Abstract
The stress response to acute disease is characterized by activation of the hypothalamic-pituitary-adrenal (HPA) axis and the sympathoadrenal system, increased serum cortisol levels, increased percentage of its free fraction and increased nuclear translocation of the glucocorticoid-receptor complex, even though many pathways may be inhibited by poorly understood mechanisms. There is no consensus about the cutoff point of serum cortisol levels for defining adrenal insufficiency. Furthermore, recent data point to the participation of tissue resistance to glucocorticoids in acute systemic inflammatory processes. In this review, we evaluate the evidence on HPA axis dysfunction during critical illness, particularly its action on the inflammatory response, during acute severe injury and some pitfalls surrounding the issue. Critical illness-related corticosteroid insufficiency was defined as a dynamic condition characterized by inappropriate cellular activity of corticosteroids for the severity of the disease, manifested by persistently elevated proinflammatory mediators. There is no consensus regarding the diagnostic criteria and treatment indications of this syndrome. Therefore, the benefits of administering corticosteroids to critically ill patients depend on improvements in our knowledge about the possible disruption of its fragile signalling structure in the short and long term.
Keywords: adrenal, cortisol, cytokines, glucocorticoid, inflammation, sepsis
Introduction
For more than 70 years, we have been studying how cortisol acts to maintain body homeostasis, and we are still far from understanding the whole picture. One example is how incomplete our knowledge is about the function of the hypothalamic-pituitary-adrenal (HPA) axis during critical illness, particularly sepsis. Hundreds of articles have been published about this issue, but it remains unclear whether glucocorticoids improve the outcome of septic patients or how they work.
It is known that under stress conditions, the need for cortisol may increase several times with a concomitant loss of the circadian rhythm pattern of production [1]. Moreover, mortality in critical patients follows a bimodal pattern, being higher when serum cortisol levels are elevated (possibly reflecting the greater severity of the disease) or decreased (confirming the idea that glucocorticoids are important for survival of a severe disease) [2]. Unfortunately, determining the appropriate adrenal response under such acute challenges is presently impossible due to considerable variability in disease severity, serum protein levels and resistance to hormone activity in different tissues.
In this short review, we evaluate the literature on HPA axis dysfunction during critical illness, particularly its action on the inflammatory response, during acute severe injury, and we aim to point out some of the pitfalls in our knowledge on the issue. Most of the studies reviewed here involve patients with sepsis. It is understandable, because use of corticosteroids in other critical illnesses is not recommended (such as acute cardiovascular syndromes or trauma) or has precise indications (such as asthma or COPD exacerbations); however, in sepsis, corticosteroid use is still a controversial matter.
Physiology of the hypothalamic-pituitary-adrenal axis
In healthy individuals, secretion of cortisol is strictly controlled by the HPA axis. In situations of imbalance of homeostasis (cold, infection, haemorrhage, hypotension, emotional stress, and burn, among others), the hypothalamus secretes corticotropin-releasing hormone (CRH), which, through a pituitary-portal system, reaches the adenohypophysis and stimulates specialized cells to release adrenocorticotrophic hormone (ACTH) [3]. This hormone acts on the adrenal cortex, stimulating the release of steroids, especially cortisol. The system is modulated by a negative feedback mechanism at both the pituitary and hypothalamic levels (Fig. 1) [3].
Fig. 1.
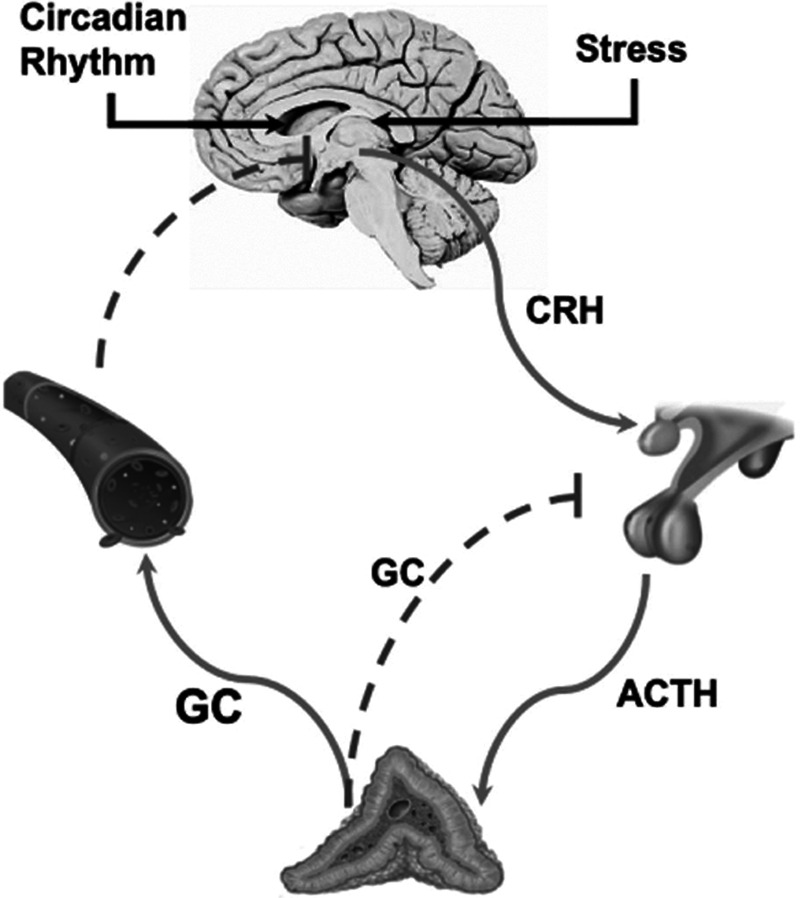
Physiology of the HPA axis. Cortisol secretion is rigorously controlled by the HPA axis, which works with a tethering mechanism of negative feedback at all levels. Stress stimulates the hypothalamus to increase the secretion of CRH, a hormone that stimulates specialized cells of the anterior pituitary gland to release ACTH. ACTH reaches the adrenal cortex and stimulates the production of steroids, such as cortisol. Cortisol contributes to the restoration and maintenance of haemostasis during stress, counteracts many steps of the inflammatory response, stimulates gluconeogenesis and glycogenolysis, and maintains vascular tone and endothelial integrity. ACTH, adrenocorticotrophic hormone; CRH, corticotropin-releasing hormone; HPA, hypothalamic-pituitary-adrenal.
