Abstract
Purpose
Combination treatment with immune checkpoint inhibitors has shown enhanced antitumor activity compared with monotherapy in tumor types such as melanoma. The open-label, parallel-cohort, dose-escalation, phase I CheckMate 016 study evaluated the efficacy and safety of nivolumab plus ipilimumab in combination, and nivolumab plus a tyrosine kinase inhibitor in metastatic renal cell carcinoma (mRCC). Safety and efficacy results from the nivolumab plus ipilimumab arms of the study are presented.
Patients and Methods
Patients with mRCC received intravenous nivolumab 3 mg/kg plus ipilimumab 1 mg/kg (N3I1), nivolumab 1 mg/kg plus ipilimumab 3 mg/kg (N1I3), or nivolumab 3 mg/kg plus ipilimumab 3 mg/kg (N3I3) every 3 weeks for four doses followed by nivolumab monotherapy 3 mg/kg every 2 weeks until progression or toxicity. End points included safety (primary), objective response rate, and overall survival (OS).
Results
All patients in the N3I3 arm (n = 6) were censored at the time of analysis as a result of dose-limiting toxicity or other reasons. Forty-seven patients were treated in both the N3I1 and the N1I3 arm, and baseline patient characteristics were balanced between arms. Grade 3 to 4 treatment-related adverse events were reported in 38.3% and 61.7% of the patients in the N3I1 and N1I3 arms, respectively. At a median follow-up of 22.3 months, the confirmed objective response rate was 40.4% in both arms, with ongoing responses in 42.1% and 36.8% of patients in the N3I1 and N1I3 arms, respectively. The 2-year OS was 67.3% and 69.6% in the N3I1 and N1I3 arms, respectively.
Conclusion
Nivolumab plus ipilimumab therapy demonstrated manageable safety, notable antitumor activity, and durable responses with promising OS in patients with mRCC.
INTRODUCTION
Renal cell carcinoma (RCC) accounts for 2.4% of total cancer cases worldwide, with 338,000 patients diagnosed each year.1 At the time of diagnosis, 25% to 30% of patients present with metastatic disease associated with high mortality.2,3 Before agents that target vascular endothelial growth factor (VEGF) and mammalian target of rapamycin pathways became available, treatment options were limited to cytokine-based therapies (interleukin-2, interferon) that were not widely applicable.4,5 Responses to VEGF and mammalian target of rapamycin inhibitors, however, usually are not durable, and significant toxicities related to the agents’ mechanisms of action can occur.4,6-8 Newly developed immunotherapies have demonstrated durable responses, improved overall survival (OS), and better tolerability in a variety of tumor types.9,10
Nivolumab is a fully human immunoglobulin G4 programmed death 1 (PD-1) immune checkpoint inhibitor antibody that selectively blocks the interaction between PD-1 expressed on activated T cells, with its ligands PD-L1/PD-L2 preventing the T cells from being inactivated.11,12 Nivolumab has demonstrated durable antitumor activity in previously treated patients with metastatic RCC (mRCC).13,14 In a phase III study, superior OS, a higher objective response rate (ORR), fewer grade 3 or 4 treatment-related adverse events (AEs), and improved quality of life were observed with nivolumab treatment versus everolimus.13,15
The immunotherapeutic agent ipilimumab, a human CTLA-4 (cytotoxic T-lymphocyte antigen-4) immune checkpoint inhibitor antibody,16 offers a different, but complementary mechanism of action to nivolumab while also preventing the inactivation of T cells.17,18 Ipilimumab had demonstrated antitumor activity in a subset of patients with mRCC in a phase II study, which suggests that mRCC can respond to CTLA-4 blockade.19 The combination of nivolumab and ipilimumab has previously achieved greater and more durable responses compared with either agent alone in patients with metastatic melanoma and lung cancer, which suggests an added benefit for this combination across various tumor types.20-23
This study (CheckMate 016) is an open-label, parallel-cohort, dose-escalation, phase I study and is the first clinical study to our knowledge to evaluate the efficacy and safety of the nivolumab and ipilimumab combination or nivolumab and a tyrosine kinase inhibitor in mRCC. We present the safety and efficacy results from the nivolumab plus ipilimumab arms of the study; the nivolumab plus tyrosine kinase inhibitor data will be presented elsewhere.
PATIENTS AND METHODS
Study Design and Treatment
CheckMate 016 is a multicenter, open-label, phase I study with 5 parallel treatment arms (results from the 2 nivolumab plus tyrosine kinase inhibitor arms will be presented elsewhere; Appendix Fig A1, online only). Eligible patients in the nivolumab plus ipilimumab arms were randomly assigned to one of three treatments: nivolumab 3 mg/kg plus ipilimumab 1 mg/kg (N3I1), nivolumab 1 mg/kg plus ipilimumab 3 mg/kg (N1I3), or nivolumab 3 mg/kg plus ipilimumab 3 mg/kg (N3I3). All patients in the N3I3 arm (n = 6) were censored at the time of analysis. Each treatment was administered by intravenous infusion every 3 weeks for up to four doses (induction) followed by nivolumab 3 mg/kg IV as monotherapy every 2 weeks until disease progression or intolerable toxicity (maintenance). Patients were administered two infusions at each induction visit: nivolumab 1 or 3 mg/kg of body weight followed 30 minutes later by ipilimumab 1 or 3 mg/kg as the second infusion. On the basis of the sponsor’s medical monitor and site investigators’ agreement, patients could switch to nivolumab monotherapy, even if they did not complete all four combination doses because of ipilimumab-related toxicity. Patients could discontinue treatment as a result of disease progression, unacceptable toxicity, withdrawal of consent, or on the basis of the investigator’s clinical judgment. Treatment beyond disease progression was permitted if patients tolerated treatment and the investigator considered the patient to benefit clinically. Treatment was discontinued if subsequent disease progression was evident (≥ 10% increase in tumor burden from the time of initial progression).
