Supplemental Digital Content is available in the text.
Keywords: coronavirus disease 2019 infection, coronavirus disease 2019 pandemic, in-hospital mortality, intubation, mechanical ventilation, respiratory failure
Abstract
Objectives:
To examine whether increasing time between admission and intubation was associated with mortality in patients with coronavirus disease 2019 who underwent mechanical ventilation.
Design:
Retrospective cohort study of patients with severe acute respiratory syndrome coronavirus 2 infection who were admitted between January 30, 2020, and April 30, 2020, and underwent intubation and mechanical ventilation prior to May 1, 2020. Patients were followed up through August 15, 2020.
Setting:
Five hospitals within the Mount Sinai Health System in New York City, NY.
Patients:
Adult patients with severe acute respiratory syndrome coronavirus 2 infection who underwent intubation and mechanical ventilation.
Interventions:
Tracheal intubation and mechanical ventilation.
Measurements and Main Results:
The primary outcome was in-hospital mortality. A hospital-stratified time-varying Cox model was used to evaluate the effect of time from admission to intubation on in-hospital death. A total of 755 adult patients out of 5,843 admitted with confirmed severe acute respiratory syndrome coronavirus 2 infection underwent tracheal intubation and mechanical ventilation during the study period. The median age of patients was 65 years (interquartile range, 56–72 yr) and 64% were male. As of the time of follow-up, 121 patients (16%) who were intubated and mechanically ventilated had been discharged home, 512 (68%) had died, 113 (15%) had been discharged to a skilled nursing facility, and 9 (1%) remained in the hospital. The median time from admission to intubation was 2.3 days (interquartile range, 0.6–6.3 d). Each additional day between hospital admission and intubation was significantly associated with higher in-hospital death (adjusted hazard ratio, 1.03; 95% CI, 1.01–1.05).
Conclusions:
Among patients with coronavirus disease 2019 who were intubated and mechanically ventilated, intubation earlier in the course of hospital admission may be associated with improved survival.
Coronavirus disease 2019 (COVID-19) is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). COVID-19 has a diverse range of clinical manifestations, ranging from entirely asymptomatic infection to severe respiratory distress. Respiratory symptoms caused by COVID-19 may be managed expectantly at home or require more advanced supportive care and hospital admission. Among patients with COVID-19 who require hospitalization, acute respiratory distress syndrome (ARDS) can occur in up to 30% (1).
The traditional management of ARDS is tracheal intubation and “lung-protective” mechanical ventilation (2). Reports from hospitals caring for patients during the initial outbreak in China indicated that at least 80% of patients with COVID-19 requiring mechanical ventilation died (1, 3). A recent meta-analysis, however, found that the mortality rate for ICU patients has fallen to approximately 40% as the pandemic has progressed, suggesting improvements in treatments and system resources (4).
Despite these improvements, there remains a paucity of evidence-based guidelines on optimizing both patient selection and timing of intubation for patients with respiratory failure from COVID-19. Late tracheal intubation may negate the potential benefits of mechanical ventilation if the intervention is delayed until a patient is at too late a stage of illness. Conversely, noninvasive respiratory support is recommended as an initial step in management (5) and may avoid intubation in some patients. Balancing these competing concerns is a challenge for clinicians caring for patients with COVID-19, particularly during resource-limited pandemic settings.
To address this critical knowledge gap, we aimed to determine whether there was an association between timing of intubation relative to hospital admission and mortality in patients with COVID-19 who underwent mechanical ventilation at the Mount Sinai Health System in New York City. The association between mechanical ventilation duration and mortality was also analyzed. We hypothesized that among patients who received mechanical ventilation, early intubation would be associated with decreased mortality.
MATERIALS AND METHODS
Patients and Data Collection
Approval was obtained from the Icahn School of Medicine at Mount Sinai Institutional Review Board (20-03520) for this retrospective study. Informed consent was waived. Patients with SARS-CoV-2 infection (confirmed by at least one positive RNA-based polymerase chain reaction) who were admitted to one of five hospitals in the Mount Sinai Health System in New York, NY between January 30, 2020, and April 30, 2020, and were tracheally intubated prior to May 1, 2020, were included.
Data were collected from the hospital electronic medical record (Epic Systems Corporation, Verona, WI), including age, gender, race, ethnicity, body mass index, medical comorbidities, and laboratory values immediately prior to intubation. Clinical status immediately prior to intubation including vital signs, type of noninvasive respiratory support, administration of antibiotics, anticoagulation, and steroids were also captured. Start of hospital admission was defined as time of presentation to the emergency department. Patients transferred from a site outside the Mount Sinai Health System were excluded. For those transferred within the system, start of hospital admission was defined as time of presentation to the emergency department at the first hospital where the patient presented. The decision to intubate was a clinical decision made at the discretion of the primary team caring for the patient. However, health system guidelines were developed with recommendations for consideration of tracheal intubation, including as follows: worsening mental status, increasing hypercarbia not resolved with noninvasive ventilation, refractory hypoxemia (oxygen saturation < 85%) without recovery on noninvasive ventilation, or increased work of breathing and tachypnea not responsive to noninvasive ventilation. Initial ventilator settings immediately after intubation were volume control with a tidal volume of 6 mL/kg of ideal body weight, with a respiratory rate of 16–24 breaths per minute, Fio2 of 1.0, and positive end-expiratory pressure of 16 cm H2O. Subsequently, the Fio2 was titrated down first and then positive end-expiratory pressure with a goal Pao2 of 55–80 torr (7.3–10.7 kPa) while maintaining plateau pressure less than 30 cm H2O and driving pressure less than 14 cm H2O. If a patient had persistent (> 12 hr) Fio2 requirements greater than 0.75 and Pao2:Fio2 ratio less than 150, prone position ventilation was recommended.
