Abstract
BRASH syndrome is characterized by bradycardia, renal failure, use of an atrioventricular nodal blocker (AVNB), shock, and hyperkalemia. These symptoms represent an ongoing vicious cycle in a patient with a low glomerular filtration rate taking an AVNB. Decreased clearance of the medication and hyperkalemia associated with renal failure synergize to cause bradycardia and hypoperfusion. This reaction causes renal function to worsen, thereby perpetuating the cycle of BRASH syndrome.
Keywords: bradycardia, renal failure, atrioventricular nodal blocker, shock, hyperkalemia, BRASH syndrome
INTRODUCTION
Although life-threatening arrhythmias can occur at any potassium level, hyperkalemia-associated arrhythmias are more common with potassium levels above 6.5 mEq/L. Electrocardiographic (ECG) abnormalities that are disproportionate to the degree of hyperkalemia, such as bradycardia, should prompt consideration of concomitant contributory factors that may be amplifying the effects of mildly elevated potassium levels.
One such under-recognized entity is BRASH syndrome, which was initially coined on social media but has recently been described in a few case reports.1–7 It is important to note that patients with BRASH syndrome typically have profound bradycardia that is out of proportion to their hyperkalemia or use of an atrioventricular nodal blocker (AVNB). Additionally, such patients are often refractory to overdose antidotes and chronotropic agents; therefore, early diagnosis is critical to avoid multiorgan failure.8 Herein we present a case of BRASH syndrome with mild hyperkalemia in a patient taking diltiazem.
CASE PRESENTATION
A 55-year-old female with a history of stage 5 chronic kidney disease (CKD) and type-2 diabetes mellitus was referred to the emergency department (ED) for worsening dyspnea, bilateral lower extremity edema, and increased drowsiness. In addition to her history of CKD and diabetes, the patient had essential hypertension on diltiazem 480 mg once daily, hydralazine 50 mg thrice daily, bumetanide 1 mg twice daily, metolazone 5 mg once before the morning dose of bumetanide, atorvastatin 40 mg once daily, and insulin glargine 18 units subcutaneously at night. In the ED, her physical exam was remarkable for blood pressure of 218/79 mm Hg, bilateral lower extremity edema to the knees, and bibasilar crackles. The rest of her vital signs were within normal limits.
Laboratory findings included creatinine of 13.5 mg/dL, blood urea nitrogen (BUN) of 116 mg/dL, bicarbonate of 15 mEq/L, and potassium of 5.0 mEq/L. The ECG was unremarkable (Figure 1), and the chest x-ray showed mild pulmonary congestion with small bilateral pleural effusions. The patient was admitted to the general medicine ward with a preliminary diagnosis of volume overload secondary to renal failure with a plan to initiate hemodialysis. In the interim, she was started on intravenous bumetanide 2 mg every 12 hrs with metolazone 5 mg before the dose of the loop diuretic. She had a net negative fluid balance of 1360 mL through her first night in the hospital with some clinical improvement in volume status.
Figure 1.
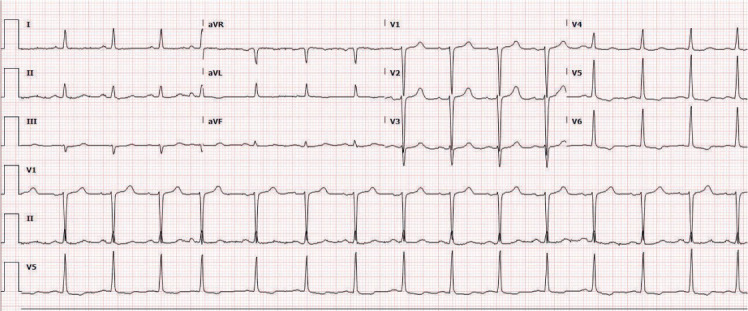
Sinus rhythm with nonspecific T wave abnormalities.
The next day, however, her clinical course was complicated by profound hypotension (82/37 mm Hg), bradycardia of 30 to 40 beats/min, and a serum potassium level of 5.4 mEq/L. She was afebrile and had no focal signs of infection, although an ECG showed narrow-complex bradycardia (Figure 2). She was transferred to the medical intensive care unit for further management and given calcium gluconate, regular insulin with dextrose, albuterol inhalation, and sodium polystyrene sulfonate for her hyperkalemia. She was also administered two doses of atropine (1 mg followed by 500 mcg) for her significant bradycardia, and transcutaneous pads were placed to facilitate transvenous pacing. When the bradycardia did not significantly improve, a dopamine infusion at 5 mcg/kg/min was begun and titrated to 20 mcg/kg/min.
Figure 2.
