Abstract
PURPOSE
The combination of lenalidomide, bortezomib, and dexamethasone (RVD) is a highly effective and convenient induction regimen for both transplantation-eligible and -ineligible patients with myeloma. Here, we present the largest cohort of patients consecutively treated with RVD induction therapy followed by risk-adapted maintenance therapy with the longest follow-up and important information on long-term outcomes.
PATIENTS AND METHODS
We describe 1,000 consecutive patients with newly diagnosed myeloma treated with RVD induction therapy from January 2007 until August 2016. Demographic and clinical characteristics and outcomes data were obtained from our institutional review board–approved myeloma database. Responses and progression were evaluated per International Myeloma Working Group Uniform Response Criteria.
RESULTS
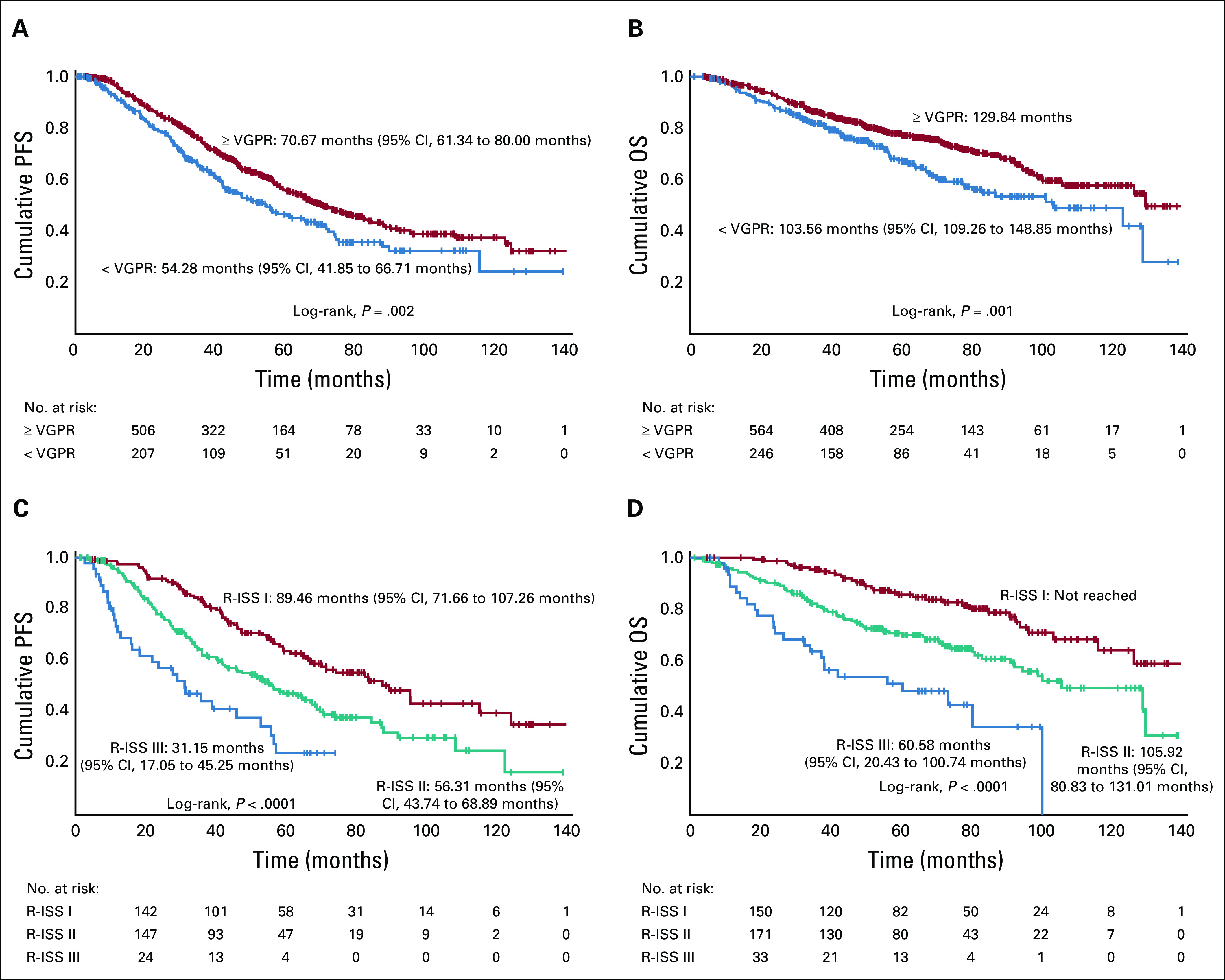
The overall response rate was 97.1% after induction therapy and 98.5% after transplantation, with 89.9% of patients achieving a very good partial response (VGPR) or better and 33.3% achieving stringent complete response after transplantation at a median follow-up time of 67 months. The estimated median progression-free survival time was 65 months (95% CI, 58.7 to 71.3 months) for the entire cohort, 40.3 months (95% CI, 33.5 to 47 months) for high-risk patients, and 76.5 months (95% CI, 66.9 to 86.2 months) for standard-risk patients. The median overall survival (OS) time for the entire cohort was 126.6 months (95% CI, 113.3 to 139.8 months). The median OS for high-risk patients was 78.2 months (95% CI, 62.2 to 94.2 months), whereas it has not been reached for standard-risk patients. Five-year OS rates for high-risk and standard-risk patients were 57% and 81%, respectively, and the 10-year OS rates were 29% and 58%, respectively.