Study Oversight
This study was approved by local institutional review boards or an independent ethics committee and conducted in accordance with International Conference on Harmonisation Good Clinical Practice guidelines. Written informed consent was obtained from all patients on the basis of Declaration of Helsinki principles before initiation of study procedures.
Patients
Patients eligible for inclusion were ≥ 18 years of age with histologically confirmed advanced RCC or mRCC with a clear-cell component and measurable disease according to Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST 1.1)24 and a Karnofsky performance status of at least 80% at study enrollment.25 Patients were required to have a baseline imaging scan for tumor burden within 28 days of study treatment assignment. Patients were assigned to the treatment arms in two cohorts: The initial cohort was designed to gain safety information, and an expansion cohort was added so that both safety and efficacy data could be interpreted.
Patients either could have been treatment naïve or had received prior systemic therapy in both the N3I1 and N1I3 initial cohorts. However, patients enrolled in the N3I1 and N1I3 expansion cohorts (and all patients in the N3I3 arm) were not permitted to have received prior systemic therapy for RCC, with the exception of one prior adjuvant or neoadjuvant therapy for localized or locally advanced RCC (provided that recurrence was ≥ 6 months after the last dose) or only prior cytokine therapy for metastatic disease. Patients were excluded if they had active CNS metastases or current/recent history of an autoimmune disorder that required systemic corticosteroids equivalent to ≥ 10 mg/kg prednisone.
Study End Points and Assessments
The primary objective was to assess overall safety and tolerability of nivolumab plus ipilimumab to determine the maximum tolerated dose and recommended phase II dose of this combination regimen. Safety and tolerability were defined by incidence of AEs or serious AEs (SAEs) that occurred up to 100 days after the last dose of study treatment and the frequency of clinical laboratory tests, including hematology, serum chemistry, and urinalysis, by worst toxicity grade. AEs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0). SAEs were defined as any medical occurrence that resulted in death, was life-threatening, required hospitalization or prolonged existing hospitalization, resulted in persistent or significant disability/incapacity, or was an important medical event (ie, could jeopardize the patient or require intervention to prevent other serious outcomes). An additional safety assessments was determination of select treatment-related AEs (any grade) defined as those with possible immune-mediated etiology.
Secondary end points were best overall response (BOR), ORR, duration of response (DOR) per RECIST 1.1,24 time to response, progression-free survival (PFS), and 24-week PFS rate. BOR was defined as the best response designation (confirmed complete or partial response or progression) over the study as a whole recorded between the date of the first dose of study medication and the date of objectively documented progression per RECIST 1.1 or the date of subsequent anticancer therapy, whichever occurred first, for an individual patient in the study. ORR was defined as the proportion of all treated patients whose investigator-assessed BOR was a complete or partial response. DOR was calculated for all treated patients who achieved a complete or partial response and defined as the time between dates of first response and of disease progression or death, whichever occurred first. PFS was defined as the time from first study medication dose to first disease progression or death. Tumor assessments were done at screening, every 6 weeks (± 1 week) from the first dose of study treatment of the first four infusions, and every 12 weeks (± 1 week) thereafter until disease progression. OS was included as an exploratory end point.
Statistical Analysis
The assignment of 20 patients per treatment arm (initial cohorts) was determined adequate to provide safety information on the basis of a 90% probability of observing one or more occurrences of any possible AE with an 11% incidence. The initial N3I1 and N1I3 cohorts were increased to 45 patients per treatment arm (expansion cohorts) to better evaluate safety and efficacy of the combination therapy. The expansion of the N3I1 and N1I3 cohorts to 45 patients was deemed adequate to provide 90% probability of observing at least one occurrence of any AE that might occur with a 5% incidence. In addition, with 45 patients treated in each arm, the maximum width of the exact two-sided 95% CI would be 28% (95% exact CI, 18% to 47%), which assumes the true ORR to be 30%.
Safety and efficacy analyses included all patients who received one or more doses of study medication. All AEs were summarized and reported by organ system, preferred term, treatment arm, and dose level and coded per the current version of the Medical Dictionary for Regulatory Activities. ORR and its 95% exact CI was determined by Clopper and Pearson26 methodology. The Kaplan-Meier method was used to analyze DOR and its 95% CI. PFS rate by treatment arm and dose level, and the associated 95% CI, were derived on the basis of the Greenwood formula.27 OS was plotted by using the Kaplan-Meier method, with median and corresponding two-sided 95% CIs reported. Statistical analyses that compare safety between arms were not performed.
