Abstract
The extraction of secondary metabolites by water, MeOH:water (8:2) containing NaF, methanol, ethanol and acetone (all of them diluted (7:3) in water)from the different parts (leaves, flowers, stems and roots) of Passiflora caerulea L., Physalis peruviana L. and Solanum muricatum Aiton via decoction and maceration methods was studied. The highest extraction yields were recorded by methanol for decoction and acetone for maceration. The total polyphenol content (TPC) obtained by decoction had the highest TPC contents, and MeOH containing NaF was the best solvent for the extraction of TPC. Maceration was suitable for flavonoid extractions, with ethanol and acetone being the best solvents. In general, the highest levels of TPC and flavonoids were obtained from Passiflora leaves regardless of the solvent or extraction method applied. Furthermore, the roots of Physalis and Solanum showed important levels of these compounds in consonance with the total antioxidant activity (TAA) evaluated in the different organs of the plant in the three species. In this study, the solvents and extraction methods applied were tools that determined significantly the level of extraction of bioactive compounds, showing a different impact on plant organs for each medicinal species studied.
Keywords: maceration, decoction, total polyphenol content, flavonoids, antioxidant activity
1. Introduction
Polyphenols are natural compounds that are widely found in plants and their importance continues to grow, in particular because of their impact on organoleptic and health benefits [1,2]. Their role as natural antioxidants is useful in the prevention and treatment of cancer, inflammation and cardiovascular diseases [2]. Indeed, in the extraction of active ingredients with high added value from plants, especially polyphenols, the extraction method applied is a very important step in the isolation, as well as in the identification, of phenolic compounds. As a result, many authors have studied the influence of different extraction methods on the extraction yields of phenolic compounds in plant sources [3]. Among others things, the solubility of the phenolic compounds is affected by the polarity of the solvent used. Further, the determination of phenolic compounds from plant materials is influenced by the extraction technique, the extraction time and the temperature. Therefore, it is difficult to develop an extraction process suitable for the extraction of all the phenolic compounds of plants [3,4,5].
Despite several disadvantages, liquid-liquid and solid-liquid extraction are still the most commonly used extraction procedures for phenolic acids and flavonoids. For many years, the conventional techniques have been widely accepted, mainly because of their ease of use, efficiency, and wide-ranging applicability [6]. Maceration and decoction techniques are very useful methods for weakening the cell wall in vegetal tissues, but in different ways. The main difference between them regards temperature. High temperatures applied with decoction can destroy bioactive compounds, such as polyphenols, so the application of milder temperatures through a gentle maceration process would be a better option [7]. However, applying high temperatures during a decoction process can also destroy enzymes, preventing the degradation of polyphenols [8]. Some modern extraction methods, such as microwave-assisted extraction, ultrahigh pressure extraction and supercritical carbon dioxide extraction, have been utilized for the preparation of plant extracts [9]. These methods have various benefits, such as the improved penetration of solvents into plant particles, a low extraction temperature and reduced extraction time, but maceration is simple, more convenient and less costly in terms of instrumentation [10]. Therefore, it is more applicable in small and medium enterprises (SMEs) and in developing countries [11]. The use of medicinal plants is the first choice of treatment for areas where access to appropriate care is difficult, while traditional herbal medicines provided by traditional healers are fast and available at all times when needed. In this sense, extraction methods based on decoction and maceration are the most used generally [12].
Bioactive compounds, including flavonoids, phenols, anthocyanins, ascorbic acid, amides, alkaloids, tannins, saponins and glycosides, from different part of medicinal species play an important role in human health, due to their biological activity [13].
Passiflora caerulea L., better known as the ‘blue passion flower’ [14], belongs to the family Passifloraceae, which is composed of 18 genera and about 630 species [15]. Passiflora is a rich source of phenolic compounds, amino acid α-alanine and organic acids, including butyric, formic, oleic, linoleic, citric, malic, myristic, linolenic and palmitic acids [16]. Passiflora plants are used in traditional medicine in South America to treat various pathologies associated with the gastrointestinal tract [17,18], and also in the Netherlands, Spain, Italy and Poland [19]. They exhibit various pharmacological properties and possess biologically active complex compounds [20].
Physalis peruviana L. is a plant of the family Solanaceae and the genus Physalis, which has about 100 species [21]. It is commonly referred to as the gooseberry [22]. Physalis peruviana is a medicinal plant widely used in traditional medicine to treat diseases such as malaria, asthma, hepatitis, dermatitis, cancer and rheumatism. It has antispasmodic, diuretic, antiseptic, sedative, analgesic, antioxidant, antifungal, antibacterial, anti-inflammatory, cataract-cleansing, antidiabetic and antiparasitic properties [23,24,25,26,27,28]. It has been described as a good source of nutrients and bioactive compounds, β-carotene and vitamins A and C, and minerals including K, Mg and Cu, with moderate fibre content, phenolic compounds and low levels of calories [29,30].
Solanum muricatum Aiton is a horticultural crop commonly known as ‘pepino’ or ‘pear melon’, and is a member of the family Solanaceae [31]. It is a plant known for its medicinal uses [32], with fruit which are very rich in minerals (Fe, Zn, Cu, Mn, Ca and P) and vitamin C but poor in starch and sugars [33]. It has also been found that the phenolic content of the pepino fruit is much higher than that of vitamin C [34]. In addition to these nutritional aspects, Solanum muricatum Aiton is known for its anti-tumor, antioxidant, antidiabetic and anti-inflammatory properties [35,36].
The investigation of these plants and their derived chemical compounds offers a different source of natural products, which could be a promising area of functional ingredient study. This type of processing is very sustainable in industrial production, as it has a secured market for the final product (food and pharmaceutical industry) and cheap raw material [37]. In this sense, during the production of passion fruit, cape gooseberry or pepino, some parts of the plant are usually disposed as waste, but could provide a source of income.
In this context, the objective of our study is to determine the best technique for extracting total polyphenols and flavonoids from different parts of the aforementioned tropical plants using different extraction solvents, and to determine their antioxidant activities.
2. Results
2.1. Extraction Yield
Both extraction methods (decoction and maceration) showed significant differences (p < 0.05) for all species and plant parts used in this research.
2.1.1. Extraction Yield by Maceration
The results of the maceration extraction yield (Table 1) show that acetone is the best extraction solvent, with an average of 20.43 ± 0.23% for Passiflora, 20.95 ± 0.15% for Physalis and 20.01 ± 0.18% for Solanum, followed by water, with averages of 19.53 ± 0.23%, 18.67 ± 0.14% and 18.32 ± 0.19% for Passiflora, Physalis and Solanum, respectively. The highest extraction in Solanum was found in the leaves for all solvents used (p < 0.05).
Table 1.
Extraction yield (% DM) of the different parts of blue passion flower (Passiflora caerulea L.), cape gooseberry (Physalis peruviana L.) and pepino (Solanum muricatum Aiton) extracted by decoction and maceration.