CONCLUSION
RVD is an induction regimen that delivers high response rates (VGPR or better) in close to 90% of patients after transplantation, and risk-adapted maintenance can deliver unprecedented long-term outcomes. This study includes the largest cohort of patients treated with RVD reported to date with long follow-up and demonstrates the ability of 3-drug induction regimens in patients with newly diagnosed multiple myeloma to result in a substantial survival benefit.
INTRODUCTION
There have been significant therapeutic advances in myeloma over the past few decades, leading to an improved survival benefit for patients during this period. The availability of novel classes of drugs, namely immunomodulatory drugs (IMIDs) and proteasome inhibitors (PIs), and the increased use of autologous stem-cell transplantation (ASCT) and continuous maintenance therapy have contributed to these sustained responses.
Improvements in pre- and post-ASCT outcomes have been a result of the evolution toward the use of carefully crafted combination therapy required for newly diagnosed myeloma. Several trials have demonstrated improvements in progression-free survival (PFS) for 3-drug regimens over 2-drug regimens, both with and without the use of high-dose therapy as consolidation. However, in nearly all cases, the duration of PFS has been longer with ASCT consolidation. The widespread adoption of the lenalidomide, bortezomib, and dexamethasone (RVD) induction regimen has changed the expectation for induction regimens and has been validated with several large phase III trials, although the follow-up on these trials is relatively short. Furthermore, the type of maintenance therapy used and the duration of maintenance (continuous v limited duration) also vary in these trials using RVD for induction, limiting accurate estimation of long-term outcomes. In this analysis, we describe a consecutive cohort of 1,000 patients treated uniformly with long-term follow-up. We describe outcomes based on genetic risk at diagnosis, PFS, overall survival (OS), and the impact of genetics on the quality and depth of response, thus providing a more comprehensive picture of the overall treatment course with RVD as induction therapy.
