Abstract
Failure of rotator cuff repair surgery can be attributed to a variety of factors, including insufficient biologic environment to support healing. The subacromial bursal tissue has been shown to have a reservoir of mesenchymal stem cells and is a potential source for biologic augmentation during rotator cuff repair. We have developed a technique to capture the subacromial bursal tissue during subacromial bursectomy and then reimplant the tissue on the bursal surface of the rotator cuff tendon after rotator cuff repair. Our goal is to describe our technique of subacromial tissue collection and reimplantation that obviates the need of suturing a whole sleeve of bursal tissue while improving cell yield for rotator cuff healing.
Rotator cuff tears are widespread in the middle-aged and elderly population and are a common cause of pain and disability.1 More than 300,000 rotator cuff repairs are performed annually in the United States, making it one of the most commonly performed orthopaedic procedures.2 In contrast to acute rotator cuff injuries (which have favorable prognoses due to good tissue quality and a favorable biologic healing environment), the postoperative course after repair of chronic cuff tears is much less predictable. Factors such as decreased tendon mobility, tendon retraction, tendon loss, fatty degeneration, and chronic insertional changes combine to create an insufficient mechanical and biologic environment to support healing.3 Good clinical outcomes have been demonstrated in the literature; however, re-rupture rates for these tears can exceed 40%.4
Biologic augmentation has been proposed to improve healing rates and quality.5 The subacromial bursa has traditionally been excised and discarded during rotator cuff repair to improve visualization.6 However, this tissue may potentially serve an important role in rotator cuff healing, by acting as a reservoir of mesenchymal stem cells (MSCs).7 The bursa has been found to contain large numbers of MSCs.8 Even without isolation of specific MSCs, subacromial bursa has been found to have increased differentiation and gene expression potential than concentrated bone marrow aspirate from the proximal humerus.9 As a result, we have developed a technique to capture the subacromial bursal tissue during subacromial bursectomy and reimplant the tissue on the bursal surface of the rotator cuff tendon after rotator cuff repair.
A technique of suturing autologous subacromial bursal tissue has previously been described.6 However, mechanical cutting or chopping has been shown to improve MSC yield compared with use of a whole bursa sleeve.10 We describe a technique for subacromial bursal tissue reimplantation that obviates the need of suturing a whole sleeve of bursal tissue while improving cell yield.
Our goal with this paper is to present our technique for autologous subacromial bursal reimplantation during arthroscopic rotator cuff repair. Our technique includes collection of subacromial tissue with a filter connected to an arthroscopic shaver (GraftNet Autologous Tissue Collector; Arthrex, Naples, FL), followed by a double-row suture repair of the rotator cuff repair, and then the insertion of collected subacromial bursal tissue over the repair site.
Surgical Technique
Preoperative Planning
A thorough history and physical examination was performed for all patients. When symptomatic rotator cuff tear was suspected, magnetic resonance imaging (MRI) of the affected shoulder was ordered. Patients with MRI confirmed rotator cuff tear that remained symptomatic after a trial of nonoperative management including nonsteroidal anti-inflammatory drugs, physical therapy, and activity modification were indicated for shoulder arthroscopy, rotator cuff repair, and subacromial bursectomy with collection and reimplantation of bursal tissue at rotator cuff repair.
Patient Positioning and Diagnostic Arthroscopy
For all our cases, general anesthesia was administered and the patient was positioned in the beach-chair position. The upper extremity was prepped and draped in the usual sterile fashion. A time out was performed. Preoperative antibiotics were administered. A standard posterior portal was made and the arthroscope was inserted into the glenohumeral joint. An anterior portal was created in the rotator interval. A diagnostic arthroscopy was performed with pictures taken of the cartilaginous surfaces of the humeral head and glenoid, the anterior, posterior, and superior labrum, the biceps, and the rotator cuff.
