Abstract
Subjective sleep assessment in cancer patients poorly correlates with actigraphy parameters that usually encompass multiple nights. We aimed to determine the objective actigraphy measures that best correlated with subjective sleep ratings on a night-by-night basis in cancer patients. Thirty-one cancer patients daily self-rated sleep disturbances using the single dedicated item of the MD Anderson Symptom Inventory (0–10 scale) with 18 other items, and continuously wore a wrist actigraph for 30 days. Objective sleep parameters were computed from the actigraphy nighttime series, and correlated with subjective sleep disturbances reported on the following day, using repeated measures correlations. Multilevel Poisson regression analysis was performed to identify the objective and subjective parameters that affected subjective sleep rating. Poor subjective sleep score was correlated with poor sleep efficiency (rrm = −0.13, p = 0.002) and large number of wake episodes (rrm = 0.12, p = 0.005) on the rated night. Multilevel analysis demonstrated that the expected sleep disturbance score was affected by the joint contribution of the wake episodes (exp(β) = 1.01, 95% confidence interval = 1.00 to 1.02, p = 0.016), fatigue (exp(β) = 1.35, 95% confidence interval = 1.15 to 1.55, p < 0.001) and drowsiness (exp(β) = 1.70, 95% confidence interval = 1.19 to 2.62, p = 0.018), self-rated the following evening, and sleep disturbance experienced one night before (exp(β) = 1.77, 95% confidence interval = 1.41 to 2.22, p < 0.001). The night-by-night approach within a multidimensional home tele-monitoring framework mainly identified the objective number of wake episodes computed from actigraphy records as the main determinant of the severity of sleep complaint in cancer patients on chemotherapy. This quantitative information remotely obtained in real time from cancer patients provides a novel framework for streamlining and evaluating interventions toward sleep improvement in cancer patients.
Keywords: actigraphy, sleep disturbance, patient-reported outcome, cancer, telecare, telemedicine, circadian rhythms
Statement of Significance.
The assessment of sleep disturbances in cancer patients has usually involved subjective self-rated questionnaires, and occasionally objective actigraphy measures. However, the relations between subjective sleep complaints and concurrent objective measures remain unclear. By performing a night-by-night analysis, for the first time in patients with cancer on treatment, we demonstrate here that the severity of subjective sleep alterations was correlated negatively with sleep efficiency and positively with the number of wake episodes. This new insight into the relationship between subjective sleep assessment and quantitative actigraphic measures provides a novel framework for the identification of effective and personalized interventions to improve sleep in cancer patients.
Introduction
Sufficiently long and restorative sleep is paramount for human well-being, in particular during stressful periods such as during anticancer treatment [1, 2]. Moreover, in cancer patients, altered sleep has been shown to be associated with poor prognosis [3–5]. Notwithstanding, sleep issues in patients with cancer are often unrecognized, overlooked, or undertreated [6, 7].
The main challenges in addressing sleep issues in cancer involve the identification of both the most relevant sleep parameters for quality of life, and the most appropriate instruments regarding feasibility and accuracy [8, 9]. Specific questionnaires have been frequently used to evaluate sleep in cancer patients, on the basis that the most legitimate approach for appraising sleep function is through capturing the patient’s perspective [10, 11]. Thus, patient-reported outcome measures (PROM) of sleep function are integrated into multidimensional health-related quality of life assessment tools used in cancer research, and altered sleep is one of the most prevalent complaints in cancer patients [12–14].
Alongside subjective questionnaire-based assessment tools of sleep, wearable activity trackers can be used for objective sleep assessment and their regular use or testing has recently increased in oncology research [10, 15–20]. The relationship between subjective sleep complaints and concurrent objective evaluation has been previously investigated in cancer patients; however, limited associations were found [21–25]. These inconsistent findings could be partially explained by the heterogeneity in patient populations, as well as in objective and subjective tools and methods. Wearable biosensors have the advantage of potential long-term remote monitoring of sleep, as well as physical activity and circadian rhythms, which also affect on sleep, in reciprocal manners [15, 26–28]. However, the identification of the most crucial features of sleep function derived from sleep trackers that capture the cancer patient’s personal perspective is still unclear [21].
Indeed, anticancer treatment as well as supportive medications can transiently or persistently affect sleep, usually resulting in a diurnal hypersomnia and/or nocturnal insomnia [8, 29]. Hence, the sleep patterns of patients with cancer or other diseases could display large intraindividual variations from night to night [30], with possible treatment effects [31, 32]. A single snapshot assessment or an averaging over a week could, therefore, result in inaccurate estimates of sleep function in patients displaying iatrogenic dynamic changes in sleep-wake cycles. We tackled this issue through harnessing remote sleep monitoring at home, using both continuous wrist-actigraphy tele-transmitted records, and daily electronic questionnaires. This study was part of the Integrated Network for Completely Assisted Senior Citizen’s Autonomy (inCASA) Domomedicine cancer pilot [30, 33]. This unique multidimensional and longitudinal dataset involved patients receiving multidrug chemotherapy—usually chronotherapy [34, 35]—at home for advanced cancer. The dataset structure enabled night-by-night analyses with shortest recall biases between awakening and subjective rating of the previous night’s sleep for 30 days in each patient. This approach highlighted the within-subject variability in subjective sleep complaints rather than the between-subjects one, which has been mostly investigated thus far [31].
The current study aimed at the determination of the main established quantitative measures that characterize sleep at night, including duration, timing, and efficiency, and their relation with the subjective rating of the sleep quality on the same night by the patient. On the basis of previous studies, we hypothesized that sleep duration and efficiency would have been the most critical objective sleep features associated with the subjective complaint of disturbed sleep [36, 37].
Patients and Methods
Study population
The patients were screened and recruited at the Chronotherapy Clinics in the Medical Oncology Department of Paul Brousse Hospital in Villejuif, France, within the framework of the European inCASA project (ICT-PSP) [30, 33, 38]. Eligible patients were informed about the study by the oncologist in charge and the research nurses explained the study in details to the enrolled patients. Patients older than 18 years, with any histologically proven cancer type requiring chemotherapy and having an Internet connection at home were eligible. During the study, patients received conventional or chronomodulated chemotherapy, either at the hospital or at home. The study was approved by the local institutional review board and conducted according to the Declaration of Helsinki on medical research involving human subjects [39].
inCASA platform
The electronic inCASA platform was installed at a patient’s home and connected to their Internet network. Each patient was instructed to use the platform and was given a form with telephone contact details for technical or health-related assistance.