PATIENTS AND METHODS
Study Design and Participants
We identified 1,000 consecutive patients with myeloma who were treated with RVD induction therapy between January 2007 and August 2016 (Fig 1). Data cutoff was May 31, 2019. Additional information regarding patient selection, dosing schedule, and the statistical methods are provided in the Data Supplement.
FIG 1.
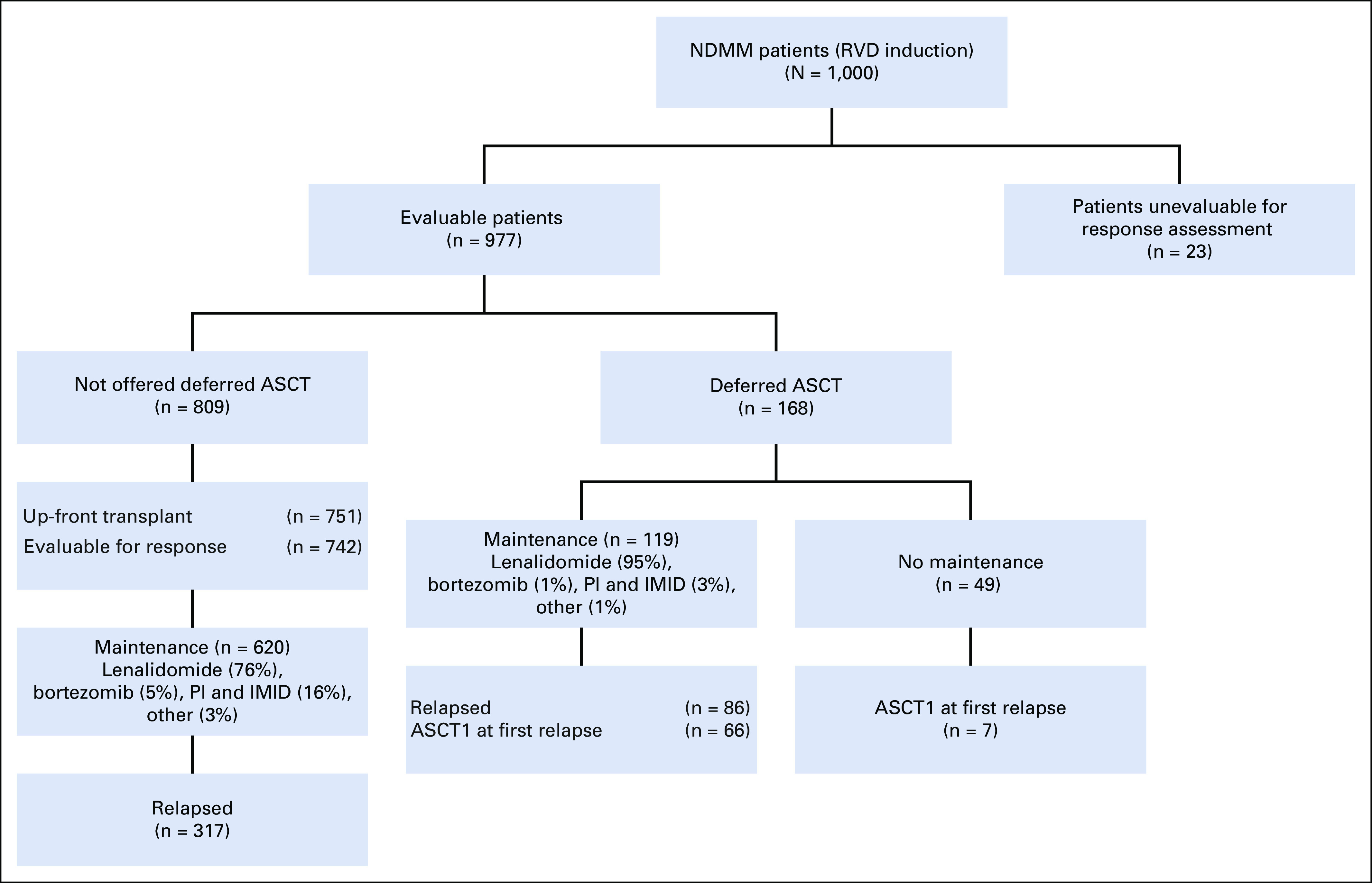
CONSORT diagram. ASCT, autologous stem-cell transplantation; ASCT1, first autologous stem-cell transplantation; IMID, immunomodulatory drug; NDMM, newly diagnosed multiple myeloma; PI, proteasome inhibitor; RVD, lenalidomide, bortezomib, and dexamethasone.
RESULTS
Patient Characteristics
Patient demographic and clinical characteristics are listed in Table 1. The median age of patients was 61 years, and 35.2% of patients were African American (AA), consistent with the demographics of the myeloma population Emory University serves.
TABLE 1.
Patient Demographics and Clinical Characteristics
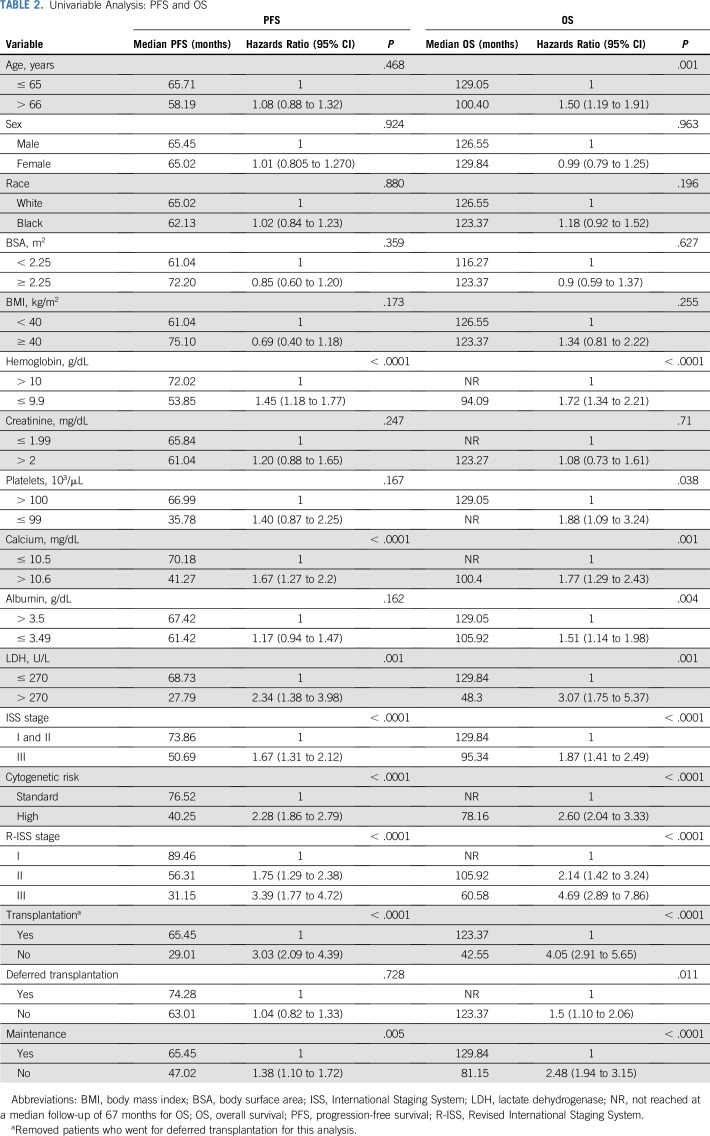
The age of diagnosis was earlier among AA patients compared with white patients; the median age of diagnosis in AA patients was 58 years compared with 63 years for white patients. This is consistent with previously published population-based studies.1 There were significant differences found in the rates of amplification of 1q and del(17p) in AA and white patients (1q, 10.8% v 18.8%, respectively; and del(17p), 6.7% v 12.2%, respectively; P = .001). The lower incidence of the deleterious higher risk features in AA patients is consistent with previously reported studies as well.2
Response Rates, PFS, and OS
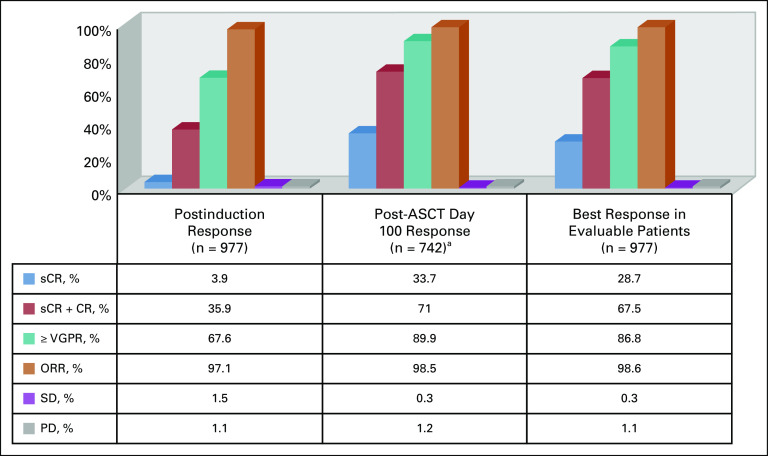
Response assessment as defined by the International Myeloma Working Group (IMWG) was available for 977 patients (consort diagram). Responses are summarized in Figure 2. The median duration of induction therapy was 3.9 months (range, 1.4 - 2.1 months). A median of 4 cycles of RVD (range, 2-15 cycles) were administered. The median time to best response from induction therapy for all patients was 3.9 months from the initiation of therapy (range, 0.4-39.5 months).