Assessment of Rupture Dimension and Tendon Mobility
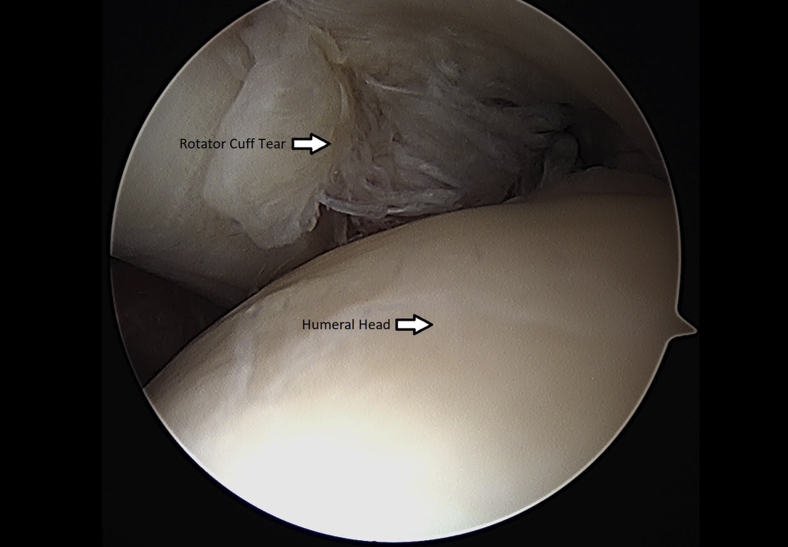
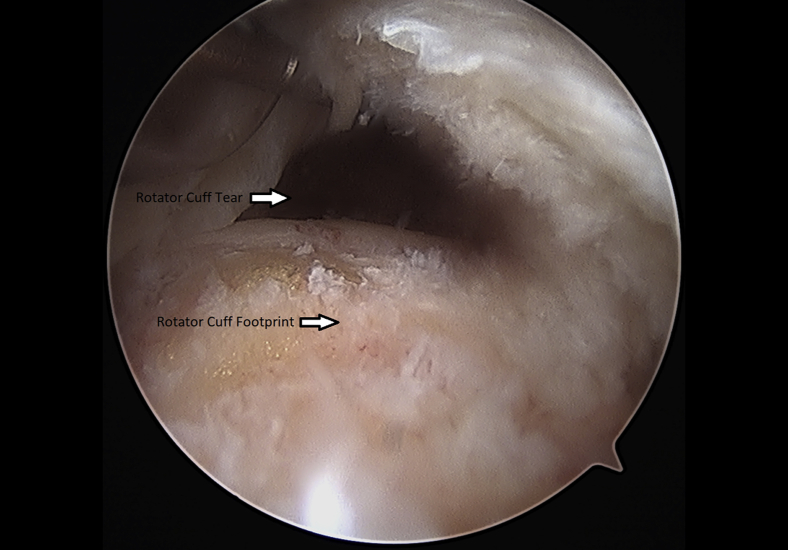
Once the rotator cuff was identified and probed through the glenohumeral joint (Fig 1), the instruments were removed and the arthroscope was inserted into the subacromial space to define the nature of the tear (Fig 2). An accessory lateral portal was created. The subacromial bursa was visualized.
Fig 1.
Left shoulder rotator cuff tear visualized through glenohumeral joint from. a posterior arthroscopic viewing portal with the patient in the beach-chair position.
Fig 2.
Left shoulder rotator cuff tear probed through subacromial space from a. lateral arthroscopic viewing portal with the patient in the beach-chair position.