Outcomes measured included discharge from the hospital alive, death, continued hospitalization, or transfer to a skilled nursing facility. Follow-up data were censored as of August 15, 2020.
Statistical Analyses
Descriptive data are presented as mean (sd), median (interquartile range [IQR]), or n (%). For group comparisons, chi-square tests were used for categorical variables and either the analysis of variance or the Kruskal-Wallis test for continuous variables, as appropriate.
Survival Analysis
A hospital-stratified time-varying Cox model was used to evaluate the effect of time to intubation on in-hospital death. The stratified Cox model allowed underlying survival distributions to differ among the five hospital sites. Time zero was defined as the time a patient was first placed on invasive mechanical ventilation. In counting the individuals at risk for mortality at a given time, patients were stratified into three possible states: still intubated, extubated, or having had a tracheostomy. Tracheostomy was treated as a distinct category from intubation as it represents a progression to chronic critical illness and both the management and expected outcome of these patients is different than that of an acutely critically ill patient. The numbers as well as the timing of subsequent extubation and reintubation episodes, as well as timing of tracheostomy placement were not fixed across patients. Start and end times of each episode were considered in the Cox model. The respective interactions between extubation and tracheostomy with cumulative duration of intubation (log-transformed to improve model fit) were tested to see whether the effect of extubation or tracheostomy were the same throughout the follow-up period. The interaction between tracheostomy and cumulative duration of intubation was not significant (> 0.05) and was removed in all subsequent analyses. Three time-to-ventilation models were considered as follows: Model 1: unadjusted for any covariate; Model 2: adjusted for hospital site, baseline demographics, and preexisting comorbidities; and Model 3: adjusted for all factors in Model 2, laboratory findings at admission, maximum vital sign values before onset of mechanical ventilation, last noninvasive respiratory device used prior to intubation, and administration of medications prior to intubation, including anticoagulation, broad-spectrum antibiotics, antiplatelet agents, and steroids.
As time to intubation was the primary focus of the analysis, we investigated the dose-response association between the time to intubation and in-hospital mortality using the restricted cubic spline (RCS) analysis (6). The RCS functional form of the time to intubation variable was used to check, visually and statistically, the assumption of linearity of the association between the log of the hazard function and the time to ventilation. The 5th, 50th, and 95th percentiles of time to intubation were chosen as the default knots for model fitting. The p value test for nonlinear association between the log of the hazard ratio and time to ventilation was 0.372, justifying the validity to use a linear term for the time to intubation variable in the analyses.
Statistical analysis was performed using SAS 9.4 (SAS Institute, Cary, NC). All tests were two-sided and statistical significance was defined as a p value of less than 0.05 unless otherwise indicated.
RESULTS
A total of 5,843 patients were admitted to five hospitals within the Mount Sinai Health System with confirmed SARS-CoV-2 infection during the study period. After exclusions for age less than 18 or greater than 110 (n = 49), there were 773 patients who were intubated and mechanically ventilated and 5,021 who were never intubated. Patients who were transferred already intubated (n = 3) and those who were intubated after April 30, 2020, were excluded (n = 15). The final cohort for analysis consisted of 755 patients intubated prior to May 1, 2020 (Fig. 1). Baseline patient characteristics are described in Table 1. The median age of patients was 65 years (IQR, 56–72 yr), 64% were male, and 80% were overweight or obese.
Figure 1.
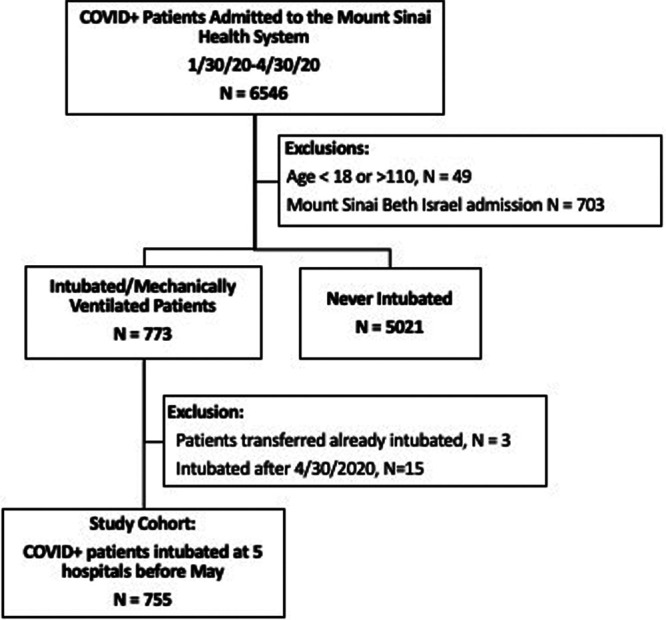
Flow chart of patient selection. COVID+ = tested positive for severe acute respiratory syndrome coronavirus 2 infection.