RESULTS
Patient Population and Baseline Characteristics
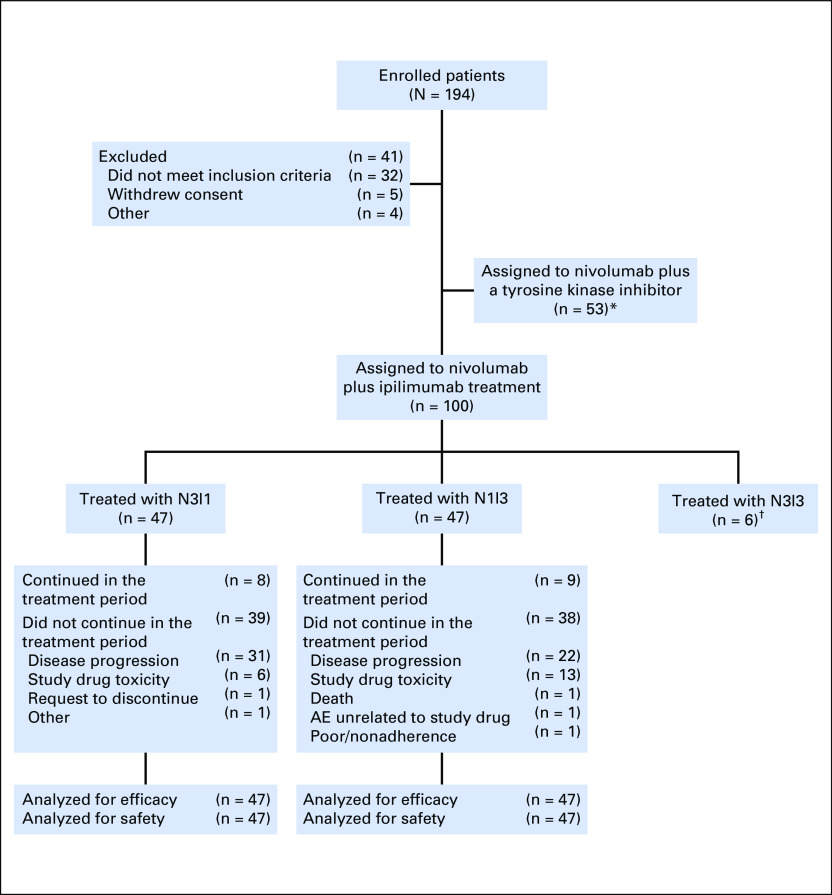
A total of 194 patients enrolled between February 2012 and May 2014, with 47 each assigned to the N3I1 and N1I3 arms (Appendix Fig A1, online only). Of the six N313 patients, one was censored at 6 months, one at 12 months, one at 18 months, and two at 24 months; one patient withdrew consent. Reasons for discontinuation were disease progression (three patients) and treatment-related toxicity (two patients). Baseline demographic and clinical characteristics for the N3I3 arm are listed in Table 1. Because of the high censoring percentage, no confirmed responses were found in the N3I3 arm, so efficacy is not presented; however, safety data are listed in Table 2 and Appendix Table A1 (online only).
Table 1.
Baseline Demographic and Clinical Characteristics of Treated Patients
Table 2.
Treatment-Related AEs Reported in 20% or More of Patients (N3I1 and N1I3 Arms), Grade 3 or 4 AEs Reported in Two or More Patients (Any Arm), and Select Treatment-Related AEs
Baseline demographic and clinical characteristics generally were balanced between the N3I1 and the N1I3 arms (Table 1). Twenty-five (53.2%) and 21 (44.7%) treatment-naïve patients were assigned to the N3I1 and the N1I3 arms, respectively. At data cutoff (March 16, 2016), median follow-up was 22.3 months for both arms, and minimum follow-up was 22 months. Forty-six (97.9%) patients in the N3I1 arm and 42 (89.4%) in the N1I3 arm received ≥ 90% of the planned nivolumab and ipilimumab dose intensity (ie, four doses) during the induction phase. A total of 63.8% and 70.2% of patients continued onto nivolumab monotherapy in the N3I1 and N1I3 arms, respectively. The median number of nivolumab doses received was 10.0 in the N3I1 arm and 7.0 in the N1I3 arm.
Safety
Dose delays.
Twenty-nine (61.7%) patients in the N3I1 arm and 35 (74.5%) in the N3I1 arm had one or more nivolumab dose delays, where hematologic toxicity accounted for five (10.6%) and eight (17.0%) patients’ delays in the N3I1 and N1I3 arms, respectively. Twelve (25.5%) and 14 (29.8%) patients had one or more ipilimumab dose delays, where hematologic toxicity accounted for three (6.4%) and two (4.3%) patients’ delays in the N3I1 and N1I3 arms, respectively.
AEs and select AEs.
Among all patients assigned to the N3I1 or N1I3 arms, 100% experienced an AE of any grade, and 71.3% experienced a grade 3 or 4 AE. Treatment-related AEs were observed in 93.6% of all patients; 50.0% of patients experienced treatment-related AEs classified as grade 3 or 4. Forty-three (91.5%) patients in the N3I1 arm experienced a treatment-related AE of any grade, and 18 (38.3%) experienced grade 3 or 4 treatment-related AEs (Table 2). Among patients in the N1I3 arm, 45 (95.7%) experienced treatment-related AEs, with 29 (61.7%) reporting grade 3 or 4 treatment-related AEs (Table 2). Select treatment-related AEs are also listed in Table 2. Treatment-related AEs of any grade that led to discontinuation occurred in five (10.6%) and 13 (27.7%) patients in the N3I1 and N1I3 arms, respectively. Grade 4 amylase and lipase not associated with symptoms or clinical manifestations of pancreatitis initially required discontinuation, but patients in the expansion cohorts could continue despite these events per site investigator discussion with the medical monitor.
SAEs.
Among all patients assigned to the N3I1 or N1I3 arms, 62.8% reported an SAE of any grade, and 46.8% reported a grade 3 or 4 SAE. Twenty-seven (28.7%) patients experienced treatment-related SAEs, with 25 (26.6%) reporting treatment-related SAEs classified as grade 3 or 4. Among patients in the N3I1 arm, 11 (23.4%) had a treatment-related SAE, with nine (19.1%) experiencing a grade 3 or 4 treatment-related SAE. Diarrhea and pyrexia (all grades) were reported for three (6.4%) patients each and were the most common treatment-related SAEs in the N3I1 arm; two (4.3%) patients had grade 3 or 4 diarrhea. Treatment-related SAEs were reported for 16 (34.0%) patients in the N1I3 arm and included colitis in six (12.8%), diarrhea in five (10.6%), elevated ALT in four (8.5%), elevated AST in four (8.5%), dehydration in two (4.3%), and increased ALT and AST levels in two (4.3%). All treatment-related SAEs in the N1I3 arm were grade 3 or 4, except one patient with diarrhea. No grade 5 treatment-related SAEs occurred in either study arm.
