Abstract
PURPOSE
The Adjuvant Paclitaxel and Trastuzumab trial was designed to address treatment of patients with small human epidermal growth factor receptor 2 (HER2)–positive breast cancer. The primary analysis of the Adjuvant Paclitaxel and Trastuzumab trial demonstrated a 3-year disease-free survival (DFS) of 98.7%. In this planned secondary analysis, we report longer-term outcomes and exploratory results to characterize the biology of small HER2-positive tumors and genetic factors that may predispose to paclitaxel-induced peripheral neuropathy (TIPN).
PATIENTS AND METHODS
In this phase II study, patients with HER2-positive breast cancer with tumors 3 cm or smaller and negative nodes received paclitaxel (80 mg/m2) with trastuzumab for 12 weeks, followed by trastuzumab for 9 months. The primary end point was DFS. Recurrence-free interval (RFI), breast cancer–specific survival, and overall survival (OS) were also analyzed. In an exploratory analysis, intrinsic subtyping by PAM50 (Prosigna) and calculation of the risk of recurrence score were performed on the nCounter analysis system on archival tissue. Genotyping was performed to investigate TIPN.
RESULTS
A total of 410 patients were enrolled from October 2007 to September 2010. After a median follow-up of 6.5 years, there were 23 DFS events. The 7-year DFS was 93% (95% CI, 90.4 to 96.2) with four (1.0%) distant recurrences, 7-year OS was 95% (95% CI, 92.4 to 97.7), and 7-year RFI was 97.5% (95% CI, 95.9 to 99.1). PAM50 analyses (n = 278) showed that most tumors were HER2-enriched (66%), followed by luminal B (14%), luminal A (13%), and basal-like (8%). Genotyping (n = 230) identified one single-nucleotide polymorphism, rs3012437, associated with an increased risk of TIPN in patients with grade 2 or greater TIPN (10.4%).
CONCLUSION
With longer follow-up, adjuvant paclitaxel and trastuzumab is associated with excellent long-term outcomes. Distribution of PAM50 intrinsic subtypes in small HER2-positive tumors is similar to that previously reported for larger tumors.
INTRODUCTION
Longer-term follow-up of the large pivotal trials has confirmed a dramatic and sustained benefit of the addition of trastuzumab to standard adjuvant chemotherapy in patients with early-stage, human epidermal growth factor receptor 2 (HER2)–positive breast cancer, reducing both the risk of recurrence and death.1-3 Moreover, the benefits of trastuzumab are independent of age, tumor size, nodal status, and hormone receptor (HR) status.
Retrospective data suggest that patients with small, node-negative HER2-positive tumors have recurrence rates that range from 5% to 30%.4,5 However, randomized trials of adjuvant trastuzumab included a limited number of patients with stage I disease. To our knowledge, the Adjuvant Paclitaxel and Trastuzumab (APT) trial6 was the first study designed to specifically address the appropriate treatment approach for patients with small, node-negative HER2-positive breast cancer. This study was a single-arm trial, because it was believed that a randomized study would not be feasible given retrospective data suggesting more than a minimal risk of recurrence for this population. Furthermore, there was no standard treatment at that time for patients with small node-negative HER2-positive tumors to define a control arm. The first report of the APT trial in 2015 after a median follow-up of 4 years showed a 3-year rate of survival free from invasive disease of 98.7% (95% CI, 97.6 to 99.8) and a low rate of serious adverse events.6 Because the majority of patients included in this trial had HR-positive, HER2-positive breast cancers, which are believed to be associated with later recurrences,1,2 long-term follow-up of this trial was important to better determine the true efficacy of this regimen.
In addition, HER2-positive breast cancer is a biologically heterogeneous disease,7 with different treatment sensitivities and survival outcomes. Work looking at larger HER2-positive breast cancers has shown that all four intrinsic molecular subtypes can be identified by gene expression analyses.8 However, molecular profiling of small HER2-positive tumors is largely unknown, and we sought to better characterize these small, node-negative tumors.
In this planned secondary analysis of the APT trial, we report the updated results on 7-year disease-free survival (DFS), recurrence-free interval (RFI), breast cancer–specific survival (BCSS), and overall survival (OS). We also report some exploratory analyses that include PAM50 intrinsic subtyping and risk of recurrence (ROR) score performed on the available archival tissue, to better characterize this patient population with small, node-negative HER2-positive disease, because it may provide deeper insights into potential therapeutic approaches. We also evaluated genotyping for the association of genetic variants and paclitaxel-induced peripheral neuropathy (TIPN), given that this is one of the most burdensome long-term effects of paclitaxel-containing regimens, and efforts to predict and prevent such toxicity are warranted.