Collection of Bursal Tissue (With Video Illustration)
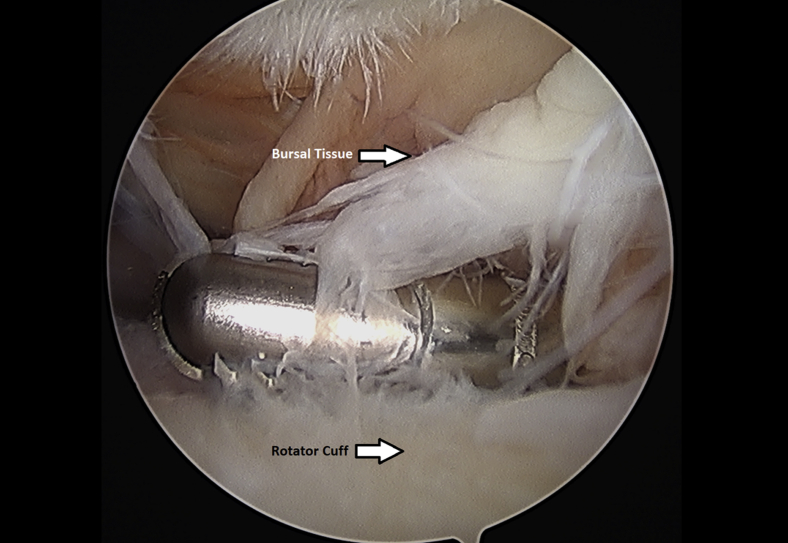
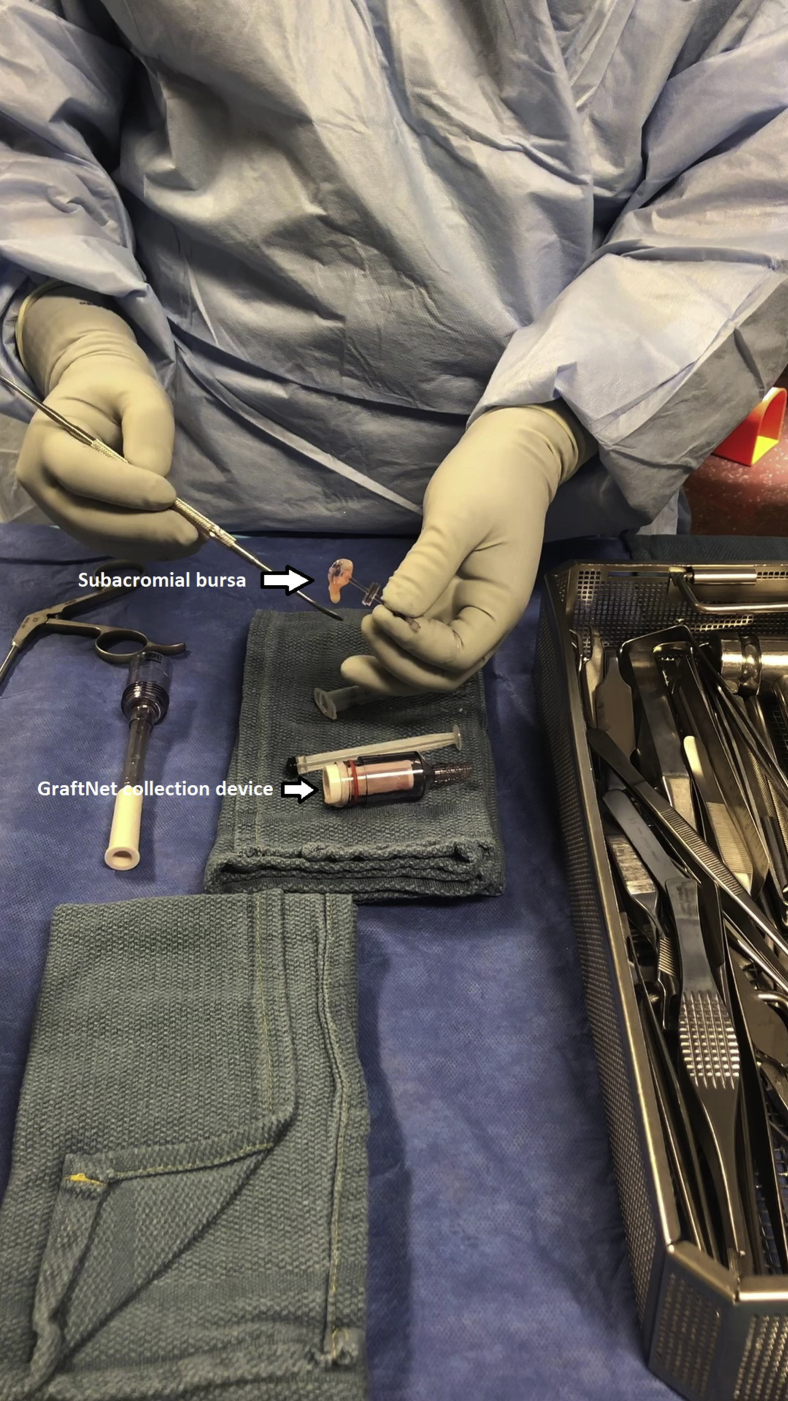
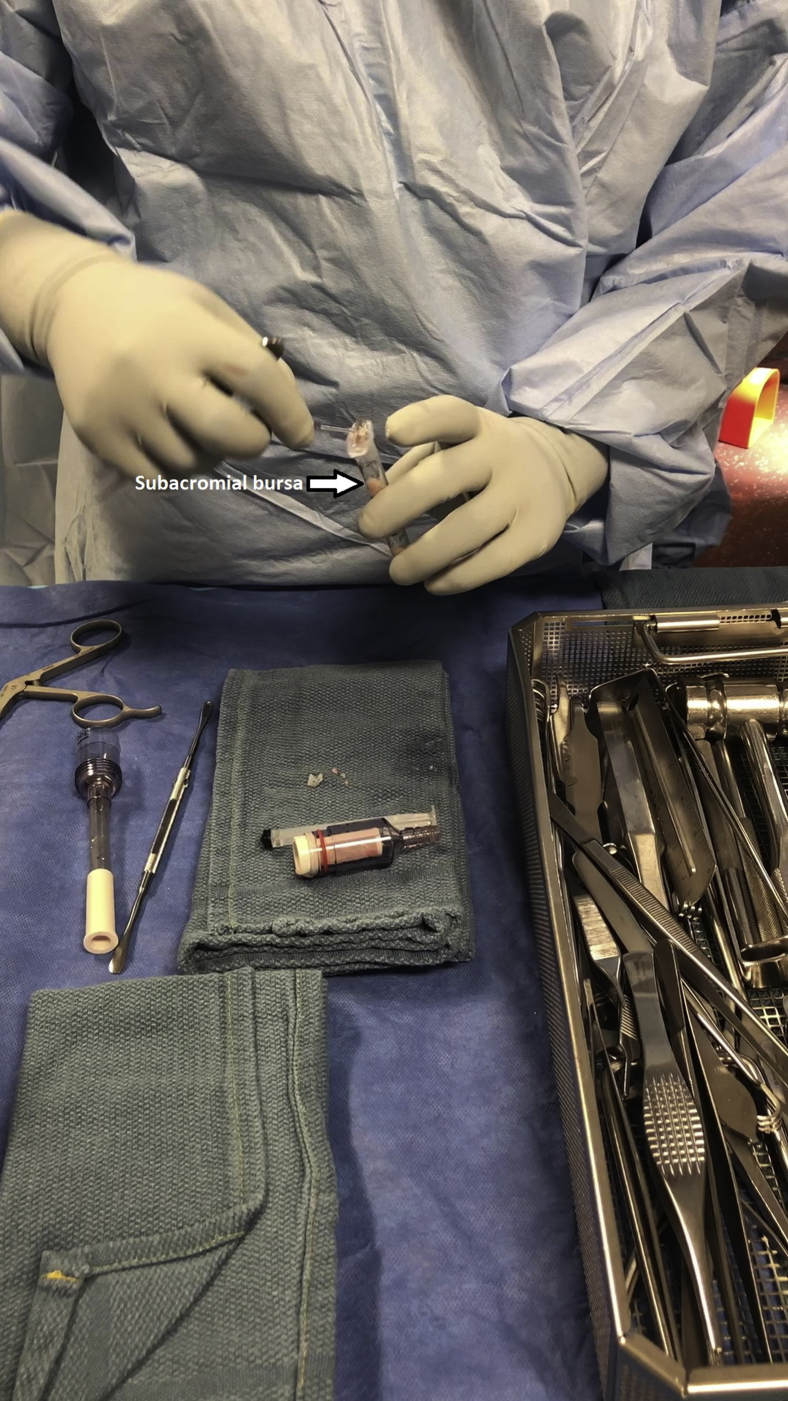
Subacromial bursectomy was performed with a 4.5-mm oscillating shaver (Fig 3) and the bursa was collected for later reimplantation at the end of the case (Video 1). The bursal collection device (GraftNet Autologous Tissue Collector; Arthrex) was attached to the suction on the shaver before subacromial bursectomy (Fig 4). The collection device was then removed and the bursa was extracted (Fig 5). The extracted bursa was then placed in a 3-cc syringe (Fig 6 and Video 1). After the bursal tissue was collected the rotator cuff tear dimensions and tendon mobility were noted. Tears that were amenable to repair were than repaired.