Table 1.
Characteristics of Patients With Coronavirus Disease 2019 Who Were Mechanically Ventilated
| Characteristic | Overall, n = 755 | Hospital A, n = 376 | Hospital B, n = 87 | Hospital C, n = 79 | Hospital D, n = 136 | Hospital E, n = 77 |
|---|---|---|---|---|---|---|
| Age, yr | 63 (13) | 61 (14) | 67 (10) | 64 (11) | 64 (13) | 66 (12) |
| BMI, kg/m2 | 31 (8) | 31 (8) | 31 (8) | 31 (8) | 32 (9) | 31 (8) |
| Male gender | 483 (64.0%) | 245 (65.2%) | 54 (62.1%) | 54 (68.4%) | 79 (58.1%) | 51 (66.2%) |
| BMI category, kg/m2 | ||||||
| Under and normal weight | 156 (20.7%) | 77 (20.5%) | 20 (23.0%) | 20 (25.3%) | 20 (14.7%) | 19 (24.7%) |
| Overweight (25–30) | 225 (29.8%) | 111 (29.5%) | 28 (32.2%) | 20 (25.3%) | 44 (32.4%) | 22 (28.6%) |
| Obese (> 30) | 374 (49.5%) | 188 (50.0%) | 39 (44.8%) | 39 (49.4%) | 72 (52.9%) | 36 (46.8%) |
| Race/ethnicity | ||||||
| Asian | 38 (5.0%) | 18 (4.8%) | 2 (2.3%) | 9 (11.4%) | 4 (2.9%) | 5 (6.5%) |
| Black | 178 (23.6%) | 68 (18.1%) | 48 (55.2%) | 6 (7.6%) | 37 (27.2%) | 19 (24.7%) |
| Hispanic | 225 (29.8%) | 118 (31.4%) | 7 (8.0%) | 35 (44.3%) | 51 (37.5%) | 14 (18.2%) |
| Other | 165 (21.9%) | 95 (25.3%) | 9 (10.3%) | 17 (21.5%) | 28 (20.6%) | 16 (20.8%) |
| White | 149 (19.7%) | 77 (20.5%) | 21 (24.1%) | 12 (15.2%) | 16 (11.8%) | 23 (29.9%) |
| Current or former smoker | 185 (33.7%) | 86 (31.7%) | 13 (18.8%) | 26 (44.1%) | 33 (36.3%) | 27 (45.8%) |
| Asthma/chronic obstructive pulmonary disease | 47 (6.3%) | 21 (5.7%) | 4 (4.6%) | 8 (10.1%) | 8 (5.9%) | 6 (7.9%) |
| Cancer | 47 (6.3%) | 24 (6.5%) | 5 (5.7%) | 5 (6.3%) | 8 (5.9%) | 5 (6.6%) |
| Congestive heart failure/valvular disease | 30 (4.3%) | 14 (4.2%) | 3 (3.8%) | 4 (5.1%) | 7 (5.7%) | 2 (2.7%) |
| Chronic kidney disease | 69 (9.2%) | 35 (9.5%) | 8 (9.2%) | 7 (8.9%) | 12 (8.8%) | 7 (9.2%) |
| Diabetes | 177 (23.7%) | 81 (21.9%) | 14 (16.1%) | 26 (32.9%) | 35 (25.7%) | 21 (27.6%) |
| Hypertension | 248 (33.1%) | 102 (27.5%) | 26 (29.9%) | 38 (48.1%) | 49 (36.0%) | 33 (43.4%) |
| Liver disease | 10 (1.4%) | 8 (2.4%) | 0 (0.0%) | 1 (1.3%) | 0 (0.0%) | 1 (1.4%) |
| Stroke or neurologic disease | 37 (4.9%) | 18 (4.8%) | 6 (6.9%) | 4 (5.1%) | 5 (3.7%) | 4 (5.2%) |
| Given anticoagulation agentsa | 552 (73.1%) | 295 (78.5%) | 52 (59.8%) | 55 (69.6%) | 94 (69.1%) | 56 (72.7%) |
| Given broad-spectrum antibioticsa | 624 (82.6%) | 299 (79.5%) | 83 (95.4%) | 75 (94.9%) | 106 (77.9%) | 61 (79.2%) |
| Given antiplatelet agentsa | 197 (26.1%) | 103 (27.4%) | 23 (26.4%) | 23 (29.1%) | 39 (28.7%) | 9 (11.7%) |
| Given corticosteroidsa | 590 (78.1%) | 315 (83.8%) | 55 (63.2%) | 63 (79.7%) | 84 (61.8%) | 73 (94.8%) |
| Maximum heart rate prior to intubation, beats/min | 120 (25) | 119 (25) | 113 (25) | 121 (25) | 121 (22) | 127 (27) |
| Maximum respiratory rate before intubation, breaths/min | 33 (7) | 34 (7) | 31 (8) | 33 (7) | 33 (8) | 36 (7) |