Concomitant immune-modulating medication for AE management.
Twenty-nine (61.7%) and 39 (83.0%) patients required treatment with an immune-modulating medication (IMM) to manage AEs in the N3I1 and N1I3 arms, respectively (Appendix Table A2, online only). Twenty-five patients (53.2%) received systemic corticosteroid, nine (19.1%) received a topical corticosteroid, and one (2.1%) required infliximab treatment in the N3I1 arm. Among the patients in the N1I3 arm, 36 (76.6%) were treated with a systemic corticosteroid, and 13 (27.7%) required a topical corticosteroid. In addition, 12 (25.5%) patients in the N1I3 arm required treatment with infliximab or mycophenolic acid. IMM use in the N3I3 arm also is listed in Appendix Table A2.
Efficacy
The confirmed ORR was 40.4% in both treatment arms. Five (10.6%) patients achieved complete response and 14 (29.8%) a partial response in the N3I1 arm. No patients achieved complete response in the N1I3 arm, but 19 (40.4%) had a partial response (Table 3). ORR and BOR for patients who received prior treatment versus treatment-naïve patients in both arms are listed in Appendix Table A3 (online only).
Table 3.
ORRs by Treatment Arm

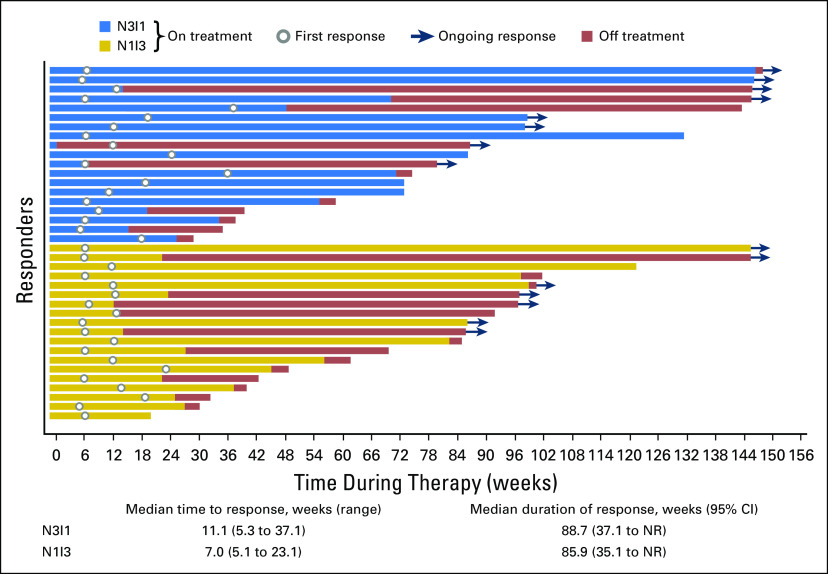
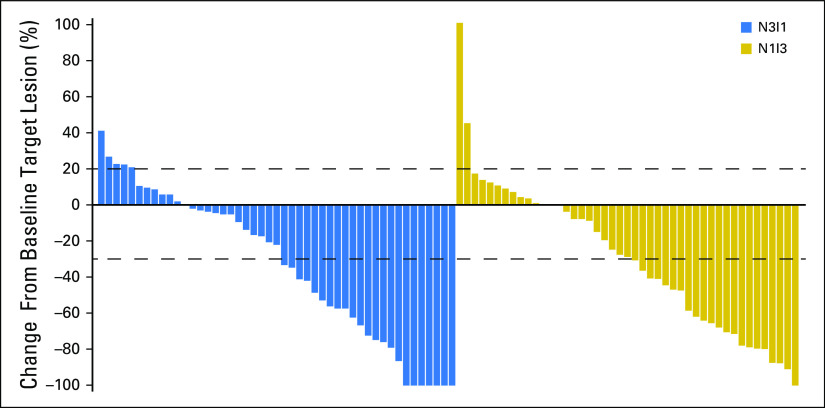
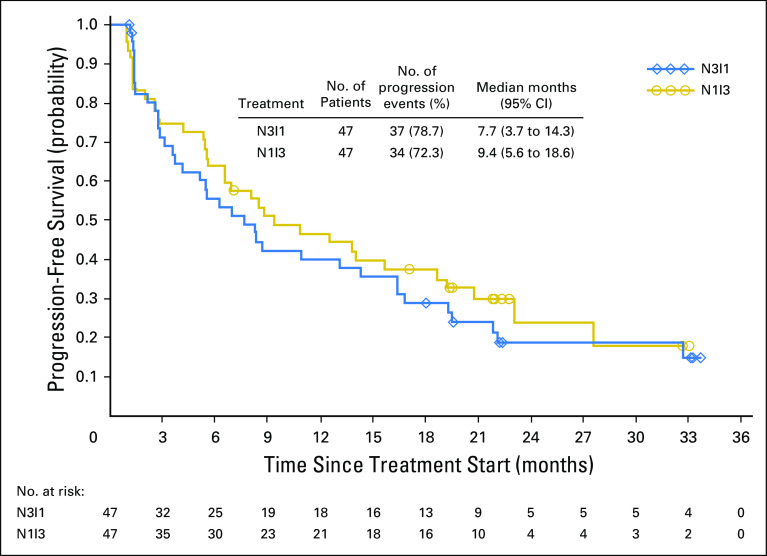
Ongoing response was observed for eight (42.1%) patients in the N3I1 arm and seven (36.8%) in the N1I3 arm. Responses were rapid and sustained (Fig 1). The majority of patients experienced either no increase or a reduction in target lesion size over time (Fig 2). In addition, approximately one half of all patients in each arm experienced a reduction in target lesion tumor burden of at least 30% (Appendix Fig A2, online only). At 6, 12, 18, and 24 months, PFS rates were 55.6%, 40.0%, 28.9%, and 18.7% for the N3I1 arm versus 63.8%, 46.4%, 37.6%, and not calculated in the N1I3 arm, respectively. Median PFS was 7.7 months (95% CI, 3.7 to 14.3 months) for the N3I1 arm versus 9.4 months (95% CI, 5.6 to 18.6 months) in the N1I3 arm (Appendix Fig A3, online only).
