Abstract
Chest CT has a potential role in the diagnosis, detection of complications, and prognostication of coronavirus disease 2019 (COVID-19). Implementation of appropriate precautionary safety measures, chest CT protocol optimization, and a standardized reporting system based on the pulmonary findings in this disease will enhance the clinical utility of chest CT. However, chest CT examinations may lead to both false-negative and false-positive results. Furthermore, the added value of chest CT in diagnostic decision making is dependent on several dynamic variables, most notably available resources (real-time reverse transcription–polymerase chain reaction [RT-PCR] tests, personal protective equipment, CT scanners, hospital and radiology personnel availability, and isolation room capacity) and the prevalence of both COVID-19 and other diseases with overlapping manifestations at chest CT. Chest CT is valuable to detect both alternative diagnoses and complications of COVID-19 (acute respiratory distress syndrome, pulmonary embolism, and heart failure), while its role for prognostication requires further investigation. The authors describe imaging and managing care of patients with COVID-19, with topics including (a) chest CT protocol, (b) chest CT findings of COVID-19 and its complications, (c) the diagnostic accuracy of chest CT and its role in diagnostic decision making and prognostication, and (d) reporting and communicating chest CT findings. The authors also review other specific topics, including the pathophysiology and clinical manifestations of COVID-19, the World Health Organization case definition, the value of performing RT-PCR tests, and the radiology department and personnel impact related to performing chest CT in COVID-19.
©RSNA, 2020
An earlier incorrect version of this article appeared online and in print. This article was corrected on October 22, 2021.
SA-CME LEARNING OBJECTIVES
After completing this journal-based SA-CME activity, participants will be able to:
■ Discuss the role of chest CT in diagnostic decision making.
■ Describe the precautionary safety measures and chest CT protocol for imaging patients with COVID-19.
■ Recognize the chest CT appearance of COVID-19 and apply standardized reporting methods.
Introduction
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (1). The first human cases of COVID-19 were first reported by officials in Wuhan, China, in December 2019 (2). The disease rapidly spread throughout the world and was declared a pandemic by the World Health Organization (WHO) on March 12, 2020 (3). On June 13, 2020, there were nearly 8 million confirmed cases and more than 425 000 confirmed deaths due to COVID-19 worldwide (4). There are currently no specific treatments or vaccines for COVID-19 (1). However, there are many ongoing clinical trials evaluating potential treatments (1), and many efforts are underway to develop vaccines (5).
The chest imaging findings of COVID-19 were first published in January 2020 and included bilateral lung involvement and ground-glass opacities in the majority of hospitalized patients (6). Since then, a myriad of articles on chest CT findings in COVID-19 have been published at a rapid pace. The appropriate use of chest CT in patients with COVID-19 should be based on experience and, above all, the scientific evidence that has emerged since the outbreak of this disease, which keeps accumulating.
In this article, we provide an overview of chest CT in imaging and managing care of patients with COVID-19 and discuss topics including (a) chest CT protocol, (b) chest CT findings in COVID-19 and its complications, (c) the diagnostic accuracy of chest CT and its role in diagnostic decision making and prognostication, and (d) reporting and communicating chest CT findings. Additional specific topics outlined in this article include the pathophysiology and clinical manifestations of COVID-19, the case definitions of COVID-19 according to the WHO, the value of performing real-time reverse transcription–polymerase chain reaction (RT-PCR) tests, and the radiology department and personnel impact related to performing chest CT for COVID-19. Knowledge of these topics is important for all radiologists to optimize their care to patients with suspected or proven COVID-19 and to those without known COVID-19 amid the ongoing pandemic.
Pathophysiology of COVID-19
Coronaviruses have a single-stranded positive-sense RNA genome of ∼30 kb (7). Cell entry of coronaviruses is dependent on the binding of the viral spike (S) proteins to cellular receptors and on S protein priming by host cell proteases (7,8). SARS-CoV-2 uses the severe acute respiratory syndrome coronavirus (SARS-CoV) receptor angiotensin converting enzyme 2 (ACE2) for cell entry and the serine protease transmembrane protease serine 2 (TMPRSS2) for S protein priming (7,8). ACE2 is highly expressed on the epithelial cells of the oral mucosa and lungs but also in the heart, blood vessels, intestine, kidney, bladder, and brain (7,9,10).TMPRSS2 is highly expressed with a broader distribution, suggesting that ACE2 rather than TMPRSS2 may be a limiting factor for viral entry at the initial infection stage (11). The nasal epithelium is one of the first sites of infection with SARS-CoV-2 (11,12). Interestingly, it was reported that ACE2 gene expression is lower in the nasal epithelium of children than that of adults, which may help explain why COVID-19 is less prevalent in children (13).
Clinical Manifestations of COVID-19
A wide spectrum of clinical manifestations can be seen with COVID-19. Fever (80.4%), cough (63.1%), fatigue (46%), and expectoration (41.8%) are the most common manifestations of COVID-19 (14). Other common symptoms include anorexia (38.8%), chest tightness (35.7%), shortness of breath (35%), dyspnea (33.9%), and muscle soreness (33%) (14). Olfactory dysfunction (41.0%) and gustatory dysfunction (38.2%) also appear to be relatively frequent symptoms (15). Other less frequently reported symptoms include headache (15.4%), pharyngalgia (13.1%), diarrhea (12.9%), shivering (10.9%), nausea and vomiting (10.2%), and abdominal pain (4.4%) (14). The list of potential symptoms for COVID-19 is so long that anything can be considered a symptom. In addition, the list of potential symptoms may expand as we learn more about COVID-19.
To our knowledge, there is no uniform definition of what constitutes symptomatic COVID-19. Most symptomatic patients with COVID-19 experience mild to moderate respiratory illness and recover without requiring special treatment (1). Importantly, more than half of patients with a positive RT-PCR test result may be asymptomatic at the time of testing (16,17). Reported fatality rates vary widely, ranging from 0.3% to 13.1% (18) and are probably dependent on several variables, including the demographics of the population, intensity of testing, available health care resources, and completeness and accuracy of mortality data. Older patients and patients with underlying disease (including diabetes mellitus, cardiovascular disease, respiratory disease, hyperlipidemia, obesity, and chronic kidney and liver disease) may be more susceptible to severe disease (7,19). Otherwise-healthy children tend to have milder symptoms (7,19). The long-term sequelae of COVID-19 are still largely unknown.
WHO Case Definitions of COVID-19 and RT-PCR Tests
The WHO has provided definitions for suspect, probable, and confirmed cases of COVID-19 (Table 1) (20). These definitions can be used as a guide for undertaking appropriate actions, including infection control measures. A confirmed case is defined as a patient with RT-PCR test–proven COVID-19, irrespective of clinical signs and symptoms (20). The RT-PCR test can be performed by using nasopharyngeal swabs to obtain nasopharyngeal specimens or by obtaining other upper respiratory tract specimens by using a throat or saliva swab (21). Unfortunately, the sensitivity of RT-PCR tests is imperfect, with a pooled estimate of 89% (95% CI: 81%, 94%) (22), and one or more negative results do not rule out COVID-19 (23).
Table 1:
WHO Definitions for Suspect, Probable, and Confirmed Cases of COVID-19
A number of factors can lead to a false-negative result, including (a) poor quality of the specimen; (b) collecting the specimen too early (eg, between exposure to SARS-CoV-2 and symptom onset, which may take up to 1 week) or late in the course of infection (eg, grossly estimated in week 4 after symptom onset and beyond [21]); (c) inappropriate handling and shipping of the specimen; and (d) technical reasons inherent in the test (23). If a negative result is obtained from a patient with a high index of suspicion for COVID-19, additional specimens should be collected and tested (23).
The specificity of most of the RT-PCR test results is theoretically 100% because the primer design is specific to the genome sequence of SARS-CoV-2 (21). However, occasional false-positive results may occur owing to technical errors and reagent contamination (21). Furthermore, it should be realized that a positive RT-PCR test result reflects only the detection of viral RNA and does not necessarily indicate the presence of viable virus (21,24).
Another disadvantage of the RT-PCR test is that it takes some time before results are available (25–28), with estimated testing times ranging from 50 minutes to 4 hours for semiautomated to fully automated, walk-away assays and 6–14 hours for manually performed assays (29). Finally, although rapid point-of-care immunodiagnostic tests are being developed and investigated, their use for patient care is currently not recommended (25–28).
Radiology Department and Personnel Preparedness
SARS-CoV-2 is optimized to disseminate rapidly and widely (30), primarily through the respiratory tract by droplets, respiratory secretions, and direct contact (31,32). It has been described that small particles containing the virus may diffuse in indoor environments covering distances up to 10 m from the emission source (33). Furthermore, SARS-CoV-2 may remain viable in aerosols for 3 hours and on plastic and stainless steel for up to 72 hours (34).
Chest CT should be performed with strict precautions to minimize hazardous exposure of patients and health care professionals to SARS-CoV-2 (35,36). When possible, chest CT is performed at sites with less traffic to avoid exposure of other patients and staff (35). Where more than one fixed CT scanner is available, dedicated use of only one CT scanner for patients with COVID-19 may be ideal. Another option is the use of a mobile CT scanner.
Patients who are referred for chest CT should be screened for COVID-19 symptoms, and symptomatic patients should be provided with a surgical mask and placed in an isolation room (35,36). The same applies to patients with proven COVID-19. A strong case can also be made for all patients to wear face masks, whether they are symptomatic or not (37). Distances between patients in waiting areas near the CT scanner should be maximized; maintaining an interpersonal distance of 2 m in combination with wearing a face mask has been reported to be effective protection (33).
Radiology personnel should use appropriate PPE, including face masks, eye protection, gown, and gloves. Following correct donning and doffing procedures of PPE is essential (35,36). Increasing the air-exchange per hour or using high-efficiency particulate air (HEPA) filtration in CT examination rooms are potential supplemental mitigation measures (35,36). Deep cleaning of the CT examination room is necessary before imaging the next patient (35,36). All material coming into or near contact with a patient with (suspected) COVID-19 should be disinfected. After chest CT is performed, the CT examination room downtime may be between 30 minutes to 1 hour to allow for room decontamination and passive air exchange, according to a policy implemented by the University of Washington (35,36). As a result, patient throughput will be limited.
Depending on local circumstances, such as the number of patients with proven or suspected COVID-19 who require chest CT, the number of patients who require CT for reasons other than COVID-19, and available CT scanners and radiology staff, this limited patient throughput may cause considerable planning and logistic challenges that need to be addressed. Of interest, a 2014 study at a hospital in Saudi Arabia that treated patients with Middle East respiratory syndrome coronavirus (MERS-CoV) infection (which has around 50% similarity to SARS-CoV-2, according to genome sequencing [38]) reported that among all health care personnel, radiology technicians were most frequently infected with MERS-CoV (39). Although these findings may not be entirely applicable to the current SARS-CoV-2 outbreak or in other hospital settings, they underline the need to take precautionary measures seriously.
Chest CT Protocol
Patients referred for CT should undergo non–contrast material–enhanced chest CT (40) unless CT pulmonary angiography is required to detect pulmonary embolism (PE). Patients of all ages can become infected with SARS-CoV-2 and may need to undergo chest imaging. In addition, although chest radiography is most frequently used for follow-up imaging, some patients with COVID-19 may need to undergo follow-up chest CT. Therefore, nonenhanced chest CT should preferably be performed by using a low-radiation-dose protocol to minimize radiation burden.
Low-radiation-dose CT images can be obtained by using lower kilovoltage settings, iterative or more recently developed deep learning–based reconstructions for noise reduction, and spectral shaping of the x-ray beam to reduce the low-energy component of the x-ray spectrum (41,42), dependent on the local availability of these technologies. For CT examinations at risk for motion artifact, lowering the rotation time of the tube detector system with high pitch and wide collimation values may be considered (41,42). Low-radiation-dose chest CT performed on the basis of these principles has been shown to be feasible for imaging patients with COVID-19, with noninferior diagnostic quality and a radiation dose reduction of around 90% compared with those of a standard CT acquisition (42). Therefore, performing low-radiation-dose CT instead of full-radiation-dose CT as standard for the evaluation of the lung parenchyma in COVID-19 can be defended on the basis of the ALARA (as low as reasonably achievable) principle.
CT images should be acquired during a single inspiratory breath hold. Expiratory phase CT increases radiation dose, and evaluation for air trapping has not been reported to increase the suspicion for COVID-19 at chest CT. Whether expiratory phase CT has any value in the follow-up of patients with COVID-19 and prognostication remains unclear. Acquired CT data should be reconstructed by using a sharp kernel.
Chest CT Appearance of COVID-19
Several studies have been published reporting chest CT findings in COVID-19 (43). However, many studies are limited by selection bias, potential blinding issues, and potential confounding of chest CT findings owing to the simultaneous presence of other lung diseases (43). Nearly all authors of studies who investigated the chest CT appearance of COVID-19 investigated CT performed in symptomatic patients (43). The pulmonary histologic findings of COVID-19, which are characterized by acute and organizing diffuse alveolar damage, resemble those observed in other coronavirus infections, including severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1) and MERS-CoV (44,45). Accordingly, the reported chest CT abnormalities in COVID-19 are similar to those seen in infections with SARS-CoV-1 and MERS-CoV (46). The prevalence of chest CT abnormalities in COVID-19 is dependent on the stage and severity of the disease. There is currently a lack of radiologic-pathologic correlation studies in the literature.
Normal Chest CT Findings
The incidence of normal chest CT findings in symptomatic patients with COVID-19 is estimated at about 10.6% (95% CI: 7.6%, 13.7%) (43). Although normal chest CT findings are more frequently visualized during the first 4–5 days after symptom onset (in 13.9%–33.3% of patients), a nonnegligible number of symptomatic cases with normal chest CT findings are observed during the later stage of the infection (in 1.2%–4.0% of patients) (47–49). The incidence of normal chest CT findings in asymptomatic patients with COVID-19 is considerably high (an estimated 46% of patients) (50). Low viral loads and confinement to the upper respiratory tract are plausible explanations for false-negative chest CT findings for COVID-19 on a patient level (21,24). In addition, there are likely host factors that lead to false-negative chest CT findings. Many patients simply do not elicit the pulmonary inflammatory response needed to produce the chest CT findings of lung injury.