Under physiological conditions, cortisol has both diurnal and pulsatile secretion patterns, with maximum circulatory levels in the morning and noticeable decreases throughout the day. The adrenal cortex secretes approximately 20–30 mg of cortisol daily, reaching serum levels ranging from 5 to 24 µg/dL [1]. Under stress conditions, secretion may increase 10- to 12-fold [4].
Once released, almost all of the cortisol (90–95%) in circulation is bound to plasma proteins [cortisol-binding globulin (CBG) and albumin]; however, only the free fraction is bioactive [3].
Lipid-soluble glucocorticoids easily cross the plasma membrane and bind to cytoplasmic glucocorticoid receptors. Once activated, the dimeric complex glucocorticoid-glucocorticoid receptor changes its conformation and enters the nucleus. Glucocorticoid exerts an anti-inflammatory action through two different mechanisms: transactivation or transrepression, as illustrated in Fig. 2.
Fig. 2.
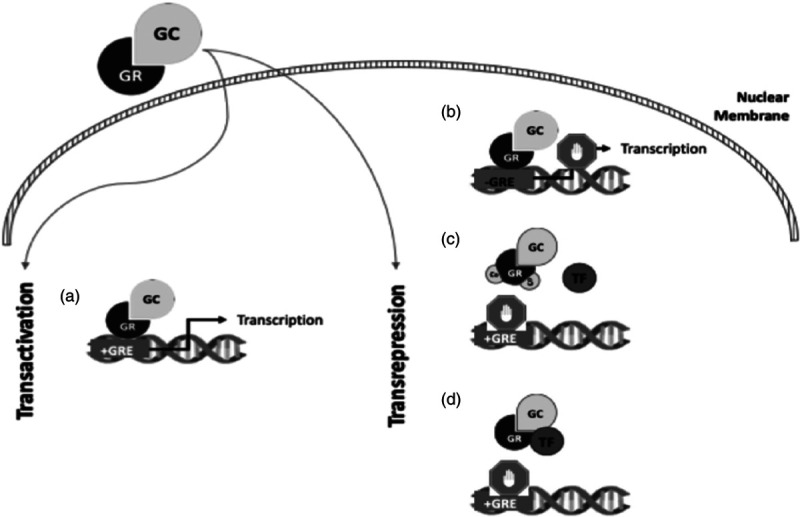
Mechanism of action of glucocorticoids at the cellular level. (a) Transactivation occurs when glucocorticoid-glucocorticoid receptor binds to specific DNA sequences [glucocorticoid-response elements (GREs)] and mediates the transcription of anti-inflammatory genes, such as IL-10. Transrepression is more complex and involves three distinct mechanisms. (b) The glucocorticoid-glucocorticoid receptor complex can bind to specific sequences that repress the transcription of genes involved in the inflammatory response [negative GREs (nGREs)]. (c) The glucocorticoid-glucocorticoid receptor complex can capture coactivators necessary for gene transcription, avoiding their use by proinflammatory transcription factors. (d) The glucocorticoid-glucocorticoid receptor complex can attach itself to proinflammatory transcription factors (e.g., NF-kappaB or AP1, preventing them from connecting to their promoters and consequently, blocking their activity).
In addition to their action inside the nucleus, the glucocorticoid-glucocorticoid receptor complex can also affect cell signalling inside the cytoplasm, acting on second messengers such as calcium or cyclic AMP. All these processes may occur together or separately, resulting in a strong anti-inflammatory response [5].
A large interindividual variability of cortisol sensitivity has been reported in population studies. Although still poorly understood, this variability seems to be correlated, at least in part, with genetic variations in glucocorticoid receptors. Three isoforms of glucocorticoid receptor were isolated: glucocorticoid receptor-α, more abundant, functionally active; glucocorticoid receptor-β, which partially inhibits glucocorticoid receptor-α; and glucocorticoid receptor-P, which optimizes the function of glucocorticoid receptor-α. Changes in the levels of each glucocorticoid receptor isoform are believed to regulate tissue sensitivity to cortisol [6]. Unfortunately, the particular expression or activity of these isoforms has not been described in acute critical patients.