PATIENTS AND METHODS
Study Design and Patient Population
The APT study was a multicenter, single-arm, investigator-initiated phase II trial. Details of the study design and study population have been previously reported.6 Eligible patients had HER2-positive breast cancer, pathologically confirmed by local testing either through immunohistochemistry 3+ intensity or amplification of the HER2 gene on fluorescence in situ hybridization 2.0 or greater. Primary invasive tumor had to measure 3.0 cm or smaller in the greatest dimension, and patients had to have node-negative disease (although the protocol was amended in June 2, 2009 after 188 patients enrolled to allow patients with a single micrometastatic node [Data Supplement]). The protocol required patients to have a left ventricular ejection fraction of at least 50% (by echocardiography or multiple-gated acquisition). Other requirements included no prior myocardial infarction or uncontrolled hypertension, no neuropathy higher than grade 1, no prior malignancy within the past 5 years, and no prior history of invasive breast cancer.
Procedures
Adjuvant treatment consisted of weekly paclitaxel (80 mg/m2) plus weekly trastuzumab for 12 weeks followed by single-agent trastuzumab every 3 weeks for a total of 13 doses. Genentech provided funding for the study, but paclitaxel and trastuzumab were commercially supplied. Adjuvant endocrine therapy was recommended as per institutional standard for women with HR-positive tumors after the completion of paclitaxel therapy. Adjuvant radiation therapy was also performed according to local institutional standards. Patients could be treated with conventional post-chemotherapy whole-breast radiation or partial breast radiation administered by external beam or brachytherapy.
All patients adhered to the same schedule of follow-up visits. Left ventricular ejection fraction by echocardiography or multitargeted acquisition scanning was required at baseline, 12 weeks, 6 months, and 1 year after the start of protocol treatment. Clinical assessments after finishing trastuzumab monotherapy were scheduled every 6 months for the first 4 years and then annually during years 5 to 10 after study enrollment or until the end point was reached. An appropriate screening of residual breast tissue performed at least yearly was required. The institutional review board at each participating institution approved the study, and written informed consent from all the trial participants was provided before the study entry. A second consent was obtained from patients for extended follow-up.
Statistical Considerations
Details of the statistical design were published previously.6 The primary end point was DFS, which is the time from study enrollment to the first of the following events that define a failure: locoregional ipsilateral recurrence, contralateral invasive breast cancer, distant recurrence, or death from any cause, as defined by the Standardized Efficacy Endpoints criteria.9 Participants who were alive and free from recurrence were censored at the date of the last follow-up. For patients who did not consent for additional follow-up, their survival time was censored at their off-study dates. Exploratory survival end points included RFI, BCSS, and OS. RFI was defined as the time from study enrollment to the first documentation of disease recurrence, including invasive locoregional recurrence or distant recurrence, and death as a result of breast cancer. BCSS was defined as the time from study enrollment until death as a result of breast cancer. OS was the time from study enrollment until death as a result of any cause.
In our primary analysis, a Poisson test was used to evaluate the failure rate at 3 years against a null hypothesis of 9.2% using a one-sided type I error of 0.05. The planned sample size was 400 patients, with interim futility analyses after 225 and 800 patient-years of follow-up and a final analysis after 1,600 patient-years of follow-up. Under this design, the probability of rejecting the null was 0.95 if the true 3-year failure rate is 5%. In this update analysis, the Kaplan-Meier method was used in estimating the survival function for primary and exploratory end points, and point estimates are reported with two-sided 95% CIs.
PAM50 Intrinsic Subtype Analysis and ROR Score Calculation
Hematoxylin and eosin–stained slides from formalin-fixed paraffin-embedded (FFPE) surgical specimens were examined to confirm the presence of invasive tumor and to determine the minimum surface area for scraping and tumor enrichment. Total RNA was isolated from 5-μm-thick FFPE slides for each tumor specimen using the Qiagen FFPE kit for RNA isolation from FFPE tissue (Qiagen, Hilde, Germany) following the manufacturer’s protocol. RNA was quantified using the NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific Waltham, MA). A minimum of 200 ng of total RNA was used to measure the expression of the PAM50 subtype predictor genes. PAM50 gene expression analysis was conducted on the nCounter gene expression platform (NanoString Technologies, Seattle, WA). Data were analyzed using the Prosigna algorithm (NanoString Technologies) to determine the intrinsic subtype calls (luminal A, luminal B, HER2-enriched, or basal-like) and to generate ROR scores by mapping data to a validated subtype-based risk model as previously described.10,11 Quality assessment and normalization were performed in nSolver 4.0 from NanoString Technologies, as per the manufacturer's instructions.