Fig 3.
Left shoulder subacromial bursectomy performed with an arthroscopic shaver from a posterior arthroscopic viewing portal with the patient in the beach-chair position.
Fig 4.
GraftNet (Arthrex) filtration device attached to suction on an arthroscopic shaver.
Fig 5.
Extraction of the minced subacromial bursa tissue from the GraftNet device.
Fig 6.
The subacromial bursa is placed into a 3-cc syringe for later implantation after completion of rotator cuff repair.
Footprint Preparation, Double-Row Repair
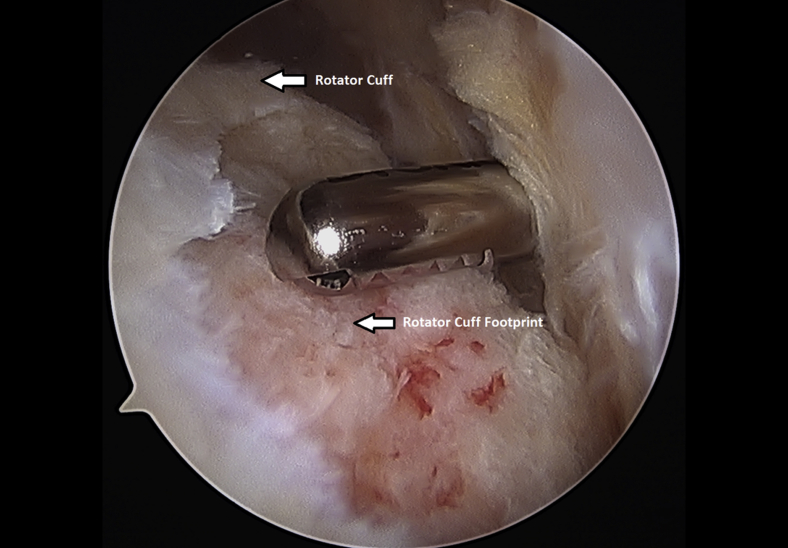
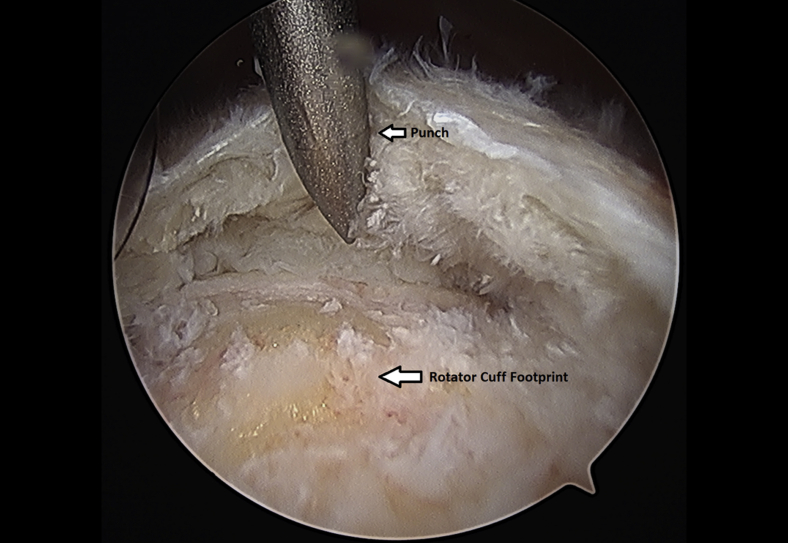
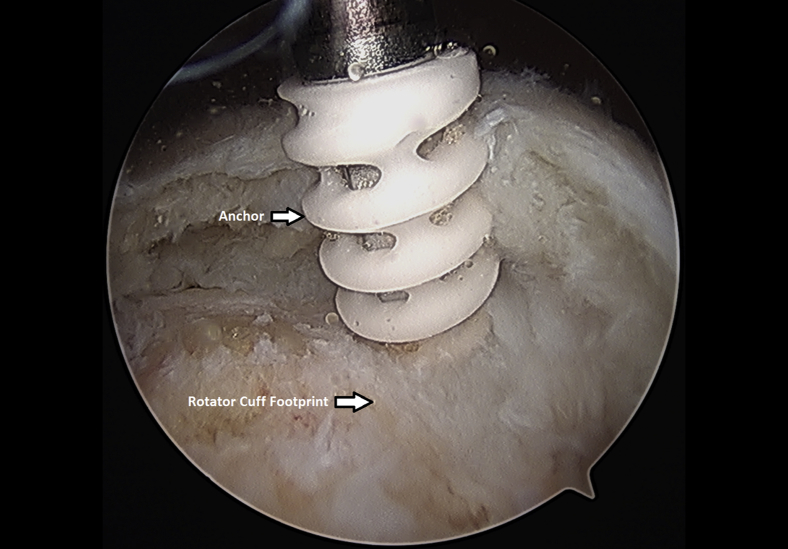
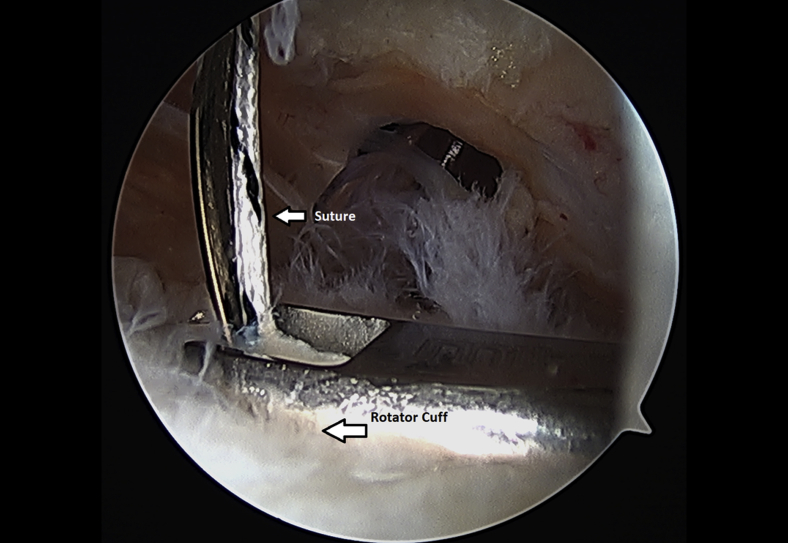