Chest CT Abnormalities with High Incidence (>70%)
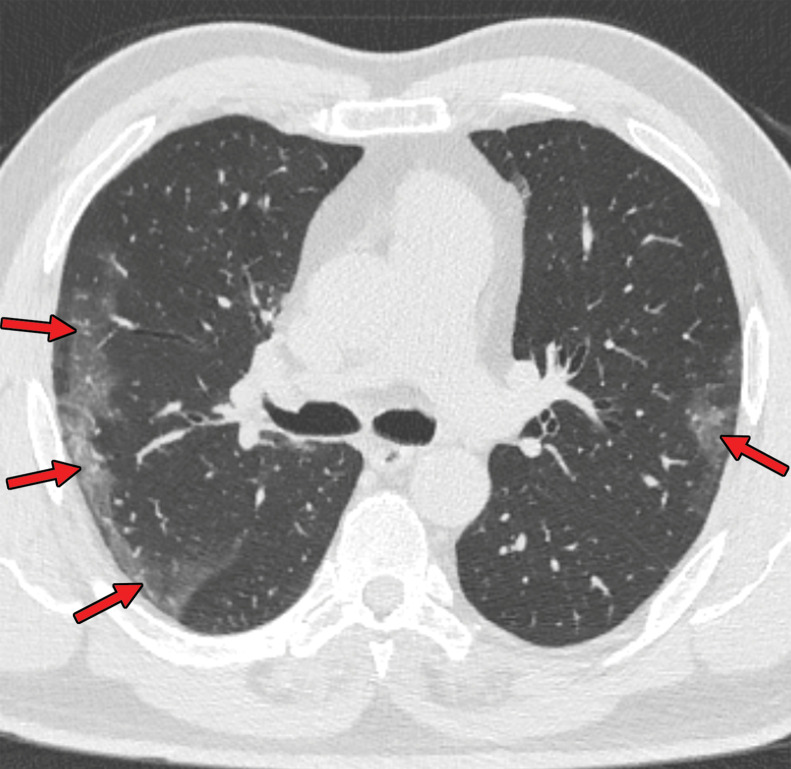
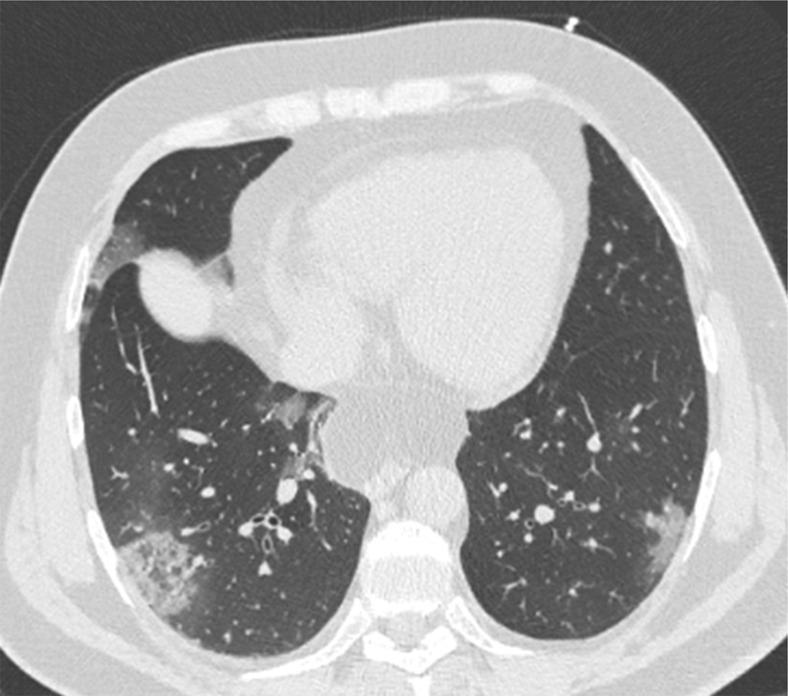
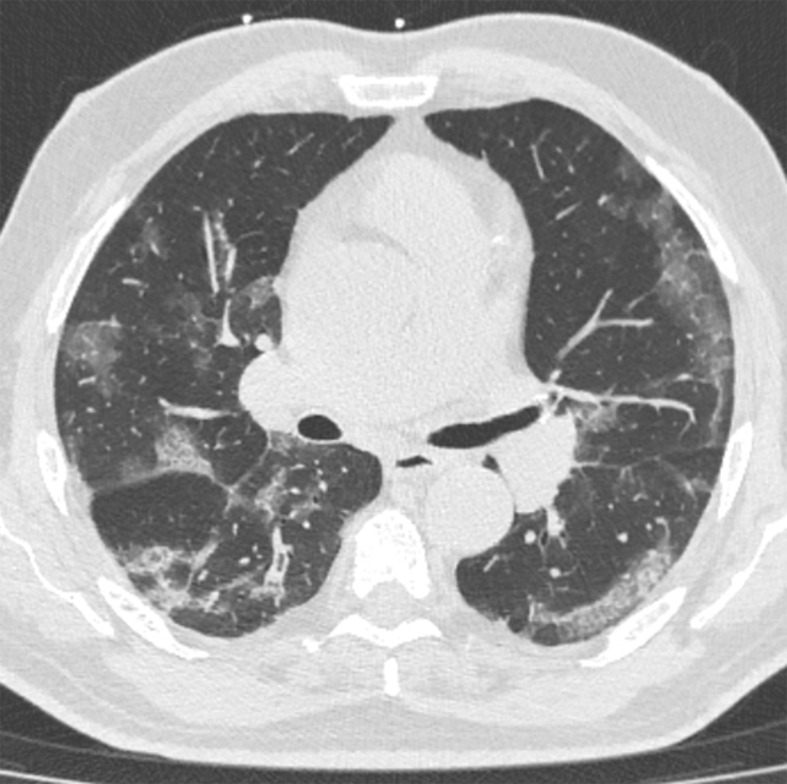
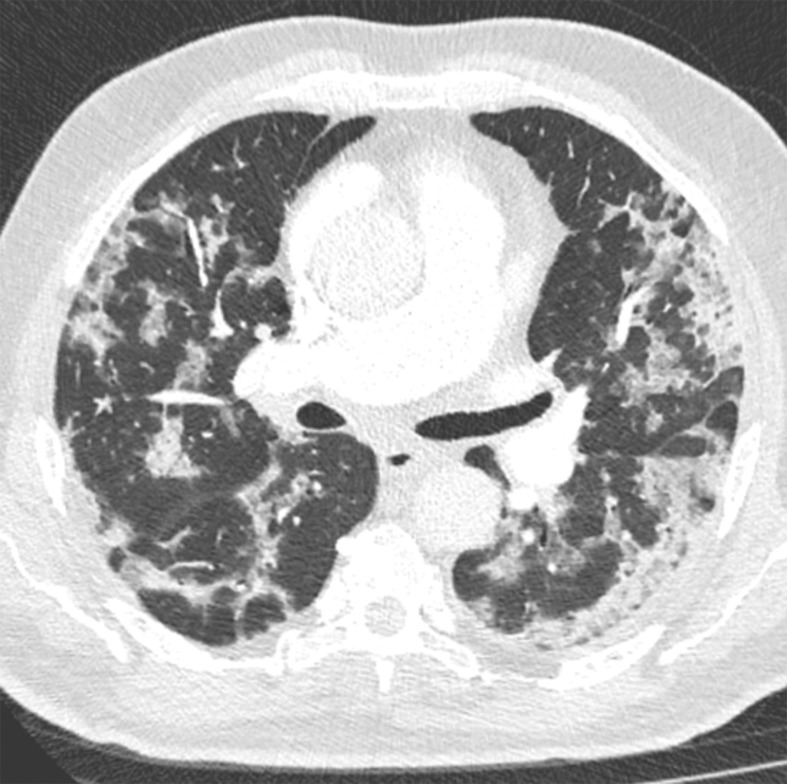
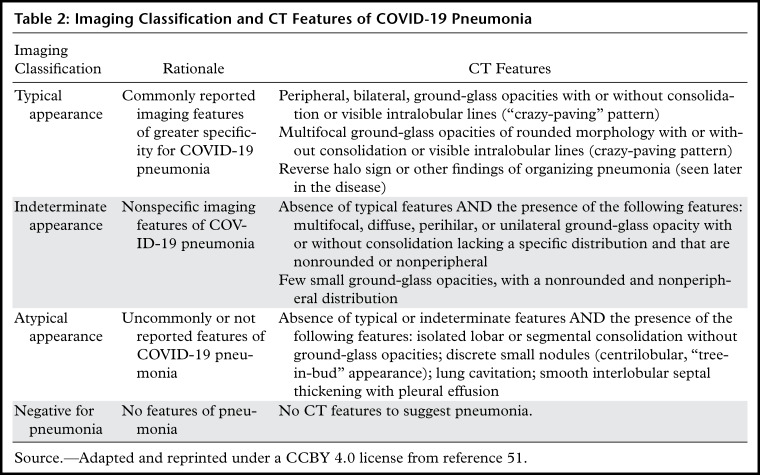
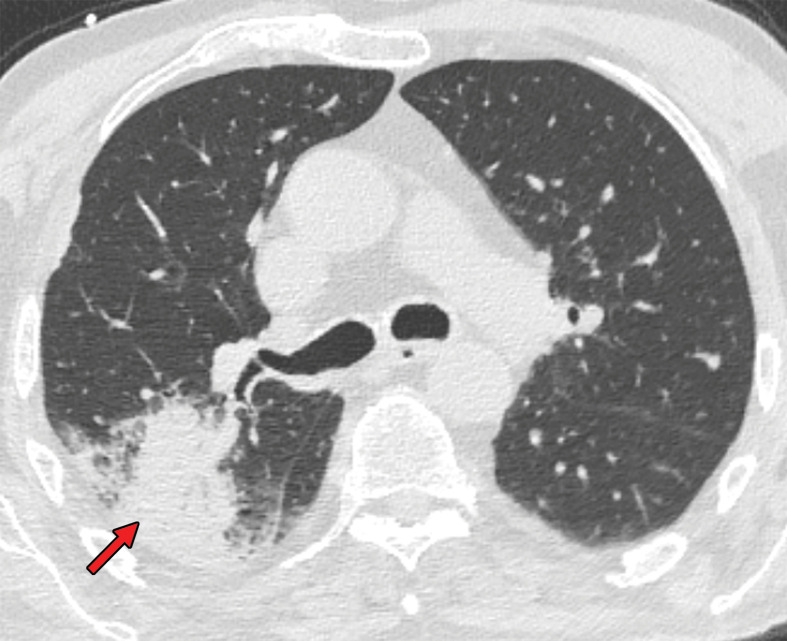
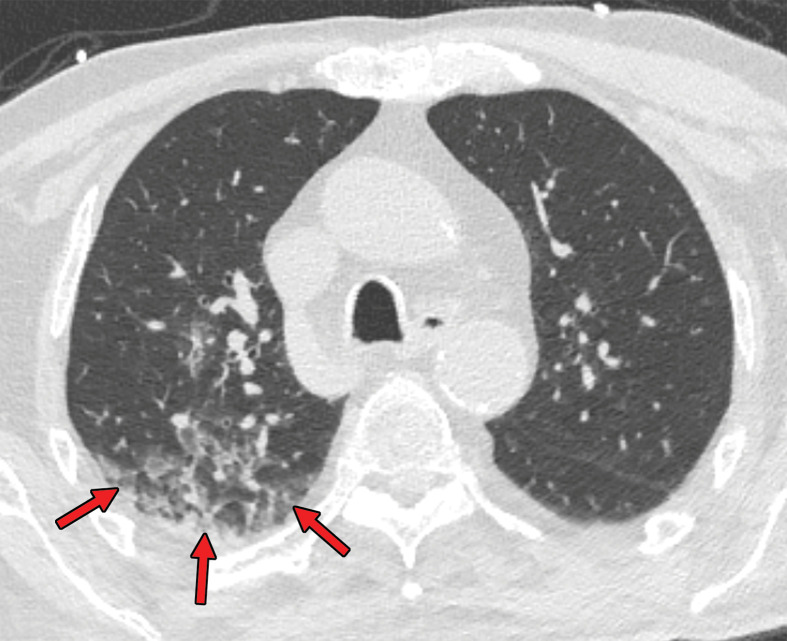
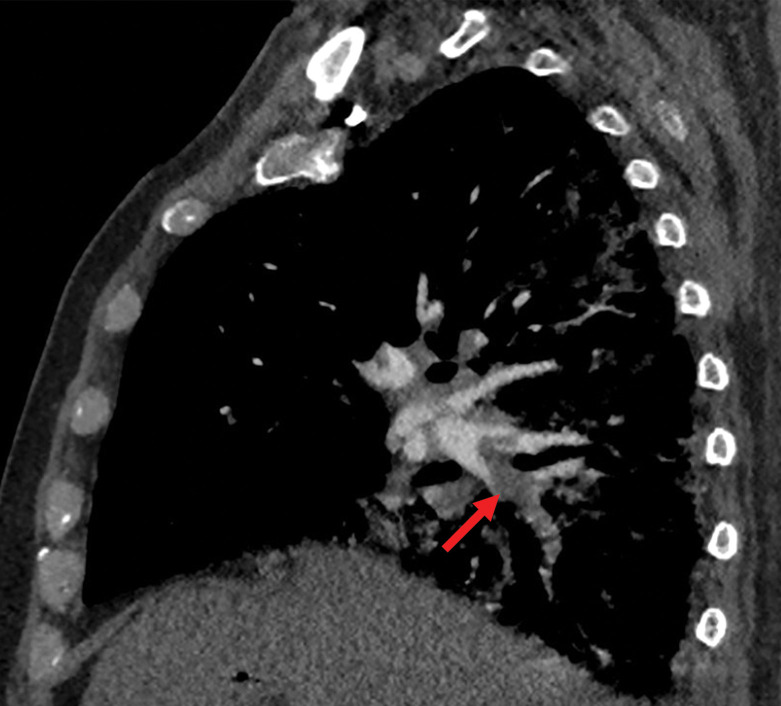
Several chest CT findings have been reported in more than 70% of RT-PCR test–proven COVID-19 cases, including ground-glass opacities (Figs 1, 2), vascular enlargement (Fig 2), bilateral abnormalities, lower lobe involvement, and posterior predilection (43). In COVID-19–endemic regions, the observation of these chest CT findings should raise the suspicion of possible COVID-19 diagnosis (43).