The equipment which was part of the platform included a touch screen computer (ASUS Eee Top ET1611; ASUSTek, Taipei, Taiwan) provided with the SARA software (Telefonica Investigacion y Desarrollo SA, Granada, Spain) and a wrist-worn actigraph (Micro MotionLogger; Ambulatory Monitoring Inc, Ardsley, NY) [40–42]. This device has been validated in sleep studies and successfully used in patients with cancer [43–46]. Actigraphy data were transmitted daily by the patient using an infrared USB dongle connected to the computer. An electronic version of the MD Anderson Symptom Inventory (MDASI) questionnaire [47, 48] was installed on the SARA software and completed every evening on the touch screen. Although diurnal variations in symptom ratings have been described [49], we opted for a pragmatic approach to administer the whole questionnaire in the evening and to assume that the fairly short recall time to the previous night’s sleep would have minimally biased the rating as compared to a morning assessment. More details about the functions and technical aspects of the platform are provided elsewhere [30, 33].
Wrist-actigraphy parameters
The actigraph recorded wrist accelerations per 1 minute epochs. Actigraphy data were analyzed with Action4 and Action W-2 software (Ambulatory Monitoring Inc). Total sleep time, sleep efficiency, sleep onset latency, latency to persistent sleep, wake after sleep onset, sleep fragmentation index, inactivity index, sleep timing (time of retiring, sleep midpoint, and time of awakening) as well as parameters related to sleep and wake episodes [4, 21, 41, 42, 50, 51] were computed with Action W-2. The full list of computed parameters with their definition is available in Supplementary Table S1.
MDASI selected items (PROM)
The MDASI requests the patient to rate the worst severity of 13 symptoms and 6 interference items experienced during the previous 24 hours on a scale from 0 (representing no symptom present) to 10 (worst possible severity) [14, 47].
The single MDASI item on sleep was selected as the main subjective measure of perceived sleep disturbance the night before [52]. This MDASI item on disturbed sleep has been shown to correlate well with the global score of the Pittsburgh Sleep Quality Index, a widely used multiple-item scale [52], thus confirming its appropriateness tool for sleep disturbance screening [53], in accordance with other evidence using different yet comparable instruments [54]. Additional selected PROM including fatigue, drowsiness, and interference with general activity were investigated for sensitivity analyses. These symptoms have been described as constituting a cluster with potentially a common pathogenic mechanism [47] and they have been shown to be associated with subjective sleep rating [55].
Study design
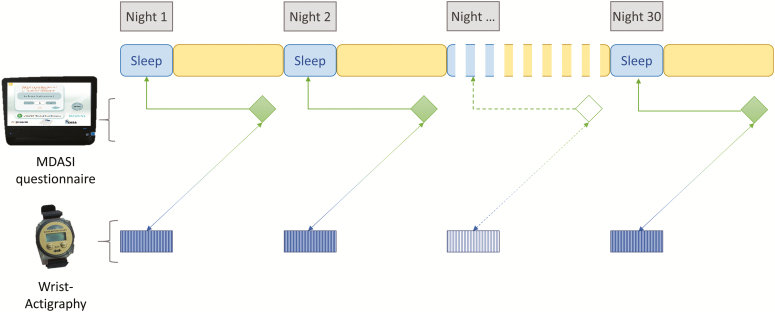
Patients were asked to wear the wrist actigraph continuously, to fill out the on-screen MDASI electronic questionnaire each evening and to tele-transmit the actigraphy data before or after questionnaire completion. All the data were then transmitted via the Internet to a secured server [30, 33]. The study duration was 30 days for each patient (Figure 1).
Figure 1.
Study design, describing the pattern of objective and subjective data collection over time.
We evaluated the relations between objective actigraphy parameters corresponding to each night, with the MDASI sleep item completed the following day, referring to the severity of sleep disturbance on the previous night [56, 57] (Figure 1).
Data analysis
Descriptive statistics were used to characterize the distribution of the various parameters within the sample. Frequencies were computed for the ordinal scales from the MDASI questionnaire (ranging from 0 to 10), whereas mean, SD, median, and interquartile range (IQR) were reported for the continuous wrist-actigraphy variables.
Inter- and intraindividual changes in MDASI sleep scores and actigraphy-derived sleep parameters were mapped through computing respective mean values, range, and coefficient of variations for each patient.
The primary endpoint was the identification of objective actigraphy parameters, assessing sleep duration, efficiency, and timing, during the night, associated with subjectively rated sleep (dedicated MDASI item) related to the same night. Thus, within-person associations were explored for each single night from all the participants, hence generating an aggregate dataset of night (actigraphy) and day (MDASI) dyads.
Given the exploratory and observational nature of the study, we tested all the available and validated sleep parameters derived from the wrist actigraph.
First, repeated measures correlations (rmcorrs) [58] were computed between the MDASI sleep scores and actigraphy sleep parameters. Rmcorr was well suited for investigating the linear association in paired repeated measures data. Correlations were considered statistically significant if p ≤ 0.05. Rmcorrs were programmed in R with package rmcorr.
Second, multivariate analyses explored the statistical significance of multiple explanatory variables on the MDASI sleep disturbance rating. Given the quantitative measures of sleep scores and the hierarchical data structure, i.e. measures (level 1) grouped within the individual patient (level 2), a two-level Poisson regression model was applied to relate the expected sleep score to a set of explanatory variables via a log-linear link [59]; see Supplementary Data for more details.
First, as the level-1 potential explanatory variables (which vary within individual patient) we considered the objective actigraphy parameters, which were found to be significantly correlated with sleep scores in rmcorrs, rating during a weekend (binary variable Y/N) and whether the patient was receiving chemotherapy that day (Y/N). Potential level-2 explanatory variables (non-nested data that only vary in different individual) included sex (M/F), age (years), marital/cohabitation status (Y/N), and existence of comorbidity (Y/N). As recommend in Hox et al. [60], a multistep model selection procedures (discussed in details in Supplementary Data) was applied to find the “best” two-level Poisson regression model based on both Akaike information criterion and Bayesian information criterion [61].
We further considered adding selected subjective MDASI items as potential explanatory variables. In addition to objective parameters, we explored the impact of fatigue, drowsiness, and interference with activity scores (following the evaluated night N), as well as the sleep score of one night before (night N−1), on the self-rating of sleep disturbance (night N). As MDASI items were reported as discrete scores ranging from 0 to 10, cutoff values previously determined in a report including 728 patients [62] were used to make binary explanatory MDASI variables (marked as *) and thus simplify the model. The same two-level Poisson regression with model selection procedures used for the main outcome of sleep disturbance was performed. The two-level Poisson regression models were programmed in R with package lme4. The multilevel modeling included datasets from 30 patients as one of them did not provided concomitant objective actigraphy parameters and subjective sleep ratings.
Results
Study population
A total of 31 patients were enrolled in the Domomedicine cancer pilot study and provided multidimensional data [33]. The majority of patients had undergone both surgery and chemotherapy treatments before enrollment, yet maintained a fairly good general condition as indicated by a performance status ranging from 0 to 2 (Table 1). None of them had a formal diagnosis of sleep disorder.
Table 1.