Fig 1.
Time to response, duration of response, and time during therapy (weeks) by treatment arm. N1I3, nivolumab 1 mg/kg plus ipilimumab 3 mg/kg; N3I1, nivolumab 3 mg/kg plus ipilimumab 1 mg/kg; NR, not reached.
Fig 2.
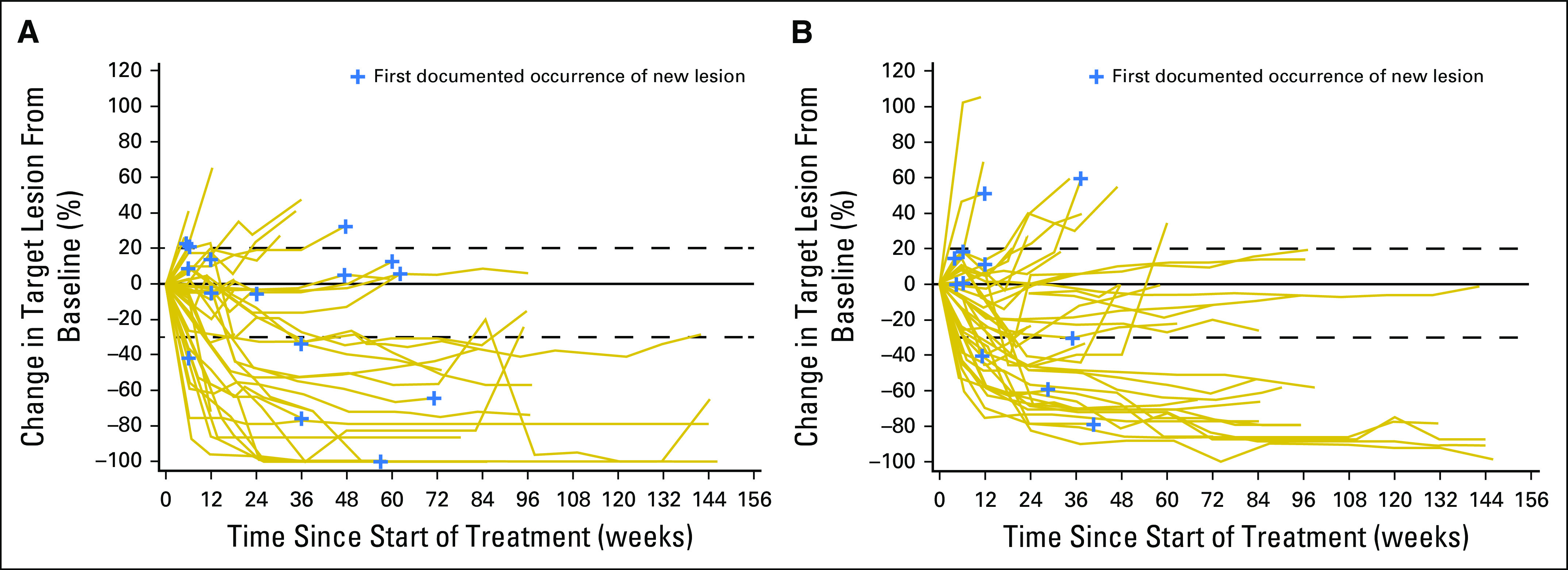
(A) Tumor burden over time on the basis of percent change in target lesion size from baseline for the nivolumab 3 mg/kg plus ipilimumab 1 mg/kg arm. (B) Tumor burden over time on the basis of percent change in target lesion from baseline for the nivolumab 1 mg/kg plus ipilimumab 3 mg/kg arm. Dashed lines denote a 20% increase (progression) or 30% decrease indicative of response.