| Extraction Method | Extraction Solvents | Blue Passion Flower (Passiflora caerulea L.) | |||
| Leaves | Flower | Stem | Roots | ||
| Maceration | MeOH (70%) | 15.44 ± 0.58 k–p | 14.34 ± 1.33 m–q | 12.07 ± 0.05 s | 15.81 ± 0.4 l–p |
| EtOH (70%) | 13.22 ± 0.07 p–s | 12.10 ± 2.14 p–s | 12.83 ± 1.27 r,s | 13.45 ± 2.7 n–r | |
| H2O | 20.98 ± 0.27 a–c | 18.73 ± 0.67 b–g | 19.56 ± 7.43 g–n | 18.81 ± 0.87 d–k | |
| Acetone (70%) | 21.95 ± 0.05 a | 20.32 ± 0.38 a–e | 18.33 ± 0.53 c–i | 21.07 ± 0.9 a–e | |
| MeOH, NaF (80%) | 19.00 ± 0.95 d–j | 15.28 ± 2.33 o–s | 16.69 ± 1.67 j–p | 18.70 ± 2.26 f–m | |
| Decoction | MeOH (70%) | 22.02 ± 0.02 a | 20.02 ± 0.05 a–f | 22.08 ± 0.96 a,b | 22.18 ± 0.06 a |
| EtOH (70%) | 20.82 ± 0.17 a–d | 18.42 ± 0.79 e–l | 20.09 ± 0.27 a–f | 20.53 ± 1.47 a–f | |
| H2O | 18.87 ± 0.81 d–j | 18.32 ± 1.63 f–m | 16.68 ± 1.34 i–o | 18.73 ± 0.54 b–g | |
| Acetone (70%) | 13.73 ± 0.27 o–s | 16.28 ± 0.07 h–o | 12.25 ± 0.28 q–s | 16.53 ± 1.93 l–p | |
| MeOH, NaF (80%) | 19.07 ± 0.31 b–h | 15.43 ± 1.11 i–o | 16.83 ± 2.76 l–p | 17.45 ± 0.1 f–n | |
| Extraction Method | Extraction Solvents | Cape Gooseberry (Physalis peruviana L.) | |||
| Leaves | Flower | Stem | Roots | ||
| Maceration | MeOH (70%) | 17.25 ± 0.14 l,m | 15.02 ± 0.19 q | 17.20 ± 0.16 l,m | 18.76 ± 0.83 i–k |
| EtOH (70%) | 11.90 ± 0.67 s | 16.02 ± 0.2 o,p | 16.04 ± 0.21 o,p | 12.81 ± 0.17 r | |
| H2O | 18.87 ± 0.91 i–k | 19.28 ± 0.39 g–i | 17.76 ± 0.87 l,m | 18.74 ± 0.83 i–k | |
| Acetone (70%) | 20.33 ± 0.00 e,f | 20.99 ± 1.25 e,f | 20.90 ± 0.1 c–e | 21.59 ± 0.29 a,b | |
| MeOH, NaF (80%) | 16.96 ± 1.07 m–p | 17.66 ± 0.82 l–n | 18.93 ± 0.29 h–k | 19.01 ± 0.23 g–j | |
| Decoction | MeOH (70%) | 19.53 ± 0.36 f,g | 21.32 ± 0.35 a–c | 20.31 ± 0.5 d,e | 22.36 ± 0.13 a |
| EtOH (70%) | 17.22 ± 0.15 m,n | 21.07 ± 0.14 b–e | 19.38 ± 0.11 g,h | 21.60 ± 0.64 b–d | |
| H2O | 17.22 ± 0.3 l–n | 17.90 ± 0.65 k,j | 17.85 ± 0.33 k,l | 18.23 ± 0.34 j,k | |
| Acetone (70%) | 17.45 ± 0.88 m,n | 13.06 ± 0.46 r | 15.62 ± 0.25 p,q | 16.41 ± 0.08 s | |
| MeOH, NaF (80%) | 17.2 ± 0.73 m,n | 17.09 ± 0.32 m–o | 17.08 ± 0.16 m,n | 16.20 ± 0.32 o,p | |
| Extraction Method | Extraction Solvents | Pepino (Solanum muricatum Aiton) | |||
| Leaves | Flower | Stem | Roots | ||
| Maceration | MeOH (70%) | 18.95 ± 0.17 e–g | 15.78 ± 0.29 h–l | 14.85 ± 0.55 o–q | 15.79 ± 0.44 k–n |
| EtOH (70%) | 17.09 ± 0.36 h | 15.44 ± 0.7 j–m | 12.86 ± 0.33 r | 14.28 ± 0.87 q | |
| H2O | 18.63 ± 0.65 g | 18.03 ± 0.35 g | 16.17 ± 0.15 h–k | 14.88 ± 0.43 n–q | |
| Acetone (70%) | 21.41 ± 0.58 b | 19.46 ± 0.4 d,e | 19.34 ± 0.14 d–f | 19.82 ± 0.01 d | |
| MeOH, NaF (80%) | 19.54 ± 0.52 d–f | 19.08 ± 0.44 d–f | 16.05 ± 0.12 h–l | 14.62 ± 0.83 m–p | |
| Decoction | MeOH (70%) | 22.67 ± 0.07 a | 21.29 ± 0.01 b | 19.63 ± 0.43 d–f | 22.69 ± 0.1 a |
| EtOH (70%) | 20.98 ± 0.16 b | 19.80 ± 0.89 e–f | 14.97 ± 0.61 l–o | 20.41 ± 0.68 c,d | |
| H2O | 18.27 ± 0.54 f,g | 18.18 ± 0.41 g | 18.20 ± 2.02 h | 18.61 ± 0.22 f,g | |
| Acetone (70%) | 13.98 ± 0.59 p,q | 14.43 ± 0.45 p,q | 14.75 ± 0.67 o–p | 12.57 ± 0.05 r | |
| MeOH, NaF (80%) | 16.36 ± 0.71 h,i | 16.09 ± 0.38 h–l | 15.46 ± 0.3 l–o | 16.64 ± 0.075 h–j | |
Different letters in the same column for each plant indicate significant differences at a p < 0.05 level of probability. Data are the mean ± standard deviation (SD).
In general, the leaves recorded the best yield for Passiflora and Solanum, averaging 18.51 ± 0.22% and 18.79 ± 0.21%, respectively, followed by roots (18.33 ± 0.21%), flowers (16.93 ± 0.22%) and stems (16.75 ± 0.20%) for Passiflora. The flowers, roots and stems had average yields of 17.76 ± 0.18%, 17.04 ± 0.21% and 16.23 ± 0.19%, respectively, for Solanum.
On the other hand, the highest yield recorded from the averaging of Physalis was that of the roots (18.57 ± 0.16%), followed by stems (18.11 ± 0.16%), flowers (17.89 ± 0.17%) and leaves (17.40 ± 0.16%).
2.1.2. Extraction Yield by Decoction
In contrast to the results of the yields obtained by maceration, the yields obtained by decoction when methanol was used as an extractant solution (Table 1) were the highest yields, with averages of 21.58 ± 0.24% for Passiflora caerulea, 20.89 ± 0.14% for Physalis peruviana and 21.57 ± 0.26% for Solanum muricatum on four samples (leaves, flowers, stems, roots), while acetone gave the lowest yield for Passiflora, Physalis and Solanum, with averages of 14.83 ± 0.15%, 14.39 ± 0.14% and 11.15 ± 0.23%, respectively. Physalis peruviana exhibited the highest extraction level from its roots when methanol was used, showing a significant difference (p < 0.05) in comparison with other parts of the plant, such as leaves and stem. On the other hand, Passiflora and Solanum in general yielded higher levels of extraction in both roots and leaves, with significant differences (p < 0.05) between these plant parts, especially for Solanum.
2.2. Total Polyphenol Content (TPC)
The data analysis showed significant differences (p < 0.05) in the total polyphenol content (TPC) among the plant parts (Table 2). After dosing and calculation of the TPC extracted by decoction and by maceration for the different parts of the Passiflora and Physalis plants, we can conclude that extraction by decoction gave better general results compared to solvents extracted by maceration.
Table 2.
Total polyphenol content (mg eq GA/100 g DM) in the different parts of blue passion flower (Passiflora caerulea L.), cape gooseberry (Physalis peruviana L.) and pepino (Solanum muricatum Aiton) extracted by decoction and maceration.
| Extraction Method | Extraction Solvents | Blue Passion Flower (Passiflora caerulea L.) | |||
| Leaves | Flower | Stem | Roots | ||
| Maceration | MeOH (70%) | 1429.13 ± 55.62 g,h | 1261.73 ± 47.89 j–m | 1233.47 ± 74.25 l,m | 1372.6 ± 60.81 h,i |
| EtOH (70%) | 1505.21 ± 65.65 c–e | 1166.08 ± 47.89 n | 1259.56 ± 75.26 k–m | 1437.82 ± 67.12 f,g | |
| H2O | 1324.78 ± 57.84 i,j | 1083.47 ± 43.18 o | 1198.69 ± 57.4 m,n | 1137.82 ± 56.07 n,o | |
| Acetone (70%) | 1476.95 ± 58.7 d–g | 1274.78 ± 47.62 j–l | 1290 ± 56.07 j–l | 1316.08 ± 57.4 i–k | |
| MeOH, NaF (80%) | 1976.95 ± 62.04 a | 1498.69 ± 28.73 c–f | 1518.26 ± 68.1 c,d | 1466.08 ± 49.69 d–g | |
| Decoction | MeOH (70%) | 1234.13 ± 26.41 l,m | 924.34 ± 24.33 r,s | 974.34 ± 19.28 p–s | 958.04 ± 24.17 p–s |
| EtOH (70%) | 1373.26 ± 32.02 h,i | 964.56 ± 24.69 p–s | 991.73 ± 15.26 p,q | 1447.17 ± 19.24 e–g | |
| H2O | 1229.78 ± 35.56 l,m | 787.39 ± 28.51 t | 918.91 ± 12.98 s | 947.17 ± 28.92 q–s | |
| Acetone (70%) | 1362.39 ± 47.41 i | 938.47 ± 23.38 q–s | 984.13 ± 9.64 p–r | 1289.56 ± 31.15 j–l | |
| MeOH, NaF (80%) | 1597.17 ± 41.45 b | 1020 ± 27.26 p | 997.17 ± 22.83 p,q | 1551.52 ± 26.88 b,c | |
| Extraction Method | Extraction Solvents | Cape Gooseberry (Physalis peruviana L.) | |||
| Leaves | Flower | Stem | Roots | ||
| Maceration | MeOH (70%) | 1340 ± 36.54 f,g | 940 ± 27.03 p | 1050.86 ± 35.05 n | 1576.95 ± 19.28 c |
| EtOH (70%) | 1774.78 ± 27.03 a | 983.08 ± 30.81 o,p | 1129.13 ± 37.14 m | 1448.69 ± 29.27 d | |
| H2O | 1205.21 ± 49.69 j,k | 829.13 ± 29.59 q | 1048.69 ± 30.94 n | 1366.08 ± 32.14 e,f | |
| Acetone (70%) | 1337.82 ± 31.25 f,g | 968.26 ± 25.96 p | 1220.43 ± 32.04 j,k | 1492.17 ± 29.27 d | |
| MeOH, NaF (80%) | 1635.65 ± 29.7 b | 1016.08 ± 28.73 n,o | 1296.52 ± 33.67 g,h | 1637.82 ± 36.46 b | |
| Decoction | MeOH (70%) | 1141.73 ± 27.72 l,m | 480.86 ± 9.39 t | 1120 ± 37.73 m | 1224.34 ± 29.05 i,j |
| EtOH (70%) | 1221.08 ± 40.53 j,k | 809.13 ± 29.38 q,r | 1177.6 ± 37.96 k,l | 1268.91 ± 24.94 h,i | |
| H2O | 1026.52 ± 31.25 n,o | 422.17 ± 18.27 u | 772.17 ± 31.15 r | 1099.34 ± 28.7 m | |