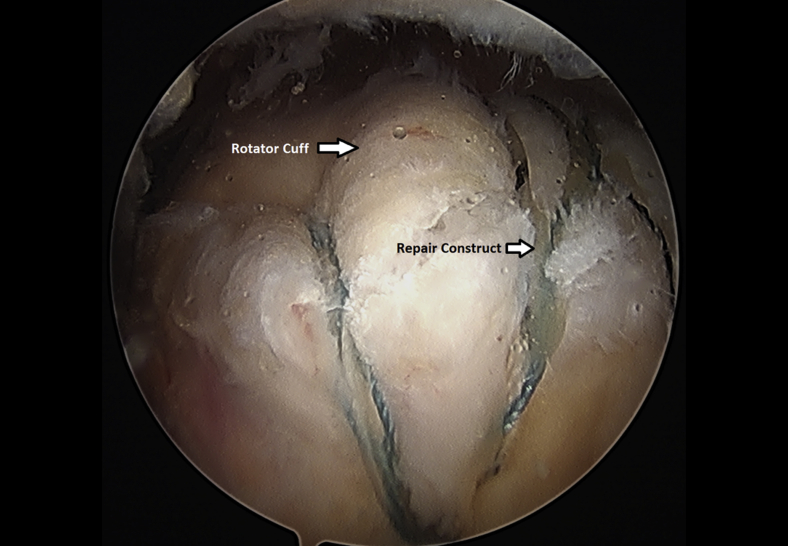
The rotator cuff footprint on the greater tuberosity of the humerus was identified and then decorticated with a shaver and burr (Fig 7). The tuberosity was than microfractured to stimulate bleeding (Fig 8 and Video 1). We placed 1 to 3 anchors at the medial aspect of the footprint depending on the nature of the tear (Fig 9 and Video 1). Sutures were passed in a horizontal mattress fashion through the tendon and knots were tied (Fig 10). Subsequently, the sutures were then passed in a crossing fashion through lateral anchors to create a transosseous equivalent double-row repair construct (Fig 11 and Video 1). The integrity of the repair was checked by taking the shoulder through range of motion (ROM).