Figure 1a.
COVID-19 pneumonia with typical imaging features according to the Radiological Society of North America (RSNA) chest CT classification system (51). Axial nonenhanced chest CT images (lung window) in a 59-year-old man (a) and a 47-year-old man (b), each with positive RT-PCR test results for SARS-CoV-2, show bilateral areas of ground-glass opacities (arrows) in a peripheral distribution.
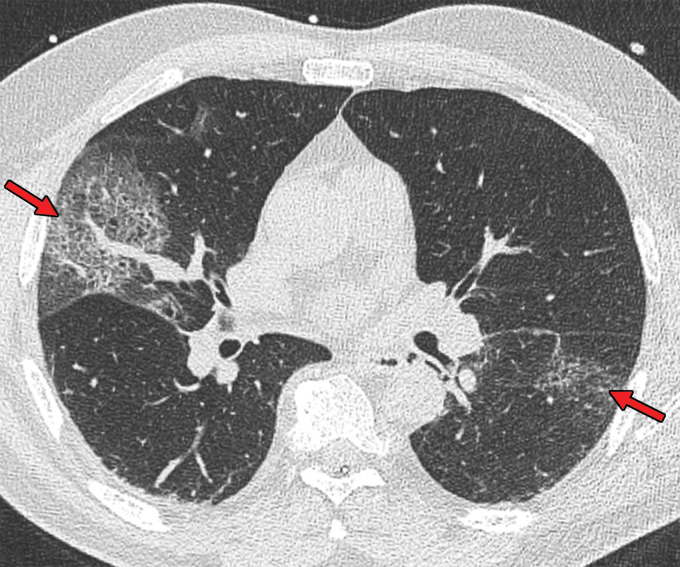
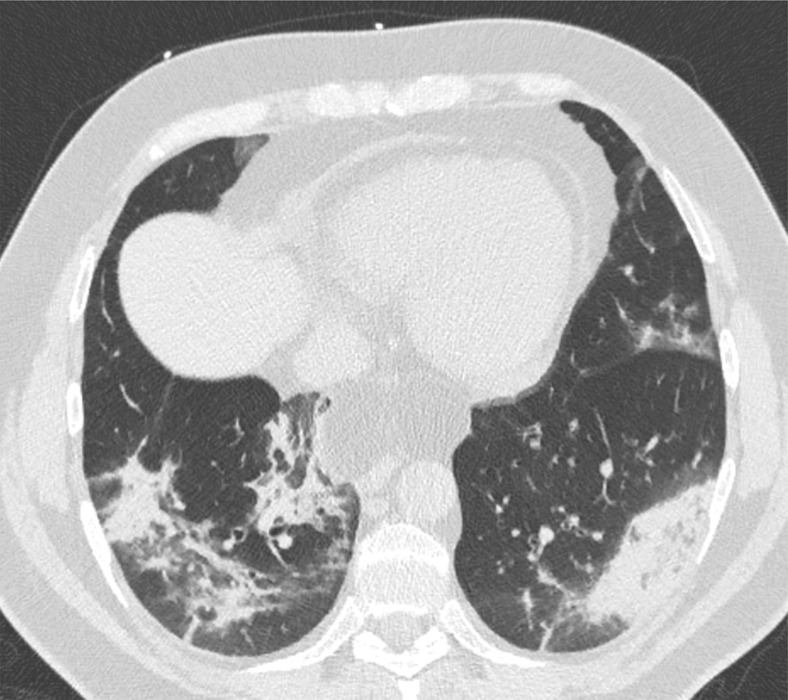
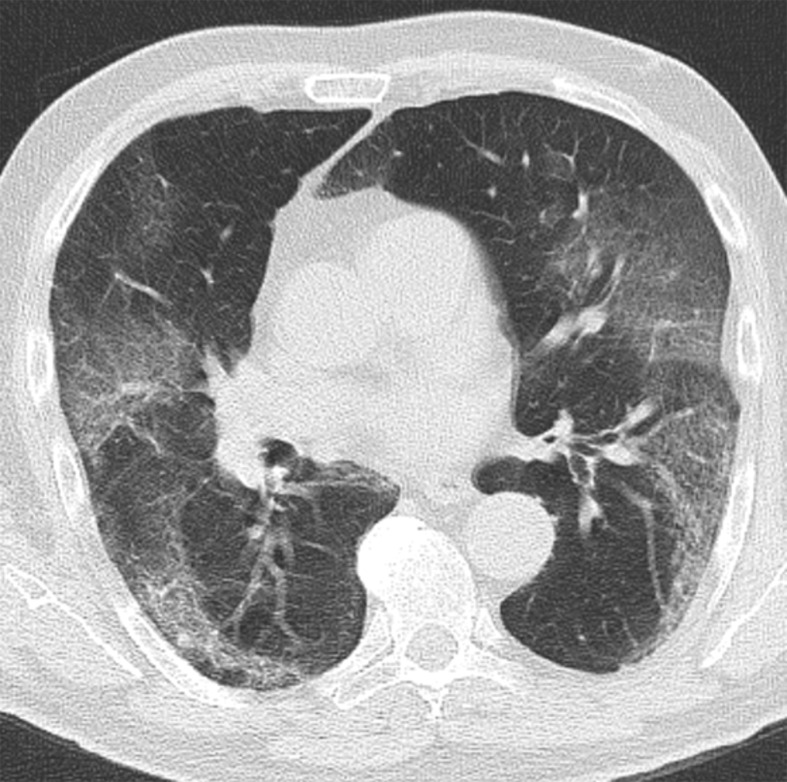
Figure 2.
Chest CT abnormalities of relatively high prevalence in COVID-19. Axial nonenhanced chest CT image (lung window) shows bilateral ground-glass opacities and dilated segmental and subsegmental vessels, mainly on the right, in a 70-year-old man with positive RT-PCR test results for SARS-CoV-2.
Figure 1b.
COVID-19 pneumonia with typical imaging features according to the Radiological Society of North America (RSNA) chest CT classification system (51). Axial nonenhanced chest CT images (lung window) in a 59-year-old man (a) and a 47-year-old man (b), each with positive RT-PCR test results for SARS-CoV-2, show bilateral areas of ground-glass opacities (arrows) in a peripheral distribution.
Chest CT Abnormalities with Intermediate Incidence (10%–70%)
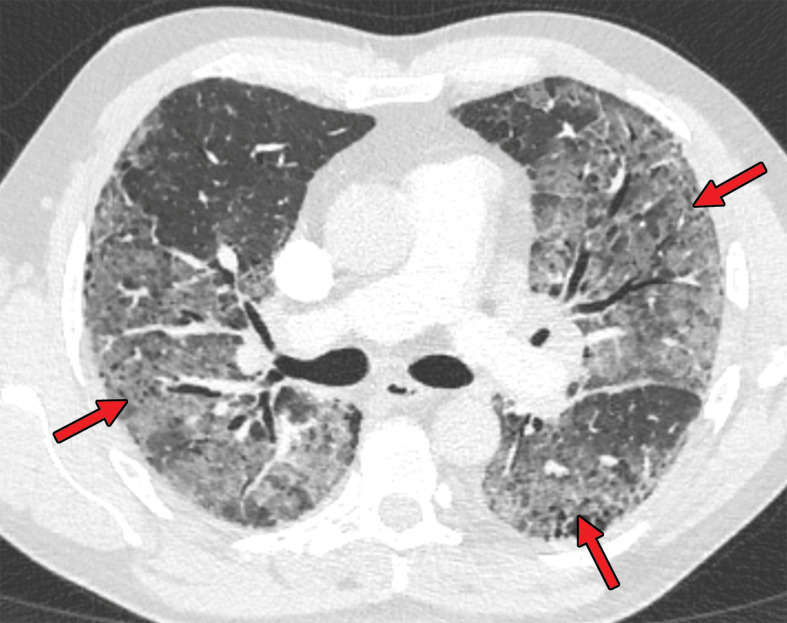
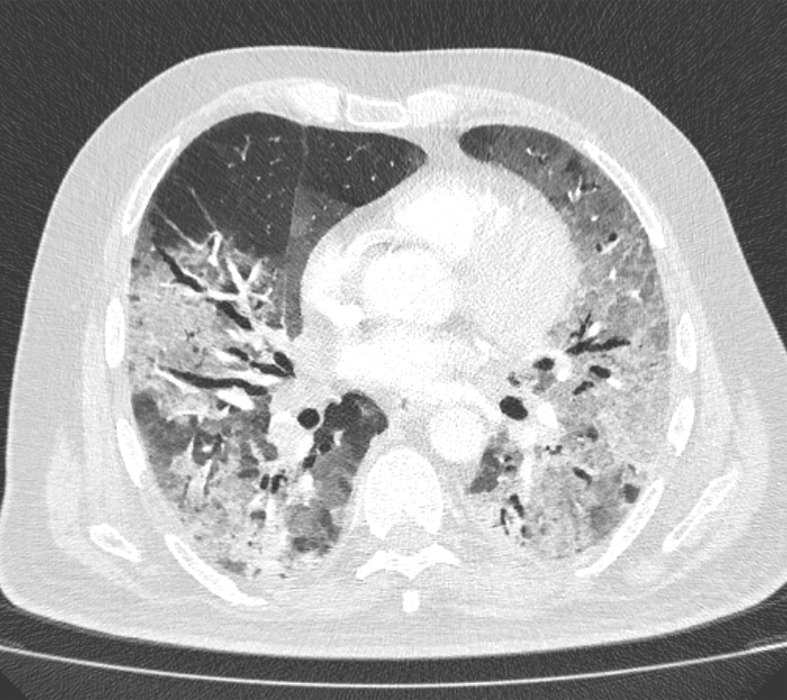
Several chest CT findings have been reported in 10%–70% of RT-PCR test–proven COVID-19 cases, including consolidation (51.5%), linear opacity (40.7%) (Fig 3), septal thickening and/or reticulation (49.6%), crazy-paving pattern (34.9%) (Fig 4), air bronchogram (40.2%), pleural thickening (34.7%), halo sign (34.5%) (Fig 5), bronchiectasis (24.2%), nodules (19.8%), bronchial wall thickening (14.3%), and reversed halo sign (11.1%) (43). The following lesion distributions have been reported: unilateral (15.0%), multifocal (63.2%), diffuse (26.4%), single and/or focal (10.5%), middle or upper lobe involvement (49.3%–55.4%), peripheral location (59.0%), and central and peripheral location (36.2%) (43).
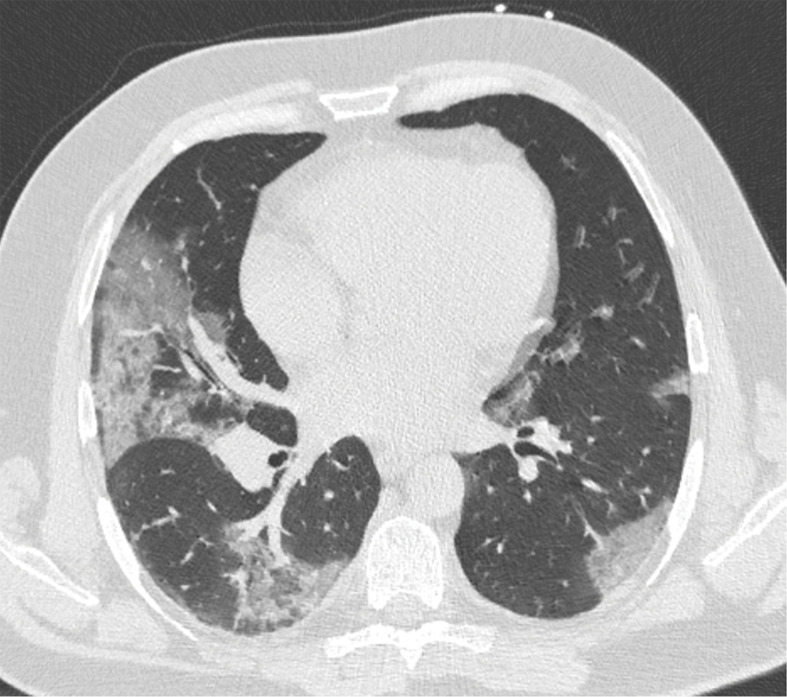
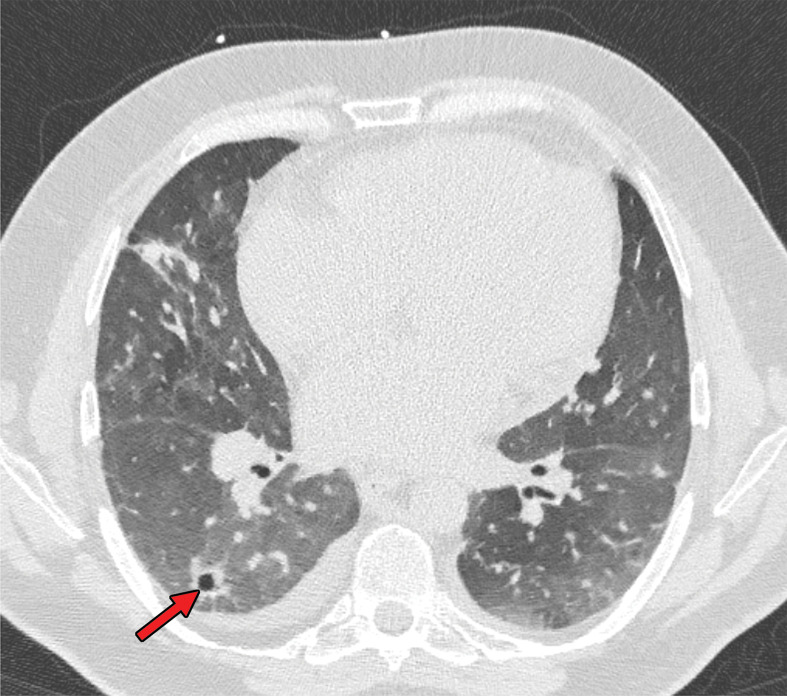
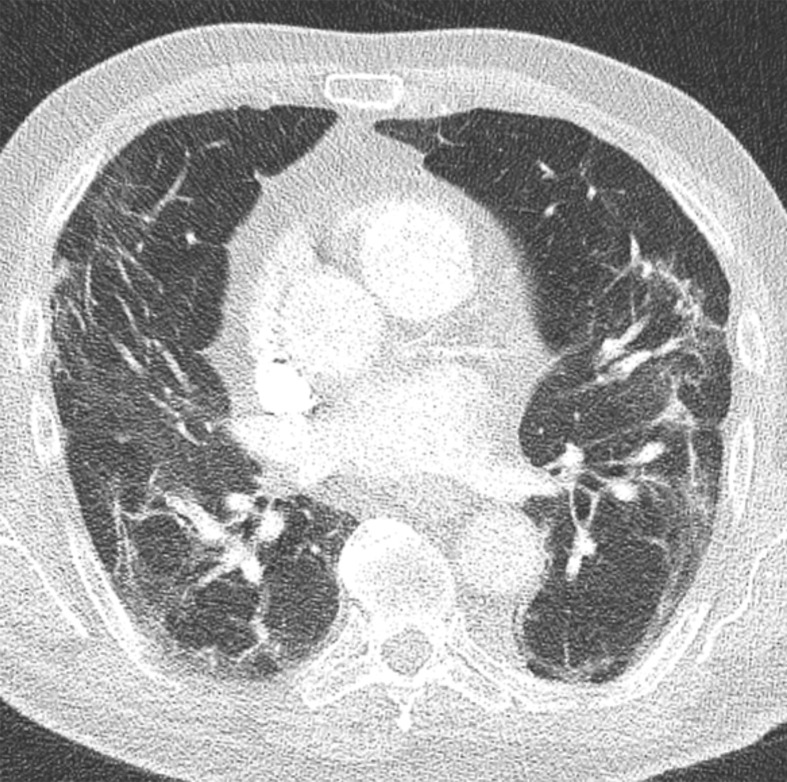
Figure 3.