Hypothalamic-pituitary-adrenal axis function during critical illness
In critical illness, inflammatory mediators (mainly IL-1α, IL-1β, IL-6 and TNF-α) activate the HPA axis at the hypothalamic, pituitary and adrenal levels in such a way that the pulsatile pattern and the circadian variability of cortisol secretion are lost [7,8]. The resulting hypercortisolism is initially protective, increasing gluconeogenesis, maintaining intravascular volume and modulating the inflammatory response. Cortisol levels remain high throughout the critical illness, despite the reduction in adrenocorticotrophic hormone (ACTH), suggesting the participation of other pathways in this regulation [9,10].
Several mechanisms have been implicated in maintaining high cortisol levels and negative feedback loss during critical illness:
-
1.
Reduction in cortisol metabolism may occur due to impaired expression and activation of enzymes associated with this process [11].
-
2.
Renal dysfunction prolongs the half-life of circulating cortisol.
-
3.
Concentrations of cortisol-binding globulin (CBG) and albumin are decreased, as well as their affinity for cortisol, increasing the hormone-free fraction [12].
-
4.
Inflammatory cytokines may increase glucocorticoid receptor affinity, increasing peripheral conversion of their precursors [12,13].
Once the critical phase of the disease has passed, cortisol levels tend to return to normal. The maintenance of hypercortisolism, however, seems to be associated with long-term complications (hyperglycaemia, myopathy, posttraumatic stress disorder, and poor wound healing) [2].
In summary, the stress response to acute disease is characterized by activation of the HPA axis and sympathoadrenal system, increased serum cortisol levels, increased percentage of its free fraction and increased nuclear translocation of the Glucocorticoid-glucocorticoid receptor complex. In many critical patients, however, this fragile balance between stimuli and response seems to be lost. The mechanisms of HPA axis dysfunction in this context are complex and still poorly understood [14].
Hypocortisolism in critically ill patients may occur due to structural damage of adrenal glands, HPA axis blockade secondary to exogenous corticosteroid replacement or HPA dysfunction.
Absolute adrenal insufficiency is uncommon in critically ill patients, with an estimated incidence of less than 3% [15]. A subgroup of patients may develop adrenal structural damage (infarction, haemorrhage) secondary to abdominal trauma, major surgeries, disseminated intravascular coagulation, heparin-induced thrombocytopenia, HIV, fungal infections and tuberculosis [14,16].
Exogenous replacement of glucocorticoids in suppressing doses is also a cause of secondary adrenal insufficiency. The time required to suppress the axis varies depending on the dose and from patient to patient. The axis is considered suppressed in patients with clinical manifestations of Cushing’s syndrome who use 20 mg/day of prednisone or the equivalent for more than three weeks or in patients taking supraphysiological doses of glucocorticoids for more than 3–6 weeks [17]. Several other drugs can also cause adrenal insufficiency. Inducers of cytochrome P-450 (rifampicin, phenytoin) increase cortisol metabolism, while ketoconazole and etomidate depress hormone secretion and synthesis [12,18].
Both of these mechanisms of adrenal insufficiency are rare. Most critically ill patients who develop adrenal insufficiency present reversible HPA axis dysfunction. A condition known as relative or functional adrenal insufficiency [14].
Functional adrenal insufficiency in critical patients
The diagnosis of adrenal insufficiency in the presence of critical illness is challenging. Unfortunately, there is no universally accepted definition, and authors differ in its diagnostic criteria. The most common methods used to classify a patient with functional adrenal insufficiency are as follows:
-
1.
Total serum cortisol: the most widely available values for serum cortisol in the literature range from 10 to 34– μg/dL [14]; however, reduced plasma protein levels may negatively impact cortisol measurements [19]. In addition, increased mortality is associated with both low levels (insufficient stress response) and high levels (reflective of greater disease severity), making cortisol levels difficult to interpret [20–22].
-
2.
Free cortisol: the free cortisol concentration more accurately reflects the activation of the HPA axis, avoiding the problem of plasma protein concentrations. Moreover, there appears to be a good correlation between the free cortisol measured and calculated by the Coolens equation (based on plasma cortisol and CBG measurements) [23]. Unfortunately, there are still insufficient data to support the use of free cortisol.
-
3.
High-dose ACTH stimulation test: This test is used to evaluate the integrity of the HPA axis. It consists of the administration of 250 μg of intravenous corticotropin and measurement of serum cortisol concentrations after 30–60 min. A diagnosis of adrenal insufficiency is suggested if poststimulus cortisol is <20 μg/dL or the increase in cortisol (Δ cortisol) is <9 μg/dL [14,24]. The main pitfall of this test is that nonresponse may not be indicative of insufficiency but rather of a cortisol secretion maximally and proportionally appropriate to the stimulus.
-
4.
Low-dose ACTH stimulation test: because 250 μg of corticotropin is considered a supraphysiological dose and capable of stimulating cortisol secretion even with a decreased adrenal reserve, 1 μg of corticotropin has been proposed as an alternative [25]. A prospective cohort of 59 septic shock patients was used to compare both tests, and adrenal insufficiency was detected in 22% of patients with 1 μg corticotropin, while it was identified in 9% with 250 μg [26].
We can find that, in the literature, functional adrenal insufficiency diagnosed by all the methods described above, which makes a comparison among them difficult.