| Maximum O2 saturation prior to intubation | 88 (6%) | 88 (6%) | 91 (7%) | 87 (6%) | 88 (7%) | 85 (7%) |
| Maximum creatinine before intubation, mg/Dl | 3.2 (3.3) | 3.2 (3.2) | 4.2 (3.9) | 3.2 (2.6) | 2.9 (3.4) | 3.2 (3.3) |
| Oxygen therapy prior to intubation | ||||||
| Room air | 48 (6.4%) | 27 (7.2%) | 9 (10.3%) | 2 (2.5%) | 8 (5.9%) | 2 (2.6%) |
| Nasal cannula | 44 (5.8%) | 22 (5.9%) | 8 (9.2%) | 5 (6.3%) | 5 (3.7%) | 4 (5.2%) |
| Mask | 192 (25.4%) | 85 (22.6%) | 38 (43.7%) | 25 (31.6%) | 30 (22.1%) | 14 (18.2%) |
| Continuous positive airway pressure/bilevel positive airway pressure | 233 (30.9%) | 86 (22.9%) | 26 (29.9%) | 39 (49.4%) | 51 (37.5%) | 31 (40.3%) |
| High-flow nasal cannula | 88 (11.7%) | 49 (13.0%) | 1 (1.1%) | 6 (7.6%) | 18 (13.2%) | 14 (18.2%) |
| Unknown | 150 (19.9%) | 107 (28.5%) | 5 (5.7%) | 2 (2.5%) | 24 (17.6%) | 12 (15.6%) |
| Week intubated | ||||||
| February 21 to March 12 | 8 (1.1%) | 2 (0.5%) | 2 (2.3%) | 2 (2.5%) | 1 (0.7%) | 1 (1.3%) |
| March 13 to March 19 | 26 (3.4%) | 11 (2.9%) | 2 (2.3%) | 5 (6.3%) | 6 (4.4%) | 2 (2.6%) |
| March 20 to March 26 | 110 (14.6%) | 50 (13.3%) | 14 (16.1%) | 5 (6.3%) | 30 (22.1%) | 11 (14.3%) |
| March 27 to April 02 | 169 (22.4%) | 90 (23.9%) | 21 (24.1%) | 14 (17.7%) | 27 (19.9%) | 17 (22.1%) |
| April 3 to April 9 | 175 (23.2%) | 94 (25.0%) | 17 (19.5%) | 22 (27.8%) | 22 (16.2%) | 20 (26.0%) |
| April 10 to April 16 | 114 (15.1%) | 57 (15.2%) | 17 (19.5%) | 13 (16.5%) | 18 (13.2%) | 9 (11.7%) |
| April 17 to April 23 | 93 (12.3%) | 49 (13.0%) | 7 (8.0%) | 12 (15.2%) | 13 (9.6%) | 12 (15.6%) |
| April 24 to April 30 | 60 (7.9%) | 23 (6.1%) | 7 (8.0%) | 6 (7.6%) | 19 (14.0%) | 5 (6.5%) |
| Time to intubation, d | 2.3 (0.6–6.3) | 2.2 (0.5–5.8) | 3.1 (0.9–6.4) | 2.8 (0.6–6.2) | 2.0 (0.5–4.9) | 2.3 (0.6–9.4) |
| Duration of intubation, d | 8.6 (3.6–17.4) | 9.2 (4.0–20.9) | 6.2 (3.5–11.4) | 6.9 (2.5–10.0) | 10.5 (4.6–20.7) | 8.4 (2.4–13.0) |
| Length of stay, d | 13.3 (6.0–24.7) | 15.8 (7.7–28.1) | 8.1 (3.9–19.7) | 8.3 (3.7–14.5) | 13.9 (6.6–27.3) | 11.9 (7.2–21.5) |
| Tracheostomy | 163 (21.6%) | 123 (32.7%) | 4 (4.6%) | 8 (10.1%) | 21 (15.4%) | 7 (9.1%) |
| Disposition | ||||||
| Died in hospital | 512 (67.8%) | 211 (56.1%) | 73 (83.9%) | 73 (92.4%) | 97 (71.3%) | 58 (75.3%) |
| Discharged home | 121 (16.0%) | 88 (23.4%) | 7 (8.0%) | 1 (1.3%) | 16 (11.8%) | 9 (11.7%) |
| Remains hospitalized | 9 (1.2%) | 4 (1.1%) | 0 (0.0%) | 0 (0.0%) | 4 (2.9%) | 1 (1.3%) |
| Discharged to long-term care facility | 100 (13.2%) | 67 (17.8%) | 7 (8.0%) | 4 (5.1%) | 14 (10.3%) | 8 (10.4%) |
| Other/unknownb | 13 (1.7%) | 6 (1.6%) | 0 (0.0%) | 1 (1.3%) | 5 (3.7%) | 1 (1.3%) |
BMI = body mass index.
aIndicates therapy given at any point during hospitalization (prior to or after intubation).
bOther includes nursing homes and rehabilitation facilities.
Values are expressed as mean (sd), median (interquartile range), or n (%).