Chest CT abnormalities of relatively intermediate prevalence in COVID-19, shown in a 63-year-old man with positive RT-PCR test results for SARS-CoV-2. Axial nonenhanced chest CT image shows a subpleural curvilinear opacity (arrow) and an area of ground-glass opacity (arrowhead) in the right upper lobe.
Figure 4.
Crazy-paving pattern in a 66-year-old man with COVID-19. Axial nonenhanced chest CT image shows ground-glass opacities, with superimposed septal thickening (arrows) in the middle lobe and left lower lobe.
Figure 5a.
Halo sign in a 55-year-old man with RT-PCR-test–proven COVID-19. Axial nonenhanced chest CT images show consolidations surrounded by ground-glass opacities (arrows) in both upper lobes, findings consistent with the halo sign. There is a ground-glass opacity in the right upper lobe (arrowhead in a) and consolidation in both lower lobes (arrowheads in b).
Figure 5b.
Halo sign in a 55-year-old man with RT-PCR-test–proven COVID-19. Axial nonenhanced chest CT images show consolidations surrounded by ground-glass opacities (arrows) in both upper lobes, findings consistent with the halo sign. There is a ground-glass opacity in the right upper lobe (arrowhead in a) and consolidation in both lower lobes (arrowheads in b).
Chest CT Abnormalities with Low Incidence (<10%)
Several chest CT findings have been reported to be uncommon in RT-PCR test–proven COVID-19 cases, and these include pleural effusion (5.2%), lymphadenopathy (5.1%), tree-in-bud sign (4.1%), central lesion distribution (3.6%), pericardial effusion (2.7%), and cavitating lung lesions (0.7%) (43). The isolated observation of one or more of these findings is more suggestive of another diagnosis than of COVID-19, although COVID-19 cannot be completely eliminated from the differential diagnosis (43). Furthermore, some of these chest CT findings may only occur in some patients later in the course of disease.
For instance, cavitating lung lesions can manifest in patients with COVID-19 (Fig 6), which may be due to mechanical ventilator–induced lung injury. Of interest, authors of a study (52) reported that barotrauma (pneumothorax, pneumomediastinum) occurs in approximately 15% of patients with COVID-19 who require invasive mechanical ventilation, and that it is more likely to occur in younger patients. Pericardial effusion may be seen as a complication in the setting of cardiac injury.
Figure 6a.
Development of cavitating lung lesions in a 47-year-old man with COVID-19. (a, b) Axial nonenhanced CT images (lung window) obtained at hospital admission show ground-glass opacities in both lungs (early progressive stage). (c, d) Axial nonenhanced CT images (lung window) obtained after 10 days show progressive organizing consolidation (peak stage). (e, f) Axial nonenhanced CT images (lung window) obtained 40 days after the baseline CT images (a, b) show cavitating lesions in both lower lobes (arrow) (late stage).
Figure 6b.
Development of cavitating lung lesions in a 47-year-old man with COVID-19. (a, b) Axial nonenhanced CT images (lung window) obtained at hospital admission show ground-glass opacities in both lungs (early progressive stage). (c, d) Axial nonenhanced CT images (lung window) obtained after 10 days show progressive organizing consolidation (peak stage). (e, f) Axial nonenhanced CT images (lung window) obtained 40 days after the baseline CT images (a, b) show cavitating lesions in both lower lobes (arrow) (late stage).
Figure 6c.
Development of cavitating lung lesions in a 47-year-old man with COVID-19. (a, b) Axial nonenhanced CT images (lung window) obtained at hospital admission show ground-glass opacities in both lungs (early progressive stage). (c, d) Axial nonenhanced CT images (lung window) obtained after 10 days show progressive organizing consolidation (peak stage). (e, f) Axial nonenhanced CT images (lung window) obtained 40 days after the baseline CT images (a, b) show cavitating lesions in both lower lobes (arrow) (late stage).
Figure 6d.
Development of cavitating lung lesions in a 47-year-old man with COVID-19. (a, b) Axial nonenhanced CT images (lung window) obtained at hospital admission show ground-glass opacities in both lungs (early progressive stage). (c, d) Axial nonenhanced CT images (lung window) obtained after 10 days show progressive organizing consolidation (peak stage). (e, f) Axial nonenhanced CT images (lung window) obtained 40 days after the baseline CT images (a, b) show cavitating lesions in both lower lobes (arrow) (late stage).
Figure 6e.
Development of cavitating lung lesions in a 47-year-old man with COVID-19. (a, b) Axial nonenhanced CT images (lung window) obtained at hospital admission show ground-glass opacities in both lungs (early progressive stage). (c, d) Axial nonenhanced CT images (lung window) obtained after 10 days show progressive organizing consolidation (peak stage). (e, f) Axial nonenhanced CT images (lung window) obtained 40 days after the baseline CT images (a, b) show cavitating lesions in both lower lobes (arrow) (late stage).
Figure 6f.
Development of cavitating lung lesions in a 47-year-old man with COVID-19. (a, b) Axial nonenhanced CT images (lung window) obtained at hospital admission show ground-glass opacities in both lungs (early progressive stage). (c, d) Axial nonenhanced CT images (lung window) obtained after 10 days show progressive organizing consolidation (peak stage). (e, f) Axial nonenhanced CT images (lung window) obtained 40 days after the baseline CT images (a, b) show cavitating lesions in both lower lobes (arrow) (late stage).
Temporal Evolution of Lung Abnormalities at Chest CT
Knowledge of the natural temporal evolution of lung abnormalities in COVID-19 may be helpful to radiologists in determining the stage of disease and in distinguishing them from potential complications when evaluating chest CT examinations. However, it should be noted that there are relatively few studies that have evaluated serial temporal changes in patients who underwent repeat CT examinations (43). In addition, these studies are limited by selection bias and potential confounding of the natural course of lung abnormalities owing to medical interventions (such as the administration of antimicrobial agents, fluid, or steroid therapy).
Roughly four stages of COVID-19 at chest CT have been described: (a) early stage (0–5 days after symptom onset), which is characterized by either normal findings or mainly ground-glass opacities; (b) progressive stage (5–8 days after symptom onset), which is characterized by increased ground-glass opacities and crazy-paving appearance (Fig 4); (c) peak stage (9–13 days after symptom onset), which is characterized by progressive consolidation (Figs 6, 7); and (d) late stage (≥14 days after symptom onset), which is characterized by a gradual decrease of consolidation and ground-glass opacities, while signs of fibrosis (including parenchymal bands, architectural distortion, and traction bronchiectasis) may manifest (Fig 8) (47,53–56). It has been reported that unilateral involvement is only present in the early and late phases (47). It should also be noted that the temporal evolution and extent of lung abnormalities are heterogeneous among different patients, dependent on the severity of the disease (53,54,57).
Figure 7a.
Transition from progressive stage to peak stage in a 69-year-old man with COVID-19. (a) Axial nonenhanced chest CT image (lung window) obtained at hospital admission shows bilateral areas of ground-glass opacities and crazy-paving appearance. (b) Axial contrast-enhanced chest CT image (lung window) obtained after 7 days shows progression from ground-glass opacities to multifocal organizing consolidation.
Figure 8a.
Occurrence of lung fibrosis in a 75-year-old man with COVID-19. (a) Axial nonenhanced CT image (lung window) obtained at hospital admission shows bilateral ground-glass opacities, which are mainly peripherally located. (b) Axial contrast-enhanced CT image (lung window) obtained after 8 weeks shows bilateral curvilinear parenchymal bands with distortion of lung architecture. Focal traction bronchiectasis (not shown) also manifested.
Figure 7b.
Transition from progressive stage to peak stage in a 69-year-old man with COVID-19. (a) Axial nonenhanced chest CT image (lung window) obtained at hospital admission shows bilateral areas of ground-glass opacities and crazy-paving appearance. (b) Axial contrast-enhanced chest CT image (lung window) obtained after 7 days shows progression from ground-glass opacities to multifocal organizing consolidation.
Figure 8b.
Occurrence of lung fibrosis in a 75-year-old man with COVID-19. (a) Axial nonenhanced CT image (lung window) obtained at hospital admission shows bilateral ground-glass opacities, which are mainly peripherally located. (b) Axial contrast-enhanced CT image (lung window) obtained after 8 weeks shows bilateral curvilinear parenchymal bands with distortion of lung architecture. Focal traction bronchiectasis (not shown) also manifested.
The temporal evolution of lung abnormalities in COVID-19 likely parallels that of other inflammatory lung injuries (58), and there are definitely patients with chest CT abnormalities that simply resolve after the acute phase. The long-term sequelae of COVID-19 and their associated lung abnormalities remain to be investigated.
Role of Chest CT in Diagnostic Decision Making
The Fleischner Society published a consensus statement on the use of chest imaging (including radiography and CT) for certain scenarios during the COVID-19 pandemic (59). The Fleischner Society provided a consensus statement rather than a guideline given the limited evidence at the time of writing. Its aim is to guide medical practitioners in the use of chest imaging in the management of COVID-19 (59).
Asymptomatic Patients and Patients with Mild Respiratory Symptoms
According to the Fleischner Society consensus statement, chest imaging is not indicated as a screening test for COVID-19 in asymptomatic patients or in patients with mild respiratory symptoms of COVID-19 (ie, absence of significant pulmonary dysfunction or damage) (59). The statement noted that uncertainty still exists whether chest CT should be used as a screening tool, either as a stand-alone screening tool or as an adjunct to RT-PCR tests, to exclude occult infection before surgery or intensive immunosuppressive therapy in regions with a high prevalence of COVID-19 (59). If used as a stand-alone screening tool in these settings, chest CT should have a near-perfect sensitivity because a false assumption of COVID-19–negativity may have a major negative impact on patient and personnel safety. However, a negative chest CT examination does not exclude COVID-19 (as discussed in a later section). Another disadvantage of using chest CT as a screening test is the nonnegligible number of incidentalomas that can be expected (60).
Preliminary data from The Netherlands show that the yield and added value of chest CT in the preoperative screening of asymptomatic patients is low (61). Therefore, the Dutch Association of Medical Specialists practice guidelines for preoperative workup for patients with COVID-19 infection do not recommend performing chest CT as a screening tool in asymptomatic patients scheduled for surgery for which they will undergo general anesthesia (61).
Patients with Moderate to Severe Respiratory Symptoms
According to the Fleischner Society consensus statement, chest imaging is indicated in patients with moderate to severe respiratory symptoms (ie, presence of significant pulmonary dysfunction or damage) and any pretest probability of COVID-19 infection, when RT-PCR test results are negative, and in any patient for whom an RT-PCR test is not performed or not readily available (59). It should be emphasized that the Fleischner Society consensus statement did not specify whether chest imaging should be preferably performed with radiography or CT. The speed of CT may support rapid triage, which is desirable in a resource-constrained environment (eg, limited access to personnel, PPE, RT-PCR testing ability, hospital beds, and/or ventilators and the urgent need to rapidly triage patients) (59).
Chest imaging can help suggest an alternative diagnosis to explain the patient’s clinical features, or it may demonstrate features of COVID-19 infection (59). If no alternative diagnosis is determined or if images demonstrate features of COVID-19 infection, then a subsequent clinical evaluation would be dependent on the pretest probability of COVID-19 infection and RT-PCR test availability (59). False-negative RT-PCR test results are more prevalent in high-pretest-probability circumstances (eg, high background prevalence of disease associated with community transmission) (59). In these cases, repeat RT-PCR tests should be considered (23,59).