In one of these studies, 46 patients with sepsis were challenged with high or low doses of corticotropin. Responsive patients (Δ cortisol > 9 μg/dL), regardless of the test, presented higher survival rates than nonresponsive patients [27]. Those who responded only to the 250-μg test showed higher mortality. It is suggested that the 1-μg corticotropin test identifies a subgroup of patients with inadequate adrenal reserve neglected by the high-dose test [27]. In another study, a cohort of 189 patients with septic shock was divided into three prognostic groups according to baseline cortisol and response to the high-dose ACTH stimulation test: (1) good prognosis (26% mortality in 28 days) – baseline cortisol ≤34 μg/dL and Δ cortisol >9 μg/dL; (2) poor prognosis (82% mortality in 28 days) – baseline cortisol >34 μg/dL and Δ cortisol ≤9 μg/dL and (3) intermediate prognosis (67% mortality in 28 days) – baseline cortisol >34 μg/dL and Δ cortisol ≥9 μg/dL or baseline cortisol <34 μg/dL and Δ cortisol ≤9 μg/dL [2].
As observed, there is no consensus about a cutoff point of serum cortisol levels for defining adrenal insufficiency. Moreover, there are other limitations of the assessment of adrenal function in critically ill patients:
-
1.
Patients may present a spontaneous increase (without stimulus) in serum cortisol ≥9 μg/dL, which calls into question the clinical validity of data obtained after the ACTH stimulation test [28].
-
2.
Reproducibility of the ACTH stimulation test is questionable, with varying results when performed in the same individual on more than one occasion [29].
-
3.
Cortisol metabolism is impaired, which is at least partially responsible for hypercortisolaemia and not only the activation of the axis [11].
-
4.
Etomidate, a drug commonly used in the context of critical illness, may interfere with the test results [30].
-
5.
The most accurate cortisol dosing methodology (liquid chromatography with mass spectrometry) is laborious and not wide.ly available and has commonly been replaced by immunoassays, which present known pitfalls [31].
In addition, serum cortisol levels may not translate into activity in tissues. In a study of 35 septic patients undergoing mechanical ventilation, a worrisome discrepancy was observed between circulating cortisol levels and interstitial cortisol concentrations, measured by a microdialysis catheter in the subcutaneous tissue of the thigh [32].
Furthermore, tissue resistance to glucocorticoids is a classically described condition in chronic inflammatory disorders (COPD, severe asthma, systemic lupus erythematosus, ulcerative colitis and rheumatoid arthritis). Recent data point to the participation of this condition in acute systemic inflammatory processes, such as sepsis and acute respiratory distress syndrome (ARDS).
Several mechanisms have been implicated in the resistance to glucocorticoid associated with systemic inflammation: reduction in glucocorticoid receptor, increase in β-isoform expression, reduction in glucocorticoid receptor affinity to ligands, altered coactivators of nuclear receptors, reduction in DNA binding, increased conversion of cortisol to cortisone, glucocorticoid receptor polymorphisms, among others. It is believed that mediators released during critical illness can both stimulate and inhibit the synthesis and action of cortisol depending on the severity of the disease, the time of evolution and the mediator pattern, interfering with the HPA axis and the glucocorticoid receptor signalling [14]. In an ex-vivo model, a reduction in nuclear translocation of the glucocorticoid receptor-glucocorticoid complex was demonstrated in patients with fatal ARDS, despite adequate plasma cortisol concentrations [33]. The results from other clinical and experimental studies indicate that the absence of improvement in sepsis and ARDS may correlate with failure to activate glucocorticoid receptor and therefore, in modulating the inflammatory response, a condition known as systemic inflammation-associated glucocorticoid resistance [16]. These findings led some authors to propose the term critical illness-related corticosteroid insufficiency (CIRCI).
Critical illness-related corticosteroid insufficiency
Adrenal insufficiency in critical patients seems to be a complex and still unknown medical entity. Considering all the pitfalls described above, it was suggested that the term absolute or relative adrenal insufficiency should be replaced by CIRCI [14].
CIRCI was then defined as a dynamic and usually reversible condition characterized by inappropriate cellular activity of corticosteroids for the severity of the disease, manifested by persistently elevated proinflammatory mediators. There is no consensus regarding the diagnostic criteria and treatment indications of this syndrome [14].
The signs and symptoms of these patients seem to be nonspecific. When classic symptoms of adrenal crisis are present in patients with other clinical signs of adrenal insufficiency, such as hyperpigmentation, the diagnosis may be more easily suspected, but only a minority of these patients show such findings.
More commonly, clinical manifestations of CIRCI are a consequence of an exacerbated inflammatory response. Hypotension refractory to volume resuscitation requiring vasopressors is a hallmark for suspicion. The laboratory may show eosinophilia and hypoglycaemia. Hyponatraemia and hyperkalaemia are uncommon [14].
In the study conducted by Annane et al. [2] cited above, the incidence of adrenal insufficiency (Δ cortisol ≤ 9 μg/dL after 250 μg of Cortrosyn) in patients with septic shock was 54%. On the other hand, a study conducted in Australia in which patients who received etomidate were excluded, the incidence of CIRCI in septic shock patients was 24.5% [34].
Although the definitions are nebulous and not consensual, the evidence showing a dysfunction in cortisol metabolism during sepsis seems convincing. Therefore, several studies have addressed how glucocorticoids should be replaced.
