Abstract
The aim of this study was to describe the outcomes of patients with coronavirus disease 2019 (COVID-19) in the outpatient setting after early treatment with zinc, low-dose hydroxychloroquine and azithromycin (triple therapy) dependent on risk stratification. This was a retrospective case series study in the general practice setting. A total of 141 COVID-19 patients with laboratory-confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in the year 2020 were included. The main outcome measures were risk-stratified treatment decision and rates of hospitalisation and all-cause death. A median of 4 days [interquartile range (IQR) 3–6 days; available for n = 66/141 patients] after the onset of symptoms, 141 patients (median age 58 years, IQR 40–67 years; 73.0% male) received a prescription for triple therapy for 5 days. Independent public reference data from 377 confirmed COVID-19 patients in the same community were used as untreated controls. Of 141 treated patients, 4 (2.8%) were hospitalised, which was significantly fewer (P < 0.001) compared with 58 (15.4%) of 377 untreated patients [odds ratio (OR) = 0.16, 95% confidence interval (CI) 0.06–0.5]. One patient (0.7%) in the treatment group died versus 13 patients (3.4%) in the untreated group (OR = 0.2, 95% CI 0.03–1.5; P = 0.12). No cardiac side effects were observed. Risk stratification-based treatment of COVID-19 outpatients as early as possible after symptom onset using triple therapy, including the combination of zinc with low-dose hydroxychloroquine, was associated with significantly fewer hospitalisations.
Keywords: SARS-CoV-2, COVID-19, Outpatients, Zinc, Hydroxychloroquine, Azithromycin
1. Introduction
In December 2019, the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) started as an outbreak in Wuhan, China. This coronavirus has spread rapidly as a pandemic around the world [1], causing coronavirus disease 19 (COVID-19) pneumonia, acute respiratory distress syndrome (ARDS), cardiac injury, liver and renal injury, thrombosis and death [2].
As of June 2020, the diagnosis and treatment of COVID-19 have been almost exclusively studied from an inpatient perspective, including intensive care with mechanical ventilation. Only one study has described the characteristics and key health outcomes of COVID-19 diagnosed patients in an outpatient setting [3]. This is surprising as primary care physicians often see COVID-19 patients first. Thus, they could play a critical role in early diagnosis, treatment and management of disease progression and virus spread. This assumption is supported by the established principle in medicine that speed of eradication is linked to the outcome of life-threatening infections [4].
The early clinical phase of COVID-19 has not been the focus of much research so far, even though timing of antiviral treatment seems to be critical [5]. The optimal window for therapeutic intervention would seem to be before the infection spreads from the upper to lower respiratory tract and before severe inflammatory reaction ensues [6]. Therefore, diagnosis and treatment of COVID-19 outpatients as early as possible, even based on clinical diagnosis only, may have been an underestimated first step to slow down or even stop the pandemic more effectively. Based on clinical application principles of antiviral therapies, as demonstrated in the case of influenza A [7], antiviral treatments should be used early in the course of infection.
Due to the lack of a vaccine or SARS-CoV-2 specific therapies, the proposed use of repurposed antiviral drugs remains a valid practical consideration [8]. One of the most controversial drugs during the current SARS-CoV-2 pandemic is the well-known oral antimalarial drug hydroxychloroquine (HCQ), routinely used in the treatment of autoimmune diseases such as rheumatoid arthritis and systemic lupus erythematosus (SLE) [9,10]. HCQ is currently listed as an essential medication for SLE by the World Health Organization (WHO) [11]. With more than 5.6 million prescriptions in the USA, HCQ was the 128th most commonly prescribed medication in 2017 [12]. In the meantime, the first observational studies concluding beneficial therapeutic effects of HCQ as monotherapy or in combination with the antibiotic azithromycin were reported just a few weeks after the start of the SARS-CoV-2 outbreak [13]. All studies that used HCQ with rather contradictory results were in hospitalised and often sicker patients [13], [14], [15], [16], and one publication was recently withdrawn [17,18]. As of June 2020, no studies of COVID-19 outpatients treated with HCQ at an early stage of the disease have been reported.
The antiviral effects of HCQ are well documented [19]. It is also known that chloroquine, and probably HCQ, have zinc ionophore characteristics, increasing intracellular zinc concentrations [20]. Zinc itself is able to inhibit coronavirus RNA-dependent RNA polymerase (RdRp) activity [21]. It has been hypothesised that zinc may enhance the efficacy of HCQ in treating COVID-19 patients [22]. The first clinical trial results confirming this hypothesis were recently published as a preprint [23]. Nevertheless, many studies with HCQ as monotherapy or in combination with the antibiotic azithromycin have been inconclusive so far [13], [14], [15], [16]. In all of these studies, HCQ was used later than 5 days after the onset of symptoms when hospitalised patients most likely had already progressed to stage II or III of the disease [6]. Regardless of the established antiviral effects of zinc and that many COVID-19 patients are prone to zinc deficiency, dependent on co-morbidities and drug treatments [22], none of these studies were designed to include zinc supplementation as combination treatment.
This first retrospective case series study of COVID-19 outpatients was done to show whether (i) a simple-to-perform outpatient risk stratification might allow for a rapid treatment decision shortly after onset of symptoms and (ii) whether the 5-day triple therapy with zinc, low-dose HCQ and azithromycin might result in fewer hospitalisations and fatalities compared with relevant public reference data of untreated patients.
2. Methods
2.1. Setting
This retrospective case series study analysed data from COVID-19 outpatients with confirmed SARS-CoV-2 infection treated in a community in New York State, USA, between 18 March 2020 and 14 May 2020. The outcome of patients treated with a specific triple therapy was compared with public reference data of patients in the same community who were not treated with this therapy.
2.2. Confirmation of COVID-19 diagnosis
The COVID-19 diagnosis was confirmed if patients tested positive for SARS-CoV-2 by PCR of nasal or pharyngeal swab specimens (majority of tests by Roche, Basel, Switzerland; 99.1% sensitivity and 99.7% specificity; other tests used with lower frequency included: DiaSorin: 500 copies/mL; Thermo Fisher: 10 genomic copy equivalents/reaction; Seegene: 1250 copies/mL; Hologic: TCID50/mL: 1 × 10–2) or retrospectively by IgG detection tests [DiaSorin: sensitivity 97.6% (≥15 days after diagnosis), specificity 99.3%; Diazyme: sensitivity 91.2%, specificity 97.3%]. Only patients who had a record of a positive test result were included in the analysis. The PCR assays were authorised by the US Food and Drug Administration (FDA) without clinical sensitivity/specificity data owing to the urgent nature of the pandemic. Only one positive test was necessary for the patient to be included in the retrospective analysis.
2.3. Patients
Sequentially consecutive COVID-19 outpatients aged >18 years at diagnosis were included in the analysis as the treatment group. All patients were White. Patients received a prescription for triple therapy only if they met one of the following risk stratification requirements during a medical office-based or telehealth consultation: Group A, age >60 years, with or without clinical symptoms; Group B, age ≤60 years and shortness of breath (SOB); or Group C, age ≤60 years, clinically symptomatic and with at least one of the following co-morbidities: hypertension, hyperlipidaemia, diabetes mellitus, obesity [body mass index (BMI) ≥ 30 kg/m2], cardiovascular disease, heart failure, history of stroke, history of deep vein thrombosis or pulmonary embolism, asthma, chronic obstructive pulmonary disease (COPD), other lung disease, kidney disease, liver disease, autoimmune disease or history of cancer. Pregnant women, if any, were also included in this group.