Additional Chest CT in Patients Who Undergo CT of Other Body Regions
This scenario was not addressed in the Fleischner Society consensus statement (59). CT is widely used in the emergency department, with the head and abdomen being among the most commonly imaged body regions (62,63). In COVID-19 endemic areas, additional chest CT may be performed to help detect COVID-19 in patients who undergo extrathoracic CT. The results of several studies in COVID-19 endemic regions have shown that incidental chest CT findings suggestive of COVID-19 pneumonia can be detected in the visualized lung parenchyma in patients who underwent CT of other body regions, such as CT angiography of the head and neck (64–66), CT of the cervical or thoracic spine (64,65), and CT of the abdomen (67–69). These patients should undergo subsequent RT-PCR tests before the diagnosis of COVID-19 can be confirmed (59).
Of interest, gastrointestinal symptoms may predominate or may even manifest without respiratory symptoms in COVID-19 (67–70). Therefore, it has been advocated in the surgical community to perform additional chest CT for COVID-19 screening in patients with acute abdomen who undergo abdominal CT in the severe acute pandemic scenario (69).
Similarly, in patients with stroke owing to acute ischemic large vessel occlusion, it has been suggested that performing low-radiation-dose chest CT at the same time as head CT with CT angiography of the head and neck should be considered, provided that pulmonary symptoms have manifested and the addition of chest CT does not cause 5 minutes or more delay to endovascular treatment (71). However, before deciding to implement additional chest CT in these settings, several factors have to be taken into account, including the diagnostic performance and yield of chest CT for COVID-19 (which is affected by both the prevalence of COVID-19 and that of other diseases in the community, such as other viral and [atypical] bacterial pneumonias [72]) and the local availability of RT-PCR tests.
Diagnostic Accuracy of Chest CT
A meta-analysis, which included six studies comprising a total of 1431 patients who were mainly symptomatic and at high risk for COVID-19, reported a chest CT pooled sensitivity of 94.6% (95% CI: 91.9%, 96.4%) and a pooled specificity of 46.0% (95% CI: 31.9%, 60.7%) in the detection of COVID-19 (73). However, the published diagnostic accuracy studies to date have methodologic quality issues, which may have led to an overestimation of sensitivity (73,74).
The results of another meta-analysis showed that 10.6% of symptomatic patients with RT-PCR test–proven COVID-19 have normal chest CT findings (73), which suggests that true sensitivity may be considerably lower than that reported by many of the initial studies on this topic. Thus, a negative chest CT examination result certainly does not exclude COVID-19. The proportion of false-positive chest CT examination results is substantial and due to overlapping imaging features with numerous other diseases, including other viral pneumonias (72,75). The interpretation of chest CT examinations may become particularly challenging during influenza season. Some studies suggest that a peripheral distribution of ground-glass opacities is a more typical finding of COVID-19 pneumonia (76–78), whereas other studies did not find these features helpful in discriminating COVID-19 pneumonia from influenza pneumonia (79).
Importantly, however, the differentiation between different types of viral pneumonias at chest CT may not be relevant from a practical point of view, because the in-hospital infection control precaution requirements for these various types are basically identical (80). At present, there are not much data on other alternative diagnoses (eg, PE, acute interstitial pneumonitis, drug-induced lung disease, alveolar hemorrhage) that may produce false-positive findings and further limit the specificity of chest CT.
The diagnostic accuracy of chest CT is dependent on reader experience and the diagnostic criteria that are used as the threshold value (76,81). However, there are currently no uniformly accepted diagnostic criteria (73,82). Furthermore, the diagnostic accuracy of chest CT is dependent on a variety of other factors, including the study population, COVID-19 prevalence, COVID-19 stage and disease severity at the time of imaging, and coexisting lung disease (82,83). It is important to realize that CT is not the standard for the diagnosis of COVID-19, but its findings help suggest the diagnosis in the appropriate setting. It is crucial to correlate chest CT findings with epidemiologic history, clinical presentation, and RT-PCR test results.
Reporting and Communicating Chest CT Findings
The RSNA has provided guidance in reporting chest CT findings potentially attributable to COVID-19 pneumonia (51). Four categories for standardized COVID-19 reporting were proposed (Table 2), with the aim to help radiologists recognize the findings of COVID-19, decrease reporting variability, reduce uncertainty in reporting findings potentially attributable to COVID-19 infection, and improve communication with referring physicians (51). The four categories include “typical appearance” (Fig 1), “indeterminate appearance” (Figs 9, 10), “atypical appearance” (Fig 11), and “negative for pneumonia.” Adherence to the American College of Radiology Practice Parameter for Communication of Diagnostic Imaging Findings is highly recommended (84).
Table 2:
Imaging Classification and CT Features of COVID-19 Pneumonia
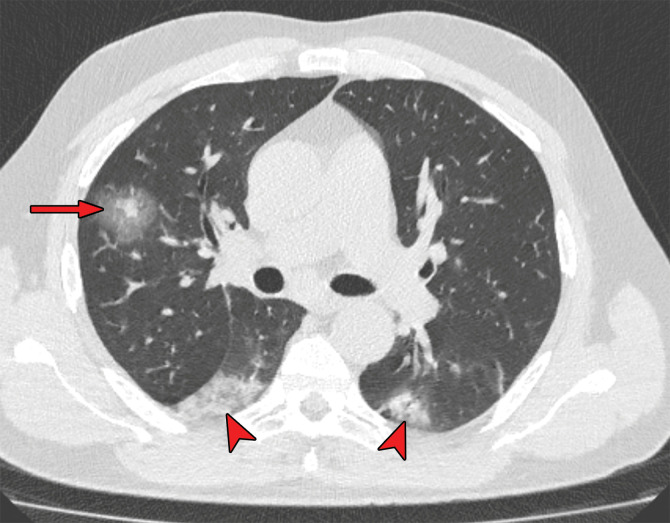
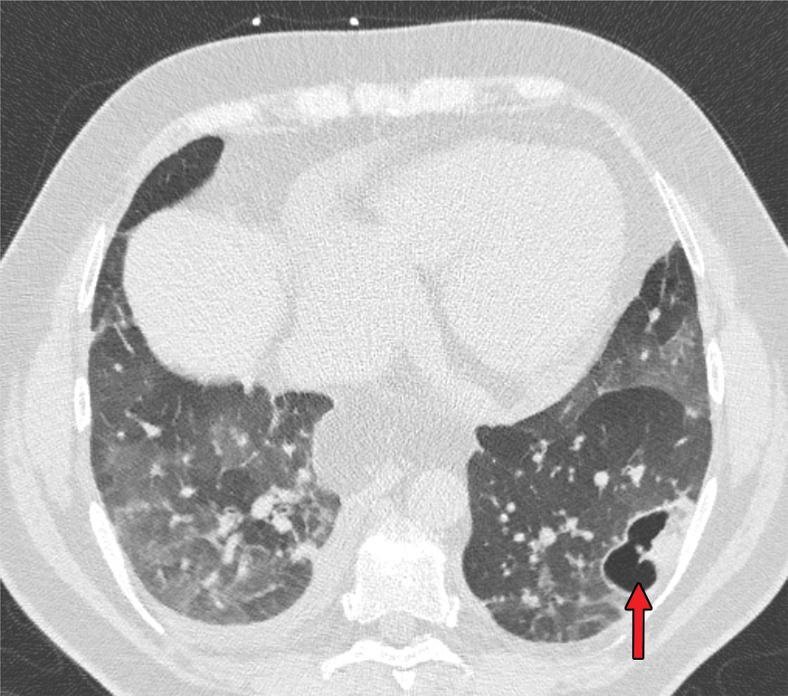
Figures 9.
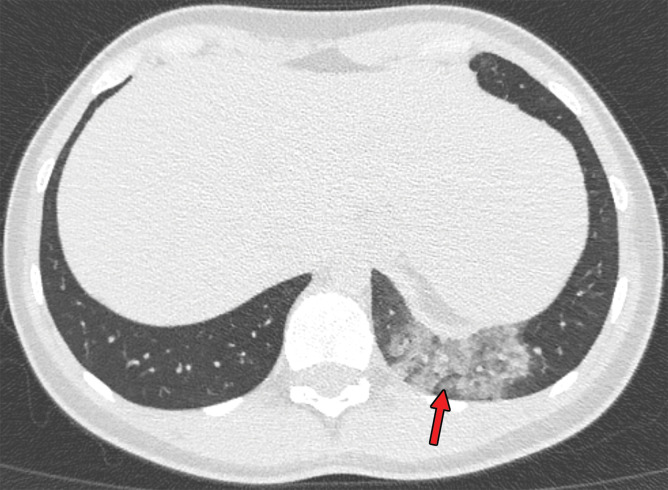
Findings classified as indeterminate appearance of COVID-19 pneumonia in a 26-year-old woman. Axial nonenhanced chest CT image (lung window) shows an area of ground-glass opacity (arrow) in the posterior basal segment of the left lower lobe. No other lung abnormalities were visualized. The RT-PCR test results were positive for SARS-CoV-2.
Figures 10.
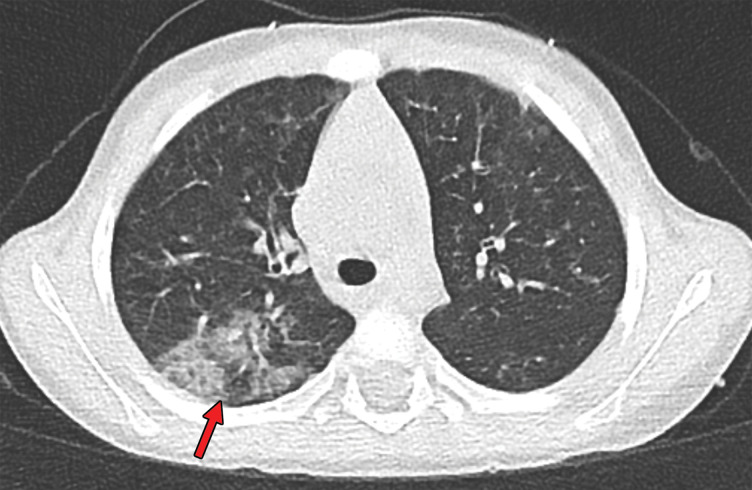
Findings classified as indeterminate appearance of COVID-19 pneumonia according to the RSNA chest CT classification system (51) in a 24-year-old woman. Axial nonenhanced chest CT image (lung window) shows ground-glass opacities (arrow) in the right upper lobe. In addition, there are discrete centrilobular opacities in the upper lobes. The RT-PCR test results were negative for SARS-CoV-2 but positive for influenza type A.
Figure 11.
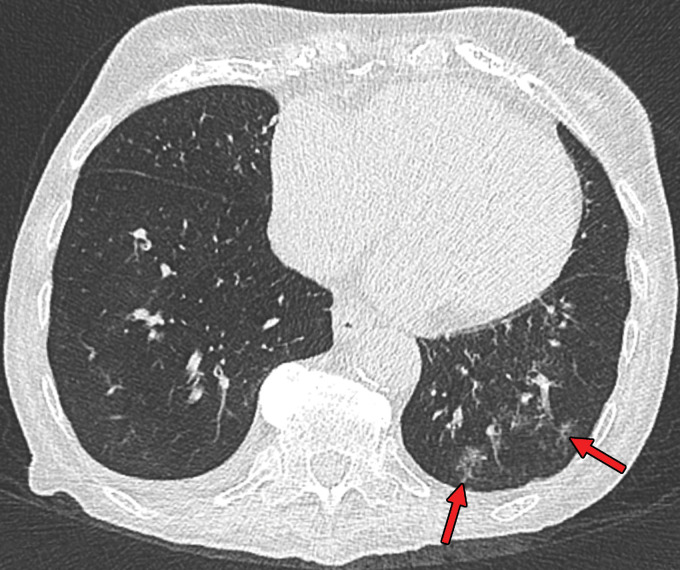
Findings classified as atypical appearance of COVID-19 pneumonia in a 94-year-old woman. Axial nonenhanced chest CT image (lung window) shows subtle centrilobular tree-in-bud opacities (arrows) in the left lower lobe. The RT-PCR test results were negative for SARS-CoV-2 but positive for influenza type A.
When typical or indeterminate features of COVID-19 pneumonia are visualized as incidental findings in patients in endemic areas, the referring physician should be urgently contacted to discuss the possibility of COVID-19 pneumonia (51). In addition, personnel in the CT examination room should be notified to initiate standard operating procedures for potential exposure (51). Incidental findings do not necessarily need to be reported as COVID-19 pneumonia, as the use of the term “viral pneumonia” is a reasonable and inclusive alternative (51). Nonroutine communication of typical or indeterminate features of COVID-19 pneumonia is less relevant in patients under investigation for COVID-19, as clinical suspicion already exists. It should be noted that the presence of mixed chest CT findings may complicate the interpretation and categorization of imaging observations (Fig 12) (51). In these cases, the radiologist will have to determine whether these findings may be part of the same process or are unrelated (51).
Figure 12a.
Mixed chest CT findings in an 86-year-old man. (a) Axial nonenhanced CT image (lung window) obtained at hospital admission shows sublobar consolidation (arrow) in the posterior segment of the right upper lobe, a finding more consistent with lobar pneumonia than COVID-19. (b) Axial CT image obtained at a more superior level shows the presence of ground-glass opacities (arrows). Altogether, the findings were classified as indeterminate for COVID-19 pneumonia, according to the RSNA chest CT classification system (51). The RT-PCR test results were positive for SARS-CoV-2.
Figure 12b.
Mixed chest CT findings in an 86-year-old man. (a) Axial nonenhanced CT image (lung window) obtained at hospital admission shows sublobar consolidation (arrow) in the posterior segment of the right upper lobe, a finding more consistent with lobar pneumonia than COVID-19. (b) Axial CT image obtained at a more superior level shows the presence of ground-glass opacities (arrows). Altogether, the findings were classified as indeterminate for COVID-19 pneumonia, according to the RSNA chest CT classification system (51). The RT-PCR test results were positive for SARS-CoV-2.