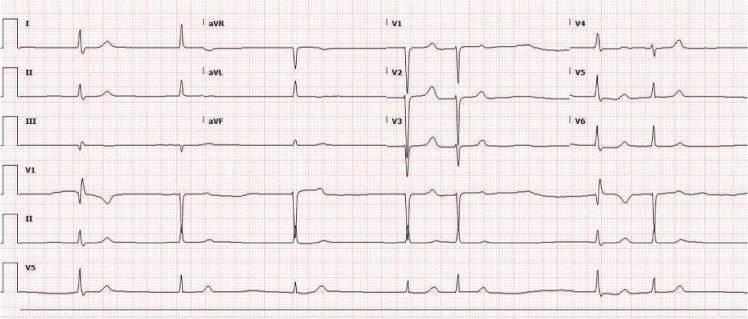
Narrow-complex bradycardia.
Because the bradycardia and hypotension were disproportionate to the hyperkalemia, and she had been on a stable dose of diltiazem for many months, a thorough infectious work-up was performed to exclude sepsis. Blood, urine cultures, and all the other infectious work-up came up negative. Diltiazem was stopped, and the constellation of bradycardia and hypotension was attributed to the combination of hyperkalemia and the atrioventricular nodal blocking effect of diltiazem. After initiating the dopamine infusion, her bradycardia and blood pressure started to improve. A temporary dialysis catheter was placed in the right femoral vein, and a session of hemodialysis was performed. She regained normal sinus rhythm and rate over the next 24 hours and was transferred to a general medicine floor for observation.
The patient was ultimately discharged as a newly declared end-stage renal disease patient requiring dialysis. Diltiazem was switched to nifedipine 60 mg daily. The patient was seen in the clinic after 2 weeks from hospital discharge and was found to be doing well on hemodialysis with a normal heart rate and blood pressure.
DISCUSSION
Given the increasing number of patients with CKD and multiple comorbidities who require polypharmacy, it is common to see an AVNB on their medication list. In such patients, recognizing BRASH syndrome becomes pertinent as it embodies conglomerate yet closely interlinked factors that could lead to an ominous clinical course if not identified early. Our case was a classic presentation of such findings; the patient began a rather benign hospital course that was unexpectedly complicated owing to the presence of all the risk factors for BRASH syndrome. Importantly, she never developed severe hyperkalemia, unlike other previously reported cases, yet the augmented effect of diltiazem therapy was remarkable.
A reasonable explanation for not initially withholding diltiazem could be its predominant hepatic metabolization, which usually does not warrant renal adjustment in patients with underlying CKD. However, this highlights the caveat that the atrioventricular nodal blocking effect of any medication can lead to marked bradycardia if compounded by even mild hyperkalemia. The result can be a significant decrease in cardiac output, causing shock with further worsening of renal function. This, in turn, can aggravate the hyperkalemia, which would further augment the AVNB effect and thus complete the vicious cycle of BRASH syndrome (Figure 3).
Figure 3.
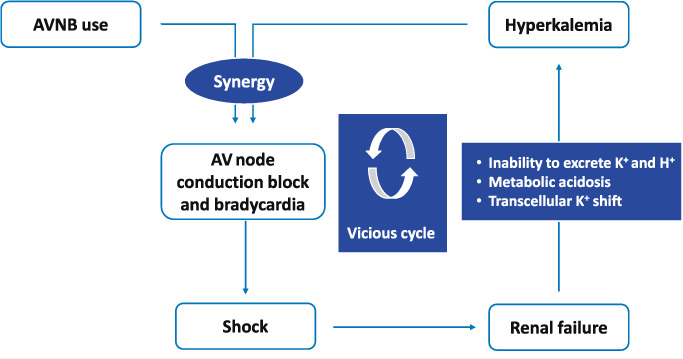
The vicious cycle of BRASH syndrome. AVNB: atrioventricular nodal blocker
Only a few cases of BRASH syndrome have been reported to date (Table 1).1–8 We postulate that there are many more cases with these findings that are not reported with this specific term. Previously reported cases show the variety of AVNBs that can have a synergistic effect in the presence of hyperkalemia.1–8 It is also noteworthy that BRASH syndrome can occur in patients taking an AVNB who do not have CKD but develop AKI that is severe enough to cause hyperkalemia.1
Table 1.