FIG 2.
Response rates to lenalidomide, bortezomib, and dexamethasone (RVD) induction therapy (evaluable patients, n = 977). ASCT, autologous stem-cell transplantation; CR, complete response; ORR, overall response rate; PD, progressive disease; sCR, stringent complete response; SD, stable disease; VGPR, very good partial response. (a) Seven hundred forty-two evaluable patients of 751 patients who underwent up-front transplantation.
A third of the patients were > 65 years old, and 45% of the patients were female. Response rates by different subgroups are summarized in the Data Supplement. The median PFS for the entire cohort was 65 months (95% CI, 58.7 to 71.3 months). The median PFS times for white males, white females, black males, and black females were 65.4, 65, 64.8, and 62.1 months, respectively (P = .830). The median OS for the entire cohort was 126.6 months (95% CI, 113.3 to 139.8 months). The median OS times for white males, white females, black males, and black females were 126.6, 129.8, 126.6, and 106.1 months, respectively (P = .986).
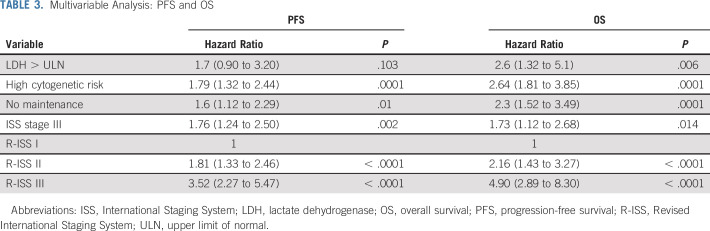
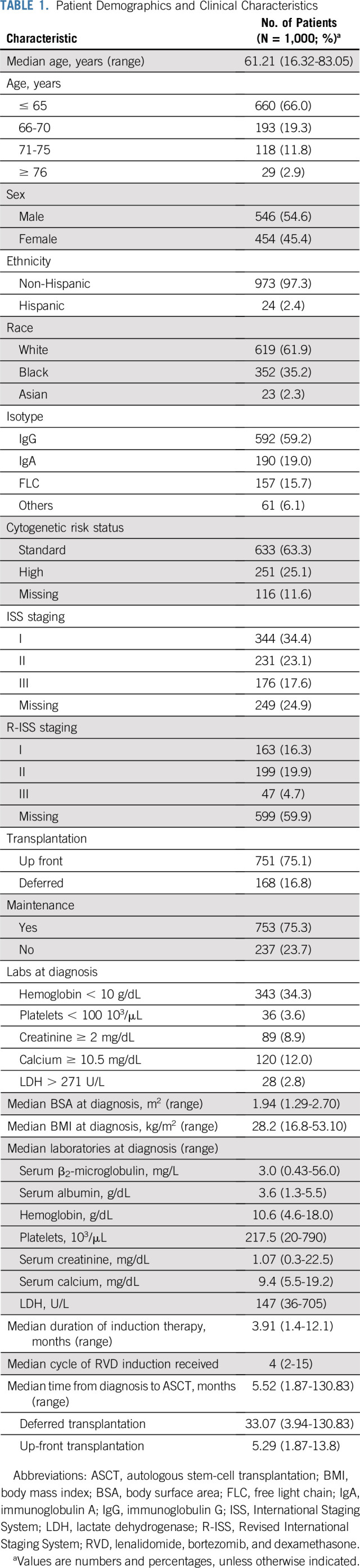
International Staging System (ISS) staging was available for 751 patients, and Revised International Staging System (R-ISS) staging was available for 409 patients. The rates of renal failure at the time of diagnosis (defined as creatinine > 2 mg/dL) were higher among patients with ISS stage III (ISS stage III v I or II: 38.3% v 3.7%, respectively; P < .0001) and patients with R-ISS stage III (R-ISS stage III v II v I: 40.5% v 9.8% v 0.6%, respectively; P < .0001). The median PFS times for patients with ISS stage I, II, and III disease were 74.3, 73.9, and 50.7 months, respectively (P < .0001), whereas the median PFS times for patients with R-ISS stage I, II, and III disease were 89.5, 56.3, and 31.2 months, respectively (P < .0001; Fig 3). Median OS times for patients with ISS stage I, II, and III disease were not reached (NR), 129.8 months, and 95.3 months, respectively (P < .0001), whereas the median OS times for patients with R-ISS stage I, II, and III disease were NR, 105.9 months, and 60.6 months, respectively (P < .0001; Fig 3). Renal failure at diagnosis did not have prognostic significance for PFS or OS (Table 2). These data substantiate ISS as a prognostic model for both PFS and OS, and R-ISS as a much more robust prognostic model even in the light of existing modern induction therapies (Table 3). PFS and OS by response rates are summarized in the Data Supplement. Achieving a stringent complete response (CR) after induction and transplantation was associated with a significant PFS and OS benefit.