Genotyping and Statistical Analysis for the Association of Genetic Variants and TIPN
Top candidate germline single-nucleotide polymorphisms (SNPs) for TIPN were selected from prior correlative genome-wide analyses of TIPN in Eastern Cooperative Oncology Group trial E510312 and further evaluated here. All 51 candidate SNPs had a P value < .001 in the E5103 analysis, had linkage disequilibrium support, were from unique regions of the genome, and were confined to patients of European ancestry.12 The SNPs were genotyped using blood DNA derived from APT trial with a QuantStudio 12K Flex OpenArray AccuFill System (Thermo Fisher Scientific) platform. SNPs were removed from the analysis if the call rate was less than 95%, minor allele frequency less than 3%, or the Hardy-Weinberg equilibrium P value < .001. A case-control analysis was performed for those of self-defined white race. Cases were defined as patients who received at least one dose of paclitaxel and experienced grade 2 or greater TIPN during treatment. Patients without TIPN served as controls. Logistic regression model was used in the statistical analysis. Age and body surface area were used as covariates, similar to our prior analyses.12
RESULTS
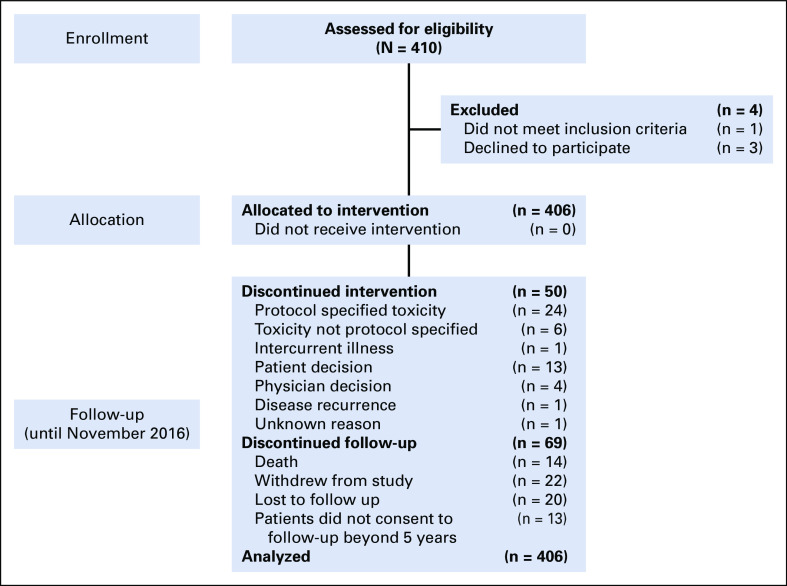
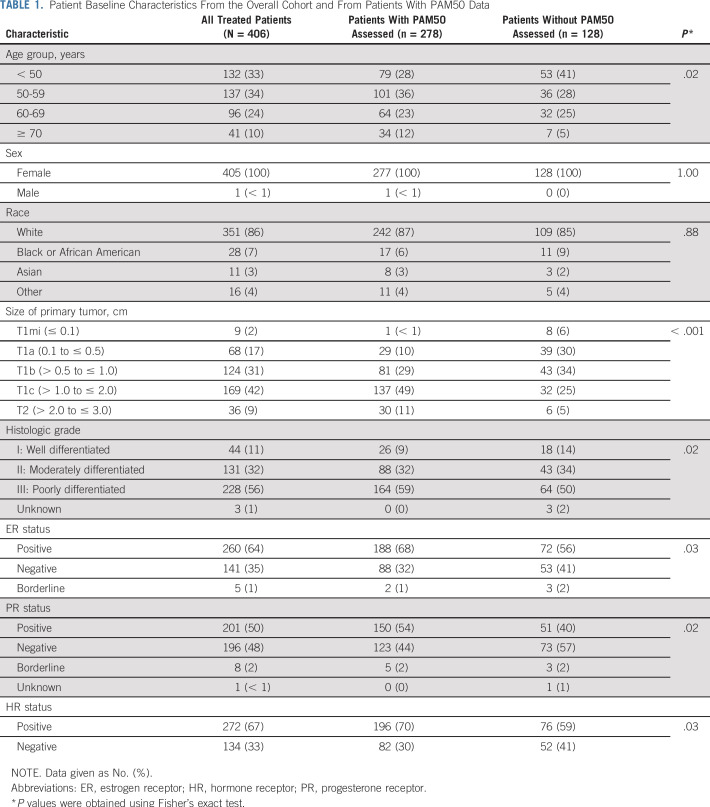
Between October 9, 2007, and September 3, 2010, 410 patients were enrolled in the study and 406 started protocol therapy (Fig 1). Baseline patient characteristics are listed in Table 1. Overall, median age at enrollment was 55 years (range, 24 to 85 years), and most patients (67%) had HR-positive disease. Fifty percent of patients had tumors 1.0 cm or smaller, most of which were T1b. Only 9% of patients had tumors larger than 2 cm and up to 3 cm. Mean tumor size was 1.1 cm.