Previously reported cases of BRASH syndrome. AKI: acute kidney injury; CKD: chronic kidney disease; AVNB: atrioventricular nodal blocker
| REFERENCE | CR* (MG/DL) | AKI† OR CKD‡ | AVNB§ USED | POTASSIUM LEVEL (MEQ/L) | SEVERITY OF BRADYCARDIA (BEATS/MIN) | RECOVERY TIME (HRS) |
|---|---|---|---|---|---|---|
| 1 | 3.3 | AKI | Carvedilol | 8.6 | 20 to 30s | 24 |
| 2 | NA# | NA | Metoprolol | 7.4 | 40 | 24 |
| 3 | 6.11 | CKD | Diltiazem and Sotalol | 8.6 | 35 | 24 |
| 4 | NA | AKI on CKD 4 | Beta blocker (name not mentioned) | 7.1 | 30s | 24 |
| 5 | 4.38 | CKD 4 | Metoprolol | 6.6 | 17 to 30s | 24 |
| 6a | 3.05 | AKI on CKD | Verapamil | 5.6 | 48 | 36 |
| 6b | 1.79 | AKI on CKD | Verapamil | 5.5 | 44 | NA |
| 7 | NA | CKD | Metoprolol | 7.4 | 40 | 24 |
| 8 | 3.01 | AKI on CKD | Carvedilol, verapamil | 6.5 | 33 | 1 |
| Our Case | 13.5 | CKD 5 | Diltiazem | 5.4 | 30 to 40 | 24 |
* serum creatinine;
† acute kidney injury;
‡ chronic kidney disease;
§ atrioventricular nodal blocker;
# information not available
The learning objectives from this case are manifold. Foremost, the propensity of patients with CKD to develop hyperkalemia warrants a meticulous review of concurrent medications. Understanding the synergistic effect of hyperkalemia and AVNB use is of utmost importance when treating patients with CKD since the burden of hyperkalemia is accentuated with a consequently high incidence of poor clinical outcomes if hyperkalemia is not prevented or treated early. Therefore, AVNB agents should not be the primary antihypertensive agents in patients with worsening CKD when other alternatives are available. Nonetheless, if a patient taking an AVNB presents with renal dysfunction and even a mild degree of hyperkalemia, BRASH syndrome should be one of the anticipated complications. Additionally, it is important to recognize that any AVNB including beta blockers and verapamil can cause BRASH syndrome in patients with CKD in the setting of hyperkalemia.
CONCLUSION
BRASH syndrome is a serious complication that warrants a high index of suspicion in patients with CKD or acute kidney injury who take an AVNB. Early recognition of this entity can help avoid development of severe bradycardia and multiorgan failure in high-risk patients. When BRASH syndrome does develop, immediate discontinuation of AVNB agents, prompt correction of hyperkalemia, and other supportive measures can rapidly reverse the hemodynamic instability and lead to a good clinical outcome.
Footnotes
Conflict of Interest Disclosure:
The authors have completed and submitted the Methodist DeBakey Cardiovascular Journal Conflict of Interest Statement and none were reported.
REFERENCES
- 1.Diribe N, Le J. Trimethoprim/Sulfamethoxazole-Induced Bradycardia, Renal Failure, AV-Node Blockers, Shock and Hyperkalemia Syndrome. Clin Pract Cases Emerg Med. 2019 Aug;3(3):282–5. doi: 10.5811/cpcem.2019.5.43118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simmons T, Blazar E. Synergistic Bradycardia from Beta Blockers, Hyperkalemia, and Renal Failure. J Emerg Med. 2019 Aug;57(2):e41–e44. doi: 10.1016/j.jemermed.2019.03.039. [DOI] [PubMed] [Google Scholar]
- 3.Sohal S, Ramachandran A. Syndrome Of Bradycardia, Renal Failure, Atrioventricular Nodal Blockers, Shock, And Hyperkalemia (Brash Syndrome): A New Clinical Entity? Chest. 2019;156(4) [Google Scholar]
- 4.Golchin A, Zhou M, Khan AH. Bradycardia, Renal Failure, AV-Nodal Blockers, Shock, and Hyperkalemia (BRASH) - A New Clinical Syndrome. Am J Respir Crit Care Med. 2018;197:A3467. (abstract) [Google Scholar]
- 5.EMRA [Internet]. Tans CJ. BRASH Syndrome: Profound Bradycardia in the Setting of Mild Hyperkalemia. Irving, TX: EMRA; c2020. 2018 Oct 15 [cited 2020 Jul 5]. Available from: https://www.emra.org/globalassets/emra/be-involved/events–activities/casecon/2018-posters/group-2/calvin_tan_final_poster.pdf. [Google Scholar]
- 6.Prabhu V, Hsu E, Lestin S, Soltanianzadeh Y, Hadi S. Bradycardia, Renal Failure, Atrioventricular Nodal Blockade, Shock, and Hyperkalemia (BRASH) Syndrome as a Presentation of Coronavirus Disease 2019. Cureus. 2020 Apr 24;12(4):e7816. doi: 10.7759/cureus.7816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hegazi MO, Aldabie G, Al-Mutairi S, Sayed AE. Junctional bradycardia with verapamil in renal failure - care required even with mild hyperkalaemia. J Clin Pharm Ther. 2012 Dec;37(6):726–8. doi: 10.1111/j.1365-2710.2012.01352.x. [DOI] [PubMed] [Google Scholar]
- 8.EMCrit [Internet]. Farkas J. PulmCrit-BRASH syndrome: Bradycardia, Renal failure, AV blocker, Shock, Hyperkalemia. Cheyenne, WY: Metasin LLC; c2020. 2016 Feb 15 [cited 2020 Jul 5]. Available from: https://emcrit.org/pulmcrit/brash-syndrome-bradycardia-renal-failure-av-blocker-shock-hyperkalemia. [Google Scholar]