Clinical features of the study population
| Characteristics | |
|---|---|
| Age (years) | |
| Median (range) | 61 (35–91) |
| Sex | |
| Male | 17 (55) |
| Female | 14 (45) |
| Marital status | |
| Never married | 2 (6) |
| Married | 20 (64) |
| Divorced | 4 (13) |
| Widowed | 3 (10) |
| Cohabiting | 2 (6) |
| Education | |
| No formal schooling | 1 (3) |
| Primary school | 2 (6) |
| Secondary school | 13 (42) |
| High school | 11 (35) |
| Postgraduate degree | 4 (13) |
| Work status (over the past 12 months before inclusion) | |
| Government employee | 2 (6) |
| Nongovernment employee | 8 (26) |
| Homemaker | 1 (3) |
| Retired | 16 (52) |
| Unemployed (able to work) | 1 (3) |
| Unemployed (unable to work) | 3 (10) |
| WHO performance status | |
| 0/1 | 9 (29)/11 (35) |
| 2/3 | 2 (6)/2 (6) |
| Not available | 7 (24) |
| Primary tumor site | |
| Colorectal | 12 (39) |
| Pancreas | 9 (29) |
| Breast | 5 (16) |
| Others | 5 (16) |
| Recurrent disease | |
| Yes/no | 11 (35)/20 (65) |
| Number of metastatic sites | |
| 0 | 7 (23) |
| 1 | 24 (77) |
| Comorbidities | |
| None | 24 (78) |
| Diabetes | 5 (16) |
| Hepatitis B | 1 (3) |
| Chronic heart failure | 1 (3) |
| Prior cancer surgery | |
| None | 11 (35) |
| Primary tumor only | 12 (39) |
| Primary tumor and metastases | 8 (26) |
| Prior chemotherapy | |
| None | 3 (10) |
| Adjuvant and metastatic | 16 (52) |
| Adjuvant only | 11 (36) |
| Metastatic only | 1 (3) |
| Number of prior chemotherapy protocols for metastatic disease | |
| None | 13 (42) |
| 1 | 4 (13) |
| ≥2 | 12 (39) |
| Unknown | 2 (6) |
| Noncancer treatments | |
| None | 25 (81) |
| Insulin | 3 (10) |
| Pain killer | 2 (6) |
| Antiretroviral | 1 (3) |
| Thyroid hormone replacement | 1 (3) |
| Beta blocker | 1 (3) |
Data presented as number (%) of patients unless otherwise indicated.
Data compliance
The per-protocol patient participation lasted 30 days, representing a total of 930 days during which actigraphy data could have been recorded with the wrist actigraph and symptoms could have been self-rated by the patient using the MDASI questionnaire. Sleep parameters were computed for a total of 730 nights (78%). MDASI questionnaire was filled out for 696 days (75%). At worst, the proportion of available concomitant data was 57% (528/930 dyads). Each analysis was performed on the largest dataset available. The most common reasons for missing data, besides planned or emergency hospitalizations, were informally reported to be technical problems, out-of-home trips, or patient forgetting or feeling too sick [33].
The median number of nights with sleep data available per patient was 26 (of 30) for actigraphy-computed parameters (87%; range, 7%–100%) and 25 for subjective sleep (85%; range, 17%–100%). Thus, individual patients provided both actigraphy sleep parameters and MDASI data concurrently for a median of 18/30 dyads (60%).
Descriptive statistics
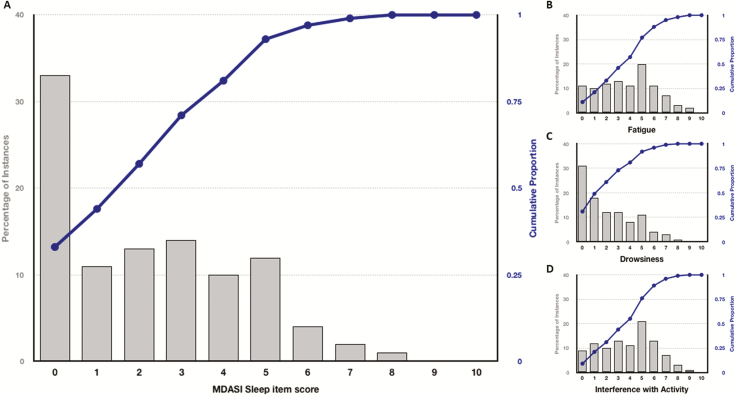
Median rating for sleep was 2 (IQR, 0–4). Bothersome sleep disturbance was reported for 57% of the nights (Figure 2A). Mean (SD) of the PROM of interest were 2.26 (2.13) for sleep disturbance, 2.18 (2.13) for drowsiness, 3.75 (2.36) for fatigue, and 3.82 (2.26) for interference with activity (Figure 2B–D).
Figure 2.
Distribution of MDASI sleep, fatigue, drowsiness, and interference with activity item scores. Panels show the percentage of occurrences (gray bars) of the MDASI sleep (A), fatigue (B), drowsiness (C), and interference with activity (D) scores (ranging 0–10), respectively, as rated by the patients and their cumulative proportion (blue line).
Sleep Efficiency ranged between 20.2% and 100%, with a median of 92.0% (IQR, 86.7%–95.0%) (Table 2; Figure 3D). Of the determinants of sleep efficiency, median sleep onset latency was 5 minutes (IQR, 3–7 minutes), median wake after sleep onset duration was 42 minutes (IQR, 25–70 minutes) and median number of wake episodes was 11 (IQR, 1–17) (Table 2; Supplementary Figure S1).
Table 2.