FIG 3.
(A) Progression-free survival (PFS) by postinduction very good partial response (VGPR) status. (B) Overall survival (OS) by postinduction VGPR status. (C) PFS by Revised International Staging System (R-ISS) stage. (D) OS by R-ISS stage.
TABLE 2.
Univariable Analysis: PFS and OS
TABLE 3.
Multivariable Analysis: PFS and OS
Seven hundred fifty-one patients underwent up-front first ASCT (ASCT1) after induction therapy, with a median time to ASCT1 of 5.5 months (range, 1.9-13.8 months) from diagnosis (Fig 1). Seven hundred forty-two patients were evaluable for response, and 71% achieved CR or better at day 100 restaging after transplantation (Fig 2). One hundred sixty-eight patients had stem cells collected but were offered deferred ASCT. This was a select group of standard-risk patients who had good responses to induction therapy compared with patients who underwent up-front ASCT, with very good partial response (VGPR) or better rates of 84.8% and 64.1%, respectively (P < .0001), and CR or better rates of 56.7% and 31.7%, respectively (P < .0001). Altogether, in the deferred group, 119 patients received maintenance therapy. Patients who had received maintenance, compared with patients who received no maintenance, had higher VGPR or better rates (92.9% v 80.4%, respectively; P = .025), higher CR or better rates (76.1% v 56.4%, respectively; P = .013), and longer OS (NR v 88.9 months, respectively; P < .0001). Median PFS times in the deferred and not deferred groups were 74.3 and 63 months, respectively (P = .728), and median OS times were NR and 123.4 months, respectively, at a median follow-up of 102 months. In the deferred group, median time for patients who experienced progression (n = 66) to receive ASCT was 36.1 months (range, 32.5-39.7 months). Twenty patients in the deferred group did not receive ASCT at relapse. Their median time to relapse was 74.3 months from diagnosis, and their median OS was NR at a median follow-up of 107 months. Among the patients who did not experience progression, the median PFS was 143.5 months.
Fifty-eight patients who received RVD induction and did not proceed to a stem-cell collection had a PFS of 29 months (maintenance v no maintenance: 37.5 v 21.39 months, respectively). An OS of 42.5 months (95% CI, 19.4 to 65.7 months) was reported for this cohort of patients.
Overall, 753 patients received maintenance. Six hundred of these patients (60.7%) received lenalidomide maintenance therapy alone based on standard-risk cytogenetics, and 107 patients (10.7%) received IMID and PI maintenance therapy, predominantly with RVD. Standard-risk patients started maintenance therapy at a median of 3.75 months after transplantation, and high-risk patients were initiated on maintenance therapy at 3 months after transplantation. Ninety-one percent of patients who were on maintenance had VGPR or better (compared with 74.4% of patients not on maintenance; P < .0001), and 71% achieved CR or better (compared with 57% of patients not on maintenance; P < .0001). The median PFS times for patients on maintenance and not on maintenance were 65.45 and 47.02 months, respectively (P = .005), and the median OS times were 129.84 and 81.15 months, respectively (P < .0001). Both in the univariable and multivariable analysis, lack of maintenance therapy was a significant predictor for progression or death (PFS: hazard ratio [HR], 1.6; 95% CI, 1.12 to 2.29; P = .01; OS: HR, 2.3; 95% CI, 1.52 to 3.49; P = .0001). The median duration of maintenance therapy among all patients was 59 months (range, 50.4-67.6 months). For high-risk patients, the median duration of maintenance therapy was 35 months (range, 25.8-44.2 months), and for standard-risk patients, it was 72 months (range, 63.7-80.3 months). The median duration of maintenance therapy also differed for patients who underwent up-front ASCT (57 months; range, 49.9-64.1 months) versus patients who deferred ASCT (71 months; range, 57.4-84.6 months).