Many randomized controlled trials evaluating glucocorticoid replacement in critically ill patients (severe sepsis, septic shock and ARDS) have been published in recent decades [20,28,31,35,36]. Different doses, drugs, strategies and treatment times were tested. Two trials must be initially mentioned; both included patients with septic shock and performed a high-dose ACTH stimulation test. In a multicentric French study, 300 patients were randomly divided into an intervention group (hydrocortisone 50 mg 6/6 h + fludrocortisone 50 μg/day) and a control group (placebo). There was a difference in mortality between the groups, 55 vs. 61%, respectively. When stratified by adrenal function, those patients with adequate adrenal reserve (Δ cortisol > 9 μg/dL) had no mortality benefit or vasopressor weaning, unlike that observed in patients with diminished adrenal reserve [20]. On the other hand, the CORTICUS study randomized 499 patients between hydrocortisone and placebo. There was no difference between the groups (28-day mortality of 35 vs. 32%, respectively), even when the subgroups were analysed for adrenal reserve. The hydrocortisone group showed a more rapid reversion of the shock (3.3 vs. 5.8 days in the placebo group). Thus, CORTICUS did not show a difference between high-dose corticosteroid responders or nonresponders to adjuvant corticosteroid treatment [31]. Although there were large methodological differences between these two studies (therapeutic window, fludrocortisone, treatment time, steroid weaning strategy, inclusion criteria, severity of patients included, percentage of surgical patients, incidence of adrenal insufficiency) and conflicting results, the sepsis and septic shock management guidelines published state that adjunctive treatment with corticosteroids should not be based on response to tests with synthetic ACTH [37].
A meta-analysis published by Minneci et al. [38] demonstrated a linear inverse relationship between disease severity and glucocorticoid effects on mortality. This finding suggests that steroids are harmful in less severe patients and that at low doses, increase the survival in septic shock with a high risk of death [38]. The heterogeneity of the included trials is frequently criticized and limits the reliability of this effect. Another meta-analysis published in the same year, considered to be of better quality, included eight studies (six randomized trials) with 1876 patients with septic shock and found that corticosteroid treatment did not result in a difference in mortality at 28 days, only in shock duration, independent of adrenal function. The meta-analysis concludes that the glucocorticoid does not increase the incidence of nosocomial infections, contrary to what was previously suggested by CORTICUS [39].
In summary, the use of intravenous hydrocortisone is recommended in cases of septic shock with poor response after volume resuscitation and vasopressor therapy, with dosages not exceeding 400 mg/day; dexamethasone should be avoided because of the possible immediate and prolonged suppression of the HPA axis; fludrocortisone is dispensable when hydrocortisone is used for its intrinsic mineralocorticoid effect and progressive weaning is suggested by the risk of increased proinflammatory mediators and haemodynamic deterioration with abrupt withdrawal [37].
Evidence for using adjunctive glucocorticoids in sepsis and septic shock
The last Surviving Sepsis Campaign, published in 2017, recommends considering adjunctive treatment with a stress dose of hydrocortisone (<400 mg/day for ≥3 days at full dose), regardless of serum total cortisol levels, in the situation of septic shock with poor response to fluid resuscitation and vasopressors. We must emphasize that this is a weak recommendation with low quality of evidence, as the precise indication and optimal regimen have not yet been established [37].
The year 2018 was emblematic for this issue because two randomized clinical trials (ADRENAL [40] and APROCCHSS [35]) enrolled more patients than all observational and interventional studies designed since the 1950s on adjunctive glucocorticoids in sepsis. A misinterpretation of these trials could restrict the analysis of their conflicting results regarding the primary outcome (90-day mortality), defining APROCCHSS as a positive study and ADRENAL as a negative one. Nonetheless, when all things are considered, the real picture is much more complex than this simple dichotomy.
Indeed, both studies included more than 5000 patients with septic shock and randomized for hydrocortisone (and fludrocortisone, in the case of APROCCHSS) vs. placebo. APROCCHSS demonstrated a lower mortality rate in the treatment arm (49.1 vs. 43%; P = 0.03), a finding not present in ADRENAL. However, both studies identified a significant reduction in secondary outcomes, such as the duration of mechanical ventilation, time to resolution of shock and time to discharge from the ICU.
It must be mentioned that the mortality observed in APROCCHSS was much higher than that in ADRENAL. Other differences could have influenced this survival benefit in APROCCHSS: sample with mean higher doses of vasopressors, older population, higher frequency of medical sepsis than surgical sepsis and a shorter time from shock onset until randomization [35,40].
Notably, it seems unlikely that in the short and medium term, another similar study would be conducted to address the use of glucocorticoids in critical patients, comparable with these very large RCTs. Therefore, the task is how to jointly interpret the lessons coming from these two trials. Moreover, even the three meta-analyses subsequently published, which already included APROCCHSS and ADRENAL, still exhibited conflicting results [41–43]. The prevailing reading of them resides on the potential benefits, including survival, of low-dose hydrocortisone restricted to the sickest population with septic shock, mainly with high doses of vasopressors (refractory shock) on the early onset of presentation (less than 12 h) [44].
Corroborating the view that only more severe patients might benefit, the capacity of preventing the development of septic shock was touched upon by the HYPRESS study, published in 2016 [45]. The researchers randomized 380 patients with sepsis to receive low-dose continuous infusion of hydrocortisone vs. placebo and found that this approach was not effective.