Descriptive statistics of the objective actigraphy sleep parameters in the whole dataset
| Actigraphy sleep parameters | Median | Quartiles (Q1, Q3) | Range (min, max) | Average | SD |
|---|---|---|---|---|---|
| Time of retiring | 23:07 | 22:11, 23:54 | 19:29, 05:00 | 23:04 | 1:21 |
| Sleep midpoint | 3:29 | 2:50, 4:09 | 00:38, 10:19 | 3:32 | 1:01 |
| Time of waking up | 8:00 | 7:14, 8:46 | 3:49, 15:39 | 8:00 | 1:14 |
| Total sleep time | 7 h 50 min | 6 h 50 min, 8 h 44 min | 1 h 1 min, 13 h 7 min | 7 h 45 min | 1 h 42 min |
| Sleep efficiency | 92.0% | 86.7%, 95.0% | 20.2%, 100% | 88.8% | 10.6% |
| Sleep onset latency | 5 min | 0 min, 7 min | 0 min, 50 min | 5 min | 5 min |
| Latency to persistent sleep | 6 min | 0 min, 12 min | 0 min, 10 h 25 min | 15 min | 42 min |
| Wake after sleep onset | 42 min | 25 min, 1 h 10 min | 0 min, 6 h 54 min | 57 min | 53 min |
| Sleep fragmentation index | 2.4% | 0.1%, 3.6% | 0.1%, 28.8% | 3.0% | 2.7% |
| Short burst inactivity index | 33 | 0, 41 | 0, 73 | 34 | 13 |
| Total time in bed | 8 h 50 min | 4 h 57 min, 9 h 51 min | 4 h 57 min, 17 h 22 min | 8 h 56 min | 1 h 34 min |
| Wake minutes | 52 min | 2 min, 81 min | 2 min, 11 h 9 min | 1 h 12 min | 1 h 11 min |
| Wake episodes | 11 | 1, 17 | 1, 58 | 13 | 7 |
| Mean duration of wake episodes | 5 min | 1 min, 7 min | 1 min, 37 min | 6 min | 4 min |
| Wake episodes ≥5 min | 4 | 0, 5 | 0, 32 | 4 | 4 |
| Longest wake episode | 17 min | 1 min, 26 min | 1 min, 3 h 19 min | 22 min | 20 min |
| Sleep episodes | 11 | 1, 16 | 1, 57 | 12 | 7 |
| Mean duration of sleep episodes | 44 min | 4 min, 1 h 5 min | 4 min, 12 h 58 min | 57 min | 1 h 3 min |
| Sleep episodes ≥5 min | 8 | 1, 11 | 1, 30 | 9 | 4 |
| Longest sleep episode | 2 h 32 min | 13 min, 3 h 33 min | 13 min, 12 h 58 min | 2 h 49 min | 1 h 32 min |
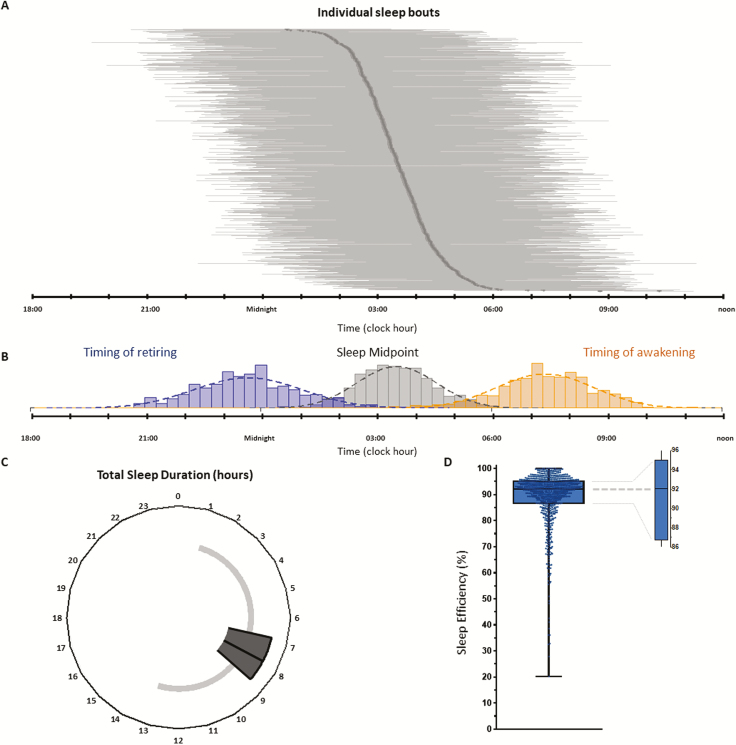
Figure 3.
Distribution of main actigraphy parameters in the study dataset. (A) The plot shows the sleep duration (horizontal gray bars) for the 735 nights of measurement. The dark gray line represents the time of mid sleep. (B) The plot combines the distribution of the time of retiring (blue bars), the time of mid sleep (gray bars), and the time of awakening (gold bars). (C) The plot illustrates the total sleep duration computed for the 735 nights of measurement. (D) The boxplot describes the distribution of the sleep efficiency measured over 735 nights. For panels C and D, the box represents the 25th, 50th (median), and 75th percentiles, whereas the whiskers display the range.
Median total sleep time was 7 hours 50 minutes (IQR, 6 h 50 min to 8 h 44 min), with extremes spanning from 1 hour 1 minute to 13 hours 7 minutes (Table 2; Figure 3C). Times of retiring were staggered over 9.5 hours (from clock hours 19:29 to 05:00), with a median occurring at 23:07 (IQR, 22:11–23:54). Its distribution partly overlapped that of the sleep midpoint, which ranged from 00:38 to 10:19, with a median value at 03:29 (IQR, 02:50–04:09). In turn, the range of sleep midpoint partly overhung the distribution of the timing of awakening, which extended over almost half of the 24-hour period, between 03:49 and 15:39, with a median timing at 08:00 (IQR, 07:14–08:46) (Table 2). Figure 3B plots the distributions of the sleep events times, together with the individual sleep periods, globally covering almost three quarters of the 24 hours (Figure 3A).
Average MDASI sleep score in each individual ranged from 0 to 5.5 between patients, but from 0 to 9 (median: 5) within patients. Similarly, the magnitude of interindividual variability in actigraphy parameters was comparable to that of the intraindividual variability (Supplementary Table S2).
Univariate associations
Rmcorr coefficients between subjectively rated sleep disturbance and objective actigraphy parameters ranged from |0.10| to |0.14|, with p values ≤0.05 (Table 3). Thus, the worse the MDASI sleep score, the lower the sleep efficiency (p = 0.002), the larger the number of wake episodes (p = 0.005), and the worse the sleep fragmentation index (p = 0.01).
Table 3.
Repeated measures correlation coefficients table and matrix (r values) among subjective symptoms (MDASI) and actigraphy parameters (only results with p ≤ 0.05 are shown for clarity)
| MDASI symptoms | ||||
|---|---|---|---|---|
| Sleep | Fatigue | Drowsiness | Interference with activity | |
| Actigraphy parameters | ||||
| Timing of retiring | −0.09 | −0.09 | ||
| Timing of waking up | 0.09 | |||
| Total sleep time | 0.11* | |||
| Sleep efficiency | −0.13* | −0.11 | ||
| Wake after sleep onset | 0.14** | 0.13* | 0.08 | |
| Sleep fragmentation index | 0.10 | |||
| Total time in bed | 0.14** | 0.11* | ||
| Wake minutes | 0.12* | 0.09 | ||
| Short burst inactivity index | 0.10 | 0.09 | ||
| Wake episodes | 0.12* | 0.10 | 0.09 | 0.09 |
| Wake episodes ≥5 min | 0.10 | |||
| Longest wake episode | 0.10 | |||
| Sleep episodes | 0.11* | 0.09 | 0.09 | 0.09 |
| Mean duration of sleep episodes | −0.09 | −0.10 | ||
| Sleep episodes ≥5 min | 0.10 | 0.11* | 0.10 | 0.11* |
| Longest sleep episode | −0.12* | −0.10 | −0.15** | |
| MDASI symptoms | ||||
| Sleep | 0.31** | 0.28** | 0.22** | |
| Fatigue | 0.31** | 0.53** | 0.54** | |
| Drowsiness | 0.28** | 0.53** | 0.42** | |
| Interference with activity | 0.22** | 0.54** | 0.42** | |
**p < 0.001, *p < 0.01.
The number of wake episodes was correlated to the PROM of interest, fatigue (p = 0.02), drowsiness (p = 0.03), and interference with activity (p = 0.03). Sleep efficiency, instead, was only associated with daytime drowsiness (p = 0.01) (Table 3).