A common theme that was consistently observed even with the most modern induction regimens suggests that high-risk patients achieve deep responses, but these are not maintained with standard maintenance. The Medical Research Council XI Group3 demonstrated that the high-risk population does not derive the same benefit as standard-risk patients from standard maintenance with lenalidomide. In this context, based on our risk-adapted algorithm for the high-risk patients, the median PFS for high-risk patients who received PIs and IMIDs (10.7%) was 40.3 months (95% CI, 33.5 to 47 months). Although we previously reported a 3-year OS rate of 93%, the median OS for the current cohort was 78.2 months (95% CI, 62.2 to 94.2 months) at a median follow-up of 74 months (Fig 4). When deletion 17p patients are specifically considered, a median PFS of 37.2 months (95% CI, 31.7 to 42.7 months) and median OS of 68.5 months are noted, and the majority of these patients received 3-drug maintenance therapy with IMID and PI.4 Putting this in perspective with current trials, the presence of deletion 17p was one of the strongest predictors for early relapse at 18 months in the FORTE trial, which used carfilzomib either with lenalidomide or cyclophosphamide.5 The median PFS times for maintenance and no maintenance among high-risk patients were 42.1 and 16.2 months, respectively (P = .007), and the median OS times were 91.3 and 23.6 months, respectively (P < .0001), a finding highlighting the importance of continued maintenance to attain a survival advantage, particularly in high-risk patients.
FIG 4.
(A) Progression-free survival (PFS) of entire cohort. (B) Overall survival (OS) of entire cohort. (C) PFS by risk. (D) OS by risk.
Secondary primary malignancies (SPMs) were observed in 33 patients (3.3%). Twenty-six SPMs (4.3%) occurred among patients receiving lenalidomide maintenance. Twenty-one SPMs (4%) occurred among patients receiving lenalidomide after transplantation.
DISCUSSION
We report long-term follow-up of the largest cohort of patients with myeloma treated consecutively with the highly active RVD induction regimen, even among patients with high-risk cytogenetics. Because of the controversial question of timing and role of transplantation in myeloma, particularly in light of evolving modern induction therapies during this time period, there were visible changes in the standard-of-care institutional practices, which explains the variability in offering ASCT among this cohort. Nevertheless, our data show that induction with RVD is highly effective in attaining deeper hematologic responses and thus positively impacts long-term survival.
A third of the patients in this data set were > 65 years old, a cohort that is often excluded from transplantation-eligible clinical trials. Among patients who underwent ASCT, postinduction and posttransplantation response rates did not differ by age (≤ v > 65 years). Moreover, age itself was not an independent predictor of decreased PFS. These findings suggest that older patients derive similar benefit as their younger counterparts if offered ASCT and that age alone should not be used as a criterion for transplantation eligibility.
One other major strength of our data set is that the patient population we serve includes a high number of AA patients. Although there is a disproportionate increase in the incidence of myeloma in AA males and females, these patients are underrepresented in studies. AA patients were diagnosed 5 years younger, were more anemic at presentation, and had lower rates of amplification of 1q and del17p compared with the white patients. Large data sets like ours with 352 AA patients receiving uniform therapy help to reassure that AA patients derive a similar benefit as their white counterparts if offered the same therapeutic care. Our data set also validated the prognostic impact of ISS and R-ISS staging on both PFS and OS.
How do the results compare with the existing randomized controlled trials in newly diagnosed myeloma? The Spanish Myeloma Group conducted a phase III trial of induction therapy with RVD followed by ASCT and RVD consolidation for patients ≤ 65 years old. The postinduction and posttransplantation rates of CR or better were similar to our reported results.6,7 Our results are also comparable to the IFM2009 study, which randomly assigned patients to RVD followed by ASCT or no ASCT for the primary end point of PFS. Patients in both groups received maintenance therapy with lenalidomide for 1 year. Eighty-eight percent of patients in the ASCT group and 77% of the patients in the RVD-alone group achieved VGPR or better as the best response, similar to our reported results.8 The DETERMINATION trial was conducted in the United States with a similar study design as the IFM2009 trial,8 with the exception that patients received maintenance therapy with lenalidomide until progression or intolerability. Our approach to offering maintenance is similar to the DETERMINATION trial, which has completed accrual and is currently awaiting results (ClinicalTrials.gov identifier: NCT01208662). Our results echo the postinduction and post-ASCT findings of the Spanish group and IFM2009 prospective phase III trials (Data Supplement). Although the PFS and OS results of the Spanish group are awaited as well, the reported median PFS in the IFM2009 trial was 36 months in the RVD-alone group versus 50 months in the ASCT group. Our median PFS was higher at 65.5 months, likely because of the fixed-duration maintenance approach in the IFM2009 trial. In addition, we used a risk-adapted approach for maintenance using combination maintenance strategies for high-risk patients, thereby preventing early relapses among these patients who are prone to experience early progression.4 We also had a deferred ASCT cohort, and long-term follow-up results in this cohort are of significance. Patients who were offered deferred ASCT were carefully chosen and had better CR rates and standard-risk features. The patients who experienced progression in this group had a median time to progression of 3 years, and the majority received ASCT. The outcomes of the patients who experienced progression and did not receive ASCT were impressive, likely reflecting the selection bias and the disease biology; median time to progression was beyond the 6-year mark, and the median OS has not been reached.