As of the time of data censoring, 121 patients (16%) who were intubated and mechanically ventilated had been discharged home, 512 (68%) had died, 113 (15%) had been discharged to a skilled nursing facility, and 9 (1%) remained in the hospital.
Timing of Intubation and Mortality
The median time from hospital admission to intubation was 2.3 days (IQR, 0.6–6.3 d). For each additional day between hospital admission and intubation, the unadjusted hazard ratio for death within the follow-up period (model 1) was 1.01 (95% CI, 1.00–1.02). After adjustment for all covariates (complete data available in 552 patients with 383 deaths, model 3), the hazard ratio was 1.03 (95% CI, 1.01–1.05) (Fig. 2). The partially adjusted model (model 2) with fewer patients excluded (690 patients with 482 deaths) showed similar results (Table 2). A survival analysis utilizing hours from admission to intubation is shown in Supplementary Table 1 (http://links.lww.com/CCX/A388). After adjustment for all covariates (model 3), the hazard ratio was 1.001 (95% CI, 1.001–1.002) for each additional hour between admission and intubation.
Table 2.
Multivariable Models for In-Hospital Mortality in COVID-19 Patients
| Variable | Model 1: Unadjusted (512/755) | Model 2a: Partially Adjusted (482/690) | Model 3a: Fully Adjusted (383/552) |
|---|---|---|---|
| Hazard Ratio (95% CI) | Hazard Ratio (95% CI) | Hazard Ratio (95% CI) | |
| Time to intubation | |||
| Per day | 1.01 (1.00–1.02) | 1.03 (1.02–1.05) | 1.03 (1.01–1.05) |
| Duration of mechanical ventilation (reference: 0–3 d) | |||
| > 3, ≤ 7 d | 4.11 (2.98–5.66) | 3.01 (2.15–4.21) | 3.31 (2.22–4.95) |
| > 7, ≤ 14 d | 2.52 (1.89–3.36) | 1.90 (1.41–2.55) | 2.03 (1.43–2.89) |
| > 14, ≤ 21 d | 1.23 (0.90–1.69) | 0.90 (0.64–1.27) | 0.96 (0.65–1.43) |
| > 21, ≤ 28 d | 0.78 (0.52–1.17) | 0.47 (0.30–0.74) | 0.61 (0.37–1.00) |
| > 28, ≤ 60 d | 0.32 (0.22–0.48) | 0.23 (0.14–0.38) | 0.21 (0.12–0.36) |
| > 2 mo | 0.04 (0.02–0.11) | 0.06 (0.02–0.16) | 0.07 (0.02–0.20) |
| Tracheostomy | 0.72 (0.51–1.01) | 0.70 (0.46–1.06) | 0.79 (0.50–1.24) |
| Discharged status | |||
| Yes | 0.01 (0.00–0.02) | 0.01 (0.00–0.02) | 0.01 (0.00–0.02) |
| Calendar week of the intubation onset | 1.23 (1.15–1.30) | 1.25 (1.15–1.36) | |
| Age | |||
| 45–64 | 2.17 (1.34–3.50) | 1.44 (0.84–2.46) | |
| 65+ | 3.12 (1.92–5.05) | 2.10 (1.23–3.58) | |
| Body mass index, kg/m2 | |||
| Overweight (25–30) | 1.35 (1.02–1.79) | 1.43 (1.04–1.98) | |
| Obese (> 30) | 1.32 (1.02–1.71) | 1.16 (0.86–1.57) | |
| Race and ethnicityb | |||
| Asian | 0.65 (0.41–1.04) | 0.53 (0.31–0.93) | |
| Black | 1.05 (0.79–1.40) | 0.91 (0.65–1.28) | |
| Hispanic | 0.85 (0.64–1.14) | 0.76 (0.54–1.06) | |
| Other | 1.34 (0.99–1.80) | 1.42 (1.0–2.01) | |
| Gender | |||
| Female | 0.85 (0.69–1.04) | 0.92 (0.72–1.17) | |
| Hypertension | 0.94 (0.75–1.19) | 0.96 (0.74–1.26) | |
| Diabetes | 0.93 (0.72–1.19) | 0.90 (0.68–1.19) | |
| Liver disease | 0.74 (0.36–1.54) | 0.76 (0.32–1.79) | |
| Asthma or chronic obstructive pulmonary disease | 1.10 (0.80–1.52) | 1.10 (0.77–1.59) | |
| Congestive heart failure or valve disorder | 1.58 (1.05–2.37) | 1.39 (0.87–2.23) | |
| Anticoagulationc | 0.39 (0.31–0.49) | 0.36 (0.27–0.48) | |
| Broad-spectrum antibioticc | 0.73 (0.56–0.95) | 0.73 (0.54–0.98) | |
| Antiplatelet agentc | 0.93 (0.74–1.16) | 0.96 (0.75–1.23) | |
| Steroidsc | 0.75 (0.59–0.96) | 0.59 (0.44–0.79) | |
| Maximum heart ratec | |||
| Per minute | 1.01 (1.01–1.01) | ||
| Maximum O2 saturationc | |||
| Per % | 0.98 (0.96–0.99) | ||
| Maximum respiratory ratec | |||
| Per minute | 1.00 (0.98–1.01) | ||
| Maximum creatininec | |||
| Maximum | 1.03 (0.99–1.06) | ||
| Last respiratory deviceb,c,d | |||
| Continuous positive airway pressure/bilevel positive airway pressure | 0.93 (0.58–1.50) | ||
| High-flow nasal cannula | 0.92 (0.54–1.57) | ||
| Mask | 1.06 (0.66–1.68) | ||
| Nasal cannula | 0.57 (0.31–1.07) | ||
| Unknown | 0.62 (0.37–1.03) | ||
aFor covariates-adjusted models, baseline survival distribution are allowed to be different for different hospitals.