Fig 7.
Decortication of the left shoulder rotator cuff footprint using an arthroscopic shaver from a posterior arthroscopic viewing portal with the patient in the beach-chair position.
Fig 8.
Left shoulder greater tuberosity is microfractured using anchor punch and mallet from a lateral arthroscopic viewing portal with the patient in the beach-chair position.
Fig 9.
Left shoulder placement of anchor at medial aspect of rotator cuff footprint from a lateral arthroscopic viewing portal with the patient in the beach-chair position.
Fig 10.
Suture is passed through the left shoulder rotator cuff tendon from a lateral arthroscopic viewing portal with the patient in the beach-chair position.
Fig 11.
Completion of a left shoulder transosseous rotator cuff repair viewing from a lateral portal with the patient in the beach-chair position.
Bursal Tissue Implantation
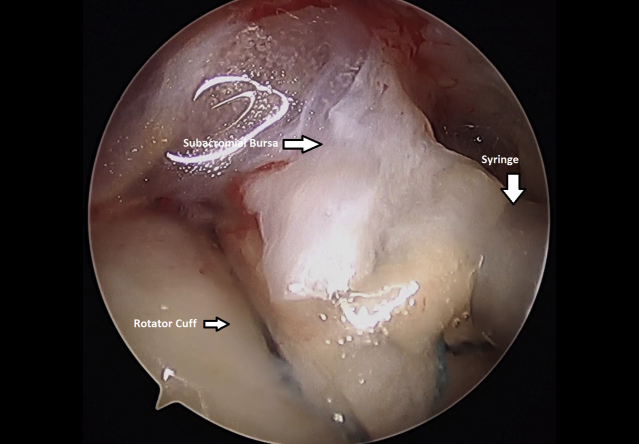
The shoulder was than evacuated of fluid by turning off the water inflow and turning on the suction on the camera. A spinal needle can be inserted percutaneously to help create an air pocket within the subacromial space, improving visualization (Table 1). Once the shoulder was dry, the 3-cc syringe contained isolated, minced subacromial bursal tissue was placed through a lateral portal and on top of the rotator cuff repair (Fig 12 and Video 1) Instruments were then removed. Incisions were closed using MONOCRYL sutures (Ethicon, Somerville, NJ). A sterile dressing was applied and the patient was placed in a sling.
Table 1.
Pearls and Pitfalls for Arthroscopic Rotator Cuff Repair Using Minced Autologous Subacromial Bursa
| Pearls | Pitfalls |
|---|---|
| To deliver bursal tissue, directly inject bursal tissue from a 3-cc syringe into lateral portal without the aid of a needle. |
Use of a needle on the end of the syringe can lead to clogging and difficulty with reimplantation of bursal tissue. |
| To improve visualization, place free spinal needle into subacromial space before suctioning water out of shoulder to help create an air pocket. |
Without an air pocket, visualization of the rotator cuff may be difficult, leading to suboptimal placement of bursal tissue. |
| To avoid filter clogging, incremental removal of bursal tissue from bursal collection device may be beneficial. |
Bursal-collection device can get clogged. |
Fig 12.
Injection of minced bursal tissue onto surface of left shoulder rotator cuff. repair. Viewed from a lateral arthroscopic portal with the patient in the beach-chair position.
Postoperative Rehabilitation
Immediately after surgery, home exercises are initiated. Initial exercises include the following; pendulum exercises (where the patient bends over at the waist letting the affected arm hang down at the side and the patient sways their body back and forth using the weight of the arm and gravity to generate small circles), elbow ROM exercises, and wrist ROM exercises. From 2 to 6 weeks, the patient can work on only passive ROM of the shoulder and grip strength. A sling is maintained for 6 weeks overall, except when the specified approved exercises are performed. From 6 to 12 weeks, the patient can initiate scapular ROM exercises and begin active assisted ROM shoulder exercises with transitioning to active ROM as tolerated. At 10 weeks, the patient can initiate strengthening with resisted motions and isometric exercises with the arm at their side. At 3 to 12 months, full ROM and advance strengthening are recommended.