Another controversial point concerns the prescription of fludrocortisone. It is not reasonable to attribute the survival benefit demonstrated in APROCCHSS to the addition of fludrocortisone, because hydrocortisone has substantial mineralocorticoid activity. Furthermore, its half-life is quite short, and its pharmacokinetics and pharmacodynamics have not been well studied in scenarios involving critically ill patients [46]. A previously published trial (COIITSS trial), which enrolled 509 patients with septic shock and multiple organ dysfunction who received adjunctive hydrocortisone, corroborated this overcited analysis, as the addition of fludrocortisone to hydrocortisone and insulin did not achieve a mortality benefit compared with hydrocortisone alone [47].
The rationale behind the prescription of adjunctive glucocorticoids in sepsis resides in the fact that the intense reaction induced by a pathogen could lead to derangements of the HPA axis associated with tissue resistance to cortisol activity (the classic description of CIRCI reported above), and this ominous and prolonged cycle of increased proinflammatory mediator secretion (leading to organ dysfunction) could potentially be interrupted by exogenous glucocorticoid administration.
Nevertheless, this view disregards the potential performance of the stress dose of hydrocortisone attributed to its mineralocorticoid activity rather than (or not only to) its anti-inflammatory properties. To our knowledge, no trials have ever been published comparing hydrocortisone vs. fludrocortisone alone in septic shock scenarios. Concerning this doubt, they would be very welcome.
Conclusion and future perspectives
We still do not have the whole picture of the HPA axis and glucocorticoid intracellular signalling during an acute disease. What we do know is that there is a very complex network of corticosteroid-secreting enzymes, receptors, signalling molecules and effectors held together with the main objective of maintaining body homeostasis.
How inflammation triggered by bacterial infections may lead to dysfunction of this network should be a primary goal in future years for all researchers interested in caring for critical patients.
The most important trials recently published in this area show conflicting results, while the prevailing view points to potential benefits restricted to the sickest subgroup of patients with septic shock. Unfortunately, these studies were not designed to correlate glucocorticoid prescriptions with CIRCI pathophysiological aspects (derangements of the HPA axis, inadequate cellular glucocorticoid activity, or alterations in cortisol metabolism or breakdown); hence, some data that could enlighten our understanding of glucocorticoid insufficiency in critical illness are not available.
Otherwise, in addition to the current evidence indicating the potential benefits of stress doses of hydrocortisone, such as refractory septic shock, for a specific subgroup of patients in the emergency department, there is a patent misinterpretation of the whole picture. Given this gap between the understanding of the pathophysiology of CIRCI and its treatment, from our perspective, it is worrisome to generalize the prescription of corticosteroids as a rule disregarding the possible disruption of this fragile intracellular signalling structure, which may be more harmful in the short and long term.
Acknowledgements
Conflicts of interest
There are no conflicts of interest.
References
- 1.Lamberts SW, Bruining HA, de Jong FH. Corticosteroid therapy in severe illness. N Engl J Med. 1997; 337:1285–1292 [DOI] [PubMed] [Google Scholar]
- 2.Annane D, Sébille V, Troché G, Raphaël JC, Gajdos P, Bellissant E. A 3-level prognostic classification in septic shock based on cortisol levels and cortisol response to corticotropin. JAMA. 2000; 283:1038–1045 [DOI] [PubMed] [Google Scholar]
- 3.Habib KE, Gold PW, Chrousos GP. Neuroendocrinology of stress. Endocrinol Metab Clin North Am. 2001; 30:695–728; vii [DOI] [PubMed] [Google Scholar]
- 4.Miller DB, O’Callaghan JP. Neuroendocrine aspects of the response to stress. Metabolism. 2002; 51:5–10 [DOI] [PubMed] [Google Scholar]
- 5.Scheinman RI, Cogswell PC, Lofquist AK, Baldwin AS., Jr Role of transcriptional activation of I kappa B alpha in mediation of immunosuppression by glucocorticoids. Science. 1995; 270:283–286 [DOI] [PubMed] [Google Scholar]
- 6.van den Akker EL, Koper JW, Joosten K, de Jong FH, Hazelzet JA, Lamberts SW, Hokken-Koelega AC. Glucocorticoid receptor mRNA levels are selectively decreased in neutrophils of children with sepsis. Intensive Care Med. 2009; 35:1247–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jäättelä M, Ilvesmäki V, Voutilainen R, Stenman UH, Saksela E. Tumor necrosis factor as a potent inhibitor of adrenocorticotropin-induced cortisol production and steroidogenic P450 enzyme gene expression in cultured human fetal adrenal cells. Endocrinology. 1991; 128:623–629 [DOI] [PubMed] [Google Scholar]