FIG 1.
Trial enrollment and follow-up.
TABLE 1.
Patient Baseline Characteristics From the Overall Cohort and From Patients With PAM50 Data
The results reported in our primary analysis published in 2015 included all data available as of April 21, 2014, with 1,605 patient-years of follow-up. The 3-year rate of DFS was 98.7% (95% CI, 97.6 to 99.8), with 12 DFS events reported (two distant recurrences). The long-term results reported here are from all data available as of November 11, 2016, which includes 2,390 patient-years of follow-up. After a median follow-up of 6.5 years (range, 0.02 to 8.9 years), there were 23 DFS events observed: five locoregional recurrences (1.2%), four distant recurrences (1%), six new contralateral breast cancers (1.5%), and eight deaths without documented recurrence (2%; Table 2). The 7-year DFS was 93.3% (95% CI, 90.4 to 96.2) for the overall population (Fig 2); in patients with HR-positive tumors, the 7-year DFS was 94.6% (95% CI, 91.8 to 97.5), and among HR-negative patients, the 7-year DFS was 90.7% (95% CI, 84.6 to 97.2).
TABLE 2.
DFS Events Observed
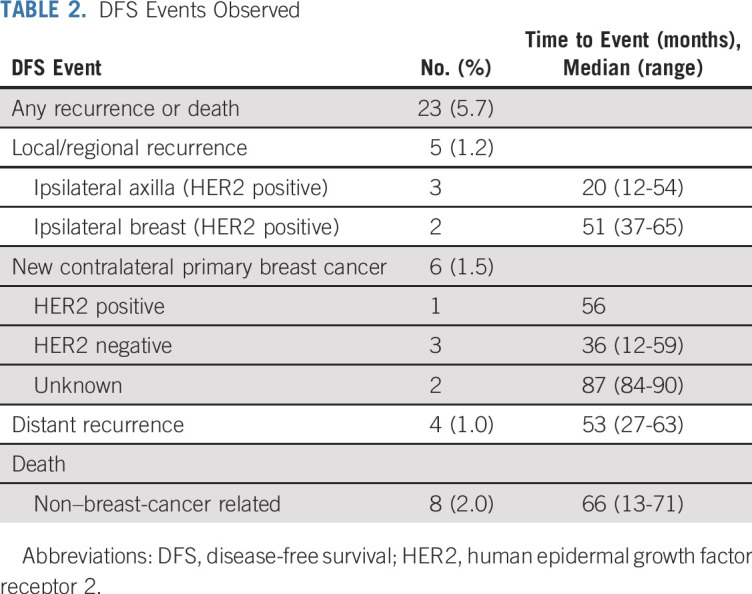
FIG 2.

Disease-free survival (DFS). (A) Kaplan-Meier plot of DFS in the intention-to-treat population. (B) DFS according to hormone-receptor status. Abbreviations: neg, negative; Point est, point estimate; pos, positive.
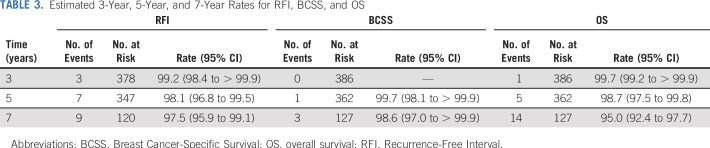
Seven-year RFI, which includes distant recurrence, death from breast cancer, and invasive locoregional recurrence, was 97.5% (95% CI, 95.9 to 99.1%). Seven-year BCSS was 98.6% (95% CI, 97.0 to 100%), and 7-year OS was 95.0% (95% CI, 92.4 to 97.7%; Table 3).
