Abstract
Of 725 consecutive hospitalized patients with coronavirus disease 2019, 108 (15%) had acute neurologic symptoms necessitating neurologic imaging.
Introduction
An outbreak of coronavirus disease 2019 (COVID-19) began in Wuhan, China, in December 2019 and has rapidly spread around the world to become a pandemic (1). Italy was the second epicenter of the spread of the disease, and at the time of writing has a total of 222 104 cases and 31 106 deaths (1). Several studies have described the spectrum of chest imaging features of COVID-19 (2). However, to date, only a few case reports have described COVID-19–associated neurologic imaging findings (3–8). The purpose of our study was to systematically characterize neurologic symptoms and neuroimaging features in hospitalized patients with COVID-19 from multiple institutions in Italy.
Materials and Methods
Study Design and Patient Population
We used a retrospective, multicenter study design from three major institutions in Italy (University of Brescia, Brescia; University of Eastern Piemonte, Novara; and University of Sassari, Sassari). Institutional review board approval and waivers for informed consent were obtained at all institutions. Our inclusion criteria included (a) hospitalized patients who were positive for COVID-19 by means of real-time reverse-transcriptase polymerase chain reaction testing (Sentinel Diagnostics, Milan, Italy) of respiratory secretions obtained by means of bronchoalveolar lavage, endotracheal aspirate, nasopharyngeal swab, or oropharyngeal swab from February 29 to April 4, 2020; (b) presence of acute neurologic symptoms during hospital stay; and (c) any neurologic imaging studies, including brain or spine imaging. We reviewed the electronic medical records to extract clinical, laboratory, and demographic data.
Image Acquisition
All images were obtained as per standard of care protocols. MRI scans of brain and spine were obtained with 1.5-T scanners with standardized protocols. Gadopentetate dimeglumine (0.1 mmol/kg gadobutrol [Gadovist; Bayer, Berlin, Germany]) was used for contrast material–enhanced studies.
Image Interpretation
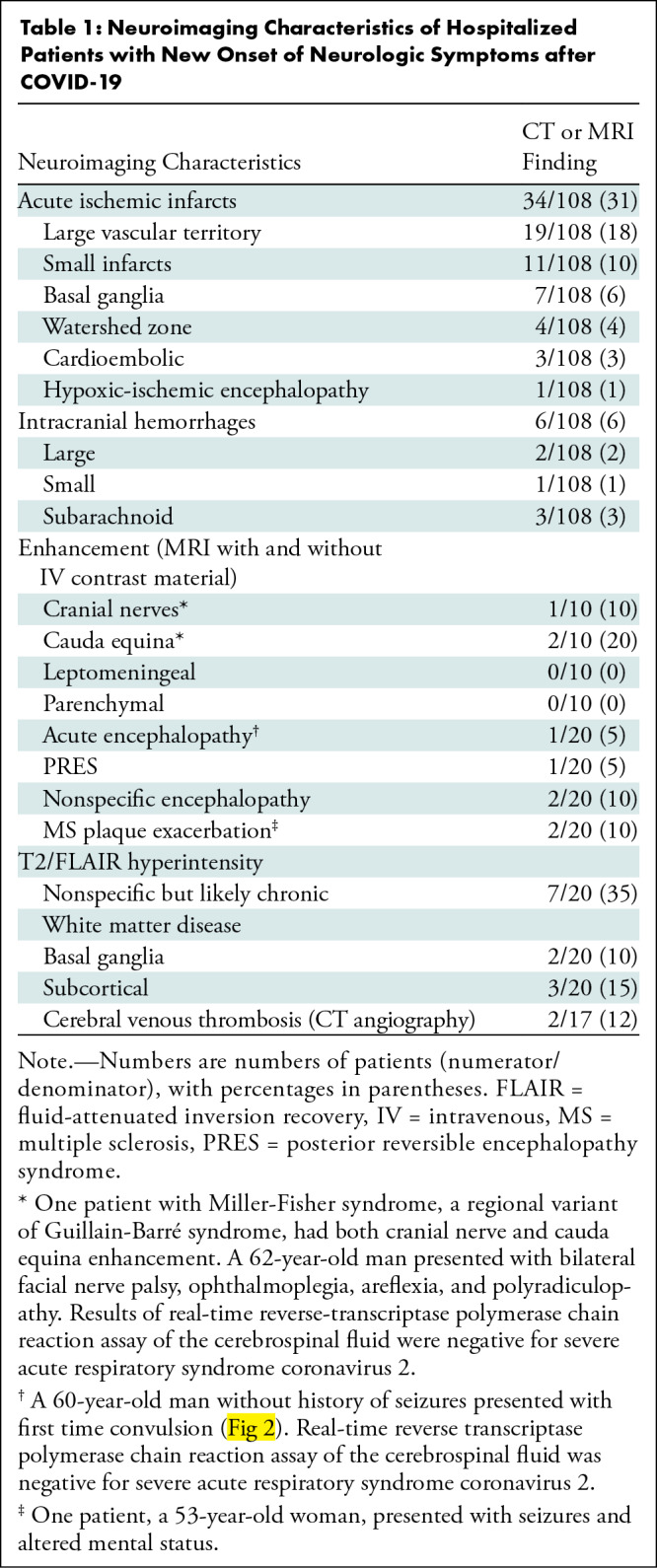
The neurologic imaging characteristics that were evaluated are listed in Table 1. All scans were initially analyzed by the institution’s own neuroradiologists. Subsequently, all images were reviewed by three neuroradiologists in consensus (R.G., L.S., and A.C., with 30, 14, and 32 years of neuroradiology experience, respectively).