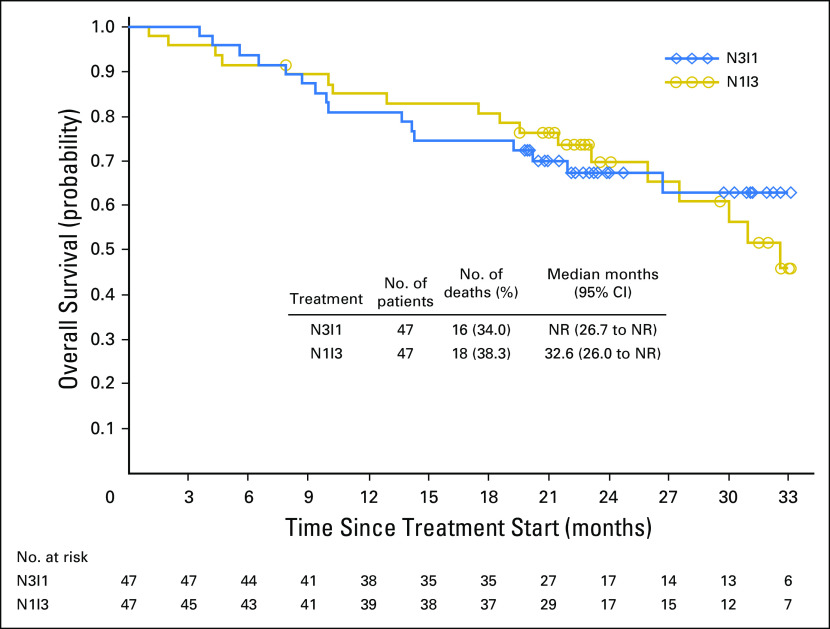
Median OS was not reached (95% CI, 26.7 months to not reached) in the N3I1 arm and 32.6 months (95% CI, 26.0 months to not reached) in patients in the N1I3 arm (Fig 3). At 12 and 24 months, OS was 81% and 67% in the N3I1 arm and 85% and 70% in the N1I3 arm, respectively.
Fig 3.
Kaplan-Meier plot of overall survival by treatment arm. N1I3, nivolumab 1 mg/kg plus ipilimumab 3 mg/kg; N3I1, nivolumab 3 mg/kg plus ipilimumab 1 mg/kg; NR, not reached.
DISCUSSION
This study is among the first to investigate the combination of two checkpoint inhibitors for the treatment of a genitourinary malignancy. These results support the safety of nivolumab plus ipilimumab combination therapy in mRCC. The study also provides preliminary support for the robust clinical activity of nivolumab plus ipilimumab combination therapy as evidenced by improved ORR relative to nivolumab monotherapy previously reported in phase II and phase III mRCC studies.13,14
Although the N3I1 and N1I3 combination therapies were similarly efficacious, the overall safety results support additional clinical development of N3I1. Relative to the other arms, patients who received the N3I1 combination regimen had lower rates of treatment-related AEs (all, and grade 3 or 4), lower rates of SAEs, and lower rates of select treatment-related AEs in a majority of categories. Furthermore, fewer patients in the N3I1 arm discontinued as a result of a treatment-related AE, and fewer patients required an IMM to manage AEs.
Treatment-related AEs occurred at similar frequencies in both the N3I1 and N1I3 arms relative to previous studies of varying nivolumab plus ipilimumab combination dosing regimens in other tumor types. In two melanoma studies, patients treated with nivolumab plus ipilimumab experienced treatment-related grade 3 or 4 AEs at rates of 55% and 53% across the dosing ranges.22,23 In addition, rates of treatment-related grade 3 or 4 AEs in patients treated with nivolumab plus ipilimumab in recent lung cancer studies ranged from 19% to 30% and 28% to 35% across dosing ranges.20,21 Of note, previously reported AEs were manageable, and the majority of select AEs reported in the melanoma studies were resolved with IMMs.20-23 Similarly, the current study reported grade 3 or 4 treatment-related AEs in 38.3% and 61.7% of patients in the N3I1 and N1I3 arms, respectively, whereas most patients (83.3%) who received the N3I3 dosing regimen experienced grade 3 or 4 treatment-related AEs. The most common select treatment-related AEs were skin, endocrine, and GI in the N3I1 and N1I3 arms, which is similar to previous reports.20-23 The rate of IMM use for the management of AEs tended to be lower in the N3I1 arm than in the N1I3 arm.22
Previous studies of varying doses of nivolumab monotherapy in patients with mRCC have reported ORRs of 20% to 22% (phase II in patients who previously received VEGF-targeted therapy) and 25% (phase III in patients who previously received antiangiogenic therapy).13,14 In a phase II study, ipilimumab monotherapy resulted in ORRs of 5% and 12.5% in patients treated with low and high doses, respectively.19 We report a confirmed ORR of 40.4% in both arms in all patients and confirmed ORRs of 45.5% (N3I1) and 38.5% (N1I3) in previously treated subgroups of patients, which suggests that nivolumab plus ipilimumab combination therapy offers improved activity over nivolumab monotherapy in this setting. Rapid responses occurred after treatment initiation for both arms, were of notable magnitude, and were durable in 42% of patients in the N3I1 arm. Both nivolumab plus ipilimumab combination regimens led to favorable OS rates at 2 years for the entire study population, which included both previously treated and treatment-naïve patients. The preliminary efficacy results of the N1I3 and N3I1 arms along with the encouraging safety results in patients treated with the N3I1 combination regimen support the additional clinical investigation of N3I1 in a phase III study for patients with mRCC (CheckMate 214; ClinicalTrial.gov identifier NCT02231749).
ACKNOWLEDGMENT
We thank the patients and their families as well as the investigators and participating study teams for making this study possible. We also thank the late Paul Gagnier, MD, PhD (central medical monitor). Professional medical writing assistance was provided by Jennifer Tyson, Maria Soushko, and Lawrence Hargett of PPSI (a PAREXEL company), funded by Bristol-Myers Squibb.
Appendix
Fig A1.
Patient distribution. (*) Data are not presented in this article. (†) All patients in the nivolumab 3 mg/kg plus ipilimumab 3 mg/kg (N3I3) arm (n = 6) were censored at the time of analysis. AE, adverse event; N3I1, nivolumab 3 mg/kg plus ipilimumab 1 mg/kg; N1I3, nivolumab 1 mg/kg plus ipilimumab 3 mg/kg.