Interobserver agreement of the RSNA chest CT classification system for reporting COVID-19 pneumonia is moderate to substantial (85). However, a nonnegligible number of cases with RT-PCR test–proven COVID-19 are classified in the categories “atypical appearance” and “negative for pneumonia” (85). Again, correlation of chest CT findings with epidemiologic history, clinical presentation, and RT-PCR test results is essential, which may be performed in a multidisciplinary team meeting.
Chest CT of COVID-19 Complications
In cases of clinical worsening, chest imaging is advised to assess for COVID-19 progression or secondary cardiopulmonary complications such as acute respiratory distress syndrome (ARDS), PE, superimposed pneumonia, or heart failure that can potentially be secondary to COVID-19–induced cardiac injury (59).
Acute Respiratory Distress Syndrome
COVID-19 may rapidly progress to ARDS (86), with older patients being at higher risk (87). ARDS seen with COVID-19 is a cytokine release syndrome, in which immune and nonimmune cells release large amounts of proinflammatory cytokines that cause damage to the host (88). ARDS is characterized by an acute onset of noncardiogenic pulmonary edema, hypoxemia, and the need for mechanical ventilation (89). Diffuse alveolar damage is the pathognomonic histologic finding (89). ARDS is the most common reason for patient admission to the intensive care unit and the main cause of mortality in patients with COVID-19 (90).
ARDS is diagnosed according to the Berlin definition (91). The imaging criterion for ARDS is fulfilled if bilateral opacities consistent with pulmonary edema manifest (Fig 13) (91). However, it should be noted that the clinical features of COVID-19–related ARDS are not fully understood and may be different from those of ARDS caused by other factors (86). For instance, it has been reported that COVID-19–related ARDS can develop after 8–12 days after symptom onset (86,92), which is longer than the 1-week onset limit according to the Berlin definition (91). Furthermore, clinical manifestations may be relatively mild, with respect to the severity of imaging findings in COVID-19 (86).
Figure 13a.
Development of ARDS in a 60-year-old man with COVID-19. (a) Axial nonenhanced CT image (lung window) obtained at hospital admission shows peripherally located ground-glass opacities (arrow), mainly in the right lung. Note the preexisting centrilobular and paraseptal emphysema. (b) Axial contrast-enhanced CT image (lung window) obtained after 3 days shows a marked progression of lung abnormalities (arrows).
Figure 13b.
Development of ARDS in a 60-year-old man with COVID-19. (a) Axial nonenhanced CT image (lung window) obtained at hospital admission shows peripherally located ground-glass opacities (arrow), mainly in the right lung. Note the preexisting centrilobular and paraseptal emphysema. (b) Axial contrast-enhanced CT image (lung window) obtained after 3 days shows a marked progression of lung abnormalities (arrows).
Pulmonary Embolism
Patients with COVID-19 are at risk for developing thromboembolic complications (93,94), which may be caused by activation of the coagulation cascade by SARS-CoV-2 or by local or systemic inflammation (95). Patients with thromboembolic complications have a more than fivefold higher risk of all-cause death (93). However, at present, there are insufficient data to recommend for or against the routine use of prophylactic thrombolytic therapy or increasing anticoagulant therapy doses in hospitalized patients with COVID-19 (96). The incidence of PE in patients with COVID-19 who underwent CT pulmonary angiography has been reported to range between 17% and 35% (93,97–101). Prevalence may be highest in critically ill patients (99), but even patients with milder disease can develop acute PE (98).
The exact contribution of PE to mortality in patients with COVID-19 is still unknown because not all patients routinely undergo CT pulmonary angiography and because of the limited number of autopsy studies available (94). In patients with suspected COVID-19 and a high clinical suspicion for PE (eg, determined on the basis of hemoptysis, unexplained tachycardia, or signs and symptoms of deep venous thrombosis and acute deterioration on patient mobilization), CT pulmonary angiography should be considered (Fig 14) (95).
Figure 14a.
PE in a 73-year-old man with COVID-19. (a) Axial nonenhanced CT image (lung window) at baseline shows peripherally diffuse ground-glass opacities in both lungs. (b) Axial contrast-enhanced CT image (lung window) obtained after 10 days shows increased consolidation in both lungs. Note the bronchial dilatation within involved portions of the lungs. (c, d) Axial contrast-enhanced CT image (mediastinal window) (c) and sagittal reconstruction (d) obtained 10 days after the baseline images show a filling defect (arrow) in a segmental pulmonary artery branch in the right lower lobe, consistent with PE.
Figure 14b.
PE in a 73-year-old man with COVID-19. (a) Axial nonenhanced CT image (lung window) at baseline shows peripherally diffuse ground-glass opacities in both lungs. (b) Axial contrast-enhanced CT image (lung window) obtained after 10 days shows increased consolidation in both lungs. Note the bronchial dilatation within involved portions of the lungs. (c, d) Axial contrast-enhanced CT image (mediastinal window) (c) and sagittal reconstruction (d) obtained 10 days after the baseline images show a filling defect (arrow) in a segmental pulmonary artery branch in the right lower lobe, consistent with PE.
Figure 14c.
PE in a 73-year-old man with COVID-19. (a) Axial nonenhanced CT image (lung window) at baseline shows peripherally diffuse ground-glass opacities in both lungs. (b) Axial contrast-enhanced CT image (lung window) obtained after 10 days shows increased consolidation in both lungs. Note the bronchial dilatation within involved portions of the lungs. (c, d) Axial contrast-enhanced CT image (mediastinal window) (c) and sagittal reconstruction (d) obtained 10 days after the baseline images show a filling defect (arrow) in a segmental pulmonary artery branch in the right lower lobe, consistent with PE.
Figure 14d.
PE in a 73-year-old man with COVID-19. (a) Axial nonenhanced CT image (lung window) at baseline shows peripherally diffuse ground-glass opacities in both lungs. (b) Axial contrast-enhanced CT image (lung window) obtained after 10 days shows increased consolidation in both lungs. Note the bronchial dilatation within involved portions of the lungs. (c, d) Axial contrast-enhanced CT image (mediastinal window) (c) and sagittal reconstruction (d) obtained 10 days after the baseline images show a filling defect (arrow) in a segmental pulmonary artery branch in the right lower lobe, consistent with PE.
There are no established age-adjusted d-dimer cutoff levels to rule out venous thromboembolism in patients with COVID-19 at this time (102). Furthermore, patients with severe COVID-19 pneumonia have markedly elevated d-dimer levels (103,104). Nevertheless, d-dimer levels have been reported to be associated with both the presence of PE and the degree of pulmonary artery obstruction in patients with COVID-19 (101). Therefore, d-dimer levels may be useful in the risk stratification of patients for PE workup (101).
Superimposed Pneumonia
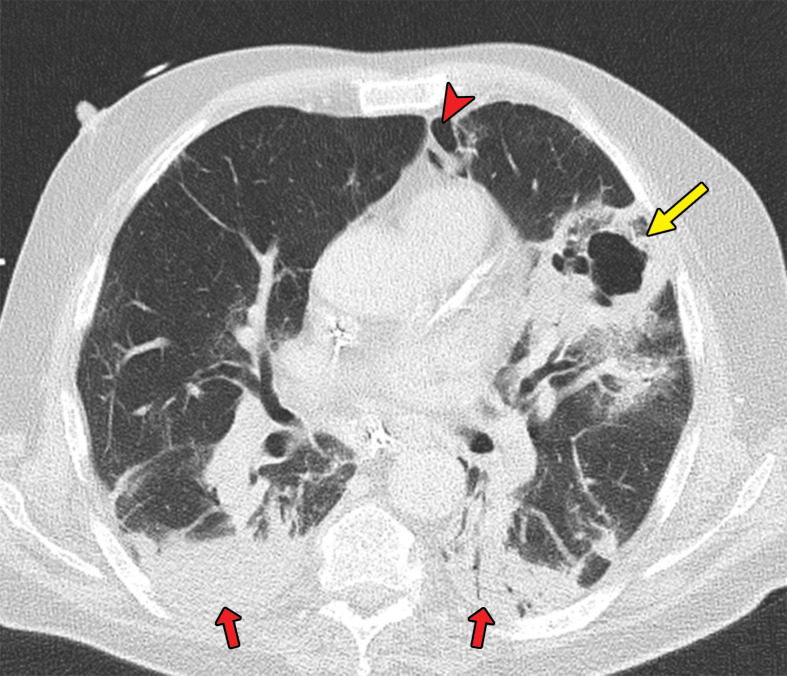
Patients with COVID-19 are vulnerable to superimposed pneumonia, which occurs in approximately 10% of hospitalized patients (6,105). Patients with COVID-19 and ARDS may die owing to superimposed bacterial or fungal infection (92,106–108). Therefore, if during COVID-19 treatment secondary respiratory worsening occurs, one should think of the possibility of superimposed pneumonia and consider obtaining lower respiratory tract cultures and performing chest imaging (105). Lobar consolidation at chest imaging may reflect a superimposed bacterial pneumonia (Fig 15) (51).
Figure 15a.
Superimposed pneumonia in a 69-year-old man with COVID-19. (a) Axial nonenhanced CT image (lung window) at baseline shows ground-glass opacities (arrows) posteriorly located in the left upper lobe and both lower lobes and an area of consolidation (arrowhead) in the right lower lobe. (b) Axial contrast-enhanced CT image (lung window) obtained after 22 days shows increased consolidation in both lower lobes (red arrows) and consolidation with central cavitation in the left upper lobe (yellow arrow). The culture of puslike bronchial fluid was positive for Staphylococcus aureus. Note the presence of pneumomediastinum (arrowhead), which is probably due to long-lasting positive-pressure ventilation.
Figure 15b.
Superimposed pneumonia in a 69-year-old man with COVID-19. (a) Axial nonenhanced CT image (lung window) at baseline shows ground-glass opacities (arrows) posteriorly located in the left upper lobe and both lower lobes and an area of consolidation (arrowhead) in the right lower lobe. (b) Axial contrast-enhanced CT image (lung window) obtained after 22 days shows increased consolidation in both lower lobes (red arrows) and consolidation with central cavitation in the left upper lobe (yellow arrow). The culture of puslike bronchial fluid was positive for Staphylococcus aureus. Note the presence of pneumomediastinum (arrowhead), which is probably due to long-lasting positive-pressure ventilation.
Cardiac Injury
Cardiac injury occurs in 12.5%–19.7% of hospitalized patients with COVID-19 and is an independent risk factor for in-hospital mortality (6,109). Pericardial effusion manifests in an estimated 5.2% of patients with COVID-19 (43), with a higher incidence in those with severe or critical illness (90,110). Pericardial effusion may also be a sign of cardiac injury in COVID-19 (111–114). Although pericardial effusion is a nonspecific finding (115), radiologists should suggest the possibility of COVID-19–related cardiac injury when pericardial effusion is depicted on chest CT images.
Role of Chest CT for Prognostication
The Fleischner Society recommends performing imaging (a) to establish a baseline pulmonary status; (b) to identify underlying cardiopulmonary abnormalities, which may facilitate risk stratification for clinical worsening in patients with mild symptoms of COVID-19, and risk factors for disease progression (eg, age >65 years, comorbidities such as cardiovascular disease, diabetes, chronic respiratory disease, hypertension, and immunocompromised status); and (c) in patients with moderate to severe symptoms of COVID-19 (59). Again, the Fleischner Society consensus statement does not specify whether radiography or CT is preferred in this setting (59).
The use of a chest CT severity score may be useful for standardized assessment of the degree of pulmonary involvement in COVID-19 for prognostication purposes (116). However, currently proposed prediction models for COVID-19, including the ones that include chest CT features, are poorly reported and are at high risk of bias, and their reported performance is probably optimistic (117). Therefore, it is currently not recommended to use any of the reported prediction models for use in clinical practice (117). More research is needed to further clarify the value of chest CT for prognostication in COVID-19, including correlation with patient outcome.
Conclusion
The clinical presentation, course, and outcome of COVID-19 are heterogeneous, and this also applies to the degree of pulmonary involvement. Performing CT in patients with suspected or proven COVID-19 requires comprehensive precautionary safety measures. Low-radiation-dose chest CT is recommended unless CT pulmonary angiography is required to evaluate for PE. Several chest CT features are commonly seen in COVID-19 (including ground-glass opacities, vascular enlargement, bilateral abnormalities, lower lobe involvement, and posterior predilection), whereas others are not, and this may help in diagnostic decision making.
The appearance of COVID-19 on chest CT images follows a somewhat predictable pattern over time. Notably, asymptomatic patients with SARS-CoV-2 infection frequently have normal chest CT examination results, and the proportion of symptomatic patients with COVID-19 and a normal chest CT examination is nonnegligible. Furthermore, lung abnormalities on chest CT images are nonspecific for COVID-19. Owing to these limitations, chest CT should not be used as an independent diagnostic tool to exclude or confirm COVID-19. RT-PCR test results are the standard for diagnosis and key component in clinical decision making.
Nevertheless, chest CT has been suggested to have potential value as a rapid triaging tool in patients with moderate to severe respiratory symptoms in a resource-constrained environment where COVID-19 is highly prevalent. In addition, chest CT may be performed if an alternative diagnosis is suspected. Typical or indeterminate features of COVID-19 pneumonia may be incidentally detected at CT performed for other reasons. In these cases, the interpreting radiologist should discuss the possibility of COVID-19 with the referring physician in a timely manner. Standardized reporting according to guidelines such as those proposed by the RSNA can facilitate this information transfer. Furthermore, chest CT may be valuable to evaluate patients with clinical deterioration for COVID-19 progression or secondary cardiopulmonary complications such as ARDS, PE, superimposed pneumonia, or heart failure. Future studies that define the prognostic role of chest CT in COVID-19 are needed.
For this journal-based SA-CME activity, the authors, editor, and reviewers have disclosed no relevant relationships.