Laboratory-confirmed COVID-19 patients from the same community who were not treated with the described triple therapy and their related outcome data represented the untreated control group, which comprised both low-risk and high-risk patients (public reference data).
2.4. Procedure and treatment
Data for treated patients were collected from electronic health records in the year 2020. Demographics, as reported by the patient, and current medical history of hypertension, hyperlipidaemia, diabetes mellitus, obesity (BMI ≥ 30 kg/m2), cardiovascular disease, heart failure, stroke, asthma, COPD, other lung disease, kidney disease, liver disease, autoimmune disease, history of cancer, thyroid disease psychiatric disorder or pregnancy were collected.
The presence of the following clinical symptoms of treated patients was documented: cough/dry cough; fever; SOB; changes to or no smell or taste; sore throat; headache; runny nose/clear rhinorrhoea; sinus congestion; diarrhoea/vomiting; cold symptoms; feeling sick; weakness; and low back pain. If reported, the number of days since onset of symptoms was documented.
The following vital signs, if available, were collected and documented: heart rate (beats/min), respiratory rate (breaths/min), systolic and diastolic blood pressure (mmHg), body temperature (°C), oxygen saturation measured by pulse oximetry (O2 %), body weight (kg) and/or BMI.
The main co-medications were characterised based on primary care prescriptions active at the time of diagnosis, documented as categorical variables, included beta-blockers, angiotensin-converting enzyme inhibitors, angiotensin 2 antagonists, calcium channel blockers, hydrochlorothiazide, statins, bronchodilators, antidiabetics and insulin.
Only diagnosed COVID-19 patients who met the defined risk stratification requirements of group A, B or C received a prescription for the following triple therapy for 5 consecutive days in addition to standard supportive care: zinc sulfate (220 mg capsule once daily, containing 50 mg elemental zinc); HCQ (200 mg twice daily); and azithromycin (500 mg once daily). No loading dose was used. Patients who did not meet the risk stratification requirements received standard of care to treat common upper respiratory tract infections. Patients were not treated with HCQ if they had known contraindications, including QT prolongation, retinopathy or glucose-6-phosphate dehydrogenase deficiency. As usual and following best practice, patients were informed about possible drug-related side effects. Reported events, if any, were documented as required.
Selection of the used zinc supplement and of drugs, dosages and the combination thereof were based on treatment guidelines, positive reports from other countries such as South Korea, emerging first clinical evidence, and based on the discretion of the treating physicians.
2.5. Outcomes
Two outcomes were studied: COVID-19 related hospital admission and all-cause death during time of follow-up of ≥28 days in the treatment group and in the untreated control group (public reference). The outcome of COVID-19 patients in the untreated control group was reported by the responsible health department.
2.6. Statistical analyses
Only patients in the treatment group who met the defined risk stratification requirements and who received at least one prescription for HCQ, with or without zinc, for 5 days were included in the retrospective analysis and were categorised accordingly. If the patient's electronic health record did not include information on a clinical characteristic, it was assumed that the characteristic was not present. In the group of the public reference data, only confirmed COVID-19 patients who were not treated in the respective general practice with triple therapy were included in the analysis. For this untreated control group, only outcome data for hospitalisation and all-cause death were available and used for the statistical comparison with the treatment group.
No sample size calculations were performed. Descriptive statistics are presented as median and interquartile range (IQR) for continuous variables and as frequencies (%) for categorical variables. For comparison with the results of other studies, the mean and standard deviation were calculated as needed. Normality of distribution for continuous variables was assessed by the Shapiro–Wilk test. A two-tailed Student's t-test was used for parametric analysis, and a Wilcoxon signed-rank test was used for non-parametric data analysis. For calculation of correlation, the point-biserial correlation coefficient was applied if one variable was dichotomous. Associations between two categorical variables were calculated with the χ2 test. The odds ratio (OR) was calculated for comparison of the outcome of the treatment group with the untreated control group. An α value of 0.05 was considered as a significance level. Data were analysed using Microsoft Excel for Microsoft 365 MSO (32-bit), the Excel add-on Real Statistics, SigmaStat 4 and Sigma Plot 14.0.
2.7. Study approval
The study was approved by the Western Institutional Review Board and was exempt under 45 CFR § 46.104(d)(4). Ref. number: D4-Excemption-Zelenko (06-16-2020). The analysis was conducted with de-identified patient data, according to the USA Health Insurance Portability and Accountability Act (HIPAA), Safe Harbor. For that reason, exact dates and locations are not mentioned in this study.
3. Results
3.1. Patients
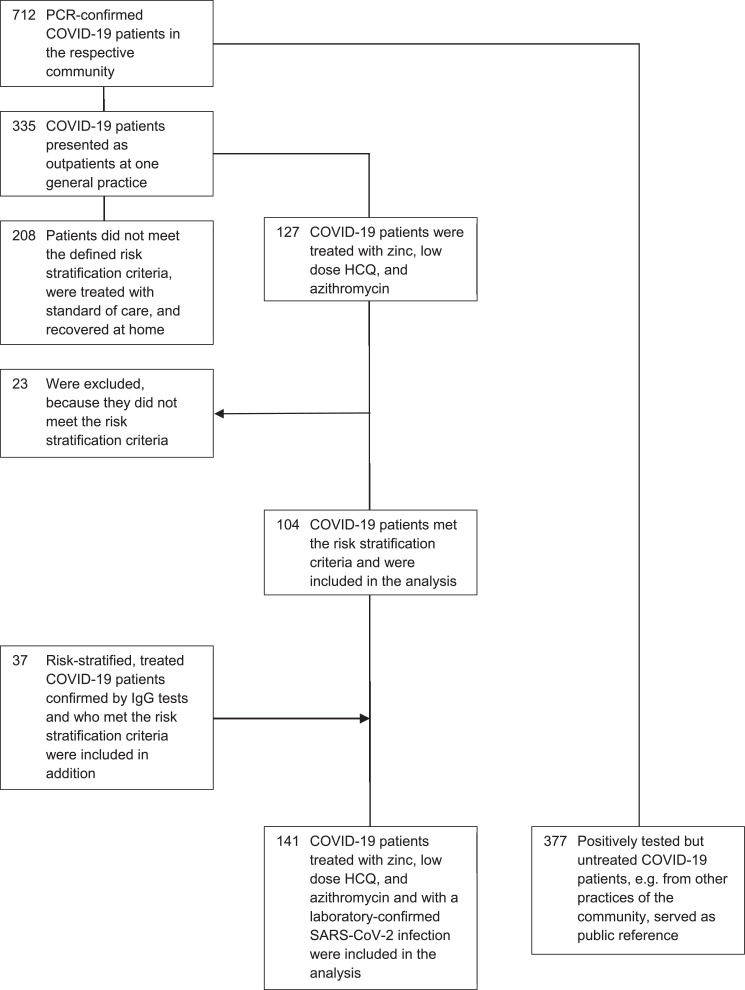
In accordance with available public reference data, 712 confirmed SARS-CoV-2 PCR-positive COVID-19 patients were reported for the respective community at the defined time point of the analysis. Of these 712 patients, 335 presented as outpatients at a general practice and 127 were treated with the triple combination therapy. Of these 127 patients, 104 met the risk stratification criteria and were included in the analysis (Table 1 ). Of the 335 patients, 208 did not meet the defined risk stratification criteria and were treated with standard of care and recovered at home. The SARS-CoV-2 infection of 37 additional patients who were clinically diagnosed with COVID-19 who met the risk stratification criteria and who were also treated with triple therapy was later confirmed by IgG tests (Table 1). These patients were included additionally in the analysis resulting in a total number of 141 patients, all with a confirmed SARS-CoV-2 infection by PCR or IgG tests. None of these patients were lost to follow-up for the defined outcome. The outcome of the remaining 377 positively tested but not treated COVID-19 patients, e.g. from other practices of the community, served as public reference (Fig. 1 ). Analysis of the 141 patients in the treatment group showed that all of these patients (100%) received a prescription of HCQ, 136 (96.5%) of zinc sulfate and 133 (94.3%) of azithromycin, while 1 patient (0.7%) received doxycycline instead. Instead of triple therapy, 1 patient (0.7%) in the treatment group received HCQ only, 7 patients (5.0%) received HCQ and zinc, and 4 patients (2.8%) received HCQ and azithromycin.
