. BMJ. 2020;368:m1036. doi: 10.1136/bmj.m1036. [DOI] [PubMed] [Google Scholar]
- 4.Liu Y., Yang Y., Zhang C., Huang F., Wang F., Yuan J., Wang Z., Li J., Li J., Feng C., Zhang Z., Wang L., Peng L., Chen L., Qin Y., Zhao D., Tan S., Yin L., Xu J., Zhou C., Jiang C., Liu L. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63:364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li H., Liu L., Zhang D., Xu J., Dai H., Tang N., Su X., Cao B. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 2020;395:1517–1520. doi: 10.1016/S0140-6736(20)30920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen W., Lan Y., Yuan X., Deng X., Li Y., Cai X., Li L., He R., Tan Y., Deng X., Gao M., Tang G., Zhao L., Wang J., Fan Q., Wen C., Tong Y., Tang Y., Hu F., Li F., Tang X. Detectable 2019-nCoV viral RNA in blood is a strong indicator for the further clinical severity. Emerg Microbes Infect. 2020;9:469–473. doi: 10.1080/22221751.2020.1732837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu F., Yan L., Wang N., Yang S., Wang L., Tang Y., Gao G., Wang S., Ma C., Xie R., Wang F., Tan C., Zhu L., Guo Y., Zhang F. Quantitative detection and viral load analysis of SARS-CoV-2 in infected patients. Clin Infect Dis. 2020;71:793–798. doi: 10.1093/cid/ciaa345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rački N., Morisset D., Gutierrez-Aguirre I., Ravnikar M. One-step RT-droplet digital PCR: a breakthrough in the quantification of waterborne RNA viruses. Anal Bioanal Chem. 2014;406:661–667. doi: 10.1007/s00216-013-7476-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization . WHO; Geneva: 2020. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: interim guidance. Available at https://www.who.int/publications/i/item/clinical-management-of-covid-19. [Google Scholar]
- 10.Ai T., Yang Z., Hou H., Zhan C., Chen C., Lv W., Tao Q., Sun Z., Xia L. Correlation of chest CT and RT-PCR testing for coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020;296:E32–E40. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stone M., Lanteri M.C., Bakkour S., Deng X., Galel S.A., Linnen J.M., Muñoz-Jordán J.L., Lanciotti R.S., Rios M., Gallian P., Musso D., Levi J.E., Sabino E.C., Coffey L.L., Busch M.P. Relative analytical sensitivity of donor nucleic acid amplification technology screening and diagnostic real-time polymerase chain reaction assays for detection of Zika virus RNA. Transfusion. 2017;57:734–747. doi: 10.1111/trf.14031. [DOI] [PubMed] [Google Scholar]
- 12.Xu Y., Li X., Zhu B., Liang H., Fang C., Gong Y., Guo Q., Sun X., Zhao D., Shen J., Zhang H., Liu H., Xia H., Tang J., Zhang K., Gong S. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020;26:502–505. doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A., Niemeyer D., Jones T.C., Vollmar P., Rothe C., Hoelscher M., Bleicker T., Brünink S., Schneider J., Ehmann R., Zwirglmaier K., Drosten C., Wendtner C. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 14.Chen J., Qi T., Liu L., Ling Y., Qian Z., Li T., Li F., Xu Q., Zhang Y., Xu S., Song Z., Zeng Y., Shen Y., Shi Y., Zhu T., Lu H. Clinical progression of patients with COVID-19 in Shanghai, China. J Infect. 2020;80:e1–e6. doi: 10.1016/j.jinf.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flowchart of study design. COVID-19, coronavirus disease 2019; D1 and D3, first and the third day after the enrollment, respectively; Dend, the day before discharge or death; Dx, the day when disease progressed.