Abbreviations:
- ARDS
- acute respiratory distress syndrome
- COVID-19
- coronavirus disease 2019
- PE
- pulmonary embolism
- PPE
- personal protective equipment
- RSNA
- Radiological Society of North America
- RT-PCR
- reverse transcription–polymerase chain reaction
- SARS-CoV-2
- severe acute respiratory syndrome coronavirus 2
- WHO
- World Health Organization
References
- 1. World Health Organization . Coronavirus . https://www.who.int/health-topics/coronavirus#tab=tab_1 . Accessed June 6, 2020 . [Google Scholar]
- 2. World Health Organization . Pneumonia of unknown caus: China . https://www.who.int/csr/don/05-january-2020-pneumonia-of-unkown-cause-china/en/ . Published January 5, 2020. Accessed June 6, 2020 . [Google Scholar]
- 3. World Health Organization . WHO announces COVID-19 outbreak a pandemic . http://www.euro.who.int/en/health-topics/health-emergencies/coronavirus-covid-19/news/news/2020/3/who-announces-covid-19-outbreak-a-pandemic . Published March 12, 2020. Accessed June 6, 2020 .
- 4. Johns Hopkins University School of Medicine . Coronavirus Resource Center . https://coronavirus.jhu.edu/ . Updated June 13, 2020. Accessed June 13, 2020 . [Google Scholar]
- 5. Thanh Le T , Andreadakis Z , Kumar A , et al. The COVID-19 vaccine development landscape . Nat Rev Drug Discov 2020. ; 19 ( 5 ): 305 – 306 . [DOI] [PubMed] [Google Scholar]
- 6. Huang C , Wang Y , Li X , et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China . Lancet 2020. ; 395 ( 10223 ): 497 – 506 [Published correction appears in Lancet 2020;395(10223):496.] . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yuki K , Fujiogi M , Koutsogiannaki S . COVID-19 pathophysiology: A review . Clin Immunol 2020. ; 215 : 108427 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hoffmann M , Kleine-Weber H , Schroeder S , et al. SARS-CoV-2 Cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor . Cell 2020. ; 181 ( 2 ): 271 – 280 . e8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xu H , Zhong L , Deng J , et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa . Int J Oral Sci 2020. ; 12 ( 1 ): 8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Verdecchia P , Cavallini C , Spanevello A , Angeli F . The pivotal link between ACE2 deficiency and SARS-CoV-2 infection . Eur J Intern Med 2020. ; 76 : 14 – 20 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sungnak W , Huang N , Bécavin C , et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes . Nat Med 2020. ; 26 ( 5 ): 681 – 687 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Patel AB , Verma A . Nasal ACE2 levels and COVID-19 in children . JAMA doi: 10.1001/jama.2020.8946. Published online May 20, 2020. Accessed June 6, 2020 . [DOI] [PubMed]
- 13. Bunyavanich S , Do A , Vicencio A . Nasal gene expression of angiotensin-converting enzyme 2 in children and adults . JAMA 2020. ; 323 ( 23 ): 2427 – 2429 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhu J , Ji P , Pang J , et al. Clinical characteristics of 3062 COVID-19 patients: A meta-analysis . J Med Virol doi: 10.1002/jmv.25884. Published online April 15, 2020. Accessed June 6, 2020 . [DOI] [PMC free article] [PubMed]
- 15. Agyeman AA , Chin KL , Landersdorfer CB , Liew D , Ofori-Asenso R . Smell and taste dysfunction in patients with COVID-19: a systematic review and meta-analysis . Mayo Clin Proc 2020. ; 95 ( 8 ): 1621 – 1631 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gao Z , Xu Y , Sun C , et al. A systematic review of asymptomatic infections with COVID-19 . J Microbiol Immunol Infect doi: 10.1016/j.jmii.2020.05.001. Published online May 15, 2020. Accessed June 6, 2020 . [DOI] [PMC free article] [PubMed]
- 17. Arons MM , Hatfield KM , Reddy SC , et al. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility . N Engl J Med 2020. ; 382 ( 22 ): 2081 – 2090 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Al-Tawfiq JA , Leonardi R , Fasoli G , Rigamonti D . Prevalence and fatality rates of COVID-19: What are the reasons for the wide variations worldwide? Travel Med Infect Dis 2020. ; 35 : 101711 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yang J , Zheng Y , Gou X , et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis . Int J Infect Dis 2020. ; 94 : 91 – 95 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. World Health Organization . Global surveillance for COVID-19 caused by human infection with COVID-19 virus . https://www.who.int/docs/default-source/coronaviruse/2020-03-20-surveillance.pdf?sfvrsn=e6be6ef1_2 . Published March 20, 2020. Accessed June 6, 2020 . [Google Scholar]
- 21. Sethuraman N , Jeremiah SS , Ryo A . Interpreting diagnostic tests for SARS-CoV-2 . JAMA doi: 10.1001/jama.2020.8259. Published online May 6, 2020. Accessed June 6, 2020 . [DOI] [PubMed]
- 22. Kim H , Hong H , Yoon SH . Diagnostic Performance of CT and Reverse Transcriptase Polymerase Chain Reaction for Coronavirus Disease 2019: A Meta-Analysis . Radiology 2020. ; 296 ( 3 ): E145 – E155 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. World Health Organization . Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases . https://www.who.int/publications-detail/laboratory-testing-for-2019-novel-coronavirus-in-suspected-human-cases-20200117 . Published March 19, 2020. Accessed June 6, 2020 .
- 24. Wölfel R , Corman VM , Guggemos W , et al. Virological assessment of hospitalized patients with COVID-2019 . Nature 2020. ; 581 ( 7809 ): 465 – 469 . [DOI] [PubMed] [Google Scholar]
- 25. Döhla M , Boesecke C , Schulte B , et al. Rapid point-of-care testing for SARS-CoV-2 in a community screening setting shows low sensitivity . Public Health 2020. ; 182 : 170 – 172 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Imai K , Tabata S , Ikeda M , et al. Clinical evaluation of an immunochromatographic IgM/IgG antibody assay and chest computed tomography for the diagnosis of COVID-19 . J Clin Virol 2020. ; 128 : 104393 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xiang F , Wang X , He X , et al. Antibody detection and dynamic characteristics in patients with COVID-19 . Clin Infect Dis doi: 10.1093/cid/ciaa461. Published online April 19, 2020. Accessed June 6, 2020 . [DOI] [PMC free article] [PubMed]
- 28. World Health Organization . Advice on the use of point-of-care immunodiagnostic tests for COVID-19 . https://www.who.int/news-room/commentaries/detail/advice-on-the-use-of-point-of-care-immunodiagnostic-tests-for-covid-19 . Published April 8, 2020. Accessed June 6, 2020 . [Google Scholar]
- 29. Ching L , Chang SP , Nerurkar VR . COVID-19 special column: principles behind the technology for detecting SARS-CoV-2, the cause of COVID-19 . Hawaii J Health Soc Welf 2020. ; 79 ( 5 ): 136 – 142 . [PMC free article] [PubMed] [Google Scholar]
- 30. Klompas M . Coronavirus Disease 2019 (COVID-19): Protecting hospitals from the invisible . Ann Intern Med 2020. ; 172 ( 9 ): 619 – 620 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guo YR , Cao QD , Hong ZS , et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak: an update on the status . Mil Med Res 2020. ; 7 ( 1 ): 11 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li Q , Guan X , Wu P , et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia . N Engl J Med 2020. ; 382 ( 13 ): 1199 – 1207 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Setti L , Passarini F , De Gennaro G , et al. Airborne transmission route of COVID-19: why 2 meters/6 feet of inter-personal distance could not be enough . Int J Environ Res Public Health 2020. ; 17 ( 8 ): 2932 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. van Doremalen N , Bushmaker T , Morris DH , et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1 . N Engl J Med 2020. ; 382 ( 16 ): 1564 – 1567 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mossa-Basha M , Medverd J , Linnau KF , et al. Policies and guidelines for COVID-19 preparedness: experiences from the University of Washington . Radiology 2020. ; 296 ( 2 ): E26 – E31 . [DOI] [PubMed] [Google Scholar]
- 36. Mossa-Basha M , Meltzer CC , Kim DC , Tuite MJ , Kolli KP , Tan BS . Radiology department preparedness for COVID-19: Radiology scientific expert review panel . Radiology 2020. ; 296 ( 2 ): E106 – E112 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee JK , Jeong HW . Wearing face masks regardless of symptoms is crucial for preventing the spread of COVID-19 in hospitals . Infect Control Hosp Epidemiol doi: 10.1017/ice.2020.202. Published online May 6, 2020. Accessed June 6, 2020 . [DOI] [PMC free article] [PubMed]
- 38. Lu R , Zhao X , Li J , et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding . Lancet 2020. ; 395 ( 10224 ): 565 – 574 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Alraddadi BM , Al-Salmi HS , Jacobs-Slifka K , et al. Risk factors for middle east respiratory syndrome coronavirus infection among healthcare personnel . Emerg Infect Dis 2016. ; 22 ( 11 ): 1915 – 1920 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rodrigues JCL , Hare SS , Edey A , et al. An update on COVID-19 for the radiologist: A British society of Thoracic Imaging statement . Clin Radiol 2020. ; 75 ( 5 ): 323 – 325 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kang Z , Li X , Zhou S . Recommendation of low-dose CT in the detection and management of COVID-2019 . Eur Radiol 2020. ; 30 ( 8 ): 4356 – 4357 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Agostini A , Floridi C , Borgheresi A , et al. Proposal of a low-dose, long-pitch, dual-source chest CT protocol on third-generation dual-source CT using a tin filter for spectral shaping at 100 kVp for CoronaVirus Disease 2019 (COVID-19) patients: a feasibility study . Radiol Med (Torino) 2020. ; 125 ( 4 ): 365 – 373 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Adams HJA , Kwee TC , Yakar D , Hope MD , Kwee RM . Chest CT Imaging Signature of Coronavirus Disease 2019 Infection: In Pursuit of the Scientific Evidence . Chest doi: 10.1016/j.chest.2020.06.025. Published online June 25, 2020. Accessed June 6, 2020 . [DOI] [PMC free article] [PubMed]
- 44. Schaller T , Hirschbühl K , Burkhardt K , et al. Postmortem examination of patients with COVID-19 . JAMA 2020. ; 323 ( 24 ): 2518 – 2520 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Barton LM , Duval EJ , Stroberg E , Ghosh S , Mukhopadhyay S . COVID-19 Autopsies, Oklahoma, USA . Am J Clin Pathol 2020. ; 153 ( 6 ): 725 – 733 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Koo HJ , Lim S , Choe J , Choi SH , Sung H , Do KH . Radiographic and CT Features of Viral Pneumonia . RadioGraphics 2018. ; 38 ( 3 ): 719 – 739 . [DOI] [PubMed] [Google Scholar]
- 47. Wang Y , Dong C , Hu Y , et al. Temporal changes of CT findings in 90 patients with COVID-19 pneumonia: a longitudinal study . Radiology 2020. ; 296 ( 2 ): E55 – E64 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ding X , Xu J , Zhou J , Long Q . Chest CT findings of COVID-19 pneumonia by duration of symptoms . Eur J Radiol 2020. ; 127 : 109009 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bernheim A , Mei X , Huang M , et al. Chest CT Findings in Coronavirus Disease-19 (COVID-19): Relationship to Duration of Infection . Radiology 2020. ; 295 ( 3 ): 200463 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Inui S , Fujikawa A , Jitsu M , et al. Erratum: Chest CT findings in cases from the cruise ship “Diamond Princess” with coronavirus disease 2019 (COVID-19) . Radiol Cardiothorac Imaging 2020. ; 2 ( 2 ): e204002 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Simpson S , Kay FY , Abbara S , et al. Radiological Society of North America expert consensus statement on reporting chest CT findings related to COVID-19: Endorsed by the Society of Thoracic Radiology, the American College of Radiology, and RSNA . Radiol Cardiothorac Imaging 2020. ; 2 ( 2 ): e200152 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. McGuinness G , Zhan C , Rosenberg N , et al. High incidence of barotrauma in patients with COVID-19 infection on invasive mechanical ventilation . Radiology doi: 10.1148/radiol.2020202352. Published online July 1, 2020. Accessed June 6, 2020 . [DOI] [PMC free article] [PubMed]
- 53. Pan F , Ye T , Sun P , et al. Time Course of Lung Changes at Chest CT during Recovery from Coronavirus Disease 2019 (COVID-19) . Radiology 2020. ; 295 ( 3 ): 715 – 721 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pan Y , Guan H , Zhou S , et al. Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): a study of 63 patients in Wuhan, China . Eur Radiol 2020. ; 30 ( 6 ): 3306 – 3309 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Guan CS , Lv ZB , Yan S , et al. Imaging Features of Coronavirus disease 2019 (COVID-19): Evaluation on Thin-Section CT . Acad Radiol 2020. ; 27 ( 5 ): 609 – 613 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lei P , Fan B , Mao J , Wei J , Wang P . The progression of computed tomographic (CT) images in patients with coronavirus disease (COVID-19) pneumonia . J Infect 2020. ; 80 ( 6 ): e30 – e31 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yu M , Xu D , Lan L , et al. Thin-section chest CT Imaging of coronavirus disease 2019 pneumonia: comparison between patients with mild and severe disease . Radiol Cardiothorac Imaging 2020. ; 2 ( 2 ): e200126 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zompatori M , Ciccarese F , Fasano L . Overview of current lung imaging in acute respiratory distress syndrome . Eur Respir Rev 2014. ; 23 ( 134 ): 519 – 530 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rubin GD , Ryerson CJ , Haramati LB , et al. The role of chest imaging in patient management during the COVID-19 pandemic: a multinational consensus statement from the Fleischner society . Chest 2020. ; 158 ( 1 ): 106 – 116 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. O’Sullivan JW , Muntinga T , Grigg S , Ioannidis JPA . Prevalence and outcomes of incidental imaging findings: umbrella review . BMJ 2018. ; 361 : k2387 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Dutch Association of Medical Specialists . Practice guideline: Pre-operative work-up for COVID-19 infection in asymptomatic patients scheduled for surgery under general anesthesia . https://www.demedischspecialist.nl/sites/default/files/leidraad_preoperatieve_workup.pdf . Published May 1, 2020. Accessed June 6, 2020 .