Fig A2.
Best percent change from baseline in target lesion tumor burden up to Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST 1.1) progression. Dashed lines denote 30% decrease and 20% increase in tumor burden. Patients whose target lesion resolved 100% may have had concurrent progression of nontarget lesions. N1I3, nivolumab 1 mg/kg plus ipilimumab 3 mg/kg; N3I1, nivolumab 3 mg/kg plus ipilimumab 1 mg/kg.
Fig A3.
Kaplan-Meier plot of progression-free survival by treatment arm. N1I3, nivolumab 1 mg/kg plus ipilimumab 3 mg/kg; N3I1, nivolumab 3 mg/kg plus ipilimumab 1 mg/kg.
Table A1.
AE Summary for Patients Treated With N3I3 (n = 6)
Table A2.
Concomitant Immune-Modulating Medications for AE Management

Table A3.
ORR by Treatment Arm and Prior Treatment Status
Footnotes
Supported by Bristol-Myers Squibb. Authors received no financial support or compensation for publication of this manuscript.
Clinical trial information: NCT01472081.
AUTHOR CONTRIBUTIONS
Conception and design: Hans J. Hammers
Provision of study materials or patients: Hans J. Hammers, Elizabeth R. Plimack, Jeffrey R. Infante, Brian I. Rini, David F. McDermott, Lionel D. Lewis, Martin H. Voss, Padmanee Sharma, Sumanta K. Pal, Albiruni R. Abdul Razak, Christian Kollmannsberger, Daniel Y.C. Heng, Jennifer Spratlin, and Asim Amin
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Safety and Efficacy of Nivolumab in Combination With Ipilimumab in Metastatic Renal Cell Carcinoma: The CheckMate 016 Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Hans J. Hammers
Consulting or Advisory Role: Bayer, Bristol-Myers Squibb, Cerulean, Exelixis, Pfizer
Research Funding: Bristol-Myers Squibb (Inst), Exelixis, GlaxoSmithKline, Newlink, Pfizer, SFJ, Tacon
Elizabeth R. Plimack
Consulting or Advisory Role: Novartis, Bristol-Myers Squibb, Acceleron Pharma, Genentech, AstraZeneca, MedImmune, Eli Lilly, Synergene
Research Funding: Bristol-Myers Squibb (Inst), Acceleron Pharma (Inst), AstraZeneca (Inst), Pfizer (Inst), Eli Lilly (Inst), Merck Sharp & Dohme (Inst), Novartis (Inst), Peloton Therapeutics (Inst)
Patents, Royalties, Other Intellectual Property: US patent No. 14/588,503 filed January 2, 2015 (Inst)
Jeffrey R. Infante
Research Funding: Celldex Therapeutics (Inst), ARMO Biosciences (Inst), BioMed Valley Discoveries (Inst), Novartis (Inst), Janssen Pharmaceuticals (Inst), GlaxoSmithKline (Inst), Immunocore (Inst), Calithera Biosciences (Inst), Phosplatin Therapeutics (Inst), Genentech (Inst), Roche (Inst), Aileron Therapeutics (Inst), AstraZeneca (Inst), eFFECTOR Therapeutics (Inst), MedImmune (Inst), Pfizer (Inst), Bristol-Myers Squibb (Inst), Tesaro (Inst), Merck (Inst)
Brian I. Rini
Consulting or Advisory Role: Accellion, Bristol-Myers Squibb, GlaxoSmithKline, Novartis, Pfizer, Roche
Research Funding: Accellion, Bristol-Myers Squibb (Inst), Genentech (Inst), Immatics, Peleton, Pfizer (Inst), Roche (Inst)
Travel, Accommodations, Expenses: Pfizer
David F. McDermott
Consulting or Advisory Role: Bristol-Myers Squibb, Merck, Genentech, Pfizer, Exelixis, Novartis, Eisai, Array BioPharm
Research Funding: Prometheus (Inst)
Lionel D. Lewis
Research Funding: The Geisel School of Medicine, The Norris Cotton Cancer Center at Dartmouth-Hitchcock Medical Center (Inst), Bristol-Myers Squibb (Inst)
Martin H. Voss
Honoraria: Novartis
Consulting or Advisory Role: Novartis, Calithera Biosciences, Natera, GlaxoSmithKline, Exelixis, Pfizer, Eisai
Research Funding: Bristol-Myers Squibb, Genentech
Travel, Accommodations, Expenses: Novartis, Takeda Pharmaceuticals
Padmanee Sharma
Stock or Other Ownership: Jounce Therapeutics, Kite Pharma, Evelo Therapeutics, Jounce Therapeutics (I), Kite Pharma (I), Neon Therapeutics (I), Neon Therapeutics, Constellation Pharmaceuticals
Consulting or Advisory Role: GlaxoSmithKline, Bristol-Myers Squibb, Amgen, AstraZeneca, Constellation Pharmaceuticals, Jounce Therapeutics, Kite Pharma, Evelo Therapeutics, Neon Therapeutics, EMD Serono
Patents, Royalties, Other Intellectual Property: Patent licensed to Jounce Therapeutics, patents licensed to Bristol-Myers Squibb, Jounce Therapeutics, and Merck (I)
Sumanta K. Pal
Honoraria: Novartis, Medivation, Astellas Pharma
Consulting or Advisory Role: Pfizer, Novartis, AVEO Pharmaceuticals, Genentech, Exelixis, Bristol-Myers Squibb, GlaxoSmithKline
Albiruni R. Abdul Razak
No relationship to disclose
Christian Kollmannsberger
Honoraria: Pfizer, Novartis, Bristol-Myers Squibb
Consulting or Advisory Role: Pfizer, Novartis, Bristol-Myers Squibb, Sanofi, Astellas, Eli Lilly
Daniel Y.C. Heng
Consulting or Advisory Role: Pfizer, Novartis, Bristol-Myers Squibb
Jennifer Spratlin
Honoraria: Eli Lilly, Celgene
Consulting or Advisory Role: Eli Lilly, Celgene
Research Funding: Celgene, Sanofi, Roche
M. Brent McHenry
Employment: Bristol-Myers Squibb
Stock or Other Ownership: Bristol-Myers Squibb
Research Funding: Bristol-Myers Squibb (Inst)