| Acetone (70%) | 1237.39 ± 43.91 I,j | 490.65 ± 20.81 t | 1134.13 ± 41.91 l,m | 1348.26 ± 24.72 f | |
| MeOH, NaF (80%) | 1393.91 ± 39.37 e | 558.04 ± 31.22 s | 1291.73 ± 25.96 h | 1554.78 ± 37.06 c | |
| Extraction Method | Extraction Solvents | Pepino (Solanum muricatum Aiton) | |||
| Leaves | Flower | Stem | Roots | ||
| Maceration | MeOH (70%) | 1266.08 ± 44.33 e | 818.26 ± 27.03 r | 887.826 ± 36.54 q | 1159.56 ± 37.82 g,h |
| EtOH (70%) | 1335.65 ± 48.67 d | 1024.78 ± 29.59 j–l | 1131.3 ± 40.47 h | 1168.26 ± 30.43 g,h | |
| H2O | 1163.91 ± 46.76 g,h | 716.08 ± 35.05 s | 840 ± 37.56 r | 966.08 ± 32.14 n–p | |
| Acetone (70%) | 1340 ± 45.73 d | 892.17 ± 31.75 q | 1016.08 ± 36.46 k–m | 1026.95 ± 27.03 j–l | |
| MeOH, NaF (80%) | 1637.82 ± 47.29 b | 1013.91 ± 31.75 l,m | 1172.6 ± 37.82 g,h | 1398.69 ± 36.46 c | |
| Decoction | MeOH (70%) | 1198.26 ± 36.89 f,g | 921.08 ± 24.94 p,q | 963.47 ± 23.81 n–p | 1045 ± 34.48 i–k |
| EtOH (70%) | 1403.69 ± 22.83 c | 950.43 ± 29.05 o,p | 1002.6 ± 24.84 i–k | 1061.3 ± 33.01 i–k | |
| H2O | 1074.34 ± 22.02 i | 920 ± 31.75 p,q | 738.47 ± 35.03 s | 900.43 ± 25.72 q | |
| Acetone (70%) | 1374.34 ± 27.61 c,d | 1009.13 ± 25.22 l–n | 977.6 ± 23.1 m–o | 1045 ± 33.93 i–k | |
| MeOH, NaF (80%) | 1824.34 ± 27.72 a | 1068.91 ± 27.81 i,j | 1268.91 ± 21.41 e | 1243.91 ± 27.15 e,f | |
Different letters in the same column for each plant indicate significant difference at a p < 0.05 level of probability. Data are the mean ± standard deviation (SD).
The highest TPC level extracted from the different parts of Passiflora caerulea and Solanum muricatum Aiton via the decoction method with methanol in combination with sodium fluoride (MeOH NaF) was recorded in general at the leaves, followed by the roots. The total polyphenol content in these two species are given in the following descending order: leaves > roots > stems > flowers). In this sense, the TPC in the leaves of Passiflora caerulea and Solanum muricatum Aiton exhibited the highest levels for decoction (1976.95 ± 62.04 and 1637.82 ± 47.29 mg eq GA/100 g DM, respectively), followed by the roots (1466.08 ± 49.69 and 1398.69 ± 36.46 mg eq GA/100 g DM, respectively). The highest TPC contents in the different parts of Passiflora caerulea and Solanum muricatum Aiton via the maceration method were obtained with MeOH NaF from the leaves (1597.17 ± 41.45 and 1824.34 ± 27.72 mg eq GA/100 g DM, respectively). In addition, regardless of the solvent used, the total polyphenol content in the different parts of Physalis peruviana were obtained in the following order: roots > leaves > stems > flowers. This order was independent of the method applied (maceration or decoction), but the TPC level obtained was in general higher when the decoction method was applied.
MeOH NaF recorded the highest polyphenol content, this being significantly different (p < 0.05) in comparison with the other solvents for the three species. The potential for extraction of TPC via maceration by the rest of the solvents could be classified in a different order, regarding the species studied, i.e., Passiflora and Solanum (ethanolic extracts > acetonic extracts > methanolic extracts) or Physalis (acetonic extracts > ethanolic extracts > methanolic extracts). During decoction, methanolic and acetonic extracts showed less differences regarding the potential for extraction between them in the three species evaluated, but especially for Passiflora and Solanum the best solvent used in a decoction process was ethanol, in comparison with acetone or methanol.
2.3. Flavonoid Content
The flavonoid contents of the decocts and macerates of the four parts of the plant under study, obtained by the different solvents (Table 3), show that maceration is preferable for extracting flavonoids in general from the leaves and flowers of Solanum and for Passiflora leaves, which recorded the highest level, with a mean of 736.10 ± 5.38 mg eq Qu/100 g DM for MeOH NaF. On the other hand, decoction was in general the best method for an optimal flavonoid extraction from the flowers, stems and roots of Physalis and Passiflora.
Table 3.
Flavonoid content (mg eq Qu/100 g DM) in the different parts of blue passion flower (Passiflora caerulea L.), cape gooseberry (Physalis peruviana L.) and pepino (Solanum muricatum Aiton) extracted by decoction and maceration.
| Extraction Method | Extraction Solvents | Blue passion flower (Passiflora caerulea L.) | |||
| Leaves | Flower | Stem | Roots | ||
| Maceration | MeOH (70%) | 532.71 ± 5.28 c | 162.84 ± 1.05 s–u | 222.46 ± 4.91 m–p | 200.48 ± 0.35 r,q |
| EtOH (70%) | 676 ± 9.82 b | 222.46 ± 10.52 m–p | 242.49 ± 1.2 k–m | 239.03 ± 4.21 k–m | |
| H2O | 329.92 ± 1.01 e | 100.6 ± 1.4 w | 132.6 ± 0.7 v | 129.44 ± 0.35 v | |
| Acetone (70%) | 717.28 ± 1.4 a | 217.48 ± 2.45 n–q | 265.57 ± 8.23 h–j | 237.82 ± 0.7 k–n | |
| MeOH, NaF (80%) | 736.1 ± 5.38 a | 166.78 ± 0.7 s–u | 154.94 ± 0.35 t,u | 233.27 ± 0.35 l–o | |
| Decoction | MeOH (70%) | 300.42 ± 19.63 f,g | 173.89 ± 0.7 s,t | 183.6 ± 0.7 r,s | 236.42 ± 1.4 k–n |
| EtOH (70%) | 318.99 ± 5.61 e,f | 301.63 ± 4.21 f | 218.82 ± 0.7 n-q | 472.85 ± 5.48 d | |
| H2O | 211.53 ± 3.51 o–q | 198.78 ± 1.4 qr | 158.71 ± 1.4 tu | 147.17 ± 2.1 u,v | |
| Acetone (70%) | 297.74 ± 2.1 fg | 246.74 ± 0.7 j-l | 255.24 ± 0.7 k-n | 238.24 ± 0.7 k–n | |
| MeOH, NaF (80%) | 273.74 ± 5.6 hi | 203.03 ± 3.51 p-r | 278.56 ± 6.83 gh | 229.14 ± 2.8 l–o | |
| Extraction Method | Extraction Solvents | Cape Gooseberry (Physalis peruviana L.) | |||
| Leaves | Flower | Stem | Roots | ||
| Maceration | MeOH (70%) | 205.34 ± 3.86 k | 49 ± 1.4 t | 115.78 ± 2.1 q | 219.6 ± 0.7 g–j |
| EtOH (70%) | 257.55 ± 2.19 e | 55.68 ± 3.51 s,t | 148.57 ± 4.91 n,o | 282.14 ± 2.8 c | |
| H2O | 116.09 ± 3.86 q | 23.8 ± 0.35 u | 73.59 ± 1.05 r | 133.39 ± 0.7 p | |
| Acetone (70%) | 277.59 ± 1.77 c | 63.26 ± 3.15 r,s | 136.43 ± 5.61 o,p | 300.66 ± 8.58 b | |
| MeOH, NaF (80%) | 230.53 ± 7.71 f,g | 45.66 ± 1.75 t | 155.55 ± 10.17 n | 279.11 ± 2.1 c | |
| Decoction | MeOH (70%) | 209.1 ± 1.93 k,j | 57.31 ± 2.1 s,t | 215.78 ± 7.01 h–k | 279.53 ± 3.51 c |
| EtOH (70%) | 323.85 ± 2.64 a | 155.07 ± 1.2 n | 217.6 ± 13.32 h–k | 272.24 ± 4.72 c,d | |
| H2O | 169.64 ± 9.82 m | 140.49 ± 1.4 o,p | 170.24 ± 2.1 g–k | 210.32 ± 0.7 i–k | |
| Acetone (70%) | 234.6 ± 2.1 e | 175.71 ± 7.01 l | 222.46 ± 4.91 f–i | 315.35 ± 2.62 a | |
| MeOH, NaF (80%) | 260.1 ± 9.11 d,e | 157.49 ± 2.8 m,n | 224.28 ± 4.21 f–h | 252.82 ± 3.51 e | |
| Extraction Method | Extraction Solvents | Pepino (Solanum muricatum Aiton) | |||
| Leaves | Flower | Stem | Roots | ||
| Maceration | MeOH (70%) | 402.17 ± 3.51 e | 120.46 ± 6.31 s,t | 97.22 ± 2.29 v–x | 171.34 ± 0.35 p,q |
| EtOH (70%) | 649.89 ± 7.71 a | 128.96 ± 6.12 r,s | 158.71 ± 1.63 q | 229.02 ± 0.35 i–k | |
| H2O | 224.89 ± 4.72 j–l | 67.03 ± 2.1 y | 112.56 ± 8.41 t,u | 137.64 ± 1.4 r | |
| Acetone (70%) | 550.32 ± 3.32 b | 127.74 ± 3.51 r,s | 187.24 ± 2.1 n,o | 236.6 ± 0.7 h–j | |
| MeOH, NaF (80%) | 440.42 ± 2.04 d | 84.03 ± 0.7 x | 99.81 ± 2.1 u–w | 202 ± 4.21 m,n | |
| Decoction | MeOH (70%) | 252.82 ± 2.33 g | 41.53 ± 0.7 y | 159.92 ± 1.40 q | 190.89 ± 3.51 n,o |
| EtOH (70%) | 502.96 ± 3.55 c | 49.42 ± 4.21 y | 178.14 ± 4.21 o,p | 214.57 ± 7.01 k–m | |
| H2O | 212.74 ± 0.7 l,m | 17.85 ± 1.4 z | 87.67 ± 0.7 w,x | 227.92 ± 5.61 i–k | |
| Acetone (70%) | 277.71 ± 4.21 f | 61.87 ± 2.56 y | 159.92 ± 2.8 q | 241.28 ± 2.8 g–i | |
| MeOH, NaF (80%) | 288.64 ± 1.4 f | 52.15 ± 2.22 y | 107.71 ± 2.8 t–v | 246.74 ± 0.7 g–h | |
Different letters in the same column for each plant indicate significant differences at a p < 0.05 level of probability. Data are the mean ± standard deviation (SD).