Table 1.
COVID-19 diagnostics by PCR and IgG tests of patients in the treatment group
| COVID-19 diagnostic [n (%)] | Risk-stratified group |
All patients (N = 141) | ||
|---|---|---|---|---|
| Group A (N = 69) | Group B (N = 48) | Group C (N = 24) | ||
| SARS-CoV-2 PCR test | 51 (74) | 39 (81) | 14 (58) | 104 (74) |
| SARS-CoV-2 IgG test | 18 (26) | 9 (19) | 10 (42) | 37 (26) |
COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Fig. 1.
Study population. N = 141 COVID-19 patients, all with a laboratory-confirmed SARS-CoV-2 infection, were included in the analysis as the treated group. N = 377 positively tested COVID-19 patients of the public reference were included in the analysis as the untreated group. COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
3.2. Baseline characteristics of the patients
Table 2 shows the baseline demographics and clinical characteristics of all 141 patients in the treatment group and for the risk stratification groups A, B and C. Of the 141 patients, 69 (48.9%) belonged to group A, 48 (34.0%) to group B and 24 (17.0%) to group C. The age ranged from 18–80 years and the median age was 58 years (IQR 40–67 years). The median age of patients in groups A, B and C was 67, 39 and 45 years, respectively. A total of 103 patients (73.0%) were male with a male-to-female ratio of 2.71. The most common co-morbidities included hypertension (28%), obesity (28%), hyperlipidaemia (23%) and diabetes mellitus (18%), whilst the least common co-morbidities were liver disease (2%), heart failure (1%) and stroke (1%). One patient (0.7%) was pregnant at initiation of treatment. There was a positive and significant correlation between age and hypertension (r = 0.3309, P = 0.001), hyperlipidaemia (r = 0.26306, P < 0.001) and cardiovascular disease (r = 0.16757, P < 0.05), whilst asthma was negatively correlated with age (r = –0.30867, P < 0.001).
Table 2.
Baseline demographic and clinical characteristics of patients in the treatment group
| Characteristic | Risk-stratified group |
All patients (N = 141) | ||
|---|---|---|---|---|
| Group A (N = 69) | Group B (N = 48) | Group C (N = 24) | ||
| Age (years) [median (IQR)] | 67 (64–69) | 39 (24–47) | 45 (36–50) | 58 (40–67) |
| Male sex [n (%)] | 46 (67) | 40 (83) | 17 (71) | 103 (73) |
| Co-morbidities/coexisting conditions [n (%)] | ||||
| Any condition | 44 (64) | 31 (65) | 24 (100) | 99 (70) |
| Hypertension | 27 (39) | 4 (8) | 8 (33) | 39 (28) |
| Hyperlipidaemia | 21 (30) | 7 (15) | 5 (21) | 33 (23) |
| Diabetes mellitus | 16 (23) | 4 (8) | 5 (21) | 25 (18) |
| Obesitya | 20 (29) | 10 (21) | 10 (42) | 40 (28) |
| Cardiovascular disease | 9 (13) | 1 (2) | 3 (13) | 13 (9) |
| Heart failure | 2 (3) | 0 (0) | 0 (0) | 2 (1) |
| Stroke | 1 (2) | 0 (0) | 0 (0) | 1 (1) |
| Asthma | 2 (3) | 9 (19) | 2 (8) | 13 (9) |
| COPD | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Other lung disease | 6 (9) | 5 (10) | 4 (17) | 15 (11) |
| Kidney disease | 1 (2) | 3 (6) | 2 (8) | 6 (4) |
| Liver disease | 1 (2) | 2 (4) | 0 (0) | 3 (2) |
| Autoimmune disease | 2 (3) | 4 (8) | 4 (17) | 10 (7) |
| History of cancer | 6 (9) | 2 (4) | 1 (4) | 9 (6) |
| Thyroid disease | 7 (10) | 4 (8) | 2(8) | 13 (9) |
| Psychiatric disorder | 7 (10) | 4 (8) | 5 (21) | 16 (11) |
| Pregnancy | – | – | 1 (4) | 1 (1) |
IQR, interquartile range; COPD, chronic obstructive pulmonary disease.
Body mass index (BMI) ≥30 kg/m2.
The median time between onset of clinical symptoms and medical consultation was 4 days (IQR 3–6 days; available for 66/141 patients; mean 4.8 ± 2.7 days) (Table 3 ). There was no significant correlation between age and days from onset of clinical symptoms to consultation (P > 0.05). Days from onset of symptoms to consultation were not significantly different between the groups (P > 0.05).
Table 3.
Patients with reported days since onset of symptoms in the treatment group
| Characteristic | Risk-stratified group |
All patients (N = 141) | ||
|---|---|---|---|---|
| Group A (N = 69) | Group B (N = 48) | Group C (N = 24) | ||
| Patients with reported days [n (%)] | 32 (46) | 25 (48) | 9 (38) | 66 (47) |
| Days since onset of symptoms [median (IQR)] | 4 (3–6) | 3 (3–6.5) | 4 (3–5.5) | 4 (3–6) |
IQR, interquartile range.
The most common clinical symptoms included cough (87.2%), fever (77.3%), SOB (46.1%) and changes to or no smell or taste (30%), whilst the least common clinical symptoms were sinus congestion (16%), diarrhoea/vomiting (5%) and low back pain (3%). Table 4 shows the symptoms of all patients and stratified by groups A, B and C. There was a significant negative correlation between age and changes to smell or taste (r = –0.43, P < 0.001). No patient had a clinical diagnosis of pneumonia.
Table 4.
COVID-19 diagnostics and baseline reported clinical symptoms of patients in the treatment group
| Clinical symptom [n (%)] | Risk-stratified group |
All patients (N = 141) | ||
|---|---|---|---|---|
| Group (N = 69) | Group B (N = 48) | Group C (N = 24) | ||
| Cough/dry cough | 60 (87) | 39 (81) | 24 (100) | 123 (87) |
| Fever | 53 (77) | 38 (79) | 18 (75) | 109 (77) |
| Shortness of breath | 17 (25) | 48 (100) | 0 (0) | 65 (46) |
| Changes to or no smell or taste | 21 (30) | 19 (40) | 2 (8) | 42 (30) |
| Sore throat | 19 (28) | 8 (17) | 7 (29) | 34 (24) |
| Headache | 19 (28) | 6 (13) | 7 (29) | 32 (23) |
| Runny nose/clear rhinorrhoea | 16 (23) | 8 (17) | 4 (17) | 28 (20) |
| Sinus congestion | 10 (15) | 9 (19) | 4 (17) | 23 (16) |
| Diarrhoea/vomiting | 1 (2) | 5 (10) | 1 (4) | 7 (5) |
| Cold symptoms | 31 (45) | 16 (33) | 12 (50) | 59 (42) |
| Feels sick | 40 (58) | 38 (79) | 17 (71) | 95 (67) |
| Weakness | 44 (64) | 22 (46) | 11 (46) | 77 (55) |
| Low back pain | 3 (4) | 0 (0) | 1 (4) | 4 (3) |
COVID-19, coronavirus disease 2019.