Subjectively rated sleep disturbance, fatigue, drowsiness, and interference with activity were all positively correlated with each other (p < 0.001) with correlation coefficients ranging from 0.22 to 0.54.
Multivariate multilevel model
Seventy-three percent of the variability in MDASI sleep scores was accounted for by inter-patient differences, as shown with an empty random intercept two-level Poisson regression model. Thus a multilevel analysis was required for the determination of within-patients variabilities (Table 4).
Table 4.
Parameter estimates with 95% confidence intervals for the two-level Poisson regression models of sleep disturbance
| Variable | Coefficient estimate β | 95% confidence interval | P |
|---|---|---|---|
| Model A Dependent variable: sleep disturbance; objective explanatory parameters |
|||
| Fixed intercept | |||
| 2.321 | (0.316 to 4.161) | ||
| Fixed effects, exp(β) | |||
| Sleep efficiency | 0.993 | (0.984 to 1.002) | 0.081 |
| Wake episodes | 1.023 | (1.007 to 1.043) | 0.024 |
| Age | 0.975 | (0.949 to 0.997) | 0.031 |
| Random effects | |||
| Intercept SD | 1.247 | (0.832 to 1.657) | |
| Wake episodes slope SD | 0.028 | (0.014 to 0.043) | |
| Model B Dependent variable: sleep disturbance; objective and subjective explanatory parameters |
|||
| Fixed intercept | |||
| 1.656 | (0.699 to 2.269) | ||
| Fixed effects, exp(β) | |||
| Wake episodes | 1.012 | (1.002 to 1.021) | 0.016 |
| Prior night sleep disturbance* (Y) | 1.774 | (1.407 to 2.216) | <0.001 |
| Fatigue* (Y) | 1.350 | (1.154 to 1.547) | <0.001 |
| Drowsiness* (Y) | 1.695 | (1.186 to 2.616) | 0.018 |
| Comorbidity (Y) | 1.352 | (1.037 to 1.896) | 0.045 |
| Sex (M) | 0.682 | (0.543, 0.819) | 0.012 |
| Age | 0.971 | (0.964 to 0.982) | <0.001 |
| Random effects | |||
| Intercept SD | 1.079 | (0.714 to 1.300) | |
| Prior night sleep disturbance* (Y) slope SD | 0.299 | (0.050 to 0.549) | |
| Drowsiness* (Y) slope SD | 0.860 | (0.513 to 1.087) |
Models A and B are based on 591 and 528 samples, respectively, from 30 patients. The subjective MDASI explanatory variables identified with a star (*) are binary (Y/N) variables.
In case of objective explanatory variables only (Table 4, model A), the model selected sleep efficiency, wake episodes, and age as explanatory variables. The estimated intercept was with a mean of 2.321 (fixed intercept) and an SD of 1.247 (random effect intercept). The number of wake episodes was found to be positively associated (p = 0.024) with sleep disturbance. An increase by one number of the wake episodes would lead to an increase of the sleep disturbance rating expected value by 2.3% (95% confidence interval = 0.7%, to 4.3%). Age had a positive impact on sleep disturbance (p = 0.031), with an increase by 1 year in age resulting in a decrease by 2.5% of the sleep disturbance expected score. Sleep efficiency was negatively associated with sleep disturbance scores but its impact was not significant (p = 0.081). Moreover, the model decided to allow the slope of wake episodes vary in different individuals, with mean value equals to the fixed slope 0.023, i.e. computed by log(1.023), and SD 0.028.
When further taking subjective MDASI items into account (Table 4, model B), the model additionally selected prior night sleep disturbance*, fatigue*, drowsiness*, comorbidity, and sex as explanatory variables. The subjective MDASI items were shown to be strongly associated with sleep disturbance. An uncomfortable fatigue indicated an increase in the expected value of sleep disturbance rating by 35.0 % (p < 0.001), an uncomfortable drowsiness led to an increase by 69.5% (p = 0.018) and a disturbed sleep experienced the prior night was associated with an increase by 77.4% (p < 0.001). In addition, sex affected on sleep disturbance (p = 0.012). As compared to females, males reported less sleep disturbance with a 68.2% expectation rating score. The model also allows the slopes of prior night sleep disturbance* and drowsiness* to vary in different patients.
We further considered taking the MDASI ratings of fatigue, drowsiness, and interference with activity as dependent variable and the resulting models are presented in Supplementary Data.
Discussion
With a night-by-night systematic approach and multilevel modeling, we have identified objectively determined nighttime awakening episodes as the most relevant correlate of subjective sleep complaint in patients with advanced cancer undergoing systemic chemotherapy. Thus, a lower number of wake episodes were associated with better rated sleep even when other PROM were included. However, the relevance of the subjective sleep the night before in predicting the rating of sleep the following night suggests that poor subjective sleep is a rather consistent within-subject complaint, which was best evaluated using the individual night approach here advocated. Sleep behavior and perceived sleep quality can vary within the same subject from night to night also in noncancerous people, thus confirming a complex interaction between endogenous preference, environmental opportunity, and subjective perception [63, 64]. Objective actigraphy parameters, usually averaging sleep parameters over multiple nights have failed to predict for subjective sleep estimation in cancer patients [65, 66]. In contrast, our single night assessment approach provides a novel insight into the objective vs subjective sleep relation [67]. Indeed, differences in subjective and objective sleep measures were of similar magnitude both between patients and within patients (Supplementary Table S2).
Actigraphy-measured sleep efficiency, determined chiefly by the latency of sleep onset and by the number and duration of nighttime awakenings, has already demonstrated clinical relevance in cancer patients’ prognosis. Thus, sleep efficiency less than 85% was independently associated with shorter overall survival in women with advanced breast cancer [4]. Moreover, sleep efficiency of cancer patients could be improved with behavioral interventions, as shown in a recent study using morning bright light exposure [68]. Conversely, pharmacological interventions with hypnotics seem to affect sleep onset latency rather than the ability of sustaining sleep [69]. This finding would, therefore, support the upfront use of cognitive behavioral therapy for insomnia in patients with cancer, as currently advocated [70].
Frequent night awakenings can not only negatively impact on the subjective perception of sleep quality as shown here, but interrupted sleep continuity has been shown to be correlated to impaired innate immunity function [71], with lower natural killer cell count being associated with circadian disruption and shorter overall survival [72].
Furthermore, our multidimensional remote monitoring allowed us to explore the impact of objective sleep parameters on daytime consequences on systemic psychophysical symptoms. These included fatigue, drowsiness, and interference with activity, and nighttime awakenings parameters were constantly associated with less severe complaints. The consistency in the finding of the relevance of sleep maintenance on various PROM, assessing frequent complaints in cancer patients [73, 74], supports a role of sleep efficiency at large (i.e. ability to sustain efficient sleep condition through the night) in patient’s well-being that deserves additional exploration.