bOverall p < 0.01.
cPrior to intubation.
dReference: Room air.
Figure 2.
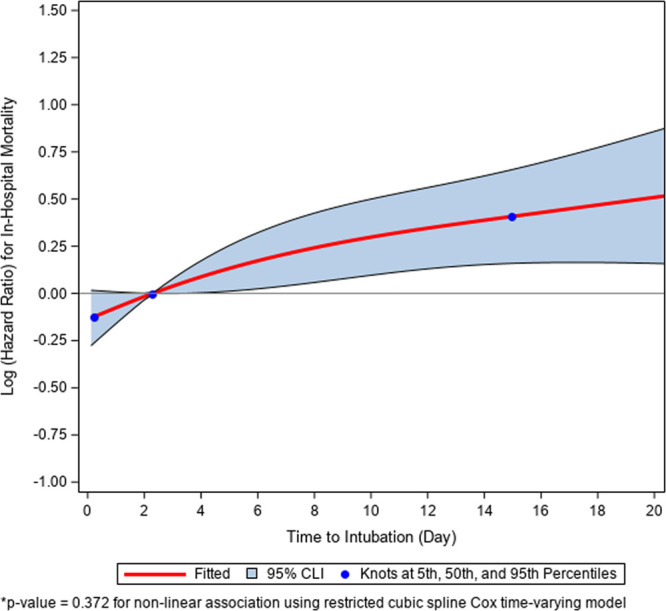
Restricted cubic spline plot demonstrating the association between days from admission to intubation and in-hospital mortality.
Timing of Intubation Throughout the Study Period and the Use of Noninvasive Devices
The median time between admission and intubation increased as the weeks progressed throughout the study period, with a median time to intubation of less than 2 days in mid to late March and 4 to 6 days by mid to late April (p < 0.001; Supplementary Table 2, http://links.lww.com/CCX/A389). There was evidence that in patients in whom mechanical ventilation occurred later in the hospitalization, less invasive measures were used to temporize deteriorating respiratory status prior to intubation. Specifically, as the time between admission and intubation increased, the use of intervening noninvasive ventilation increased. Of the 266 patients who were intubated within the first day of admission, a trial of continuous positive airway pressure (CPAP)/bilevel positive airway pressure (BiPAP) had occurred in 66 (24.8%) and a trial of high-flow nasal cannula had occurred in 5 (5.6%). In contrast, of the 162 patients who were intubated on days 2–3 of admission, 55 (34.0%) received a trial of CPAP/BiPAP and 32 (19.8%) had received a trial of high-flow nasal cannula. Among the 234 patients who were intubated on day 5 or later, 121 (51.7%) had received a trial of CPAP/BiPAP and 101 (43.2%) had received a trial of high-flow nasal cannula prior to intubation (p < 0.001; Table 3).
Table 3.
Noninvasive Respiratory Support Used and Timing of Intubation
| Covariate | Timing of Intubation | pa | |||
|---|---|---|---|---|---|
| 0–1 d, n = 265 | 2–3 d, n = 160 | 3–5 d, n = 96 | 5+ d, n = 234 | ||
| Last device before intubation | |||||
| Missing | 82 (30.94) | 26 (16.25) | 8 (8.33) | 34 (14.53) | < 0.001 |
| Room air | 30 (11.32) | 10 (6.25) | 2 (2.08) | 6 (2.56) | |
| Nasal cannula | 11 (4.15) | 14 (8.75) | 6 (6.25) | 13 (5.56) | |
| Mask | 70 (26.42) | 49 (30.63) | 34 (35.42) | 39 (16.67) | |
| HFNC | 9 (3.4) | 19 (11.88) | 15 (15.63) | 45 (19.23) | |
| CPAP/BiPAP | 63 (23.77) | 42 (26.25) | 31 (32.29) | 97 (41.45) | |
| Number of devices trialed before intubation | |||||
| Missing | 82 (30.94) | 26 (16.25) | 8 (8.33) | 34 (14.53) | < 0.001 |
| 0 | 22 (8.3) | 3 (1.88) | 0 (0) | 1 (0.43) | |
| 1 | 88 (33.21) | 34 (21.25) | 15 (15.63) | 11 (4.7) | |
| 2 | 60 (22.64) | 60 (37.5) | 41 (42.71) | 56 (23.93) | |
| 3 | 11 (4.15) | 34 (21.25) | 29 (30.21) | 91 (38.89) | |
| 4 | 2 (0.75) | 3 (1.88) | 3 (3.13) | 41 (17.52) | |
| CPAP/BiPAP ever used | |||||
| Missing | 82 (30.94) | 26 (16.25) | 8 (8.33) | 34 (14.53) | < 0.001 |
| No | 117 (44.15) | 79 (49.38) | 55 (57.29) | 79 (33.76) | |
| Yes | 66 (24.91) | 55 (34.38) | 33 (34.38) | 121 (51.71) | |
| HFNC ever used | |||||
| Missing | 82 (30.94) | 26 (16.25) | 8 (8.33) | 34 (14.53) | < 0.001 |
| No | 168 (63.4) | 102 (63.75) | 61 (63.54) | 99 (42.31) | |
| Yes | 15 (5.66) | 32 (20) | 27 (28.13) | 101 (43.16) | |
| Mask ever used | |||||
| Missing | 82 (30.94) | 26 (16.25) | 8 (8.33) | 34 (14.53) | < 0.001 |
| No | 54 (20.38) | 30 (18.75) | 16 (16.67) | 19 (8.12) | |
| Yes | 129 (48.68) | 104 (65) | 72 (75) | 181 (77.35) | |
| Nasal cannula ever used | |||||
| Missing | 82 (30.94) | 26 (16.25) | 8 (8.33) | 34 (14.53) | < 0.001 |
| No | 144 (54.34) | 57 (35.63) | 24 (25) | 43 (18.38) | |
| Yes | 39 (14.72) | 77 (48.13) | 64 (66.67) | 157 (67.09) | |
BiPAP = bilevel positive airway pressure, CPAP = continuous positive airway pressure, HFNC = high-flow nasal cannula.
aThe parametric p is calculated by χ2 test.
Duration of Ventilation and Mortality