Table 5 shows the vital signs, if available, for all patients. Many patients consulted the general practice during the COVID-19 crisis via telehealth so vital signs were not available for all of these patients. The highest proportion of patients had available measurements for heart rate (63%) and pulse oximetry (60%). Vital signs were not significantly different between risk stratification groups (P > 0.05) except for systolic blood pressure of groups A and B (P < 0.05).
Table 5.
Physical examination: vital signs of patients in the treatment group
| Parameter | Median (IQR) | Patients with available parameters [n (%) of N = 141] |
|---|---|---|
| Heart rate (beats/min) | 86 (80–94) | 89 (63) |
| Respiratory rate (breaths/min) | 16 (15–18) | 43 (31) |
| Systolic blood pressure (mmHg) | 126 (120–139) | 66 (47) |
| Diastolic blood pressure (mmHg) | 80 (74–85.5) | 66 (47) |
| Body temperature (°C) | 37.2 (37–37.8) | 79 (56) |
| Pulse oximetry (O2 %) | 97 (96–98) | 85 (60) |
| Body weight (kg) | 88 (72.6–98.4) | 43 (31) |
| BMI (kg/m2) | 32.2 (28.5–36.3) | 30 (21) |
IQR, interquartile range; BMI, body mass index.
Table 6 summarises the most important co-medications. Of the patients, 16% were taking angiotensin-converting enzyme inhibitors, angiotensin 2 antagonists, hydrochlorothiazide or a combination thereof. The most common long-term therapies at the time of COVID-19 diagnosis were statins (20%), beta-blockers (12%) and insulin (18%). A few patients had chronic prescriptions for oral corticosteroids (9%) for co-morbidities such as asthma or autoimmune diseases, and 3 patients (2.1%) received an additional antibiotic (levofloxacin) because of superinfections.
Table 6.
Co-medications of patients in the treatment group
| Drug class | Patients [n (%) of N = 141] |
|---|---|
| Beta-blockers | 17 (12) |
| Angiotensin-converting enzyme inhibitors | 8 (6) |
| Angiotensin 2 antagonists | 13 (9) |
| Calcium channel blockers | 8 (6) |
| Hydrochlorothiazide | 6 (4) |
| Statins | 28 (20) |
| Bronchodilators | 10 (7) |
| Antidiabetics | 11 (8) |
| Insulin | 26 (18) |
| Oral corticosteroids | 13 (9) |
| Antibiotics | 3 (2) |
3.3. Hospitalisations and all-cause death
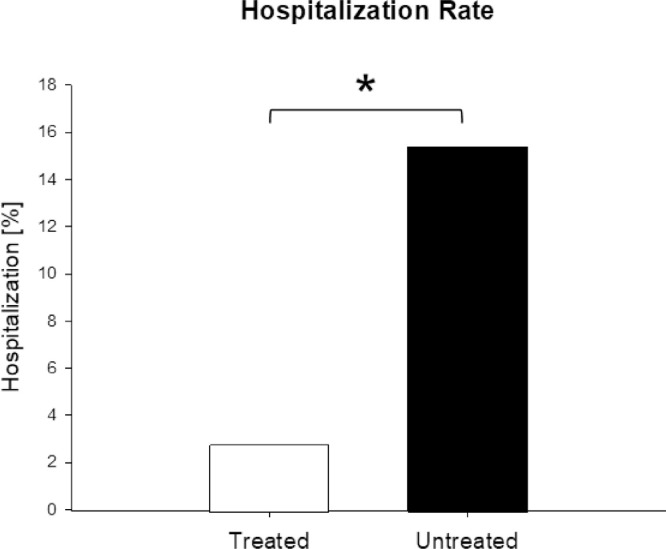
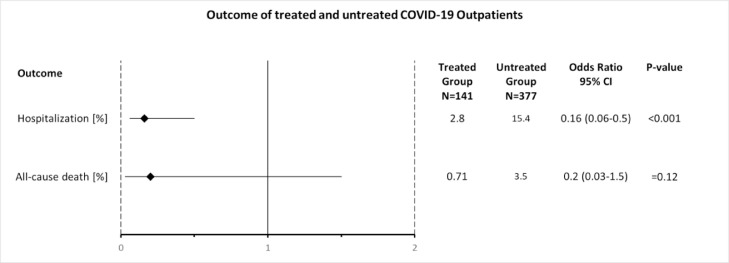
In the treatment group, 4 (2.8%) of 141 patients were hospitalised, which was significantly fewer than the 58 (15.4%) of 377 patients in the untreated group (Fig. 2 ) [OR = 0.16, 95% confidence interval (CI) 0.06–0.5; P < 0.001] (Table 7 ; Fig. 3 ). Therefore, the odds of hospitalisation of treated patients was 84% less than in the untreated patients. All hospitalised patients were male, with one in his twenties, two in their forties and one in his seventies. Three (75%) of the four hospitalised patients belonged to risk stratification group B and one (25%) to group A. All patients (100%) reported SOB at the time of consultation. The median time from onset of symptoms to consultation was 4 days. In the treatment group, one patient had to stay only 1 day in hospital, two other patients were discharged as cured and one patient died (see below). No patient was on a ventilator.
Fig. 2.
Hospitalisation. Treatment with triple therapy of zinc, low-dose hydroxychloroquine and azithromycin was associated with significantly fewer hospitalisations compared with untreated patients of the public reference data. χ2 (1, N = 518) = 14.17; * P < 0.001.
Table 7.
Clinical outcomes in the treated patient group versus the untreated patient group
| Outcome | Treated group [n (%) of N = 141] | Untreated group [n (%) of N = 377] | OR (95% CI) | P-value |
|---|---|---|---|---|
| Hospitalisation | 4 (2.8) | 58 (15.4) | 0.16 (0.06–0.5) | <0.001 |
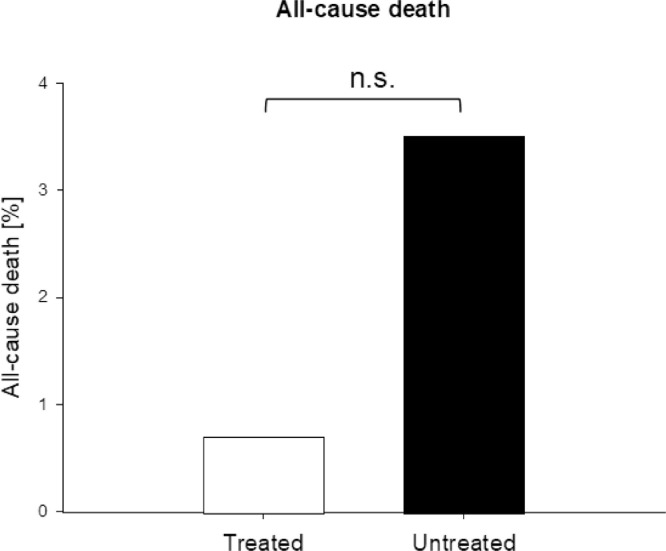
| All-cause death | 1 (0.71) | 13 (3.5) | 0.2 (0.03–1.5) | 0.12 |
OR, odds ratio; CI, confidence interval.