- 8.Schroeder S, Wichers M, Klingmüller D, Höfer M, Lehmann LE, von Spiegel T, et al. The hypothalamic-pituitary-adrenal axis of patients with severe sepsis: altered response to corticotropin-releasing hormone. Crit Care Med. 2001; 29:310–316 [DOI] [PubMed] [Google Scholar]
- 9.Naito Y, Fukata J, Tamai S, Seo N, Nakai Y, Mori K, Imura H. Biphasic changes in hypothalamo-pituitary-adrenal function during the early recovery period after major abdominal surgery. J Clin Endocrinol Metab. 1991; 73:111–117 [DOI] [PubMed] [Google Scholar]
- 10.Goodman S, Sprung CL, Ziegler D, Weiss YG. Cortisol changes among patients with septic shock and the relationship to ICU and hospital stay. Intensive Care Med. 2005; 31:1362–1369 [DOI] [PubMed] [Google Scholar]
- 11.Boonen E, Vervenne H, Meersseman P, Andrew R, Mortier L, Declercq PE, et al. Reduced cortisol metabolism during critical illness. N Engl J Med. 2013; 368:1477–1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooper MS, Stewart PM. Corticosteroid insufficiency in acutely ill patients. N Engl J Med. 2003; 348:727–734 [DOI] [PubMed] [Google Scholar]
- 13.Franchimont D, Martens H, Hagelstein MT, Louis E, Dewe W, Chrousos GP, et al. Tumor necrosis factor alpha decreases, and interleukin-10 increases, the sensitivity of human monocytes to dexamethasone: potential regulation of the glucocorticoid receptor. J Clin Endocrinol Metab. 1999; 84:2834–2839 [DOI] [PubMed] [Google Scholar]
- 14.Marik PE, Pastores SM, Annane D, Meduri GU, Sprung CL, Arlt W, et al. ; American College of Critical Care Medicine. Recommendations for the diagnosis and management of corticosteroid insufficiency in critically ill adult patients: consensus statements from an international task force by the American College of Critical Care Medicine. Crit Care Med. 2008; 36:1937–1949 [DOI] [PubMed] [Google Scholar]
- 15.Burry LD, Wax RS. Role of corticosteroids in septic shock. Ann Pharmacother. 2004; 38:464–472 [DOI] [PubMed] [Google Scholar]
- 16.Arlt W, Allolio B. Adrenal insufficiency. Lancet. 2003; 361:1881–1893 [DOI] [PubMed] [Google Scholar]
- 17.Kuperman H, Damiani D, Chrousos GP, Dichtchekenian V, Manna TD, Filho VO, Setian N. Evaluation of the hypothalamic-pituitary-adrenal axis in children with leukemia before and after 6 weeks of high-dose glucocorticoid therapy. J Clin Endocrinol Metab. 2001; 86:2993–2996 [DOI] [PubMed] [Google Scholar]
- 18.Malerba G, Romano-Girard F, Cravoisy A, Dousset B, Nace L, Lévy B, Bollaert PE. Risk factors of relative adrenocortical deficiency in intensive care patients needing mechanical ventilation. Intensive Care Med. 2005; 31:388–392 [DOI] [PubMed] [Google Scholar]
- 19.Redington AE, Meng QH, Springall DR, Evans TJ, Créminon C, Maclouf J, et al. Increased expression of inducible nitric oxide synthase and cyclo-oxygenase-2 in the airway epithelium of asthmatic subjects and regulation by corticosteroid treatment. Thorax. 2001; 56:351–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Annane D, Sébille V, Charpentier C, Bollaert PE, François B, Korach JM, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002; 288:862–871 [DOI] [PubMed] [Google Scholar]
- 21.Bernard F, Outtrim J, Menon DK, Matta BF. Incidence of adrenal insufficiency after severe traumatic brain injury varies according to definition used: clinical implications. Br J Anaesth. 2006; 96:72–76 [DOI] [PubMed] [Google Scholar]
- 22.Sam S, Corbridge TC, Mokhlesi B, Comellas AP, Molitch ME. Cortisol levels and mortality in severe sepsis. Clin Endocrinol (Oxf). 2004; 60:29–35 [DOI] [PubMed] [Google Scholar]
- 23.Ho JT, Al-Musalhi H, Chapman MJ, Quach T, Thomas PD, Bagley CJ, et al. Septic shock and sepsis: a comparison of total and free plasma cortisol levels. J Clin Endocrinol Metab. 2006; 91:105–114 [DOI] [PubMed] [Google Scholar]
- 24.Clark PM, Neylon I, Raggatt PR, Sheppard MC, Stewart PM. Defining the normal cortisol response to the short Synacthen test: implications for the investigation of hypothalamic-pituitary disorders. Clin Endocrinol (Oxf). 1998; 49:287–292 [DOI] [PubMed] [Google Scholar]
- 25.Marik P, Zaloga G. Prognostic value of cortisol response in septic shock. JAMA. 2000; 284:308–309; author reply 309 [DOI] [PubMed] [Google Scholar]
- 26.Marik PE, Zaloga GP. Adrenal insufficiency during septic shock. Crit Care Med. 2003; 31:141–145 [DOI] [PubMed] [Google Scholar]
- 27.Siraux V, De Backer D, Yalavatti G, Mélot C, Gervy C, Mockel J, Vincent JL. Relative adrenal insufficiency in patients with septic shock: comparison of low-dose and conventional corticotropin tests. Crit Care Med. 2005; 33:2479–2486 [DOI] [PubMed] [Google Scholar]
- 28.Venkatesh B, Mortimer RH, Couchman B, Hall J. Evaluation of random plasma cortisol and the low dose corticotropin test as indicators of adrenal secretory capacity in critically ill patients: a prospective study. Anaesth Intensive Care. 2005; 33:201–209 [DOI] [PubMed] [Google Scholar]