There was a significant difference (p < 0.05) in flavonoid content among plant parts, as was expected. The great differentiation between parts of the plant appears to be related to the high levels of flavonoids in some parts of these plants, especially in the leaves and roots of the species in this study. The flavonoid content in the different parts of Passiflora and Solanum was higher in the leaves than in the rest of the parts of the plant regardless of the solvent applied to the macerated and decocted samples. In general, for Physalis under maceration conditions, ethanolic and acetonic extracts were the extraction solvents that recorded the highest flavonoid levels, regardless of the part of the plant, in almost all samples studied. The flavonoid levels derived from the different parts of Physalis, in both the macerated and decocted samples, were given in the following descending order: roots and leaves > stems > flowers (Table 3).
2.4. Total Antioxidant Activity
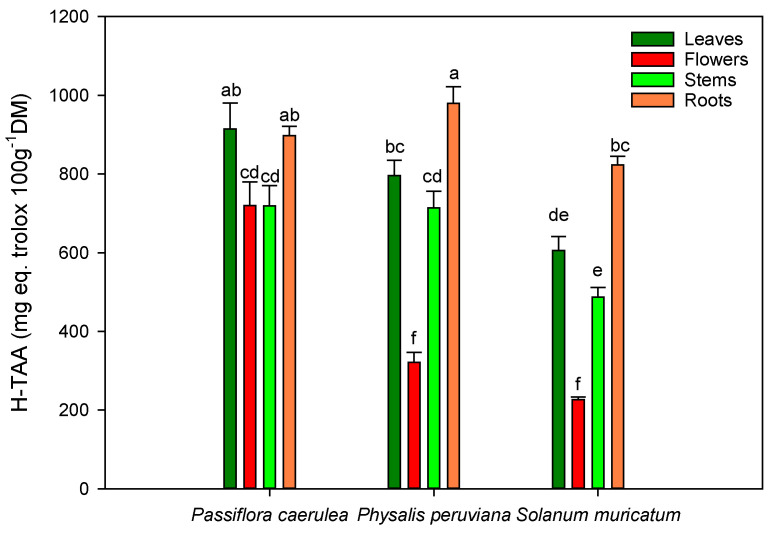
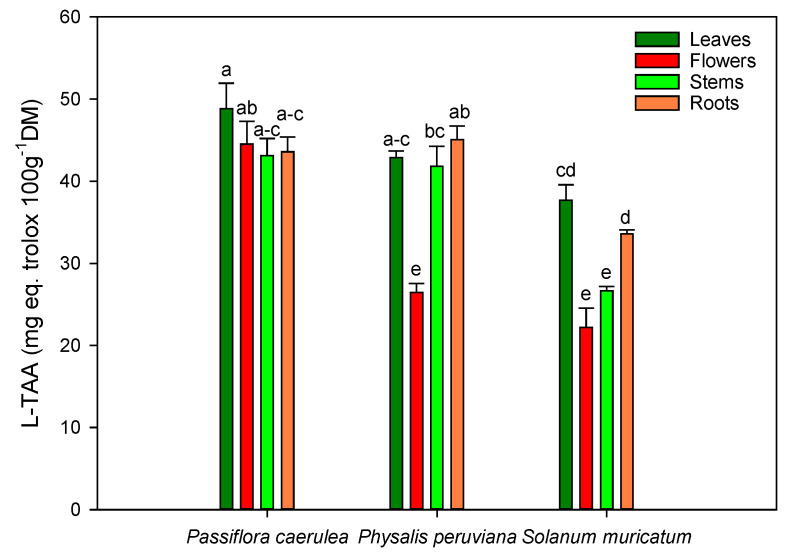
In our samples, total antioxidant activity (TAA) was measured in the hydrophilic (H-TAA) and lipophilic (L-TAA) fractions, shown in Figure 1 and Figure 2. The results showed that the H-TAA fractions of Passiflora, Physalis and Solanum obtained the highest levels of H-TAA in the leaves and roots. These levels were significantly higher (p < 0.05) in the roots of Physalis and Solanum (979.54 ± 42.43 and 823.62 ± 22.06 mg eq trolox/100 g DM, respectively) than in other parts of the plant (Figure 1). The leaves and flowers of Passiflora showed the highest level of H-TAA between all the species studied in this research. On the other hand, for L-TAA, Passiflora and Physalis both had a higher balance of lipophilic antioxidants in the stems and roots, in comparison with Solanum (Figure 2). In this sense, the lowest levels of L-TAA were found in Solanum, regardless the part of the plant evaluated.
Figure 1.
Antioxidant activity of the different parts of Passiflora caerulea L., Physalis peruviana L. and Solanum muricatum Aiton in the hydrophilic soluble phase. Different letters on the bars indicate significant differences at a p < 0.05 level of probability. Data are the mean ± standard error (SE).
Figure 2.
Antioxidant activity of the different parts of Passiflora caerulea L., Physalis peruviana L. and Solanum muricatum Aiton in the lipophilic soluble phase. Different letters on the bars indicate significant differences at a p < 0.05 level of probability. Data are the mean ± standard error (SE).
3. Discussion
3.1. Extraction Yield
Between the two extraction methods, the best yields were recorded by decoction, which gave averages of 18.35 ± 0.20% for Passiflora, 17.86 ± 0.16% for Physalis and 17.24 ± 0.20% for Solanum, versus 16.94 ± 0.94%, 17.81 ± 0.16% and 17.10 ± 0.19% (Passiflora, Physalis and Solanum, respectively) with maceration. These results were comparable to those obtained by Didi, A. [38] from Arbutus unedo, Dapline gaidium and Cynara scolymus [3] and Salem et al. [39] from Nitraria retusa, or Ozarowski et al. [40] from Passiflora species.
3.2. Total Polyphenol Content (TPC)
The TPC extracted by the decoction method was higher than that extracted by the maceration method for Passiflora and Physalis, and there were significant differences between the two methods of extraction. These results confirm that a moderate temperature would act in favor of the extraction; this fact has been confirmed by some authors who specify that the techniques using higher or lower temperatures and/or pressures would considerably increase the efficiency of the polyphenol extraction [39,41,42].