TABLE 3.
Estimated 3-Year, 5-Year, and 7-Year Rates for RFI, BCSS, and OS
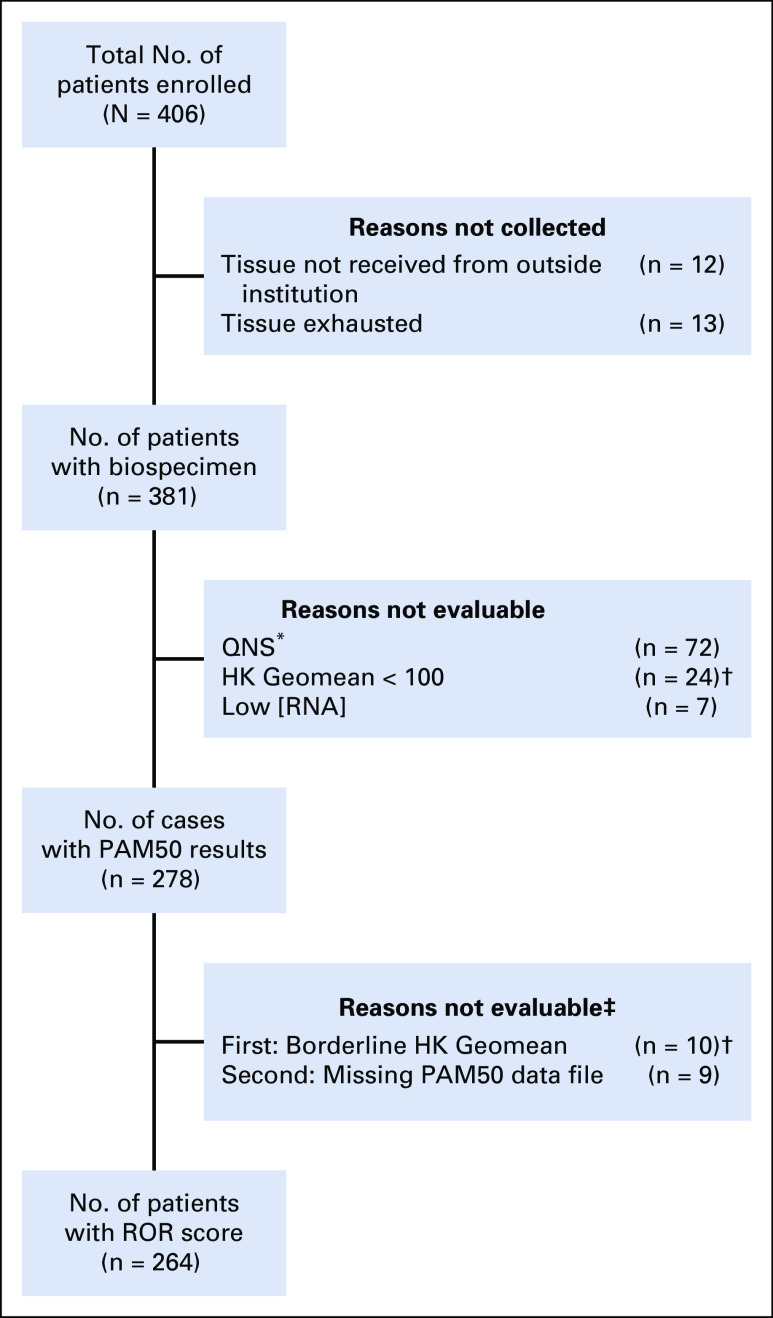
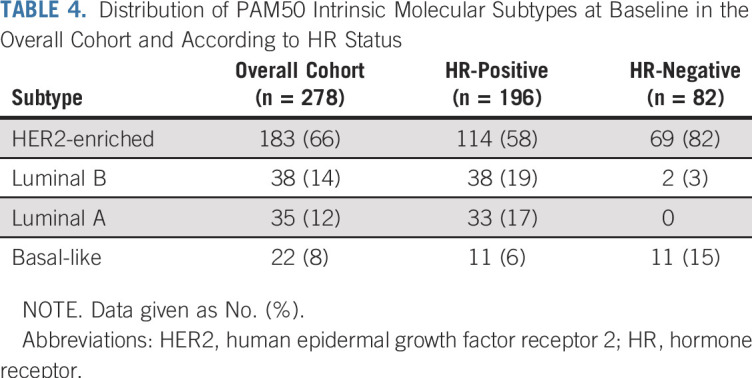
PAM50 gene expression profiling was successfully performed in 278 patients (Appendix Fig A1, online only). The overall cohort and the cohort of the subset of patients with PAM50 data presented similar baseline characteristics (Table 1), although there were significantly more patients with larger and poorly differentiated tumors in the cohort of PAM50 assessed than in the overall cohort. Distribution of PAM50 subtypes is listed in Table 4. The majority of tumors were classified as HER2 enriched (65.5%), followed by luminal B (13.7%), luminal A (12.6%), and basal-like (7.9%). Subtype distribution differed significantly by HR status (Table 4). A greater percentage of HR-negative patients were HER2 enriched (84% v 58%, P < .001) and basal-like (13% v 6%; P < .001) than in the HR-positive cohort, and no cases of luminal B were found in HR-negative tumors. The low event rate observed prevents making statistical inferences on the prognostic value of molecular intrinsic subtypes. Appendix Table A1 (online only) lists the pathologic characteristics and molecular intrinsic subtype of patients with an RFI event.