Asim Amin
Honoraria: Bristol-Myers Squibb, Merck, Pfizer
Consulting or Advisory Role: Merck, Bristol-Myers Squibb
Speakers’ Bureau: Bristol-Myers Squibb, Merck, Pfizer
Research Funding: Bristol-Myers Squibb, Merck, Dynavax
REFERENCES
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. : Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136:E359-E386, 2015 [DOI] [PubMed] [Google Scholar]
- 2.Cairns P: Renal cell carcinoma. Cancer Biomark 9:461-473, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta K, Miller JD, Li JZ, et al. : Epidemiologic and socioeconomic burden of metastatic renal cell carcinoma (mRCC): A literature review. Cancer Treat Rev 34:193-205, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Bedke J, Gauler T, Grunwald V, et al: Systemic therapy in metastatic renal cell carcinoma. World J Urol, 35:179-188, 2017. [DOI] [PMC free article] [PubMed]
- 5.Koneru R, Hotte SJ: Role of cytokine therapy for renal cell carcinoma in the era of targeted agents. Curr Oncol 16:S40-S44, 2009. (suppl 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fisher R, Gore M, Larkin J: Current and future systemic treatments for renal cell carcinoma. Semin Cancer Biol 23:38-45, 2013 [DOI] [PubMed] [Google Scholar]
- 7. Schmidinger M: Understanding and managing toxicities of vascular endothelial growth factor (VEGF) inhibitors. EJC Suppl 11:172-191, 2013. [DOI] [PMC free article] [PubMed]
- 8.Albiges L, Chamming’s F, Duclos B, et al. : Incidence and management of mTOR inhibitor-associated pneumonitis in patients with metastatic renal cell carcinoma. Ann Oncol 23:1943-1953, 2012 [DOI] [PubMed] [Google Scholar]
- 9. Escudier B: Emerging immunotherapies for renal cell carcinoma. Ann Oncol 23:viii35-viii40, 2012 (suppl 8) [DOI] [PubMed]
- 10.Raman R, Vaena D: Immunotherapy in metastatic renal cell carcinoma: A comprehensive review. BioMed Res Int 2015:367354, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Opdivo (nivolumab) injection [prescribing information]. Princeton, NJ, Bristol-Myers Squibb, 2016.
- 12.Buchbinder EI, Desai A: CTLA-4 and PD-1 pathways: Similarities, differences, and implications of their inhibition. Am J Clin Oncol 39:98-106, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Motzer RJ, Escudier B, McDermott DF, et al. : Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 373:1803-1813, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Motzer RJ, Rini BI, McDermott DF, et al. : Nivolumab for metastatic renal cell carcinoma: Results of a randomized phase II trial. J Clin Oncol 33:1430-1437, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cella D, Grünwald V, Nathan P, et al. : Quality of life in patients with advanced renal cell carcinoma given nivolumab versus everolimus in CheckMate 025: A randomised, open-label, phase 3 trial. Lancet Oncol 17:994-1003, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yervoy (ipilimumab) injection [prescribing information]. Princeton, NJ, Bristol-Myers Squibb, 2015.
- 17.Chambers CA, Kuhns MS, Egen JG, et al. : CTLA-4-mediated inhibition in regulation of T cell responses: Mechanisms and manipulation in tumor immunotherapy. Annu Rev Immunol 19:565-594, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Wolchok JD, Saenger Y: The mechanism of anti-CTLA-4 activity and the negative regulation of T-cell activation. Oncologist 13:2-9, 2008. (suppl 4) [DOI] [PubMed] [Google Scholar]
- 19.Yang JC, Hughes M, Kammula U, et al. : Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J Immunother 30:825-830, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Antonia SJ, López-Martin JA, Bendell J, et al. : Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): A multicentre, open-label, phase 1/2 trial. Lancet Oncol 17:883-895, 2016 [DOI] [PubMed] [Google Scholar]
- 21. Hellman MD, Gettinger SN, Goldman JW, et al: CheckMate 012: Safety and efficacy of first-line (1L) nivolumab (nivo; N) and ipilimumab (ipi; I) in advanced (adv) NSCLC. J Clin Oncol 34, 2016 (suppl; abstr 3001) [Google Scholar]
- 22.Larkin J, Hodi FS, Wolchok JD: Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 373:1270-1271, 2015 [DOI] [PubMed] [Google Scholar]
- 23.Wolchok JD, Kluger H, Callahan MK, et al. : Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 369:122-133, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eisenhauer EA, Therasse P, Bogaerts J, et al. : New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 45:228-247, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Schag CC, Heinrich RL, Ganz PA: Karnofsky performance status revisited: Reliability, validity, and guidelines. J Clin Oncol 2:187-193, 1984 [DOI] [PubMed] [Google Scholar]
- 26.Clopper CJ, Pearson ES: The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 26:404-413, 1934 [Google Scholar]
- 27.Greenwood M: The natural duration of cancer. Rep Public Health Med Subj (Lond) 33:1-26, 1926 [Google Scholar]