The median duration of mechanical ventilation was 8.6 days (IQR, 3.6–17.4 d). The relationship between the duration of mechanical ventilation and mortality was complex. For patients on mechanical ventilation for greater than 3–7 days, extubation was associated with an adjusted hazard ratio (model 3) of 3.31 (95% CI, 2.22–4.95) relative to those intubated for 0–3 days. Hazard ratios for mortality decreased as duration of mechanical ventilation increased, and patients on mechanical ventilation for greater than 28 to less than or equal to 60 days had an adjusted hazard ratio (model 3) of 0.21 (95% CI, 0.12–0.36) relative to those intubated for 0–3 days.
DISCUSSION
In this large, multihospital, retrospective cohort study of COVID-19 positive patients who underwent mechanical ventilation during the peak of the COVID-19 pandemic in New York City, we found that timing of intubation had a small but significant association with improved survival. Specifically, the adjusted hazard ratio for mortality was 1.03 (95% CI, 1.01–1.05) for each day of delay in intubation following initial hospital presentation. Furthermore, patients who were extubated after a more prolonged course of mechanical ventilation (> 3 to 4 wk) had a lower hazard ratio for death relative to patients who were on mechanical ventilation for a shorter duration. Together, these observations suggest that supportive care consisting of early intubation and a conservative extubation strategy may be associated with improved outcomes. A systems approach to avoid delays in tracheal intubation and an inability to provide prolonged periods of invasive mechanical ventilation during a pandemic may improve survival.
Similar observations between timing of mechanical ventilation and mortality have been made in ARDS from causes other than COVID-19. In a prospective observational study, patients who were initially not intubated when meeting ARDS criteria but subsequently intubated within the following 3 days had greater mortality compared with both those intubated early and those never intubated; a difference that persisted for 2 years of follow-up (7).
The challenge for clinicians is to determine which hypoxemic COVID-19 patients are at greatest risk of further respiratory decompensation in order to optimize timing of intubation and initiation of mechanical ventilation, without intubating patients who could have done well on noninvasive ventilation. We found that as the weeks progressed during the COVID-19 crises, intubation was more frequently delayed further from admission. It is possible that this trend occurred because clinicians were observing high mortality rates in intubated patients, and therefore had a higher threshold to intubate as time went on, hoping to avoid mechanical ventilation if possible. Alternatively, limitations in critical care bed and ventilator availability may have influenced clinician decision-making.
High-flow nasal cannula has been shown to reduce the occurrence of intubation of patients with ARDS from other causes, without an impact on mortality (8). Noninvasive respiratory support, including CPAP/BiPAP and/or high-flow nasal cannula were commonly used prior to intubation in this cohort, and the proportion of patients on noninvasive respiratory support increased as the length of time between admission and intubation increased. This current study only included patients who underwent intubation and mechanical ventilation; thus, it does not provide insight into those patients who did well on noninvasive respiratory support and never required intubation. Whether the use of noninvasive respiratory support can reduce the need for intubation in patients with respiratory distress from COVID-19, and how it may impact mortality, are important questions that warrant future study.
In distinction to several other contemporary reports from integrated hospital systems impacted in the early phase of the SARS-CoV-2 pandemic (9–11), only 16% of patients (122/755) remained in the hospital or a skilled nursing facility at the time of follow-up. Our cohort included 121 patients (16%) who were discharged home alive and 512 patients (68%) who had died at the time of follow-up.