Small extraction yields could limit the use of some extracts of Passiflora, Physalis and Solanum (and plants in general) as sources of bioactive compounds. In addition, these compounds can sometimes be present in a very low concentration. On the other hand, and in consonance with Orlando et al. [43], we observed that the polyphenol extraction yields were considerably varied between different plant parts. In a recent study, and in agreement with our results, Salih et al. [44] did not obtain a good polyphenol extraction from macerated or decocted samples when using water as an extractant, in comparison with methanol and ethanol. These results were attributed to the lower polyphenol extraction yield obtained when water was used. However, the use of other extracting agents in these conventional techniques entails an optimal extraction of bioactive compounds [44]. In this sense, methanol’s polarity is higher than ethanol’s; for this reason, very polar phenolic acids, such as cinnamic or benzoic acids, could be extracted more easily, increasing the total polyphenol content in the extracts obtained [6]. This effect was in consonance with our results, since when higher methanol concentration were used, the TPC extraction became higher in comparison with methanol (70%) and the rest of the extractant agents for maceration or decoction in all plant species studied.
On the other hand, the work done by Martínez-Esplá et al. [45] on Prunus avium L. and Tomás-Barberán et al. [46] on nectarines, peaches and plums confirms our results by indicating that MeOH containing NaF allows for inactivated polyphenol oxidases, and prevents phenolic degradation due to browning. On the other hand, the high extraction potential of ethanol evaluated during decoction has also been observed by Katalinić et al. [47], Koffi et al. [48] and Mahmoudi et al. [3]. In these studies, ethanol in combination with water allowed for the better extraction of total polyphenols, because the addition of water to organic solvents increases the solubility of polyphenols and allows for maximum extraction [49].
3.3. Flavonoid Content
In these species studied, the majority of the secondary metabolites belong to flavonoids, and in particular, glycosides of the flavonols quercetin, rutine and myricetin [50,51,52]. In this study, and despite differences between the different parts of the plant, the leaves appear as the organ with the highest level of total flavonoids for all species studied. Leaves are always more exposed to sunlight than other plant organs. In fact, flavonoids protect plant tissues against the harmful effects of solar radiation [53]. The presence of flavonoids in the four organs of the plant could suggest that the plant has anti-inflammatory properties, and could thus play a positive role in the treatment of cardiovascular and neurodegenerative diseases, also perhaps having antitumor properties [54]. On the other hand, following our results, ethanolic solvents would be able to extract the highest amounts of total flavonoids from Passiflora, Physalis and Solanum. Mulinacci et al. [55] showed that an ethanol:water solution (7:3) increases the amount of flavonoids in the extraction. In this sense, when the aerial parts of Passiflora caerulea were evaluated using methanol, more flavonoid structures were detected (isoorientin, orientin, vitexin, saponarin and rutin) than when ethanol was used (isoorientin, orientin, vitexin, isovitexin) [56]. These findings could explain the higher level of total polyphenols and flavonoid content, which we found in our manuscript with methanol as an extractant agent in Passiflora showing the highest extractant polyphenol yield, followed by ethanol. However, ethanol and water are preferable because they have the advantage of being non-polluting, less expensive and non-toxic compared to other solvents, such as methanol [3,57]. The literature is scarce with respect to the plant parts of Physalis peruviana. However, in leaves, flavonoids such as quercetin, kaempferol and rutin were the most important secondary metabolites, while the main hydroxycinnamic acids detected were p-coumaric and caffeoyl-quinic acids [58]. Regarding Solanum muricatum, quercetin was the most important flavonoid, while ferulic and cinnamic acids were the most abundant phenolic acids detected [59].
3.4. Total Antioxidant Activity
The different plant parts of Passiflora caerulea, Physalis peruviana and Solanum muricatum Aiton are considered healthy because of their high levels of bioactive compounds, such as the phenolic compounds (total polyphenols, flavonoids), which contribute to the total antioxidant activity (TAA) [50,60,61]. The flowers and stems showed similar levels of H-TAA for Passiflora, reaching higher levels in its flowers as compared to all the values obtained in the rest of the flowers of this study. It is likely that the high levels of anthocyanins that Passiflora flowers contain could explain the significant differences between the flowers studied in this research [62,63].
The high levels of TAA in some plant parts and the low levels in other parts are related to their chemical compositions, functional groups of major compounds, and polyphenol content [54,64,65]. In fact, in Physalis the total polyphenol content in the leaves is twice as much as that in the flowers when applying most of the solvents studied in this research, especially with the decoction method. A similar pattern was observed in samples obtained after maceration, but the level of TPC extracted using this method was lower in the Physalis flowers, suggesting that maceration could extract the lower levels of these bioactive compounds located in the Physalis flowers (Table 2). Antioxidant activity generally depends on the number and position of the hydroxyl groups relative to the functional carboxyl groups [66]. In this sense, the TPC and flavonoid levels were in consonance with the TAA evaluated. Phenolic compounds are not the only contributors to the antioxidant activity in the water-soluble phase, since other compounds, such as ascorbic acid, show antioxidant activity in the water-soluble fraction. On the other hand, in the lipophilic fraction, different bioactive compounds, such as tocopherols, carotenoids and terpenes, also contribute to the total antioxidant activity of a particular sample [67]. In all the flowers evaluated, anthocyanins are present, conferring their characteristic purple color on some parts of this organ and contributing to the hydrophilic fraction with other phenolic compounds, such as isoorientin and orientin for Passiflora, or quercetin for Physalis and Solanum, especially in the leaves and roots. On the other hand, Salih et al. [44], in a different study, showed that lipophilic extracts were richer in tannins (mainly ellagic acid derivatives). In this study, the lipophilic fraction could also provide the antioxidant activity of other compounds, such as myristic acid for Passiflora [16] or β-carotene for Physalis [29] and Solanum [68].
In summary, these results show that in the three plants studied, the leaves and roots, as well as the flowers in the case of Passiflora, represent a very rich source of natural antioxidants.
4. Materials and Methods
4.1. Plant Material
Blue passion flower (Passiflora caerulea L.), cape gooseberry (Physalis peruviana L.) and pepino (Solanum muricatum Aiton) were harvested in September 2018 in Oran (Algeria). These plant species were harvested early in the morning with pruning shears and were tagged and transported in plastic bags. Leaves, flowers, stems and roots were separated, then dried at room temperature in darkness for 21 days and crushed using a grinder. Then samples were packaged in sealed polyethylene bags and transported to Spain at the University Miguel Hernández.
4.2. Chemical Products
The polyphenolic standards (gallic acid, hydrated quercetin-3-rutinoside), H2O2, ABTS peroxidase and trolox were provided by Sigma-Aldrich (Berlin, Germany). The reagents (Folin-Ciocalteu, aluminium trichloride, acetate of sodium) were provided by Sigma-Aldrich (Berlin, Germany), and the other solvents were obtained from Panreac (Barcelona, Spain), and Lab-Scan (Warsaw, Poland).
4.3. Extraction of Total Polyphenols and Flavonoids
Extraction by maceration—In order to extract the total polyphenols of the different plant parts by the maceration method described by Romani et al. [69], the following was carried out: 10 to 30 g of each of the crushed different parts (leaves, flowers, stems, roots) of the three plants were macerated for 2.5 h in triplicate at room temperature in 100 mL of aqueous solutions of the solvents (methanol, ethanol, acetone) diluted 7:3 in water, water and MeOH/water (8:2) containing 2 mM NaF according to Tomás-Barberán et al. [46]. After filtration through a muslin cloth, the filtrates were recovered and centrifuged at 4000 rpm at 4 °C for 20 min and then stored at −80 °C until use.
Extraction by decoction—In order to extract bioactive compounds by decoction, we performed the protocol described by Chavan et al. [70]. Quantities of 1 g of the powders of the different parts of the three plants were added to 40 mL of the different extraction solvents asmethanol, ethanol, acetone (diluted 7:3 in water) and MeOH/water (8:2), containing 2 mM NaF. Each mixture was boiled inside hermetically-sealed flasks in triplicate for 30 min in a water bath before it was filtered using a muslin cloth. The filtrates were recovered and centrifuged at 4000 rpm at 4 °C for 20 min and then stored at −80 °C until analysis of the extracted sample.
4.4. Extraction Yield
The yield of the two extraction methods for the three plants was calculated using the formula described by Falleh et al. [71]: Y (%) = 100 Mext/Mech, where Y is the yield of extraction in %, Mext is the mass of the extract after the evaporation (using the rotary evaporator) of the extraction solvent in mg, and Mech is the mass of the plant sample in mg.
4.5. Determination of Total Polyphenols and Flavonoids
Total polyphenols—Total polyphenols were determined by the Folin-Ciocalteu (FC) method [72,73]. In total, 200 μL of each extract was taken in duplicate to be analyzed and was added to 300 μL of 50 mM phosphate buffer solution, 2.5 mL of FC reagent and 2 mL of Na2CO3 at 1 N. The mixture was vortexed and then placed in a water bath at 50 °C for 5 min. The blank was performed by replacing the extract with the solution of phosphate buffer. The absorbance was measured at 760 nm using a UV-1700 Pharma spectrophotometer (Shimadzu). The results were expressed in mg gallic acid equivalent/g of dry vegetable material with reference to the calibration curve of gallic acid. All samples were analyzed in duplicate (n = 12).