A first limitation of our study was the lack of evaluation of chronotype using validated questionnaires [75, 76], thus missing the subjective perspective to the phase assessment of the body circadian clock [5]. Another study limitation was due to the limited number of patients, despite the conspicuous number of individual night datasets, and their heterogeneity regarding cancer type, stage, and treatment. Finally, a single question was used for the subjective assessment of sleep. From the one hand, specific multiple-item questionnaires are used to accurately determine sleep [10, 53, 77]. Yet, on the other hand, the MDASI item hereby used has demonstrated its usefulness in comparison with more complex measures [52], and has the great advantage of referring to the previous night only, thus with a single-night recall precision [47, 78].
Nonetheless, we believe we provided here a first insight into the relevance of multimodal modern tele-monitoring with regard to sleep troubles in patients with cancer, with a potential opening to bespoken and dynamic interventions in the individual night of need. Indeed, patient-empowering self-management plans are increasingly becoming pivotal in the holistic management of cancer patients [79–81] and rely to a great extent upon dedicated mobile health solutions and multisensor wearable monitors collecting biometric data [16, 18, 78, 82, 83].
In order to achieve and maintain satisfactory sleep, this study’s results support the combined use of continuous objective monitoring and repeated subjective PROM. The accuracy of objective sleep assessment can be further improved by complementing activity tracking with additional physiological biorhythms, such as skin temperature or heart rate [84–86]. Indeed, we have recently demonstrated the relevance of such multidimensional monitoring in cancer using a second-generation e-Health Domomedicine platform [87]. The widespread diffusion of smartphones could permit the development of dedicated apps for regularly capturing PROM, allowing for a temporal accuracy of a single night at next morning’s awakening, particularly apt for sleep evaluation [82, 83, 88, 89].
To conclude, our results here provide evidence for both feasibility and relevance of combined objective and subjective remote monitoring of sleep and other symptoms in cancer patients with a single-night precision. This dynamic approach could trigger the development of novel therapeutic concepts, whose testing is warranted in cancer patients.
Supplementary material
Methods details for the two-level Poisson regression model and results of the multilevel analyses of the items of fatigue, drowsiness, and interference with activity (including Supplementary Table S3). Supplementary material is available at SLEEP online.
Acknowledgments
The work was performed in the Chronotherapy Unit, Department of Medical Oncology, Paul Brousse Hospital, Assistance Publique–Hopitaux de Paris, Villejuif, France (data collection) and in Warwick Medical School, Coventry, UK (data analyses). We thank all the patients who participated in this study, and A. Arbaud, J. Fursse, and J. Rovira-Simon for their contribution to the development of the Integrated Network for Completely Assisted Senior Citizen’s Autonomy (inCASA) platform. We are grateful to the Registered Nurses R. Bossevot-Desmaris, M. Mocquery, and V. Plessis for their involvement in patient recruitment and follow-up.
This work was presented in part at the First Conference of the European Association of Systems Medicine, held in Berlin, Germany, on October 26–28, 2016, and at the Annual Meeting of the Associated Professional Sleep Societies, held in Boston, MA, United States of America, on June 3–7, 2017.
Funding
Support from European Commission through the Information and Communication Technologies Policy Support Programme project inCASA (Contract CIP 250505, FP7) and CASyM (Coordinating Action Systems Medicine).
Conflict of interest statement. The authors Sandra Komarzynski, Qi Huang, Oxana G. Palesh, Ayhan Ulusakarya, Mohamed Bouchahda, Mazen Haydar, Nicholas I. Wreglesworth, and Jean-François Morère declared no conflict of interest. Francis A. Lévi discloses the following: Research funding: Philips Respironics (Inst); patents, royalties, other intellectual property: Institut National de la Santé et de la Recherche Médicale, patent on circadian dichotomy index automatic computation (Inst); travel, accommodations, expenses: Merck Serono. René Adam has consulted or advised for Merck and Amgen; honoraria: Amgen, Merck, Sanofi, and Astellas; travel, accommodations, expenses: Amgen, Merck, Sanofi, and Astellas. Pasquale F. Innominato discloses the research funding from Philips Healthcare (Inst).
References
- 1. Spiegel D. Losing sleep over cancer. J Clin Oncol. 2008;26(15):2431–2432. [DOI] [PubMed] [Google Scholar]
- 2. Redeker NS, et al. Incorporating measures of sleep quality into cancer studies. Support Care Cancer. 2015;23(4):1145–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Innominato PF, et al. Subjective sleep and overall survival in chemotherapy-naïve patients with metastatic colorectal cancer. Sleep Med. 2015;16(3):391–398. [DOI] [PubMed] [Google Scholar]
- 4. Palesh O, et al. Actigraphy-measured sleep disruption as a predictor of survival among women with advanced breast cancer. Sleep. 2014;37(5):837–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hahm BJ, et al. Bedtime misalignment and progression of breast cancer. Chronobiol Int. 2014;31(2):214–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ancoli-Israel S. Recognition and treatment of sleep disturbances in cancer. J Clin Oncol. 2009;27(35):5864–5866. [DOI] [PubMed] [Google Scholar]
- 7. Savard J, et al. Insomnia in the context of cancer: a review of a neglected problem. J Clin Oncol. 2001;19(3):895–908. [DOI] [PubMed] [Google Scholar]
- 8. Palesh O, et al. Prevalence, putative mechanisms, and current management of sleep problems during chemotherapy for cancer. Nat Sci Sleep. 2012;4:151–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Otte JL, et al. Systematic review of sleep disorders in cancer patients: can the prevalence of sleep disorders be ascertained? Cancer Med. 2015;4(2):183–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen D, et al. Measurements and status of sleep quality in patients with cancers. Support Care Cancer. 2018;26(2):405–414. [DOI] [PubMed] [Google Scholar]
- 11. Efficace F, et al. ; European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Group. Overcoming barriers to the implementation of patient-reported outcomes in cancer clinical trials: the PROMOTION Registry. Health Qual Life Outcomes. 2014;12:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kirkova J, et al. Cancer symptom assessment instruments: a systematic review. J Clin Oncol. 2006;24(9):1459–1473. [DOI] [PubMed] [Google Scholar]
- 13. Reilly CM, et al. A literature synthesis of symptom prevalence and severity in persons receiving active cancer treatment. Support Care Cancer. 2013;21(6):1525–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cleeland CS, et al. The symptom burden of cancer: evidence for a core set of cancer-related and treatment-related symptoms from the Eastern Cooperative Oncology Group Symptom Outcomes and Practice Patterns study. Cancer. 2013;119(24):4333–4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Madsen MT, et al. Actigraphy for measurements of sleep in relation to oncological treatment of patients with cancer: a systematic review. Sleep Med Rev. 2015;20:73–83. [DOI] [PubMed] [Google Scholar]