Discussion
The belief that subacromial bursa provides healing potential for rotator cuff repair has long been known.5 Rotator cuff repairs in which radical subacromial bursa resection have been performed have shown lower success rates in the literature. In contrast, the subacromial bursa traditionally has been thought of as a source of shoulder pain and may be removed in symptomatic patients.11 Although more studies are required to elucidate the role of the subacromial bursa on the healing process, we presume that the reservoir of MSCs and inflammatory cells may play an important role in the initiation and regulation of the healing process.12
Significant interest exists in the use of MSCs for rotator cuff healing. These cells are multipotent and may contribute to healing directly through differentiation into various mesenchymal tissues including osteocytes, chondrocytes, tenocytes, and adipocytes.13 They also signal surrounding cells and help modulate the overall healing environment as a whole. Although there has been support in the basic science literature for MSCs promoting healing, there are limited clinical studies on its use in shoulder disorders. In one such clinical study, bone marrow aspirate harvested from the iliac crest was used to augment the repair of complete rotator cuff tears. Of the 14 patients in the study, all had evidence by MRI of tendon integrity at 12 months postoperatively.14 While this Level IV case series is encouraging, there was no control group, and there is significant morbidity associated with iliac crest bone marrow aspirate.
Techniques also have been described of harvesting MSCs from the proximal humerus.15 However, MSCs isolated from synovial tissue have shown to have greater proliferation and differentiation potential than MSCs harvested from bone marrow or adipose tissue.9 Furthermore, local tissues in the shoulder including synovium, subacromial bursa, ruptured tendon, and enthesis have been studied for their suitability for MSCs.16 The subacromial bursa yielded the greatest number of MSCs of all tissues. The ease of harvesting the subacromial bursal tissue with its proximity to the rotator cuff lends further support to the use of subacromial bursal tissue as a biological supplement to rotator cuff repair.
It is well known that the rotator cuff insertion on to the humerus has a fibrocartilage column. An in vivo study by Song et al.2 reported that bursal derived MSCs can form bone, fibrocartilage, and tendon-like tissue in the absence of supplemental growth factors.2 We hypothesize that MSCs contained within bursal tissue may play a role in tendon reconstitution and remodeling, potentially improving rotator cuff repair healing.
Subacromial bursal tissue augmentation to rotator cuff repair has the theoretical potential to decrease rotator cuff retear rates by aiding in the biology of tendon healing. Results of bursal tissue reimplantation have not yet been reported, so clinical outcomes and effect on rotator cuff healing remains unknown. In addition, the behavior of the reimplanted minced bursa tissue is unknown. Theoretical risks of use include increased inflammation or pain. It is also possible that the tissue displaces from the site of the repair (Table 2). The tissue is very adherent, and reimplantation occurs at the end of the case to minimize this. MSCs have several mechanisms of action, including stimulation of other cells and immune response modulation. We presume that this behavior still occurs even in the setting of potential tissue displacement. We report a simple technique for arthroscopic subacromial bursa collection and reimplantation that can be used in conjunction with any type of rotator cuff repair construct.
Table 2.
Advantages and Disadvantages of Arthroscopic Rotator Cuff Repair Using Minced Autologous Subacromial Bursa
| Advantages | Disadvantages |
|---|---|
| Most surgeons have comfort performing a subacromial bursectomy. | Requires some time for surgical assistant to transfer bursal tissue to syringe. |
| Tissue is minced which improves MSC yield. | Must have a dry shoulder at time of reimplantation of bursal tissue. |
| No additional device or suture is required for fixation. | Requires use of a graft collection device. |
| Can be used with any type of rotator cuff repair. | Unproven efficacy. |
| Can be used with any type of rotator cuff repair. | |
| Less costly than other forms of biologic augmentation. | Still adds cost. |
| Applied on bursal surface of tendon, so can be used in conjunction with tendon/bone interface biologics or marrow stimulation. | No evidence that tissue remains where it is placed. |
MSC, mesenchymal stem cells.
Footnotes
The authors report the following potential conflicts of interest or sources of funding: J.M.G. reports personal fees from Wright Medical and Arthrex, and another relationship with Sparta Biomedical, outside the submitted work. Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Supplementary Data
Initial arthroscopic subacromial bursectomy of the left shoulder is performed with an arthroscopic shaver from a posterior arthroscopic viewing portal with the patient in the beach-chair position. The bursa collected in the GraftNet is then placed into a 3-cc syringe. Next, the rotator cuff is repaired per the surgeon's preferred technique. In this patient, we decorticated the greater tuberosity using a shaver, while viewing from a posterior arthroscopic viewing portal. While viewing through a lateral portal, we placed a medial row of suture anchors into the greater tuberosity, then completed a transosseous equivalent double-row rotator cuff repair. Once the repair is complete, while viewing through a lateral portal, arthroscopic fluid is evacuated from the shoulder, and a syringe is used to reimplant bursal tissue on the surface of the rotator cuff repair.