- 62. Bellolio MF , Heien HC , Sangaralingham LR , et al. Increased computed tomography utilization in the emergency department and its association with hospital admission . West J Emerg Med 2017. ; 18 ( 5 ): 835 – 845 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bellolio MF , Bellew SD , Sangaralingham LR , et al. Access to primary care and computed tomography use in the emergency department . BMC Health Serv Res 2018. ; 18 ( 1 ): 154 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Jain R , Young M , Dogra S , Kennedy H , Nguyen V , Raz E . Surprise diagnosis of COVID-19 following neuroimaging evaluation for unrelated reasons during the pandemic in hot spots . AJNR Am J Neuroradiol 2020. ; 41 ( 7 ): 1177 – 1178 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kwee RM , Krdzalic J , Fasen BACM , de Jaegere TMH ; COVID-19 CT Investigators South-East Netherlands (CISEN) Study Group. CT Scanning in Suspected Stroke or Head Trauma: Is it Worth Going the Extra Mile and Including the Chest to Screen for COVID-19 Infection? AJNR Am J Neuroradiol 2020. ; 41 ( 7 ): 1165 – 1169 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kihira S , Schefflein J , Chung M , et al. Incidental COVID-19 related lung apical findings on stroke CTA during the COVID-19 pandemic . J Neurointerv Surg 2020. ; 12 ( 7 ): 669 – 672 . [DOI] [PubMed] [Google Scholar]
- 67. Hossain R , Lazarus MS , Roudenko A , et al. CT Scans obtained for nonpulmonary indications: associated respiratory findings of COVID-19 . Radiology 2020. ; 296 ( 3 ): E173 – E179 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Dane B , Brusca-Augello G , Kim D , Katz DS . Unexpected findings of coronavirus disease (COVID-19) at the lung bases on abdominopelvic CT . AJR Am J Roentgenol doi: 10.2214/AJR.20.23240. Published online April 22, 2020. Accessed June 6, 2020 . [DOI] [PubMed]
- 69. Siegel A , Chang PJ , Jarou ZJ , et al. Lung base findings of coronavirus disease (COVID-19) on abdominal CT in patients with predominant gastrointestinal symptoms . AJR Am J Roentgenol doi: 10.2214/AJR.20.23232. Published online April 17, 2020. Accessed June 6, 2020 . [DOI] [PubMed]
- 70. Lima DS , Ribeiro MAF Jr , Gallo G , Di Saverio S . Role of chest CT in patients with acute abdomen during the COVID-19 era . Br J Surg 2020. ; 107 ( 7 ): e196 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Nguyen TN , Abdalkader M , Jovin TG , et al. Mechanical thrombectomy in the era of the COVID-19 pandemic: emergency preparedness for neuroscience teams—a guidance statement from the Society of Vascular and Interventional Neurology . Stroke 2020. ; 51 ( 6 ): 1896 – 1901 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Nishino M , Itoh H , Hatabu H . A practical approach to high-resolution CT of diffuse lung disease . Eur J Radiol 2014. ; 83 ( 1 ): 6 – 19 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Adams HJA , Kwee TC , Yakar D , Hope MD , Kwee RM . Systematic Review and Meta-Analysis on the Value of Chest CT in the Diagnosis of Coronavirus Disease (COVID-19): Sol Scientiae, Illustra Nos . AJR Am J Roentgenol doi: 10.2214/AJR.20.23391. Published online June 1, 2020. Accessed June 6, 2020 . [DOI] [PubMed]
- 74. Raptis CA , Hammer MM , Short RG , et al. Chest CT and coronavirus disease (COVID-19): a critical review of the literature to date . AJR Am J Roentgenol doi: 10.2214/AJR.20.23202. Published online April 16, 2020. Accessed June 6, 2020 . [DOI] [PubMed]
- 75. Franquet T . Imaging of pulmonary viral pneumonia . Radiology 2011. ; 260 ( 1 ): 18 – 39 . [DOI] [PubMed] [Google Scholar]
- 76. Bai HX , Hsieh B , Xiong Z , et al. Performance of Radiologists in Differentiating COVID-19 from Non-COVID-19 Viral Pneumonia at Chest CT . Radiology 2020. ; 296 ( 2 ): E46 – E54 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wang H , Wei R , Rao G , Zhu J , Song B . Characteristic CT findings distinguishing 2019 novel coronavirus disease (COVID-19) from influenza pneumonia . Eur Radiol 2020. ; 30 ( 9 ): 4910 – 4917 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Liu M , Zeng W , Wen Y , Zheng Y , Lv F , Xiao K . COVID-19 pneumonia: CT findings of 122 patients and differentiation from influenza pneumonia . Eur Radiol doi: 10.1007/s00330-020-06928-0. Published online May 12, 2020. Accessed June 6, 2020 . [DOI] [PMC free article] [PubMed]
- 79. Yin Z , Kang Z , Yang D , Ding S , Luo H , Xiao E . A Comparison of clinical and chest CT findings in patients with influenza A (H1N1) virus infection and coronavirus disease (COVID-19) . AJR Am J Roentgenol doi: 10.2214/AJR.20.23214. Published online May 26, 2020. Accessed June 6, 2020 . [DOI] [PubMed]
- 80. Centers for Disease Control and Prevention . Prevention strategies for seasonal influenza in healthcare settings . https://www.cdc.gov/flu/professionals/infectioncontrol/healthcaresettings.htm . Updated October 30, 2018. Accessed June 6, 2020 . [Google Scholar]
- 81. Adams HJA , Kwee TC , Kwee RM . COVID-19 and chest CT: do not put the sensitivity value in the isolation room and look beyond the numbers . Radiology doi: 10.1148/radiol.2020201709. Published online April 27, 2020. Accessed June 6, 2020 . [DOI] [PMC free article] [PubMed]
- 82. Hammer MM , Raptis CA , Henry TS , Shah A , Bhalla S , Hope MD . Challenges in the interpretation and application of typical imaging features of COVID-19 . Lancet Respir Med 2020. ; 8 ( 6 ): 534 – 536 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Eng J , Bluemke DA . Imaging publications in the COVID-19 pandemic: applying new research results to clinical practice . Radiology doi: 10.1148/radiol.2020201724. Published online April 23, 2020. Accessed June 6, 2020 . [DOI] [PMC free article] [PubMed]
- 84. ACR Practice Parameter for Communication of Diagnostic Imaging Findings . https://www.acr.org/-/media/ACR/Files/Practice-Parameters/communicationdiag.pdf?la=en . Updated 2014. Accessed June 6, 2020 .
- 85. De Jaegere TM , Krdzalic J , Fasen BA , Kwee RM ; COVID-19 CT Investigators South-East Netherlands (CISEN) Study Group. Radiological Society of North America chest CT classification system for reporting COVID-19 pneumonia: interobserver variability and correlation with RT-PCR . Radiol Cardiothorac Imaging 2020. ; 2 ( 3 ): e200213 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Li X , Ma X . Acute respiratory failure in COVID-19: is it “typical” ARDS? Crit Care 2020. ; 24 ( 1 ): 198 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wu C , Chen X , Cai Y , et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China . JAMA Intern Med 2020. ; 180 ( 7 ): 1 – 11 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Hirano T , Murakami M . COVID-19: A new virus, but a familiar receptor and cytokine release syndrome . Immunity 2020. ; 52 ( 5 ): 731 – 733 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Matthay MA , Zemans RL , Zimmerman GA , et al. Acute respiratory distress syndrome . Nat Rev Dis Primers 2019. ; 5 ( 1 ): 18 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Salehi S , Abedi A , Balakrishnan S , Gholamrezanezhad A . Coronavirus disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients . AJR Am J Roentgenol 2020. ; 215 ( 1 ): 87 – 93 . [DOI] [PubMed] [Google Scholar]
- 91. ARDS Definition Task Force ; Ranieri VM , Rubenfeld GD , et al. Acute respiratory distress syndrome: the Berlin Definition . JAMA 2012. ; 307 ( 23 ): 2526 – 2533 . [DOI] [PubMed] [Google Scholar]
- 92. Tay MZ , Poh CM , Rénia L , MacAry PA , Ng LFP . The trinity of COVID-19: immunity, inflammation and intervention . Nat Rev Immunol 2020. ; 20 ( 6 ): 363 – 374 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Klok FA , Kruip MJHA , van der Meer NJM , et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: An updated analysis . Thromb Res 2020. ; 191 : 148 – 150 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Deshpande C . Thromboembolic findings in COVID-19 autopsies: pulmonary thrombosis or embolism? Ann Intern Med doi: 10.7326/M20-3255. Published online May 15, 2020. Accessed June 6, 2020 . [DOI] [PMC free article] [PubMed]
- 95. Oudkerk M , Büller HR , Kuijpers D , et al. Diagnosis, prevention, and treatment of thromboembolic complications in COVID-19: report of the National Institute for Public Health of the Netherlands . Radiology doi: 10.1148/radiol.2020201629. Published online April 23, 2020. Accessed June 6, 2020 . [DOI] [PMC free article] [PubMed]
- 96. National Institutes of Health . COVID-19 Treatment Guidelines: Antithrombotic Therapy in Patients with COVID-19 . https://www.covid19treatmentguidelines.nih.gov/antithrombotic-therapy . Updated May 12, 2020. Accessed June 6, 2020 .
- 97. Helms J , Tacquard C , Severac F , et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study . Intensive Care Med 2020. ; 46 ( 6 ): 1089 – 1098 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Poyiadji N , Cormier P , Patel PY , et al. Acute pulmonary embolism and COVID-19 . Radiology doi: 10.1148/radiol.2020201955. Published online May 14, 2020. Accessed June 6, 2020 . [DOI] [PMC free article] [PubMed]
- 99. Bompard F , Monnier H , Saab I , et al. Pulmonary embolism in patients with COVID-19 pneumonia . Eur Respir J 2020. ; 56 ( 1 ): 2001365 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Grillet F , Behr J , Calame P , Aubry S , Delabrousse E . Acute Pulmonary Embolism Associated with COVID-19 Pneumonia Detected with Pulmonary CT Angiography . Radiology 2020. ; 296 ( 3 ): E186 – E188 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Kaminetzky M , Moore W , Fansiwala K , et al. Pulmonary embolism on CTPA in COVID-19 patients . Radiol Cardiothorac Imaging 2020. ; 2 ( 4 ): e200308 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Roncon L , Zuin M , Zonzin P . Age-adjusted D-dimer cut-off levels to rule out venous thromboembolism in COVID-19 patients . Thromb Res 2020. ; 190 : 102 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Lippi G , Favaloro EJ . D-dimer is associated with severity of coronavirus disease 2019: a pooled analysis . Thromb Haemost 2020. ; 120 ( 5 ): 876 – 878 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Tang N , Li D , Wang X , Sun Z . Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia . J Thromb Haemost 2020. ; 18 ( 4 ): 844 – 847 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Huttner BD , Catho G , Pano-Pardo JR , Pulcini C , Schouten J . COVID-19: don’t neglect antimicrobial stewardship principles! Clin Microbiol Infect 2020. ; 26 ( 7 ): 808 – 810 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Menter T , Haslbauer JD , Nienhold R , et al. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction . Histopathology doi: 10.1111/his.14134. Published online May 4, 2020. Accessed June 6, 2020 . [DOI] [PMC free article] [PubMed]
- 107. Tian S , Xiong Y , Liu H , et al. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies . Mod Pathol 2020. ; 33 ( 6 ): 1007 – 1014 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Wu JH , Li X , Huang B , et al. Pathological changes of fatal coronavirus disease 2019 (COVID-19) in the lungs: report of 10 cases by postmortem needle autopsy [in Chinese] . Zhonghua Bing Li Xue Za Zhi 2020. ; 49 ( 6 ): 568 – 575 . [DOI] [PubMed] [Google Scholar]
- 109. Shi S , Qin M , Shen B , et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China . JAMA Cardiol 2020. ; 5 ( 7 ): 802 – 810 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Li K , Wu J , Wu F , et al. The clinical and chest CT features associated with severe and critical COVID-19 pneumonia . Invest Radiol 2020. ; 55 ( 6 ): 327 – 331 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Guzik TJ , Mohiddin SA , Dimarco A , et al. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options . Cardiovasc Res 2020. ; 116 ( 10 ): 1666 – 1687 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Cizgici AY , Zencirkiran Agus H , Yildiz M . COVID-19 myopericarditis: It should be kept in mind in today’s conditions . Am J Emerg Med 2020. ; 38 ( 7 ): 1547.e5 – 1547.e6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Inciardi RM , Lupi L , Zaccone G , et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) . JAMA Cardiol 2020. ; 5 ( 7 ): 1 – 6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Hua A , O’Gallagher K , Sado D , Byrne J . Life-threatening cardiac tamponade complicating myo-pericarditis in COVID-19 . Eur Heart J 2020. ; 41 ( 22 ): 2130 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Wang ZJ , Reddy GP , Gotway MB , Yeh BM , Hetts SW , Higgins CB . CT and MR imaging of pericardial disease . RadioGraphics 2003. ; 23 ( Spec no ): S167 – S180 . [DOI] [PubMed] [Google Scholar]
- 116. Yang R , Li X , Liu H , et al. Chest CT severity score: an imaging tool for assessing severe COVID-19 . Radiol Cardiothorac Imaging 2020. ; 2 ( 2 ): e200047 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Wynants L , Van Calster B , Collins GS , et al. Prediction models for diagnosis and prognosis of covid-19 infection: systematic review and critical appraisal . BMJ 2020. ; 369 : m1328 [Published correction appears in BMJ 2020;369:m2204.] . [DOI] [PMC free article] [PubMed] [Google Scholar]