- 16. Beg MS, et al. Promise of wearable physical activity monitors in oncology practice. J Oncol Pract. 2017;13(2):82–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dicker AP, et al. Intersection of digital health and oncology. JCO Clin Cancer Inform. 2018;2:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wright AA, et al. The HOPE pilot study: harnessing patient-reported outcomes and biometric data to enhance cancer care. JCO Clin Cancer Inform. 2018;2:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cox SM, et al. Use of wearable, mobile, and sensor technology in cancer clinical trials. JCO Clin Cancer Inform. 2018;2:1–11. [DOI] [PubMed] [Google Scholar]
- 20. Gupta A, et al. Feasibility of wearable physical activity monitors in patients with cancer. JCO Clin Cancer Inform. 2018;2:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Palesh O, et al. Relationship between subjective and actigraphy-measured sleep in 237 patients with metastatic colorectal cancer. Qual Life Res. 2017;26(10):2783–2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bernatchez MS, et al. Feasibility of a cognitive-behavioral and environmental intervention for sleep-wake difficulties in community-dwelling cancer patients receiving palliative care. J Pain Symptom Manage. 2018;56(6):e117. [DOI] [PubMed] [Google Scholar]
- 23. Bernatchez MS, et al. Sleep-wake difficulties in community-dwelling cancer patients receiving palliative care: subjective and objective assessment. Palliat Support Care. 2018;16(6):756–766. [DOI] [PubMed] [Google Scholar]
- 24. Bernatchez MS, et al. Correlates of disrupted sleep-wake variables in patients with advanced cancer. BMJ Support Palliat Care. 2018. doi:10.1136/bmjspcare-2018-001505 [DOI] [PubMed] [Google Scholar]
- 25. Miaskowski C, et al. Sleep-wake circadian activity rhythm parameters and fatigue in oncology patients before the initiation of radiation therapy. Cancer Nurs. 2011;34(4):255–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Innominato PF, et al. The circadian timing system in clinical oncology. Ann Med. 2014;46(4):191–207. [DOI] [PubMed] [Google Scholar]
- 27. Gresham G, et al. Wearable activity monitors in oncology trials: current use of an emerging technology. Contemp Clin Trials. 2018;64:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bernard P, et al. Temporal relationships between sleep and physical activity among breast cancer patients with insomnia. Health Psychol. 2016;35(12):1307–1315. [DOI] [PubMed] [Google Scholar]
- 29. Parker KP, et al. Sleep/Wake patterns of individuals with advanced cancer measured by ambulatory polysomnography. J Clin Oncol. 2008;26(15):2464–2472. [DOI] [PubMed] [Google Scholar]
- 30. Innominato P, et al. Home-based e-Health platform for multidimensional telemonitoring of symptoms, body weight, sleep, and circadian activity: relevance for chronomodulated administration of irinotecan, fluorouracil-leucovorin, and oxaliplatin at home-results from a pilot study. JCO Clin Cancer Inform. 2018;2:1–15. [DOI] [PubMed] [Google Scholar]
- 31. Bei B, et al. Beyond the mean: a systematic review on the correlates of daily intraindividual variability of sleep/wake patterns. Sleep Med Rev. 2016;28:108–124. [DOI] [PubMed] [Google Scholar]
- 32. Buysse DJ, et al. Night-to-night sleep variability in older adults with and without chronic insomnia. Sleep Med. 2010;11(1):56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Innominato PF, et al. Clinical relevance of the first domomedicine platform securing multidrug chronotherapy delivery in metastatic cancer patients at home: the inCASA european project. J Med Internet Res. 2016;18(11):e305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dallmann R, et al. Dosing-time makes the poison: circadian regulation and pharmacotherapy. Trends Mol Med. 2016;22(5):430–445. [DOI] [PubMed] [Google Scholar]
- 35. Sulli G, et al. Training the circadian clock, clocking the drugs, and drugging the clock to prevent, manage, and treat chronic diseases. Trends Pharmacol Sci. 2018;39(9):812–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lemola S, et al. Variability of sleep duration is related to subjective sleep quality and subjective well-being: an actigraphy study. PLoS One. 2013;8(8):e71292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fontes F, et al. Reliability and validity of the Pittsburgh Sleep Quality Index in breast cancer patients. Support Care Cancer. 2017;25(10):3059–3066. [DOI] [PubMed] [Google Scholar]
- 38. Kapsalis AP, et al. The inCASA project: improving the quality of life and social care for the ageing population. International Journal of Integrated Care. 2012;12:e112. [Google Scholar]
- 39. World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. [DOI] [PubMed] [Google Scholar]
- 40. Ancoli-Israel S, et al. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26(3):342–392. [DOI] [PubMed] [Google Scholar]
- 41. Smith MT, et al. Use of actigraphy for the evaluation of sleep disorders and circadian rhythm sleep-wake disorders: an American academy of sleep medicine systematic review, meta-analysis, and GRADE assessment. J Clin Sleep Med. 2018;14(7):1209–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Smith MT, et al. Use of actigraphy for the evaluation of sleep disorders and circadian rhythm sleep-wake disorders: an American academy of sleep medicine clinical practice guideline. J Clin Sleep Med. 2018;14(7):1231–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rupp TL, et al. Comparison of Motionlogger Watch and Actiwatch actigraphs to polysomnography for sleep/wake estimation in healthy young adults. Behav Res Methods. 2011;43(4):1152–1160. [DOI] [PubMed] [Google Scholar]
- 44. Levin RD, et al. Circadian function in patients with advanced non-small-cell lung cancer. Br J Cancer. 2005;93(11):1202–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mormont MC, et al. Marked 24-h rest/activity rhythms are associated with better quality of life, better response, and longer survival in patients with metastatic colorectal cancer and good performance status. Clin Cancer Res. 2000;6(8):3038–3045. [PubMed] [Google Scholar]
- 46. Ma CL, et al. Rest/activity rhythm is related to the coexistence of pain and sleep disturbance among advanced cancer patients with pain. Support Care Cancer. 2014;22(1):87–94. [DOI] [PubMed] [Google Scholar]
- 47. Cleeland CS, et al. Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer. 2000;89(7):1634–1646. [DOI] [PubMed] [Google Scholar]
- 48. Basch E, et al. Recommendations for incorporating patient-reported outcomes into clinical comparative effectiveness research in adult oncology. J Clin Oncol. 2012;30(34):4249–4255. [DOI] [PubMed] [Google Scholar]
- 49. Dhruva A, et al. Differences in morning and evening fatigue in oncology patients and their family caregivers. Eur J Oncol Nurs. 2013;17(6):841–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Natale V, et al. Actigraphy in the assessment of insomnia: a quantitative approach. Sleep. 2009;32(6):767–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Martin JL, et al. Wrist actigraphy. Chest. 2011;139(6):1514–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. George GC, et al. Sleep quality and its association with fatigue, symptom burden, and mood in patients with advanced cancer in a clinic for early-phase oncology clinical trials. Cancer. 2016;122(21):3401–3409. [DOI] [PubMed] [Google Scholar]