References
- 1.Smith K.M., Le A.D.K., Costouros J.G., Dragoo J.L. Biologics for rotator cuff repair. A critical analysis review. J Bone Joint Surg Rev. 2018;6:e8. doi: 10.2106/JBJS.RVW.17.00185. [DOI] [PubMed] [Google Scholar]
- 2.Song N., Armstrong A.D., Li F., Ouyang H., Niyibizi C. Multipotent mesenchymal stem cells from human subacromial bursa: Potential for cell based tendon tissue engineering. Tissue Eng Part A. 2014;20:239–249. doi: 10.1089/ten.tea.2013.0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greenspoon J.A., Moulton S.G., Millett P.J., Petri M. The role of platelet rich plasma (PRP) and other biologics for rotator cuff repair. Open Orthop J. 2016;10:309–314. doi: 10.2174/1874325001610010309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amoo-Achampong K., Krill M.K., Acheampong D. Evaluating strategies and outcomes following rotator cuff tears. Shoulder Elbow. 2019;11(suppl 1):4–18. doi: 10.1177/1758573218768099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uhthoff H.K. Surgical repair of rotator cuff ruptures. The importance of the subacromial bursa. J Bone Joint Surg Br. 1991;73:399–401. doi: 10.1302/0301-620X.73B3.1670436. [DOI] [PubMed] [Google Scholar]
- 6.Freislederer F., Dittrich M., Scheibel M. Biological augmentation with subacromial bursa in arthroscopic rotator cuff repair. Arthrosc Tech. 2019;8:e741–e747. doi: 10.1016/j.eats.2019.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Utsunomiya H., Uchida S., Sekiya I., Sakai A., Moridera K., Nakamura T. Isolation and characterization of human mesenchymal stem cells derived from shoulder tissues involved in rotator cuff tears. Am J Sports Med. 2013;41:657–668. doi: 10.1177/0363546512473269. [DOI] [PubMed] [Google Scholar]
- 8.Lhee S.H., Jo Y.H., Kim B.Y. Novel supplier of mesenchymal stem cell: Subacromial bursa. Transplant Proc. 2013;45:3118–3121. doi: 10.1016/j.transproceed.2013.07.052. [DOI] [PubMed] [Google Scholar]
- 9.Morikawa D., Johnson J.D., Kia C. Examining the potency of subacromial bursal cells as a potential augmentation for rotator cuff healing: An in vitro study. Arthroscopy. 2019;35:2978–2988. doi: 10.1016/j.arthro.2019.05.024. [DOI] [PubMed] [Google Scholar]
- 10.Morikawa D., Muench L.N., Baldino J.B. Comparison of preparation techniques for isolating subacromial bursa-derived cells as a potential augment for rotator cuff repair. Arthroscopy. 2020;36:80–85. doi: 10.1016/j.arthro.2019.07.024. [DOI] [PubMed] [Google Scholar]
- 11.Steinert AF, Kunz M, Prager P, et al. Characterization of bursa subacromialis-derived mesenchymal stem cells. Stem Cell Res Ther 2015;6:1:114. [DOI] [PMC free article] [PubMed]
- 12.Lorbach O., Baums M.H., Kostuj T. Advances in biology and mechanics of rotator cuff repair. Knee Surg Sports Traumatol Arthrosc. 2015;23:530–541. doi: 10.1007/s00167-014-3487-2. [DOI] [PubMed] [Google Scholar]
- 13.Beitzel K., Solovyova O., Cote M.P. The future role of mesenchymal stem cells in the management of shoulder disorders. Arthroscopy. 2013;29:1702–1711. doi: 10.1016/j.arthro.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 14.Ellera Gomes J.L., da Silva R.C., Silla L.M., Abreu M.R., Pellanda R. Conventional rotator cuff repair complemented by the aid of mononuclear autologous stem cells. Knee Surg Sports Traumatol Arthrosc. 2012;20:373–377. doi: 10.1007/s00167-011-1607-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beitzel K., McCarthy M.B., Cote M.P. Comparison of mesenchymal stem cells (osteoprogenitors) harvested from proximal humerus and distal femur during arthroscopic surgery. Arthroscopy. 2013;29:301–308. doi: 10.1016/j.arthro.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 16.Jia Z., Wang S., Liu Q. Identification of differentially expressed genes by single-cell transcriptional profiling of umbilical cord and synovial fluid mesenchymal stem cells. J Cell Mol Med. 2020;24:1945–1957. doi: 10.1111/jcmm.14891. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Initial arthroscopic subacromial bursectomy of the left shoulder is performed with an arthroscopic shaver from a posterior arthroscopic viewing portal with the patient in the beach-chair position. The bursa collected in the GraftNet is then placed into a 3-cc syringe. Next, the rotator cuff is repaired per the surgeon's preferred technique. In this patient, we decorticated the greater tuberosity using a shaver, while viewing from a posterior arthroscopic viewing portal. While viewing through a lateral portal, we placed a medial row of suture anchors into the greater tuberosity, then completed a transosseous equivalent double-row rotator cuff repair. Once the repair is complete, while viewing through a lateral portal, arthroscopic fluid is evacuated from the shoulder, and a syringe is used to reimplant bursal tissue on the surface of the rotator cuff repair.