Fig. 3.
All-cause deaths. Treatment with triple therapy of zinc, low-dose hydroxychloroquine and azithromycin was associated with numerically fewer all-cause deaths compared with untreated patients of the public reference data. n.s., not significant. χ2 (1, N = 518) = 1.98; P = 0.12.
Of the 141 patients, 1 (0.7%) in treatment group A died after being hospitalised. This patient had a history of cancer and only took one daily dose of the triple therapy before hospital admission. More patients (13/377; 3.4%) died in the untreated group (Fig. 4 ) (OR = 0.2, 95% CI 0.03–1.5) (Table 7; Fig. 3). Although the odds of all-cause death of treated patients was 80% less than in the untreated group, this difference did not reach statistical significance (P = 0.12).
Fig. 4.
Odds ratios (ORs). The odds of hospitalisation in the treated patient group was 84% less than in the untreated patient group and was statistically significant (P < 0.001). The odds of all-cause death in the treated patient group was 80% less than in the untreated patient group but did not reach statistical significance (P = 0.12). COVID-19, coronavirus disease 2019; CI, confidence interval.
All patients in the treatment group with the clinical outcome of hospitalisation or all-cause death received a prescription for the complete triple therapy including zinc, low-dose HCQ and azithromycin.
The outcome of the three different risk-stratified groups (A, B and C) was not significantly different.
The 208 patients presenting at the general practice who did not meet the risk stratification requirements and who were not treated with the triple therapy recovered at home and no hospital admissions or deaths were reported.
3.4. Safety
In general, triple therapy with zinc, low-dose HCQ and azithromycin was well tolerated. After initiation of treatment in the 141 patients, 30 (21.3%) reported weakness, 20 (14.2%) nausea, 15 (10.6%) diarrhoea and 2 (1.4%) rash (Table 8 ). No patient reported palpitations or any cardiac side effects.
Table 8.
Summary of adverse events in the treatment group
| Event | Patients [n (%) of N = 141] |
|---|---|
| Any adverse event | 67 (48) |
| Weakness | 30 (21) |
| Nausea | 20 (14) |
| Diarrhoea | 15 (11) |
| Rash | 2 (1) |
4. Discussion
This first retrospective case series study of COVID-19 outpatients in a primary care setting showed that risk-stratified treatment early after onset of clinical symptoms with triple therapy of zinc, low-dose HCQ and azithromycin was associated with significantly fewer hospitalisations (OR = 0.16; P < 0.001) in comparison with untreated patients (public reference data) of the same community. Based on the performed risk stratification, the prevalences of the co-morbidities hypertension, hyperlipidaemia and diabetes mellitus were the highest in group A (>60 years and clinical symptoms), asthma and other lung diseases were the highest in group B (≤60 years and SOB), and obesity and autoimmune disease were the highest in group C (<60 years, clinical symptoms and defined co-morbidities). The most frequent symptoms of these COVID-19 patients were cough followed by fever while available median body temperature measurements were in a normal range. Almost 50% of risk-stratified and treated patients were suffering from SOB while breaths per minute and blood oxygen saturation were still in the normal range. The median time from onset of symptoms to first medical consultation was 4 days (IQR 3–6 days). Approximately 16% of patients received co-medications known to be associated with zinc deficiency, such as antihypertensive drugs. No patient experienced any known severe adverse events that were considered drug-related during treatment or follow-up.
A growing number of reports provide evidence for the effectiveness or otherwise of a range of COVID-19 drug treatments. Therefore, a living systematic review and network meta-analysis was published to assess how trustworthy the evidence is using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach [24]. Based on their most recent update from 21 July 2020, the authors conclude that glucocorticoids probably reduce mortality and mechanical ventilation in patients with COVID-19 compared with standard care. However, the effectiveness of most interventions is uncertain because most of the randomised controlled trials so far have been small and have important study limitations [24].
Another meta-analysis focused on the effectiveness of chloroquine derivatives in COVID-19 therapy [25]. The authors concluded that chloroquine derivatives are effective in improving clinical and virological outcomes and may reduce mortality by a factor of 3 in patients affected with COVID-19. They further conclude that big data are lacking basic treatment definitions and are the subject of conflict of interest [25]. At the time of this manuscript submission, only one peer-reviewed study had analysed the key health outcomes of COVID-19 patients diagnosed in a primary care setting [3]. Because of this gap in the data, the value of this study is multifold. It provides much needed recommendations for risk stratification and a treatment regimen to prevent hospitalisation and death of COVID-19 patients. The diagnosis of COVID-19 for all patients in this analysis was confirmed by PCR or IgG tests compared with a recent study in which <3% had a diagnosis confirmed by laboratory tests [26]. Starting triple therapy as early as possible after symptom onset is critical for treatment success because SARS-CoV-2 viral load appears to peak at Days 5–6 after symptom onset [27], [28], [29] and severe cases progress to ARDS after only 8–9 days [30,31]. Early antiviral treatment is an established protocol to manage severe disease progression, as was shown, for example, by a cumulative case–control study during the 2009 H1N1 influenza pandemic in Canada [32]. For patients at high risk for severe viral disease progression, it is recommended to start antiviral therapy as early as possible [33,34]. Early treatment might be also critically important to effectively reduce the SARS-CoV-2 viral load [5] and this underscores the role of early intervention by primary care physicians as reported herein.
A further strength of this approach was the simple risk stratification of symptomatic outpatients to determine the need for therapy, a strategy not yet applied in COVID-19 primary care [35] but routinely implemented in primary care for other diseases [36]. Underlying assumptions of the risk stratification used in this setting are different to other recommendations [37]. Here, age-stratified high risk was defined as >60 years (typically defined as >65 years) to encompass the common increase of co-morbidity incidences in this age group [38]. Patients ≤60 years with SOB, even without reduced pulse oximetry values, were treated because it was assumed the virus will likely spread from the upper to lower respiratory tract [39]. Also treated were patients ≤60 years with clinical symptoms and prognostically relevant co-morbidities [37]. By applying this risk stratification approach, respective care was tailored to patients with a higher likelihood for hospitalisation or fatality, which ensured that the medical principles of ‘patient first’ and ‘doing no harm’ were maintained [40]. As a result, 61.8% of COVID-19 patients were treated with standard of care only and recovered at home, and only 37.9% needed treatment with the triple therapy.
The antiviral potential of HCQ has been broadly described in vitro and in vivo [41], [42], [43]. HCQ has a long terminal elimination half-life of 32 days in plasma and 50 days in blood [44]. Therefore, the treatment approach was conservative, with the starting dose being the same as the maintenance dose and with a short treatment duration of only 5 days, being even more conservative than other recommendations [42]. HCQ-dependent intracellular increases in pH might directly interfere with pH-dependent SARS-CoV-2 replication [19]. Also, chloroquine and probably HCQ have characteristics of a zinc ionophore resulting in increasing intracellular zinc concentrations [20]. The dose of elementary zinc in this study was similar to doses previously studied to successfully prevent infections in the elderly [45]. The antiviral effects of zinc against a variety of viruses have been demonstrated during the last decades [46]. Zinc, in addition to its role as a general stimulant of antiviral immunity, is known to specifically inhibit coronavirus RNA-dependent RNA polymerase (RdRp) [21]. Based on the ionophore properties of HCQ, it has been hypothesised that zinc may enhance the efficacy of HCQ in treating COVID-19 patients [22]. In addition, zinc might inhibit the serine protease furin [47]. Furin is expressed on endothelial cells, monocytes/macrophages and smooth muscle cells in human atherosclerotic plaques [48] and therefore might play a critical role for the severe cardiovascular complications of COVID-19. As furin might be responsible to favour SARS-CoV-2 spread compared with other Betacoronaviruses [49,50] and as furin inhibition protects from certain viral-dependent infections [51], it may be important to evaluate the potential role of zinc in inhibiting this pathway.