- 53. Howell D, et al. Sleep disturbance in adults with cancer: a systematic review of evidence for best practices in assessment and management for clinical practice. Ann Oncol. 2014;25(4):791–800. [DOI] [PubMed] [Google Scholar]
- 54. Savard J, et al. Screening for clinical insomnia in cancer patients with the Edmonton Symptom Assessment System-Revised: a specific sleep item is needed. Support Care Cancer. 2019. doi:10.1007/s00520-019-4662-2 [DOI] [PubMed] [Google Scholar]
- 55. Lin YY, et al. Longitudinal study on the impact of physical activity on the symptoms of lung cancer survivors. Support Care Cancer. 2015;23(12):3545–3553. [DOI] [PubMed] [Google Scholar]
- 56. Tang NK, et al. Deciphering the temporal link between pain and sleep in a heterogeneous chronic pain patient sample: a multilevel daily process study. Sleep. 2012;35(5):675–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Russell C, et al. Subjective but not actigraphy-defined sleep predicts next-day fatigue in chronic fatigue syndrome: a prospective daily diary study. Sleep. 2016;39(4):937–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bakdash JZ, et al. Repeated measures correlation. Front Psychol. 2017;8:456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Austin PC, et al. Measures of clustering and heterogeneity in multilevel Poisson regression analyses of rates/count data. Stat Med. 2018;37(4):572–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hox JJ, et al. Multilevel Analysis: Techniques and Applications. 3rd ed.New York, NY: Routledge; 2017. [Google Scholar]
- 61. Vrieze SI. Model selection and psychological theory: a discussion of the differences between the Akaike information criterion (AIC) and the Bayesian information criterion (BIC). Psychol Methods. 2012;17(2):228–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hui D, et al. Personalized symptom goals and response in patients with advanced cancer. Cancer. 2016;122(11):1774–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pilz LK, et al. Time to rethink sleep quality: PSQI scores reflect sleep quality on workdays. Sleep. 2018;41(5):zsy029. [DOI] [PubMed] [Google Scholar]
- 64. Lavie P. Sleep-wake as a biological rhythm. Annu Rev Psychol. 2001;52:277–303. [DOI] [PubMed] [Google Scholar]
- 65. Dhruva A, et al. A longitudinal study of measures of objective and subjective sleep disturbance in patients with breast cancer before, during, and after radiation therapy. J Pain Symptom Manage. 2012;44(2):215–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Moore CM, et al. Actigraphy and sleep diary measurements in breast cancer survivors: discrepancy in selected sleep parameters. Behav Sleep Med. 2015;13(6):472–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Dijk DJ, et al. Integration of human sleep-wake regulation and circadian rhythmicity. J Appl Physiol (1985). 2002;92(2):852–862. [DOI] [PubMed] [Google Scholar]
- 68. Wu LM, et al. The effect of systematic light exposure on sleep in a mixed group of fatigued cancer survivors. J Clin Sleep Med. 2018;14(1):31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Huedo-Medina TB, et al. Effectiveness of non-benzodiazepine hypnotics in treatment of adult insomnia: meta-analysis of data submitted to the Food and Drug Administration. BMJ. 2012;345:e8343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Garland SN, et al. Sleeping well with cancer: a systematic review of cognitive behavioral therapy for insomnia in cancer patients. Neuropsychiatr Dis Treat. 2014;10:1113–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Irwin MR, et al. Sleep health: reciprocal regulation of sleep and innate immunity. Neuropsychopharmacology. 2017;42(1):129–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sephton SE, et al. Diurnal cortisol rhythm as a predictor of breast cancer survival. J Natl Cancer Inst. 2000;92(12):994–1000. [DOI] [PubMed] [Google Scholar]
- 73. Teunissen SC, et al. Symptom prevalence in patients with incurable cancer: a systematic review. J Pain Symptom Manage. 2007;34(1):94–104. [DOI] [PubMed] [Google Scholar]
- 74. Miaskowski C, et al. Advancing symptom science through symptom cluster research: expert panel proceedings and recommendations. J Natl Cancer Inst. 2017;109(4):djw253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Roenneberg T. Having trouble typing? What on earth is chronotype? J Biol Rhythms. 2015;30(6):487–491. [DOI] [PubMed] [Google Scholar]
- 76. Levandovski R, et al. Chronotype: a review of the advances, limits and applicability of the main instruments used in the literature to assess human phenotype. Trends Psychiatry Psychother. 2013;35(1):3–11. [DOI] [PubMed] [Google Scholar]
- 77. Palesh O, et al. Sleep disruption in breast cancer patients and survivors. J Natl Compr Canc Netw. 2013;11(12):1523–1530. [DOI] [PubMed] [Google Scholar]
- 78. Low CA, et al. Estimation of symptom severity during chemotherapy from passively sensed data: exploratory study. J Med Internet Res. 2017;19(12):e420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. McCorkle R, et al. Self-management: enabling and empowering patients living with cancer as a chronic illness. CA Cancer J Clin. 2011;61(1):50–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Girgis A, et al. ; PROMPT-Care Pathways Working Group. Development of health pathways to standardize cancer care pathways informed by patient-reported outcomes and clinical practice guidelines. JCO Clin Cancer Inform. 2018;2:1–13. [DOI] [PubMed] [Google Scholar]
- 81. Henry NL, et al. Pilot study of an internet-based self-management program for symptom control in patients with early-stage breast cancer. JCO Clin Cancer Inform. 2018;2:1–12. [DOI] [PubMed] [Google Scholar]
- 82. Odeh B, et al. Optimizing cancer care through mobile health. Support Care Cancer. 2015;23(7):2183–2188. [DOI] [PubMed] [Google Scholar]
- 83. Kessel KA, et al. Mobile health in oncology: a patient survey about app-assisted cancer care. JMIR Mhealth Uhealth. 2017;5(6):e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. de Zambotti M, et al. The sleep of the ring: comparison of the OURA sleep tracker against polysomnography. Behav Sleep Med. 2017:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ortiz-Tudela E, et al. Ambulatory circadian monitoring (ACM) based on thermometry, motor activity and body position (TAP): a comparison with polysomnography. Physiol Behav. 2014;126:30–38. [DOI] [PubMed] [Google Scholar]
- 86. Muzet A, et al. Assessing sleep architecture and continuity measures through the analysis of heart rate and wrist movement recordings in healthy subjects: comparison with results based on polysomnography. Sleep Med. 2016;21:47–56. [DOI] [PubMed] [Google Scholar]
- 87. Komarzynski S, et al. Relevance of a mobile internet platform for capturing inter- and intrasubject variabilities in circadian coordination during daily routine: pilot study. J Med Internet Res. 2018;20(6):e204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lunde P, et al. The effectiveness of smartphone apps for lifestyle improvement in noncommunicable diseases: systematic review and meta-analyses. J Med Internet Res. 2018;20(5):e162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Hesse BW, et al. The role of Internet resources in clinical oncology: promises and challenges. Nat Rev Clin Oncol. 2016;13(12):767–776. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.