Flavonoids—The flavonoid assay was performed according to the method detailed by Woisky and Salatino [74]. In total, 500 μL of each extract to be analyzed was mixed in duplicate with 1500 μL of methanol at 95%, 100 μL of AlCl3 at 10% (m/v), 100 μL of 1 M sodium acetate and 2.8 mL of distilled water. The whole mixture was vortexed and then incubated at room temperature in a dark condition for 30 min. The blank was made by replacing the extract with methanol at 95%, and the absorbance was measured at 415 nm by a UV-1700 Pharma spectrophotometer (Shimadzu). The results were expressed in mg equivalents of quercetin-3-rutinoside/100 g of dry matter with reference to the calibration curve of quercetin-3-rutinoside. All samples were analyzed in duplicate (n = 12). The selection of these methods allowed for comparison with other investigations, since they are the most common methods used to quantify these compounds with a good specificity and without additional equipment requirements.
4.6. Total Antioxidant Activity (TAA)
The determination of antiradical activity by the ABTS test was carried out using the method described by Cano et al. [75], slightly modified. In total, 10 mL of 50 mM phosphate buffer solution and 6 mL of ethyl acetate were added to 0.5 g of plant tissue (leaves, flowers, stems, and roots of the three plants) in three replicates and the mixture was homogenized for 1 min and then centrifuged at 10,000 rpm for 20 min at 4 °C. The hydrophilic and lipophilic phases of the different parts of the plants were separated.
Determination of the antioxidant activity in the hydrophilic phase (H-TAA)—In total, 890 μL of 50 mM glycine buffer solution was mixed with 30 μL of 10 mM ABTS solution, 30 μL of 1 mM H2O2 and 25 μL of 10 µM peroxidase. The absorbance of this mixture was measured at 730 nm against a blank prepared from the glycine solution buffer instead of a sample. In total, 25 μL of each water-soluble phase was added to the preceding mixture by duplicate (n = 6) and the absorbance was measured again at 730 nm after 1 min. The results were expressed in mg trolox equivalent/100 g of dry vegetable material with reference to the trolox calibration curve [75].
Determination of the antioxidant activity in the lipophilic phase (L-TAA)—In total, 30 μL of 10 mM ABTS solution was mixed with 30 μL of 1 mM H2O2, 25 μL of 10 µm peroxidase and 850 μL of ethanol. The absorbance of this mixture was measured at 730 nm against a blank prepared from ethanol. In total, 25 μL of each soluble lipid phase in duplicate (n = 6) was added to the preceding mixture and the absorbance was measured again at 730 nm after 1 min. The results were expressed in mg trolox equivalent/100 g of dry vegetable material with reference to the trolox calibration curve [75].
4.7. The Statistical Analyses
The treatments were distributed according to a complete randomized design (CRD). The data were analyzed via tree factors analysis of variance (methods, solvents and plant parts) procedures. Statistical analyses were performed with SAS software version 9.4 for Windows. Mean comparisons were performed using least significant difference (LSD) tests (p = 0.05) with standard deviation (SD) for the tables and standard error (SE) for the graphs.
5. Conclusions
The present work is the first study reporting information on these species of medicinal plants, with respect to the different plant parts. The extraction of total phenolic compounds and flavonoids is an important step in the evaluation of bioactive compounds and functional properties. The choice of the appropriate solvent and extraction technique is necessary in order to preserve the biological properties of these bioactive substances. From this work, decoction with methanol:water (8:2) containing 2 mM NaF was the preferred method of extraction of the total polyphenols. On the other hand, maceration with this solvent, and also with ethanol and acetone, were successful techniques for extracting flavonoids, especially from leaves (Passiflora and Solanum) and flowers for Solanum. This study reveals the difficulty of determining a single extraction process suitable for all different parts of the plant, showing significant differences between the three species studied. In all of them the results showed that the leaves and roots contain higher amounts of bioactive compounds, increasing their total antioxidant activity in comparison with other organs of the plant. In conclusion, with this research and regarding the medicinal possibilities of the different plant parts, the knowledge obtained increases the potential use of these medicinal plants in the prevention or treatment of many diseases affecting human health. In this sense, further investigation should be addressed elucidating the individual compounds with antioxidant properties that these species can provide.
Acknowledgments
We would like to thank the Algerian Ministry of Higher Education and Scientific Research for supporting Nour El-Houda Lezoul’s visit to the University Miguel Hernandez, with a scholarship from Program National Exceptional (PNE).
Author Contributions
Conceptualization, methodology, and writing subsequent drafts of the manuscript, N.E.H.L., M.B. and F.G.; statistical analysis and the results analysis, F.H., N.E.H.L. and F.G. Writing—original draft preparation. N.E.H.L. and F.G.; critical advice and supervised the study, M.B. and F.G.; writing—review and editing N.E.H.L., F.H., M.B. and F.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are not available from the authors.
References
- 1.Koechlin-Ramonatxo C. Oxygène, stress oxydant et supplémentations antioxydantes ou un aspect différent de la nutrition dans les maladies respiratoires. Nutr. Clin. Metab. 2006;20:165–177. doi: 10.1016/j.nupar.2006.10.178. [DOI] [Google Scholar]
- 2.Papoutsis K., Pristijono P., Golding J.B., Stathopoulos C.E., Bowyer M.C., Scarlett C.J., Vuong Q.V. Screening the effect of four ultrasound-assisted extraction parameters on hesperidin and phenolic acid content of aqueous citrus pomace extracts. Food Biosci. 2018;21:20–26. doi: 10.1016/j.fbio.2017.11.001. [DOI] [Google Scholar]
- 3.Mahmoudi S., Khali M., Mahmoudi N. Etude de l’extraction des composés phénoliques de différentes parties de la fleur d’artichaut (Cynara scolymus L.) Nat. Technol. 2013;9:35. [Google Scholar]
- 4.Garcia-Salas P., Morales-Soto A., Segura-Carretero A., Fernández-Gutiérrez A. Phenolic-compound-extraction systems for fruit and vegetable samples. Molecules. 2010;15:8813–8826. doi: 10.3390/molecules15128813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brglez-Mojzer E., Knez-Hrnčič M., Škerget M., Knez Ž., Bren U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules. 2016;21:901. doi: 10.3390/molecules21070901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stalikas C.D. Extraction, separation, and detection methods for phenolic acids and flavonoids. J. Sep. Sci. 2007;30:3268–3295. doi: 10.1002/jssc.200700261. [DOI] [PubMed] [Google Scholar]
- 7.Wojdyło A., Samoticha J., Chmielewska J. Effect of different pre-treatment maceration techniques on the content of phenolic compounds and color of Dornfelder wines elaborated in cold climate. Food Chem. 2020;339:1278–1288. doi: 10.1016/j.foodchem.2020.127888. [DOI] [PubMed] [Google Scholar]
- 8.Paranjpe S.S., Ferruzzi M., Morgan M.T. Effect of flash vacuum expansion process on grape juice yield and quality. Food Sci. Technol. 2012;48:147–155. doi: 10.1016/j.lwt.2012.02.021. [DOI] [Google Scholar]
- 9.Zhang Q., Lin L., Ye W. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018;13:20. doi: 10.1186/s13020-018-0177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sithisarn P., Supabphol R., Gritsanapan W. Comparison of free radical scavenging activity of Siamese neem tree (Azadirachta indica A. Juss var. Siamensis Valeton) leaf extracts prepared by different methods of extraction. Med. Princ. Pract. 2006;15:219–222. doi: 10.1159/000092185. [DOI] [PubMed] [Google Scholar]