Azithromycin was added to the treatment regimen as preliminary data provided evidence for more efficient or synergic virus elimination in conjunction with bacterial superinfection [13,52]. Although there is a synergistic antiviral effect between zinc, HCQ and azithromycin, zinc supplementation may be instrumental for the outcome of patient populations with severe clinical courses. Zinc deficiency was confirmed in a large number of healthy elderly [53] and in diabetic patients [54]. In addition, it has been documented that the antihypertensive drugs hydrochlorothiazide, angiotensin-converting enzyme inhibitors and angiotensin 2 receptor antagonists can result in increased urinary excretion of zinc with subsequent systemic zinc deficiency [55]. Age, co-morbidities and relevant co-medications align well with the majority of described COVID-19 patients at high risk, including the risk-stratified population of this analysis. Zinc deficiency might explain why certain patient groups seem not to benefit from HCQ monotherapy. During the 5-day treatment with the triple therapy and during follow-up, no severe adverse events were observed and no cases of cardiac arrhythmia were reported in this general practice, which is in accordance with available safety data for more than 300 000 patients [56].
Inherent to all retrospective analyses, our study has certain limitations, such as non-randomisation and blinding of treatment. Also, only the outcome data of the untreated control group based on the public reference were available; because no other data on patient characteristics or clinical symptoms were available, no risk adjustment was possible. Therefore, confounding factors and selection bias, among other issues, might exist. The demographic composition of the treatment group might also have had an influence on our findings. Because many physician appointments had to be managed by telehealth, vital parameters were not available for the majority of patients. Viral load and electrocardiogram (ECG) data were not analysed. Treatment with the triple therapy resulted in a numerically lower rate of all-cause death. In the absence of clinical details about the untreated patient group, the lower rate of all-cause death in the treated group was not statistically significant. However, patients in the treated group were all positively risk-stratified while the risk of the untreated group was obviously lower as this group included high- and low-risk patients. When we compared the outcome of all risk-stratified patients in the study group (treated and non-treated) with the control patients (not stratified, treated with standard therapy), hospitalisation and all-cause death were significantly less in the study group (P < 0.0001 and P = 0.0154, respectively). These data were not shown in the results section because relevant clinical information was not completely available for all patients in the control group to allow risk adjustment between groups.
In this study, the ratio of males and average age was comparable with a relevant number of other studies, but the distribution of co-morbidities was not [57]. The latter was expected because outpatients usually have a different distribution of age and especially of co-morbidities than critically ill inpatients. As expected, the prevalence of hypertension, hyperlipidaemia and cardiovascular disease correlated positively with age, while asthma correlated negatively. Approximately 50% of risk-stratified and treated patients presented with SOB, while the parameters breaths per minute and blood oxygen saturation were still within the normal range. These patients would usually not be considered for hospital admission, although SOB might be considered an alarming early sign of disease progression. Based on the implemented risk stratification, these patients were identified and treated immediately.
Indeed, three of four hospitalised patients were in risk stratification group B including patients especially with SOB, and also the hospitalised patient of group A reported SOB at the time of consultation. This supports the assumption that COVID-19 patients with SOB are at much higher risk for disease progression and need to be monitored closely.
In contrast to many other studies, the most frequent symptom was cough and not fever [58,59]. Changes in smell or taste in one-third of patients and a negative correlation with age were similar to findings from other groups [60]. While mean time from onset of symptoms to treatment was only 4.8 days (median 4 days), previously reported time spans range from 6.3 days [61] to 8 days [16], up to 16.6 days [14], or it was often even not reported [62]. In most of these studies, COVID-19 disease had most likely already progressed at the time of presentation to stages II or even stage III of the disease [6]. In many studies, often only limited information is provided about co-medications and specifically about clinical symptoms at admission [62]. The latter would be very important to better understand the differences in clinical presentation between inpatients and outpatients and thus the urgency for early anti-COVID-19 treatment in the outpatient setting [63]. The potential of zinc to enhance the antiviral efficacy of HCQ was already described in detail elsewhere [22]. This hypothesis was recently confirmed in a study using a similar triple therapy and treatment duration [23]. Zinc added to HCQ and azithromycin resulted in a significantly increased number of patients being discharged, a reduction in mortality, or transfer to hospice. In another study, when a lower dose of 200 mg of HCQ twice daily was added to basic treatment, mortality of even critically ill patients was significantly reduced [64]. These and our findings indicate that proper dosing of HCQ with its long half-life might be key for a favourable outcome of COVID-19 patients. In critical care, drugs with short half-lives are usually preferred. Especially in critically ill COVID-19 patients, higher doses of HCQ may have unforeseeable effects, e.g. on insulin sensitivity in obese patients [65] and on glucose levels in diabetics [66,67]. Besides glucose levels, it is important to closely monitor renal function, which is increasingly affected during progression of COVID-19 [68]. Because HCQ is substantially excreted by the kidneys, the risk of toxic reactions is greater in patients with impaired renal function [69].
4.1. Potential implications for clinicians and policy-makers
Clinical experience from severely ill inpatients with pneumonia who were treated with high-dose HCQ is not readily transferable to the outpatient setting with upper respiratory tract disease only. For outpatients with a median of only 4 days after onset of symptoms, COVID-19 represents a totally different disease and needs to be managed and treated differently [63]. A simple-to-perform outpatient risk stratification, as shown here, allows for rapid treatment decisions and treatment with the triple therapy of zinc, low-dose HCQ and azithromycin and may prevent a large number of hospitalisations and probably deaths during the SARS-CoV-2 pandemic. This might also help to avoid overwhelming of healthcare systems.
Acknowledgments
Acknowledgments
The authors thank all of the patients and families involved in this study; the practitioners Dr Rosy Joseph, Dr Avery Knapp, Dr Hillel Isseroff, Dr William Grace, Dr Sam Sandowski and Dr James Todaro for medical support; Chandra Duggirala and Manoj Duggirala for operational and technical support; Mendel Mochkin (CrowdProtocol Foundation) for supporting the Institutional Review Board submission; and the reviewers Vjosa C. Mujko (Invivo Brands LLC) and Tzvi Jacobs who improved the language of this publication.
Funding
None.
Competing interests
RD is/was at the time of writing an employee of Alexion Pharma Germany GmbH, and his engagement and contribution to this study and publication was private and independent from his employer; MS is/was at the time of writing External Senior Advisor for the company LEUKOCARE (Munich, Germany) and is/was Manging Director at Starts- and -Ups Consulting (Frankfurt, Germany); VZ is/was a general practitioner in New York State (USA).
Ethical approval
This study was approved by the Western Institutional Review Board and was exempt under 45 CFR § 46.104(d)(4). Ref. Number: D4-Excemption-Zelenko (06-16-2020).