- 29.Loisa P, Uusaro A, Ruokonen E. A single adrenocorticotropic hormone stimulation test does not reveal adrenal insufficiency in septic shock. Anesth Analg. 2005; 101:1792–1798 [DOI] [PubMed] [Google Scholar]
- 30.Jabre P, Combes X, Lapostolle F, Dhaouadi M, Ricard-Hibon A, Vivien B, et al. ; KETASED Collaborative Study Group. Etomidate versus ketamine for rapid sequence intubation in acutely ill patients: a multicentre randomised controlled trial. Lancet. 2009; 374:293–300 [DOI] [PubMed] [Google Scholar]
- 31.Briegel J, Sprung CL, Annane D, Singer M, Keh D, Moreno R, et al. ; for the CORTICUS Study Group. Multicenter comparison of cortisol as measured by different methods in samples of patients with septic shock. Intensive Care Med. 2009; 35:2151–2156 [DOI] [PubMed] [Google Scholar]
- 32.Vassiliadi DA, Ilias I, Tzanela M, Nikitas N, Theodorakopoulou M, Kopterides P, et al. Interstitial cortisol obtained by microdialysis in mechanically ventilated septic patients: correlations with total and free serum cortisol. J Crit Care. 2013; 28:158–165 [DOI] [PubMed] [Google Scholar]
- 33.Meduri GU, Muthiah MP, Carratu P, Eltorky M, Chrousos GP. Nuclear factor-kappab- and glucocorticoid receptor alpha- mediated mechanisms in the regulation of systemic and pulmonary inflammation during sepsis and acute respiratory distress syndrome. Evidence for inflammation-induced target tissue resistance to glucocorticoids. Neuroimmunomodulation. 2005; 12:321–338 [DOI] [PubMed] [Google Scholar]
- 34.Jones D, Hayes M, Webb S, French C, Bellomo R. Relative adrenal insufficiency in etomidate-naïve patients with septic shock. Anaesth Intensive Care. 2006; 34:599–605 [DOI] [PubMed] [Google Scholar]
- 35.Annane D, Renault A, Brun-Buisson C, Megarbane B, Quenot JP, Siami S, et al. ; CRICS-TRIGGERSEP Network. Hydrocortisone plus fludrocortisone for adults with septic shock. N Engl J Med. 2018; 378:809–818 [DOI] [PubMed] [Google Scholar]
- 36.Steinberg KP, Hudson LD, Goodman RB, Hough CL, Lanken PN, Hyzy Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med. 2006; 354:1671–1684 [DOI] [PubMed] [Google Scholar]
- 37.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017; 43:304–377 [DOI] [PubMed] [Google Scholar]
- 38.Minneci PC, Deans KJ, Eichacker PQ, Natanson C. The effects of steroids during sepsis depend on dose and severity of illness: an updated meta-analysis. Clin Microbiol Infect. 2009; 15:308–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sligl WI, Milner DA, Jr, Sundar S, Mphatswe W, Majumdar SR. Safety and efficacy of corticosteroids for the treatment of septic shock: a systematic review and meta-analysis. Clin Infect Dis. 2009; 49:93–101 [DOI] [PubMed] [Google Scholar]
- 40.Venkatesh B, Finfer S, Cohen J, Rajbhandari D, Arabi Y, Bellomo R, et al. ; ADRENAL Trial Investigators and the Australian–New Zealand Intensive Care Society Clinical Trials Group. Adjunctive glucocorticoid therapy in patients with septic shock. N Engl J Med. 2018; 378:797–808 [DOI] [PubMed] [Google Scholar]
- 41.Fang F, Zhang Y, Tang J, Lunsford LD, Li T, Tang R, et al. Association of corticosteroid treatment with outcomes in adult patients with sepsis: a systematic review and meta-analysis. JAMA Intern Med. 2019; 179:213–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rygård SL, Butler E, Granholm A, Møller MH, Cohen J, Finfer S, et al. Low-dose corticosteroids for adult patients with septic shock: a systematic review with meta-analysis and trial sequential analysis. Intensive Care Med. 2018; 44:1003–1016 [DOI] [PubMed] [Google Scholar]
- 43.Rochwerg B, Oczkowski SJ, Siemieniuk RAC, Agoritsas T, Belley-Cote E, D’Aragon F, et al. Corticosteroids in sepsis: an updated systematic review and meta-analysis. Crit Care Med. 2018; 46:1411–1420 [DOI] [PubMed] [Google Scholar]
- 44.Marik PE. The role of glucocorticoids as adjunctive treatment for sepsis in the modern era. Lancet Respir Med. 2018; 6:793–800 [DOI] [PubMed] [Google Scholar]
- 45.Keh D, Trips E, Marx G, Wirtz SP, Abduljawwad E, Bercker S, et al. ; SepNet–Critical Care Trials Group. Effect of hydrocortisone on development of shock among patients with severe sepsis: the HYPRESS randomized clinical trial. JAMA. 2016; 316:1775–1785 [DOI] [PubMed] [Google Scholar]
- 46.Polito A, Hamitouche N, Ribot M, Polito A, Laviolle B, Bellissant E, et al. Pharmacokinetics of oral fludrocortisone in septic shock. Br J Clin Pharmacol. 2016; 82:1509–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Annane D, Cariou A, Maxime V, Azoulay E, D'honneur G, Timsit JF, et al. ; COIITSS Study Investigators. Corticosteroid treatment and intensive insulin therapy for septic shock in adults: a randomized controlled trial. JAMA. 2010; 303:341. [DOI] [PubMed] [Google Scholar]