This study has some important limitations. Most importantly, given the retrospective nature of this study, we are unable to determine whether a delayed intubation itself resulted in the observed increase in mortality. It is possible that delayed intubation resulted in fewer intubated patients overall, leaving only the most severely ill patients in the cohort receiving mechanical ventilation. We also cannot determine the factors that influenced the clinicians’ decision on timing of intubation with this study design, so it is difficult to draw conclusions on whether a limitation of resources or a belief that delayed intubation was a better treatment course predominated. There was significant variation between hospitals with regards to in-hospital mortality in our study. Notably, the hospitals with higher mortality had fewer ICU beds and ventilators available at the start of the crisis (data not shown). This suggests that a limitation of resources likely explains at least a portion of the increased mortality with delayed intubation found in this study. Aggressive efforts were made at the system level to offload patients from these overwhelmed hospitals.
The retrospective data used was not sufficiently granular to determine whether patients received therapeutic or prophylactic doses of anticoagulation, or to examine the impact of pharmacologic therapies prior to versus after intubation. In addition, once patients received a tracheostomy, there was not precise enough data available regarding whether mechanical ventilation continued or was weaned to evaluate these outcomes. Finally, approximately one-third of patients had missing data and could not be included in the fully adjusted model which impacts the reliability of our results.
Therapeutic modalities have evolved as the pandemic progressed, and outcomes from this early period of the pandemic may not be generalizable to current care. Treatment protocols have been updated as new data and recommendations have been released. Since the approval of remdesivir for COVID-19 (12), its use has been expanded. Dexamethasone use has been adopted for most patients at well (13). As the thromboembolic complications of COVID-19 were recognized, more aggressive anticoagulation was used (14). Following the peak prevalence of COVID-19 cases in New York City in April 2020, the number of new cases has rapidly declined and has remained low at the Mount Sinai Health System since early June 2020. While fortunate, the low numbers of patients admitted to the hospital with COVID-19 in recent months make measuring the impact of changes in therapeutic modalities as well as decreased strain on the system difficult.
CONCLUSIONS
Despite these limitations, our analysis indicated that increasing time from admission to intubation was associated with higher mortality in a cohort of patients requiring mechanical ventilation for severe COVID-19 early in the pandemic. Duration of mechanical ventilation greater than 3 to 4 weeks was also associated with a lower risk of in-hospital death relative to those extubated earlier in the hospital course. Based on the results of this study, delaying intubation for COVID-19 patients with acute respiratory failure should be undertaken with caution. Delayed intubation, probably driven by resource constraints during the early phase of the pandemic, may have contributed to the high in-hospital mortality in this study. A systems approach is needed to address this issue to potentially improve survival in future pandemic waves.
Supplementary Material
Footnotes
This work was performed at the Icahn School of Medicine at Mount Sinai.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Supported, in part, by grant by the Institute for Critical Care Medicine, Icahn School of Medicine at Mount Sinai, New York, NY.
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir Med. 2020; 8:475–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fan E, Del Sorbo L, Goligher EC, et al. An official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine Clinical Practice Guideline: Mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017; 195:1253–1263 [DOI] [PubMed] [Google Scholar]
- 3.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020; 395:1054–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armstrong RA, Kane AD, Cook TM. Outcomes from intensive care in patients with COVID-19: A systematic review and meta-analysis of observational studies. Anaesthesia. 2020; 75:1340–1349 [DOI] [PubMed] [Google Scholar]
- 5.Poston JT, Patel BK, Davis AM. Management of critically ill adults with COVID-19. JAMA. 2020; 323:1839–1841 [DOI] [PubMed] [Google Scholar]
- 6.Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med. 2010; 29:1037–1057 [DOI] [PubMed] [Google Scholar]
- 7.Kangelaris KN, Ware LB, Wang CY, et al. Timing of intubation and clinical outcomes in adults with acute respiratory distress syndrome. Crit Care Med. 2016; 44:120–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rochwerg B, Granton D, Wang DX, et al. High flow nasal cannula compared with conventional oxygen therapy for acute hypoxemic respiratory failure: A systematic review and meta-analysis. Intensive Care Med. 2019; 45:563–572 [DOI] [PubMed] [Google Scholar]
- 9.Auld SC, Caridi-Scheible M, Blum JM, et al. ICU and ventilator mortality among critically ill adults with coronavirus disease 2019. Crit Care Med. 2020; 48:e799–e804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020; 323:1574–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richardson S, Hirsch JS, Narasimhan M, et al. ; the Northwell COVID-19 Research Consortium. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020; 323:2052–2059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beigel JH, Tomashek KM, Dodd LE, et al. ; ACTT-1 Study Group Members. Remdesivir for the treatment of Covid-19 - final report. N Engl J Med. 2020. October 8 [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horby P, Lim WS, Emberson JR, et al. ; RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19 - preliminary report. New Engl J Med. 2020. July 17 [online ahead of print] [Google Scholar]
- 14.Nadkarni GN, Lala A, Bagiella E, et al. Anticoagulation, mortality, bleeding and pathology among patients hospitalized with COVID-19: A single health system study. J Am Coll Cardiol. 2020; 76:1815–1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