The SWOG S0777 trial randomly assigned patients to receive either RVD or lenalidomide plus dexamethasone (RD) induction followed by maintenance with RD until progression. Although the study met the primary end point of PFS superiority for RVD relative to RD, in the RVD arm, the VGPR or better rate was 43.5% and median PFS and OS were 43 and 75 months, respectively. Our results starkly differ from these results, with median PFS and OS for the entire cohort reported at 65.5 and 126 months, respectively. Other phase III experiences6,8 suggest that RVD induction can achieve significantly better results than the SWOG S0777 trial findings. Nevertheless, SWOG S0777 is the first ever randomized prospective trial to show an OS benefit of 13 months for patients receiving RVD therapy (P = .025).
How do our results compare with the other maintenance trials with longer follow-up? A meta-analysis was done of 3 randomized controlled trials (CALGB 100104,9 GIMEMA RV-MM-PI-209,10 and IFM 2005-0211) in patients with newly diagnosed myeloma receiving ASCT followed by lenalidomide maintenance versus placebo or observation.12 This meta-analysis confirmed the benefit of maintenance lenalidomide, with a median PFS of 52.8 months. At a median follow-up of 79.5 months for all surviving patients, the median OS had not been reached for the lenalidomide maintenance group, whereas it was 86.0 months for the placebo or observation group (HR, 0.75; 95% CI, 0.63 to 0.90; P = .001). The induction regimens and the maintenance durations varied widely in these trials, and these could have influenced the magnitude of improvement in PFS and OS. In our data set, the PFS and OS times simulating this scenario of RVD induction followed by ASCT and lenalidomide maintenance until progression were 72 and 123.4 months, respectively.
The strengths of our study include the size of the group of consecutive patients treated with RVD induction therapy and the long duration of follow-up. Although the response rates after induction therapy and after ASCT reflect prospective trial data (Data Supplement), an inherent limitation of this analysis is that it is retrospective, and thus, results need to be interpreted with caution. Once a treatment plan was made for a patient, induction treatment was delivered by the patient’s community physician in many cases. Patients were seen and evaluated at Emory University at sentinel points in their care to assess for response, PFS, and OS, but adverse events cannot be formally described in this article. Although we have several standard time points for uniform response assessments (ie, day 100, day 180, and yearly after ASCT restaging), our assessments are limited when compared with a prospective clinical trial setting. There were 23 patients who did not have evaluable responses with induction therapy because they were lost to follow-up, but given the study design we implemented to include consecutive patients receiving RVD induction therapy, these patients were included in the analysis. This data set incorporating a collaboration of community and academic management of patients with myeloma is a true real-world reflection of the management of a large population of patients with myeloma and serves as the new benchmark for long-term survival advantages with modern induction regimens.
Our cohort included patients treated over a decade. During this period, the minimal residual disease (MRD) assessment tools have evolved from low-sensitivity flow-based assessments to the currently available US Food and Drug Administration–approved next-generation sequencing techniques. We did not report rates of MRD negativity because they were not available for all patients at a uniform time point.
Patients with IMWG-defined high-risk cytogenetics were more likely to receive a combination of PI and IMID at our institution, as we have described previously.4 Our previously reported cohort had a PFS benefit of 42.1 months, with longer follow-up. Notably, high-risk patients who did not receive maintenance therapy for various reasons had a PFS of 16.2 months and median OS of 23.6 months (compared with 91.3 months for patients receiving maintenance). The shorter time from progression to death in the high-risk patients who did not receive maintenance also signifies that saving drugs for a later time may not be the right approach and that the same therapies used at later stages in the disease course may be less effective perhaps because of changes in the disease biology.
The OS benefit with RVD induction therapy in SWOG S0777, although present, was relatively modest because of what we feel is simply the immaturity of the data. Our expectation is that the aforementioned prospective trials will eventually mature with longer follow-up and may show similar results to ours. In the interim, our analysis, in addition to the currently available literature, provides a strong rationale for adapting multidrug combination strategies in the up-front treatment of patients with myeloma and makes a strong case for risk-adapted maintenance.
PRIOR PRESENTATION
Presented in part at the 60th Annual Meeting of the American Society of Hematology, San Diego, CA, December 1-4, 2018.