- 11.Vongsak B., Sithisarn P., Gritsanapan W. Bioactive contents and free radical scavenging activity of Moringa oleifera leaf extract under different storage conditions. Ind. Crops Prod. 2013;49:419–421. doi: 10.1016/j.indcrop.2013.05.018. [DOI] [Google Scholar]
- 12.Ngoua-Meye-Misso R.L., Sima-Obiang C., Ndong J.D.L.C., Ndong-Atome G.R., Privat-Ondo J., Ovono J., Abessolo F., Obame-Engonga L.C. Medicinal plants used in management of cancer and other related diseases in Woleu-Ntem province, Gabon. Eur. J. Integr. Med. 2019;29:1009–1024. doi: 10.1016/j.eujim.2019.05.010. [DOI] [Google Scholar]
- 13.Chandrasekara A., Shahidi F. Herbal beverages: Bioactive compounds and their role in disease risk reduction-A review. J. Tradit. Complement. Med. 2018;8:451–458. doi: 10.1016/j.jtcme.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quiroga O., Bou S., Vigo M., Nolasco S. Chemical characteristics of Passiflora Caerulea seed oil and residual seed meal. Molecules. 2000;5:376–378. doi: 10.3390/50300376. [DOI] [Google Scholar]
- 15.Pérez J.O. Diversity of Colombian Passifloraceae: Biogeography and an updated list for conservation. Biota Colombiana. 2007;8 doi: 10.21068/bc.v8i1.181. [DOI] [Google Scholar]
- 16.Song Y., Wei X.Q., Li M.Y., Duan X.W., Sun Y.M., Yang R.L., Su X.D., Huang R.M., Wang H. Nutritional composition and antioxidant properties of the fruits of a Chinese wild Passiflora foetida. Molecules. 2018;23:459. doi: 10.3390/molecules23020459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anzoise M., Marrassini C., Bach H., Gorzalczany S. Beneficial properties of Passiflora caerulea on experimental colitis. J. Ethnopharmacol. 2016;194:137–145. doi: 10.1016/j.jep.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Dhawan K., Dhawan S., Sharma A. Passiflora: A review update. J. Ethnopharmacol. 2004;94:1–23. doi: 10.1016/j.jep.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 19.Ozarowski M., Thiem B. Progress in micropropagation of Passiflora spp. to produce medicinal plants: A mini-review. Revista Brasileira Farmacognosia. 2013;23:937–947. doi: 10.1590/S0102-695X2013000600011. [DOI] [Google Scholar]
- 20.Miroddi M., Calapai G., Navarra M., Minciullo P., Gangemi S. Passiflora incarnata L.: Ethnopharmacology, clinical application, safety and evaluation of clinical trials. J. Ethnopharmacol. 2013;150:791–804. doi: 10.1016/j.jep.2013.09.047. [DOI] [PubMed] [Google Scholar]
- 21.Legge A. Notes on the history, cultivation and uses of Physalis peruviana L. J. R. Hortic. Soc. 1974;99:310–314. [Google Scholar]
- 22.Barirega A. Potential for Value Chain Improvement and Commercialization of Cape Gooseberry (Physalis peruviana L.) for Livelihood Improvement in Uganda. Ethnobot. Res. Appl. 2014;12:131–140. doi: 10.17348/ERA.12.0.131-140. [DOI] [Google Scholar]
- 23.Çakir Ö., Pekmez M., Çepni E., Candar B., Fidan K. Evaluation of biological activities of Physalis peruviana ethanol extracts and expression of Bcl-2 genes in HeLa cells. Food Sci. Technol. 2014;34:422–430. doi: 10.1590/fst.2014.0060. [DOI] [Google Scholar]
- 24.Chang L.C., Sang-Ngern M., Pezzuto J.M., Ma C. The Daniel, K. Inouye college of pharmacy scripts: Poha Berry (Physalis peruviana) with potential anti-inflammatory and cancer prevention activities. Hawaii J. Med. Public Health. 2016;75:353. [PMC free article] [PubMed] [Google Scholar]
- 25.Higaki R., Chang L.C., Sang-Ngern M. Antibacterial activity of extracts from Physalis peruviana (Poha Berry) J. Health Dispar. Res. Pract. 2016;9:57–58. [Google Scholar]
- 26.Joshi K., Joshi I. Nutritional composition and biological activities of rasbhari: An overview. Int. J. Recent Sci. Res. 2015;7:7508–7512. [Google Scholar]
- 27.Kasali F.M., Kadima J.N., Mpiana P.T., Tshibangu D.S.-T. Assessment of antidiabetic activity and acute toxicity of leaf extracts from Physalis peruviana L. in guinea-pig. Asian Pac. J. Trop. Biomed. 2013;3:841–846. doi: 10.1016/S2221-1691(13)60166-5. [DOI] [Google Scholar]
- 28.Lashin I., Elhaw M. Evaluation of secondary metabolites in callus and tissues of Physalis peruviana. J. Inter. Modern Botany. 2016;1:10–17. doi: 10.5923/j.ijmb.20160601.03. [DOI] [Google Scholar]
- 29.Ozturk A., Özdemir Y., Albayrak B., Simşek M., Yildirim K.C. Some nutrient characteristics of golden berry (Physalis peruviana L.) cultivar candidate from Turkey. Sci. Papers Ser. B Hortic. 2017;61:293–297. [Google Scholar]
- 30.Pereda M.S.B., Nazareno M.A., Viturro C.I. Nutritional and antioxidant properties of Physalis peruviana L. fruits from the Argentinean northern Andean region. Plant Foods Hum. Nutr. 2017;74:68–75. doi: 10.1007/s11130-018-0702-1. [DOI] [PubMed] [Google Scholar]
- 31.Council N.R. Lost Crops of the Incas: Little-Known Plants of the Andes with Promise for Worldwide Cultivation. National Academies Press; Washington, DC, USA: 1989. [Google Scholar]
- 32.Yildiz T., Kalkan F. Some color and physical properties of pepino (Solanum muricatum Aiton) fruit. Bulg. J. Agric. Sci. 2014;20:988–992. [Google Scholar]
- 33.Huyskens-Keil S., Widayat H., Lüdders P., Schreiner M., Peters P. Physiological changes of pepino (Solanum muricatum Ait.) during maturation and ripening; Proceedings of the II ISHS Conference on Fruit Production in the Tropics and Subtropics; Bonn, Germany. 1 May 1999; pp. 251–256. [Google Scholar]
- 34.Di Scala K., Vega-Gálvez A., Uribe E., Oyanadel R., Miranda M., Vergara J., Quispe I., Lemus-Mondaca R. Changes of quality characteristics of pepino fruit (Solanum muricatum Ait) during convective drying. Int. J. Food Sci. Technol. 2011;46:746–753. doi: 10.1111/j.1365-2621.2011.02555.x. [DOI] [Google Scholar]
- 35.Ren W., Tang D. Extract of Solanum muricatum (Pepino/CSG) inhibits tumor growth by inducing apoptosis. Anticancer Res. 1999;19:403–408. [PubMed] [Google Scholar]
- 36.Sudha G., Sangeetha Priya M., Indhu Shree R.B., Vadivukkarasi S. Antioxidant activity of ripe and unripe pepino fruit (Solanum muricatum Aiton) J. Food Sci. 2012;77:C1131–C1135. doi: 10.1111/j.1750-3841.2012.02944.x. [DOI] [PubMed] [Google Scholar]
- 37.Putnik P., Barba F.J., Španić I., Zorić Z., Dragović-Uzelac V., Kovačević D.B. Green extraction approach for the recovery of polyphenols from Croatian olive leaves (Olea europea) Food Bioprod. Process. 2017;106:19–28. doi: 10.1016/j.fbp.2017.08.004. [DOI] [Google Scholar]
- 38.Didi A. Study of the Antioxidant Activity of the Flavonoids of Arbutus unedo and Daplin gaidium L. from the Tlemcen Region. University of Tlemcen; Tlemcen, Algeria: 2009. [Google Scholar]
- 39.Salem J.H., Chevalot I., Harscoat-Schiavo C., Paris C., Fick M., Humeau C. Biological activities of flavonoids from Nitraria retusa (Forssk.) Asch. and their acylated derivatives. Food Chem. 2011;124:486–494. doi: 10.1016/j.foodchem.2010.06.059. [DOI] [Google Scholar]
- 40.Ozarowski M., Piasecka A., Paszel-Jaworska A., Chaves D.S., Romaniuk A., Rybczynska M., Gryszczynska A., Sawikowska A., Kachlicki P., Mikolajczak P.L. Comparison of bioactive compounds content in leaf extracts of Passiflora incarnata, P. caerulea and P. alata and in vitro cytotoxic potential on leukemia cell lines. Bras. Farmacogn. 2018;28:179–191. doi: 10.1016/j.bjp.2018.01.006. [DOI] [Google Scholar]
- 41.Vuong Q.V., Golding J.B., Stathopoulos C.E., Nguyen M.H., Roach P.D. Optimizing conditions for the extraction of catechins from green tea using hot water. J. Sep. Sci. 2011;34:3099–3106. doi: 10.1002/jssc.201000863. [DOI] [PubMed] [Google Scholar]
- 42.Khan S.A., Aslam R., Makroo H.A. High pressure extraction and its application in the extraction of bio-active compounds: A review. J. Food Process Eng. 2019;42 doi: 10.1111/jfpe.12896. [DOI] [Google Scholar]
- 43.Orlando G., Zengin G., Ferrante C., Ronci M., Recinella L., Senkardes I., Gevrenova R., Zheleva-Dimitrova D., Chiavaroli A., Leone S. Comprehensive Chemical Profiling and Multidirectional Biological Investigation of Two Wild Anthemis Species (Anthemis tinctoria var. Pallida and A. cretica subsp. tenuiloba): Focus on Neuroprotective Effects. Molecules. 2019;24:2582. doi: 10.3390/molecules24142582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salih E.Y.A., Julkunen-Tiitto R., Luukkanen O., Sipi M., Fahmi M.K.M., Fyhrquist P.J. Potential anti-tuberculosis activity of the extracts and their active components of Anogeissus leiocarpa (Dc.) guill. and perr. with special emphasis on polyphenols. Antibiotics. 2020;9:364. doi: 10.3390/antibiotics9070364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martínez-Esplá A., Zapata P.J., Valero D., García-Viguera C., Castillo S., Serrano M. Preharvest application of oxalic acid increased fruit size, bioactive compounds, and antioxidant capacity in sweet cherry cultivars (Prunus avium L.) J. Agric. Food Chem. 2014;62:3432–3437. doi: 10.1021/jf500224g. [DOI] [PubMed] [Google Scholar]