References
- 1.Wu D., Wu T., Liu Q., et al. The SARS-CoV-2 outbreak: what we know. Int J Infect Dis. 2020;94:44–48. doi: 10.1016/j.ijid.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atri D., Siddiqi H.K., Lang J., et al. COVID-19 for the cardiologist: a current review of the virology, clinical epidemiology, cardiac and other clinical manifestations and potential therapeutic strategies. JACC Basic Transl Sci. 2020;5:518–536. doi: 10.1016/j.jacbts.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prieto-Alhambra D., Ballo E., Coma-Redon E., et al. Hospitalization and 30-day fatality in 121,263 COVID-19 outpatient cases. medRxiv. May 2020;8 doi: 10.1101/2020.05.04.20090050. [DOI] [Google Scholar]
- 4.Talan D.A., Guterman J.J., Overturf G.D., et al. Analysis of emergency department management of suspected bacterial meningitis. Ann Emerg Med. 1989;18:856–862. doi: 10.1016/s0196-0644(89)80213-6. [DOI] [PubMed] [Google Scholar]
- 5.Gonçalves A., Bertrand J., Ke R., et al. Timing of antiviral treatment initiation is critical to reduce SARS-CoV-2 viral load. medRxiv. 21 June 2020 doi: 10.1101/2020.04.04.20047886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siddiqi H.K., Mehra M.R. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020;39:405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fry A.M., Goswami D., Nahar K., et al. Efficacy of oseltamivir treatment started within 5 days of symptom onset to reduce influenza illness duration and virus shedding in an urban setting in Bangladesh: a randomised placebo-controlled trial. Lancet Infect Dis. 2014;14:109–118. doi: 10.1016/s1473-3099(13)70267-6. [DOI] [PubMed] [Google Scholar]
- 8.Schlagenhauf P., Grobusch M.P., Maier J.D., et al. Repurposing antimalarials and other drugs for COVID-19. Travel Med Infect Dis. 2020;34 doi: 10.1016/j.tmaid.2020.101658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wallace D.J. The use of chloroquine and hydroxychloroquine for non-infectious conditions other than rheumatoid arthritis or lupus: a critical review. Lupus. 1996;5(Suppl 1):S59–S64. [PubMed] [Google Scholar]
- 10.Gordon C., Amissah-Arthur M.B., Gayed M., et al. The British Society for Rheumatology guideline for the management of systemic lupus erythematosus in adults. Rheumatology (Oxford) 2018;57:e1–45. doi: 10.1093/rheumatology/kex286. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization (WHO) WHO; 2020. Model list of essential medicines. https://list.essentialmeds.org/ [Google Scholar]
- 12.Kane S.P.Hydroxychloroquine sulfate. Drug usage statistics, United States, 2007–2017. ClinCalc DrugStats Database, Version 20.1.https://clincalc.com/DrugStats/Drugs/HydroxychloroquineSulfate [accessed 23 May 2020].
- 13.Gautret P., Lagier J.C., Parola P., et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang W., Cao Z., Han M., et al. Hydroxychloroquine in patients mainly with mild to moderate COVID-19: an open-label, randomized, controlled trial. medRxiv. 7 May 2020 doi: 10.1101/2020.04.10.20060558. [DOI] [Google Scholar]
- 15.Chen J., Liu D., Liu L., et al. A pilot study of hydroxychloroquine in treatment of patients with moderate COVID-19. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020;49:215–219. doi: 10.3785/j.issn.1008-9292.2020.03.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahevas M., Tran V.-T., Roumier M., et al. No evidence of clinical efficacy of hydroxychloroquine in patients hospitalized for COVID-19 infection with oxygen requirement: results of a study using routinely collected data to emulate a target trial. medRxiv. 14 April 2020 doi: 10.1101/2020.04.10.20060699. [DOI] [Google Scholar]
- 17.Mehra M.R., Ruschitzka F., Patel A.N. Retraction—Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet. 2020;395:1820. doi: 10.1016/S0140-6736(20)31324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The Lancet Editors Expression of concern: Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet. 2020;395:E102. doi: 10.1016/S0140-6736(20)31290-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang N., Shen H.M. Targeting the endocytic pathway and autophagy process as a novel therapeutic strategy in COVID-19. Int J Biol Sci. 2020;16:1724–1731. doi: 10.7150/ijbs.45498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xue J., Moyer A., Peng B., et al. Chloroquine is a zinc ionophore. PLoS One. 2014;9 doi: 10.1371/journal.pone.0109180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.te Velthuis A.J., van den Worm S.H., Sims A.C., et al. Zn2+ inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Derwand R., Scholz M. Does zinc supplementation enhance the clinical efficacy of chloroquine/hydroxychloroquine to win today's battle against COVID-19? Med Hypotheses. 2020;142 doi: 10.1016/j.mehy.2020.109815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carlucci P., Ahuja T., Petrilli C.M., et al. Hydroxychloroquine and azithromycin plus zinc vs hydroxychloroquine and azithromycin alone: outcomes in hospitalized COVID-19 patients. medRxiv. 8 May 2020 doi: 10.1101/2020.05.02.20080036. [DOI] [Google Scholar]
- 24.Siemieniuk R.A.C., Bartoszko J.J., Ge L., et al. Drug treatments for COVID-19: living systematic review and network meta-analysis. BMJ. 2020;370:m2980. doi: 10.1136/bmj.m2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Million M., Gautret P., Colson P., et al. Clinical efficacy of chloroquine derivatives in COVID-19 infection: comparative meta-analysis between the big data and the real world. New Microbes New Infect. 2020;38 doi: 10.1016/j.nmni.2020.100709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boulware D.R., Pullen M.F., Bangdiwala A.S., et al. A randomized trial of hydroxychloroquine as postexposure prophylaxis for COVID-19. N Engl J Med. 2020;383:517–525. doi: 10.1056/NEJMoa2016638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan Y., Zhang D., Yang P., et al. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect Dis. 2020;20:411–412. doi: 10.1016/s1473-3099(20)30113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim J.Y., Ko J.H., Kim Y., et al. Viral load kinetics of SARS-CoV-2 infection in first two patients in Korea. J Korean Med Sci. 2020;35:e86. doi: 10.3346/jkms.2020.35.e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zou L., Ruan F., Huang M., et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/s0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zarychanski R., Stuart T.L., Kumar A., et al. Correlates of severe disease in patients with 2009 pandemic influenza (H1N1) virus infection. CMAJ. 2010;182:257–264. doi: 10.1503/cmaj.091884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.US Centers for Disease Control and Prevention (CDC) CDC; 2020. Influenza (flu). For clinicians: antiviral medication. https://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm. [Google Scholar]
- 34.US Centers for Disease Control and Prevention (CDC) CDC; 2020. Managing people at high risk for severe varicella. https://www.cdc.gov/chickenpox/hcp/index.html?CDC_AA_refVal=https%3A%2F%. [Google Scholar]
- 35.Esper R.B., da Silva R.S., Oikawa F.T.C., et al. Prevent Senior Institute; São Paulo, SP, Brazil: 2020. Empirical treatment with hydroxychloroquine and azithromycin for suspected cases of COVID-19 followed-up by telemedicine. [Google Scholar]