AUTHOR CONTRIBUTIONS
Conception and design: Nisha S. Joseph, Jonathan L. Kaufman, Sagar Lonial, Ajay K. Nooka
Provision of study materials or patients: Jonathan L. Kaufman, Leonard T. Heffner, Sagar Lonial
Collection and assembly of data: Nisha S. Joseph, Jonathan L. Kaufman, Dhwani K. Almaula, Leonard T. Heffner, Sagar Lonial, Ajay K. Nooka
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Long-Term Follow-Up Results of Lenalidomide, Bortezomib, and Dexamethasone Induction Therapy and Risk-Adapted Maintenance Approach in Newly Diagnosed Multiple Myeloma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Jonathan L. Kaufman
Honoraria: Tecnofarma
Consulting or Advisory Role: Janssen, Takeda, Celgene, Bristol-Myers Squibb, Karyopharm Therapeutics, TG Therapeutics, Sanofi, Amgen, Tecnofarma
Research Funding: Merck (Inst), Celgene (Inst), Janssen (Inst), Sutro Biopharma (Inst), Fortis Therapeutics (Inst), Amgen (Inst), AbbVie/Genentech (Inst), Bristol-Myers Squibb (Inst)
Travel, Accommodations, Expenses: Janssen, Celgene, Bristol-Myers Squibb, Sanofi, Amgen, Takeda
Madhav V. Dhodapkar
Consulting or Advisory Role: Genentech, Amgen, Kite Pharma, Lava Therapeutics, Janssen Oncology, Celgene
Craig C. Hofmeister
Consulting or Advisory Role: Sanofi Pasteur, Imbrium, Celgene, Nektar, Janssen Oncology, Karyopharm Therapeutics, Oncopeptides
Research Funding: Celgene, Bristol-Myers Squibb, Cellularity
Leonard T. Heffner
Research Funding: Pharmacyclics (Inst), Genentech (Inst), Kite Pharma (Inst), ADC Therapeutics (Inst), Astex Pharmaceuticals (Inst)
Lawrence H. Boise
Honoraria: AstraZeneca
Consulting or Advisory Role: AbbVie/Genentech, AstraZeneca
Research Funding: AstraZeneca (Inst)
Sagar Lonial
Consulting or Advisory Role: Celgene, Bristol-Myers Squibb, Janssen Oncology, Novartis, GlaxoSmithKline, Amgen, AbbVie, Takeda, Merck, Juno Therapeutics
Research Funding: Celgene, Bristol-Myers Squibb, Takeda
Ajay K. Nooka
Consulting or Advisory Role: Amgen, Janssen Oncology, Celgene, Spectrum Pharmaceuticals, Bristol-Myers Squibb, GlaxoSmithKline, Takeda, Oncopeptides, Karyopharm Therapeutics
Research Funding: Amgen (Inst), Janssen Oncology (Inst), Takeda (Inst)
Travel, Accommodations, Expenses: GlaxoSmithKline
No other potential conflicts of interest were reported.
REFERENCES
- 1.Waxman AJ, Mink PJ, Devesa SS, et al. Racial disparities in incidence and outcome in multiple myeloma: A population-based study. Blood. 2010;116:5501–5506. doi: 10.1182/blood-2010-07-298760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Munjuluri A, Fillmore N, Cirstea D, et al. With equal access, African Americans with non-del17p multiple myeloma have superior overall survival, but del17p still carries poor prognosis across race: A VA study. Blood. 2019;134(suppl 1):4388. [Google Scholar]
- 3.Kaiser MF, Jenner M, Cairns D, et al. Outcomes of transplant-eligible newly diagnosed ultra-high risk myeloma patients treated in the NCRI Myeloma XI Trial indicate the need for early treatment stratification and novel treatment approaches. Blood. 2019;134(suppl 1):604. [Google Scholar]
- 4.Nooka AK, Kaufman JL, Muppidi S, et al. Consolidation and maintenance therapy with lenalidomide, bortezomib and dexamethasone (RVD) in high-risk myeloma patients. Leukemia. 2014;28:690–693. doi: 10.1038/leu.2013.335. [DOI] [PubMed] [Google Scholar]
- 5.Zaccaria GM, Capra A, Petrucci MT, et al. Predictive model of early relapse in newly diagnosed multiple myeloma: Analysis from a pooled dataset. Blood. 2019;134(suppl 1):2130. [Google Scholar]
- 6.Rosinol L, Oriol A, Rios R, et al. Bortezomib, lenalidomide and dexamethasone (VRD-GEM) as induction therapy prior autologous stem cell transplantation (ASCT) in multiple myeloma (MM): Results of a prospective phase III Pethema/GEM Trial. Blood. 2017;130:2017. (suppl; abstr) [Google Scholar]
- 7.Rosiñol L, Oriol A, Rios R, et al. Bortezomib, lenalidomide, and dexamethasone as induction therapy prior to autologous transplant in multiple myeloma. Blood. 2019;134:1337–1345. doi: 10.1182/blood.2019000241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Attal M, Lauwers-Cances V, Hulin C, et al. Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. N Engl J Med. 2017;376:1311–1320. doi: 10.1056/NEJMoa1611750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCarthy PL, Owzar K, Hofmeister CC, et al. Lenalidomide after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012;366:1770–1781. doi: 10.1056/NEJMoa1114083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palumbo A, Cavallo F, Gay F, et al. Autologous transplantation and maintenance therapy in multiple myeloma. N Engl J Med. 2014;371:895–905. doi: 10.1056/NEJMoa1402888. [DOI] [PubMed] [Google Scholar]
- 11.Attal M, Lauwers-Cances V, Marit G, et al. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012;366:1782–1791. doi: 10.1056/NEJMoa1114138. [DOI] [PubMed] [Google Scholar]
- 12.McCarthy PL, Holstein SA, Petrucci MT, et al. Lenalidomide maintenance after autologous stem-cell transplantation in newly diagnosed multiple myeloma: A meta-analysis. J Clin Oncol. 2017;35:3279–3289. doi: 10.1200/JCO.2017.72.6679. [DOI] [PMC free article] [PubMed] [Google Scholar]