Table 1:
Neuroimaging Characteristics of Hospitalized Patients with New Onset of Neurologic Symptoms after COVID-19
Statistical Analysis
Continuous variables are presented as means ± standard deviations and were compared between patients with altered mental status by using the Student t test; categoric variables are presented as frequencies with percentages. All statistical analyses were performed by using software (Stata, version 15; StataCorp, College Station, Tex). P < .05 was indicative of a statistically significant difference.
Results
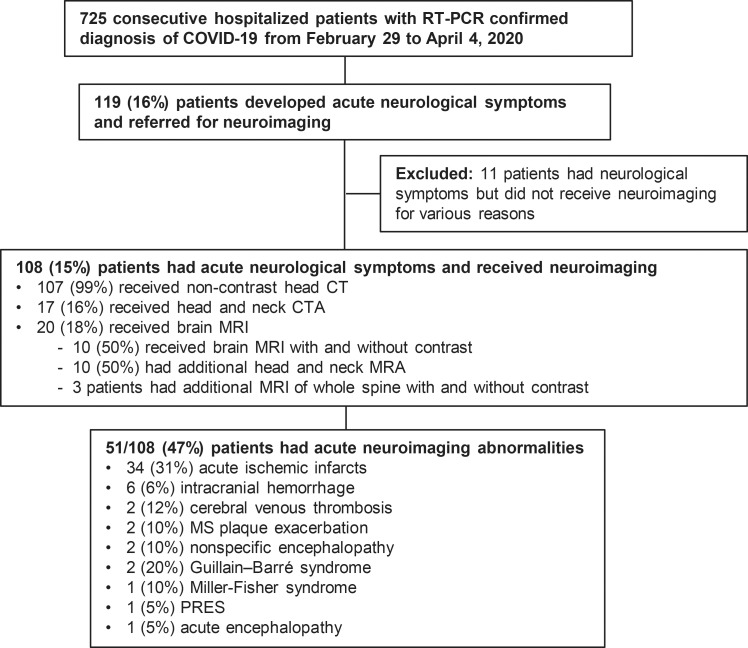
A total of 725 consecutive hospitalized patients with COVID-19 were reviewed. Of these 725 patients, 108 (15%) met the eligibility criteria (Fig 1). Of the 108 patients, 107 (99%) were examined with unenhanced brain CT, 17 (16%) with head and neck CT angiography, and 20 (18%) with brain MRI. Of the 20 patients who underwent brain MRI, 10 (50%) underwent MRI with and without intravenous contrast material, 10 (50%) underwent head and neck MR angiography, and three underwent additional MRI of the whole spine for evaluation of lower extremity weakness. Table 2 summarizes the demographic characteristics, medical history, and neurologic characteristics. The most common neurologic symptoms were altered mental status in 64 of the 108 patients (59%) and ischemic stroke in 34 (31%). Of the 108 patients, 31 (29%) had no known past medical history and 77 (71%) had at least one of the following chronic disorders: coronary artery disease (n = 25, 23%), cerebrovascular disease (n = 15, 14%), hypertension (n = 55, 51%), and diabetes (n = 30, 28%). Of the 31 patients without known past medical history (age range, 16–62 years) (29%), 10 had acute ischemic infarcts and two had intracranial hemorrhage. Seventy-one of the 108 patients (66%) had no acute findings on brain CT scans; seven of the 20 patients who underwent MRI (35%) had acute abnormalities on brain MRI scans. There was a statistically significant association between the prevalence of altered mental status and patient age (mean age, 72 years ± 11 vs 64 years ± 18; P = .007).
Figure 1:
Study flowchart. COVID-19 = coronavirus disease 2019, CTA = CT angiography, MRA = MR angiography, MS = multiple sclerosis, PRES = posterior reversible encephalopathy syndrome, RT-PCR = real-time reverse-transcriptase polymerase chain reaction.
Table 2:
Characteristics of Hospitalized Patients with COVID-19

Figure 2:
Images of acute encephalopathy in a 60-year-old-man without history of seizures who presented with convulsion. A, B, Fluid-attenuated inversion-recovery images show multifocal areas of hyperintensity in the right cerebellum (arrow in A), left anterior cingular cortex, and superior frontal gyrus (arrows in B). C–E, Diffusion-weighted images show restricted diffusion in the left anterior cingulate cortex and superior frontal gyrus (arrows in C), superior frontal and middle temporal gyrus (arrows in D), and right cerebellum (arrows in E), consistent with cerebellar diaschisis. F, MRI scan obtained with gradient-echo sequence shows no hemosiderin deposits.