- 46.Tomás-Barberán F.A., Gil M.I., Cremin P., Waterhouse A.L., Hess-Pierce B., Kader A.A. HPLC-DAD-ESIMS analysis of phenolic compounds in nectarines, peaches, and plums. J. Agric. Food Chem. 2001;49:4748–4760. doi: 10.1021/jf0104681. [DOI] [PubMed] [Google Scholar]
- 47.Katalinić V., Možina S.S., Skroza D., Generalić I., Abramovič H., Miloš M., Boban M. Polyphenolic profile, antioxidant properties and antimicrobial activity of grape skin extracts of 14 Vitis vinifera varieties grown in Dalmatia (Croatia) Food Chem. 2010;119:715–723. doi: 10.1016/j.foodchem.2009.07.019. [DOI] [Google Scholar]
- 48.Koffi E., Sea T., Dodehe Y., Soro S. Effect of solvent type on extraction of polyphenols from twenty three Ivorian plants. J. Anim. Plant Sci. 2010;5:550–558. [Google Scholar]
- 49.Sripad G., Prakash V., Rao M.N. Extractability of polyphenols of sunflower seed in various solvents. J. Biosci. 1982;4:145–152. doi: 10.1007/BF02702723. [DOI] [Google Scholar]
- 50.Azmir J., Zaidul I.S.M., Rahman M.M., Sharif K.M., Mohamed A., Sahena F., Omar A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013;117:426–436. doi: 10.1016/j.jfoodeng.2013.01.014. [DOI] [Google Scholar]
- 51.Licodiedoff S., Koslowski L.A., Ribani R. Flavonol rates of gosseberry fruits Physalis peruviana determined by HPLC through the optimization and validation of the analytic method. Int. J. Food Sci. Nutr. 2013;3:1–6. doi: 10.5923/j.food.20130301.01. [DOI] [Google Scholar]
- 52.Ahmad A.R., Wisdawati S., Asrifa W.O. Study of antioxidant activity and determination of phenol and flavonoid content of pepino’s leaf extract (Solanum muricatum aiton) Int. J. Pharm. Tech. Res. 2014;6:600–606. [Google Scholar]
- 53.Gehin A., Guyon C., Nicod L. Glyphosate-induced antioxidant imbalance in HaCaT: The protective effect of Vitamins C and E. Environ. Toxicol. Pharmacol. 2006;22:27–34. doi: 10.1016/j.etap.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 54.Evenamede K.S., Kpegba K., Simalou O., Boyode P., Agbonon A., Gbeassor M. Etude comparative des activités antioxydantes d’extraits éthanoliques de feuilles, d’écorces et de racines de Cassia sieberiana. Int. J. Biol. Chem. Sci. 2017;11:2924–2935. doi: 10.4314/ijbcs.v11i6.29. [DOI] [Google Scholar]
- 55.Mulinacci N., Prucher D., Peruzzi M., Romani A., Pinelli P., Giaccherini C., Vincieri F. Commercial and laboratory extracts from artichoke leaves: Estimation of caffeoyl esters and flavonoidic compounds content. J. Pharm. Biomed. 2004;34:349–357. doi: 10.1016/S0731-7085(03)00552-1. [DOI] [PubMed] [Google Scholar]
- 56.Gadioli I.L., da Cunha M.S., de Carvalho M.V., Costa A.M., Pineli L.L. A systematic review on phenolic compounds in Passiflora plants: Exploring biodiversity for food, nutrition, and popular medicine. Crit. Rev. Food Sci. Nutr. 2016;58:785–807. doi: 10.1080/10408398.2016.1224805. [DOI] [PubMed] [Google Scholar]
- 57.Jokić S., Velić D., Bilić M., Bucić-Kojić A., Planinić M., Tomas S. Modelling of solid-liquid extraction process of total polyphenols from soybeans. Czech J. Food Sci. 2010;28:206–212. doi: 10.17221/200/2009-CJFS. [DOI] [Google Scholar]
- 58.Medina S., Collado-González J., Ferreres F., Londoño-Londoño J., Jiménez-Cartagena D., Guy A., Durand T., Galano J.-M., Gil-Izquierd A. Potential of Physalis peruviana calyces as a low-cost valuable resource of phytoprostanes and phenolic compounds. J. Sci. Food Agric. 2019;99:2194–2204. doi: 10.1002/jsfa.9413. [DOI] [PubMed] [Google Scholar]
- 59.Hsu C.C., Guo Y.R., Wang Z.H., Yin M.C. Protective effects of an aqueous extract from pepino (Solanum muricatum Ait.) in diabetic mice. J. Sci. Food Agric. 2011;91:1517–1522. doi: 10.1002/jsfa.4345. [DOI] [PubMed] [Google Scholar]
- 60.Chemat F., Abert-Vian M., Fabiano-Tixier A.S., Strube J., Uhlenbrock L., Gunjevic V., Cravotto G. Green extraction of natural products. Origins, current status, and future challenges. TrAC Trend Anal. Chem. 2019 doi: 10.1016/j.trac.2019.05.037. [DOI] [Google Scholar]
- 61.Farnsworth N.R., Akerele O., Bingel A.S., Soejarto D.D., Guo Z. Medicinal plants in therapy. Bull. World Health Organ. 1985;63:965. doi: 10.1016/0378-8741(87)90016-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aizza L.C.B., Sawaya A.C.H.F., Dornelas M.C. Identification of anthocyanins in the corona of two species of Passiflora and their hybrid by ultra-high performance chromatography with electrospray ionization tandem mass spectrometry (UHPLC-ESI-MS/MS) Biochem. Syst. Ecol. 2019;85:60–67. doi: 10.1016/j.bse.2019.05.003. [DOI] [Google Scholar]
- 63.Aizza L.C., Dornelas M.C.A. genomic approach to study anthocyanin synthesis and flower pigmentation in passion flowers. J. Nucleic Acids. 2011:371517. doi: 10.4061/2011/371517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Athamena S., Chalghem I., Kassah-Laouar A., Laroui S., Khebri S. Activité antioxydante et antimicrobienne d’extraits de Cuminum cyminum L. Lebanese Sci. J. 2010;11:69–81. [Google Scholar]
- 65.Wong C.C., Li H.B., Cheng K.W., Chen F. A systematic survey of antioxidant activity of 30 Chinese medicinal plants using the ferric reducing antioxidant power assay. Food Chem. 2006;97:705–711. doi: 10.1016/j.foodchem.2005.05.049. [DOI] [Google Scholar]
- 66.Hayes J., Allen P., Brunton N., O’Grady M., Kerry J. Phenolic composition and in vitro antioxidant capacity of four commercial phytochemical products: Olive leaf extract (Olea europaea L.), lutein, sesamol and ellagic acid. Food Chem. 2011;126:948–955. doi: 10.1016/j.foodchem.2010.11.092. [DOI] [Google Scholar]
- 67.Wong Y.S., Sia C.M., Khoo H.E., Ang Y.K., Chang S.K., Yim H.S. Influence of extraction conditions on antioxidant properties of passion fruit (Passiflora edulis) peel. Acta Sci. Pol. Technol. Aliment. 2014;13:257–265. doi: 10.17306/J.AFS.2014.3.4. [DOI] [PubMed] [Google Scholar]
- 68.Duman S., Sivaci A. Investigation of drought stress in pepino (Solanum muricatum Ait. cv. Miski) leaves. Pak. J. Botan. 2015;47:1621–1627. [Google Scholar]
- 69.Romani A., Pinelli P., Cantini C., Cimato A., Heimler D. Characterization of Violetto di Toscana, a typical Italian variety of artichoke (Cynara scolymus L.) J. Food Chem. 2006;95:221–225. doi: 10.1016/j.foodchem.2005.01.013. [DOI] [Google Scholar]
- 70.Chavan U., Shahidi F., Naczk M. Extraction of condensed tannins from beach pea (Lathyrus maritimus L.) as affected by different solvents. Food Chem. 2001;75:509–512. doi: 10.1016/S0308-8146(01)00234-5. [DOI] [Google Scholar]
- 71.Falleh H., Ksouri R., Chaieb K., Karray-Bouraoui N., Trabelsi N., Boulaaba M., Abdelly C. Phenolic composition of Cynara cardunculus L. organs, and their biological activities. Comptes Rendus Biol. 2008;331:372–379. doi: 10.1016/j.crvi.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 72.Singleton V.L., Rossi J.A., Jr. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965;16:144–158. [Google Scholar]
- 73.Scalbert A., Monties B., Janin G. Tannins in wood: Comparison of different estimation methods. J. Agric. Food Chem. 1989;37:1324–1329. doi: 10.1021/jf00089a026. [DOI] [Google Scholar]
- 74.Woisky R.G., Salatino A. Analysis of propolis: Some parameters and procedures for chemical quality control. J. Apicult. Res. 2015;37:99–105. doi: 10.1080/00218839.1998.11100961. [DOI] [Google Scholar]
- 75.Cano A., Hernández-Ruiz J., García-Cánovas F., Acosta M., Arnao M.B. An end-point method for estimation of the total antioxidant activity in plant material. Phytochem. Anal. 1998;9:196–202. doi: 10.1002/(SICI)1099-1565(199807/08)9:4<196::AID-PCA395>3.0.CO;2-W. [DOI] [Google Scholar]