- 36.Johnson M., Rigge L., Culliford D., et al. Primary care risk stratification in COPD using routinely collected data: a secondary data analysis. NPJ Prim Care Respir Med. 2019;29:42. doi: 10.1038/s41533-019-0154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.US Centers for Disease Control and Prevention (CDC) CDC; 2020. People at increased risk and other people who need to take extra precautions. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-at-higher-risk.html. [Google Scholar]
- 38.van den Akker M., Buntinx F., Metsemakers J.F., et al. Multimorbidity in general practice: prevalence, incidence, and determinants of co-occurring chronic and recurrent diseases. J Clin Epidemiol. 1998;51:367–375. doi: 10.1016/s0895-4356(97)00306-5. [DOI] [PubMed] [Google Scholar]
- 39.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323:1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 40.Ghaly R.F., Knezevic N.N. What happened to ‘patient first’ and ‘do no harm’ medical principles? Surg Neurol Int. 2018;9:176. doi: 10.4103/sni.sni_447_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu J., Cao R., Xu M., et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:16. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yao X., Ye F., Zhang M., et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020;71:732–739. doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meo S.A., Klonoff D.C., Akram J. Efficacy of chloroquine and hydroxychloroquine in the treatment of COVID-19. Eur Rev Med Pharmacol Sci. 2020;24:4539–4547. doi: 10.26355/eurrev_202004_21038. [DOI] [PubMed] [Google Scholar]
- 44.Tett S.E., Cutler D.J., Day R.O., et al. Bioavailability of hydroxychloroquine tablets in healthy volunteers. Br J Clin Pharmacol. 1989;27:771–779. doi: 10.1111/j.1365-2125.1989.tb03439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prasad A.S., Beck F.W., Bao B., et al. Zinc supplementation decreases incidence of infections in the elderly: effect of zinc on generation of cytokines and oxidative stress. Am J Clin Nutr. 2007;85:837–844. doi: 10.1093/ajcn/85.3.837. [DOI] [PubMed] [Google Scholar]
- 46.Read S.A., Obeid S., Ahlenstiel C., et al. The role of zinc in antiviral immunity. Adv Nutr. 2019;10:696–710. doi: 10.1093/advances/nmz013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Podsiadlo P., Komiyama T., Fuller R.S., et al. Furin inhibition by compounds of copper and zinc. J Biol Chem. 2004;279:36219–36227. doi: 10.1074/jbc.M400338200. [DOI] [PubMed] [Google Scholar]
- 48.Stawowy P., Kallisch H., Borges Pereira Stawowy N., et al. Immunohistochemical localization of subtilisin/kexin-like proprotein convertases in human atherosclerosis. Virchows Arch. 2005;446:351–359. doi: 10.1007/s00428-004-1198-7. [DOI] [PubMed] [Google Scholar]
- 49.Coutard B., Valle C., de Lamballerie X., et al. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antiviral Res. 2020;176 doi: 10.1016/j.antiviral.2020.104742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Millet J.K., Whittaker G.R. Host cell entry of Middle East respiratory syndrome coronavirus after two-step, furin-mediated activation of the spike protein. Proc Natl Acad Sci U S A. 2014;111:15214–15219. doi: 10.1073/pnas.1407087111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shiryaev S.A., Remacle A.G., Ratnikov B.I., et al. Targeting host cell furin proprotein convertases as a therapeutic strategy against bacterial toxins and viral pathogens. J Biol Chem. 2007;282:20847–20853. doi: 10.1074/jbc.M703847200. [DOI] [PubMed] [Google Scholar]
- 52.Andreani J., Le Bideau M., Duflot I., et al. In vitro testing of combined hydroxychloroquine and azithromycin on SARS-CoV-2 shows synergistic effect. Microb Pathog. 2020;145 doi: 10.1016/j.micpath.2020.104228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ervin R.B., Kennedy-Stephenson J. Mineral intakes of elderly adult supplement and non-supplement users in the third National Health and Nutrition Examination Survey. J Nutr. 2002;132:3422–3427. doi: 10.1093/jn/132.11.3422. [DOI] [PubMed] [Google Scholar]
- 54.Anderson R.A., Roussel A.M., Zouari N., et al. Potential antioxidant effects of zinc and chromium supplementation in people with type 2 diabetes mellitus. J Am Coll Nutr. 2001;20:212–218. doi: 10.1080/07315724.2001.10719034. [DOI] [PubMed] [Google Scholar]
- 55.Braun L.A., Rosenfeldt F. Pharmaco-nutrient interactions—a systematic review of zinc and antihypertensive therapy. Int J Clin Pract. 2013;67:717–725. doi: 10.1111/ijcp.12040. [DOI] [PubMed] [Google Scholar]
- 56.Lane J.C.E., Weaver J., Kostka K., et al. Safety of hydroxychloroquine, alone and in combination with azithromycin, in light of rapid wide-spread use for COVID-19: a multinational, network cohort and self-controlled case series study. medRxiv. 31 May 2020 doi: 10.1101/2020.04.08.20054551. [DOI] [Google Scholar]
- 57.Richardson S., Hirsch J.S., Narasimhan M., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lovato A., de Filippis C. Clinical presentation of COVID-19: a systematic review focusing on upper airway symptoms. Ear Nose Throat J. 2020;99:569–576. doi: 10.1177/0145561320920762. [DOI] [PubMed] [Google Scholar]
- 59.Chen T., Wu D., Chen H., et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Giacomelli A., Pezzati L., Conti F., et al. Self-reported olfactory and taste disorders in SARS-CoV-2 patients: a cross-sectional study. Clin Infect Dis. 2020;71:889–890. doi: 10.1093/cid/ciaa330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Million M., Lagier J.C., Gautret P., et al. Early treatment of COVID-19 patients with hydroxychloroquine and azithromycin: a retrospective analysis of 1061 cases in Marseille, France. Travel Med Infect Dis. 2020;35 doi: 10.1016/j.tmaid.2020.101738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Geleris J., Sun Y., Platt J., et al. Observational study of hydroxychloroquine in hospitalized patients with COVID-19. N Engl J Med. 2020;382:2411–2418. doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Risch H.A. Early outpatient treatment of symptomatic, high-risk COVID-19 patients that should be ramped-up immediately as key to the pandemic crisis. Am J Epidemiol. 2020 May 27 doi: 10.1093/aje/kwaa093. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yu B., Li C., Chen P., et al. Low dose of hydroxychloroquine reduces fatality of critically ill patients with COVID-19. Sci China Life Sci. 2020;63:1515–1521. doi: 10.1007/s11427-020-1732-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mercer E., Rekedal L., Garg R., et al. Hydroxychloroquine improves insulin sensitivity in obese non-diabetic individuals. Arthritis Res Ther. 2012;14:R135. doi: 10.1186/ar3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pareek A., Chandurkar N., Thomas N., et al. Efficacy and safety of hydroxychloroquine in the treatment of type 2 diabetes mellitus: a double blind, randomized comparison with pioglitazone. Curr Med Res Opin. 2014;30:1257–1266. doi: 10.1185/03007995.2014.909393. [DOI] [PubMed] [Google Scholar]
- 67.Gupta A. Real-world clinical effectiveness and tolerability of hydroxychloroquine 400 mg in uncontrolled type 2 diabetes subjects who are not willing to initiate insulin therapy (HYQ-Real-World Study) Curr Diabetes Rev. 2019;15:510–519. doi: 10.2174/1573399815666190425182008. [DOI] [PubMed] [Google Scholar]
- 68.Ronco C., Reis T., Husain-Syed F. Management of acute kidney injury in patients with COVID-19. Lancet Respir Med. 2020;8:738–741. doi: 10.1016/s2213-2600(20)30229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Concordia Pharmaceuticals Inc . Concordia Pharmaceuticals Inc; St Michael, Barbados: 2017. Plaquenil drug monograph. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/009768s037s045s047lbl.pdf. [Google Scholar]