The main neurologic imaging hallmark was acute ischemic infarcts, which were present in 34 of the 108 patients (31%) (30 [28%] on CT scans and four [20%] on MRI scans). Of these infarcts, 19 (18%) were large (15 in the middle cerebral artery territory, two in the posterior cerebral artery territory, two in the anterior cerebral artery territory), 11 (10%) were small, three (3%) were cardioembolic, and one (1%) had an hypoxic-ischemic encephalopathy pattern. Six of the 108 patients (6%) had intracranial hemorrhages, with subarachnoid hemorrhage being the most common (n = 3, 3%). Additional neurologic imaging findings are shown in Table 1.
Discussion
Our study demonstrated that the neurologic imaging features of hospitalized patients with COVID-19 were variable, without a specific pattern but dominated by acute ischemic infarcts and intracranial hemorrhages. We also showed that the neurologic MRI spectrum may include posterior reversible encephalopathy syndrome, hypoxic-ischemic encephalopathy, exacerbation of demyelinating disease, and nonspecific cortical pattern of T2 fluid-attenuated inversion-recovery hyperintense signal with associated restriction diffusion that may be caused by systemic toxemia, viremia, and/or hypoxic effects (9). Currently, we have a poor mechanistic understanding of the neurologic symptoms in patients with COVID-19, whether these are arising from critical illness or from direct central nervous system invasion of severe acute respiratory syndrome coronavirus 2 (10). Accumulating evidence suggests that a subgroup of patients with severe COVID-19 might have a cytokine storm syndrome that could be a trigger for ischemic strokes, probably related to the prothrombotic effect of the inflammatory response (3,11).
Our results showed a lower prevalence of central nervous system symptoms than the Wuhan experience (8) (15% vs 25%, respectively); however, the prevalence of ischemic strokes was higher in our study (31% vs 11%). Furthermore, our findings also support the suggested potential for COVID-19–associated Guillain-Barré syndrome and variants (12). None of our patients showed abnormal parenchymal or leptomeningeal enhancement. In conclusion, neurologists and neuroradiologists should be familiar with the broad spectrum of neurologic imaging patterns associated with COVID-19.
Acknowledgments
Acknowledgments
We thank all patients and their families involved in the study.
Footnotes
Disclosures of Conflicts of Interest: A.M. disclosed no relevant relationships. L.S. disclosed no relevant relationships. A.V. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: institution has grants/grants pending rom Cerenovus. Other relationships: disclosed no relevant relationships. M.L. disclosed no relevant relationships. A.R. disclosed no relevant relationships. M.G. disclosed no relevant relationships. S.S. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is a paid consultant for Novocure; has a grant pending from Sontag. Other relationships: has patents planned, pending, or issued. B.Z. disclosed no relevant relationships. A.C. disclosed no relevant relationships. S.B. disclosed no relevant relationships. P.C. disclosed no relevant relationships. A. Paschè disclosed no relevant relationships. E.P. disclosed no relevant relationships. A. Padovani disclosed no relevant relationships. R.G. disclosed no relevant relationships.
A.M. and R.G. contributed equally to this work.
References
- 1.Coronavirus disease 2019 (COVID-19) Situation Report – 115. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200514-covid-19-sitrep-115.pdf?sfvrsn=3fce8d3c_6. Published May 14, 2020. [Google Scholar]
- 2.Chung M, Bernheim A, Mei X, et al. CT Imaging Features of 2019 Novel Coronavirus (2019-nCoV). Radiology 2020;295(1):202–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Y, Wang M, Zhou Y, et al. Acute Cerebrovascular Disease Following COVID-19: A Single Center, Retrospective, Observational Study. SSRN Electronic Journal 2020 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7371480/. Published online July 2, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B. COVID-19-associated Acute Hemorrhagic Necrotizing Encephalopathy: CT and MRI Features. Radiology doi:10.1148/radiol.2020201187. Published online March 31, 2020. [DOI] [PMC free article] [PubMed]
- 5.Filatov A, Sharma P, Hindi F, Espinosa PS. Neurological Complications of Coronavirus Disease (COVID-19): Encephalopathy. Cureus 2020;12(3):e7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Helms J, Kremer S, Merdji H, et al. Neurologic Features in Severe SARS-CoV-2 Infection. N Engl J Med 2020;382(23):2268–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moriguchi T, Harii N, Goto J, et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis 2020;94:55–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mao L, Jin H, Wang M, et al. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol doi:10.1001/jamaneurol.2020.1127. Published online April 10, 2020. [DOI] [PMC free article] [PubMed]
- 9.Wu Y, Xu X, Chen Z, et al. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun doi:10.1016/j.bbi.2020.03.031. Published online March 30, 2020. [DOI] [PMC free article] [PubMed]
- 10.Zochodne DW. SARS, SIRS, and neurological disease. Arch Neurol 2004;61(11):1647–1648. [DOI] [PubMed] [Google Scholar]
- 11.Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020;395(10229):1033–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao H, Shen D, Zhou H, Liu J, Chen S. Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol 2020;19(5):383–384. [DOI] [PMC free article] [PubMed] [Google Scholar]