TABLE 4.
Distribution of PAM50 Intrinsic Molecular Subtypes at Baseline in the Overall Cohort and According to HR Status
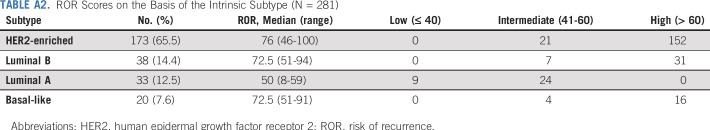
Finally, we evaluated the ROR scores, available in 264 patients. Only nine samples (3.4%) were categorized as low risk, 56 (21.2%) were classified as intermediate risk, and the majority of tumors (199; 75.4%) were categorized as high risk. ROR scores on the basis of the intrinsic subtype are listed in Appendix Table A2 (online only). As expected, luminal A tumor samples showed significantly lower median ROR scores than HER2-enriched, luminal B, and basal-like tumor samples (50 v 76, 72.5, and 72.5, respectively; P < .001). All tumor samples categorized as low risk by ROR score were luminal A. However, most luminal A tumors had an intermediate ROR score, and none of the luminal A tumors had high ROR score. In contrast, the majority of HER2-enriched, luminal B, and basal-like tumor samples were classified as high risk by ROR score.
Regarding TIPN, 230 patients had evaluable genetic data. In this subgroup, there were 24 cases of grade 2 or greater TIPN (10.4%); no grade 4 neurotoxicity was reported (in this cohort of patients or in the overall population of the study). Fifty of the planned 51 SNPs passed quality control and were evaluated. One SNP, rs3012437, was associated with an increased risk of TIPN for grade 2 or greater TIPN after correction for age and body surface area (P = .024; odds ratio, 2.1).
DISCUSSION
This 7-year follow-up report of paclitaxel and trastuzumab for patients with small, node-negative HER2-positive breast cancer demonstrates few disease recurrences with even longer follow-up and further supports the use of this regimen for adjuvant therapy for this patient population. The 3-year and 7-year DFS in the APT trial were 98.7% and 93%, respectively, with just four distant recurrences at 7 years (1%). Many of the DFS events were contralateral cancers or unrelated deaths. When evaluating outcomes in relatively low-risk patients, it is important to acknowledge that DFS captures events that do not necessarily reflect a recurrence from the initial breast cancer. As we follow patients over time, the frequency of these events increases. As such, RFI, which includes invasive locoregional and distant recurrences and deaths as a result of breast cancer, may better describe the relevant event rate in this patient population. The 7-year RFI in the APT trial was 97.5%, suggesting few events related to the initial breast cancer diagnosis.
Molecular profiling of this subset of patients with small HER2-positive breast cancers by PAM50 identified all four intrinsic molecular subtypes. Most tumors were identified as HER2 enriched (66%), and, as expected, subtype distribution differed significantly by HR status. A greater percentage of HR-negative tumors were HER2-enriched and basal-like. However, the HER2-enriched subtype was the predominant subtype not only in the HR-negative cohort but also in HR-positive tumors (84% and 58%, respectively). These results are in accordance with the PAMELA (PAM50 HER2-enriched Subtype as a Predictor of Response to Dual HER2 Blockade in HER2-positive Early Breast Cancer) trial,13 where most tumors were classified as HER2 enriched (67%) as well. In contrast, the percentage of HER2-enriched subtype at baseline in the Cancer and Leukemia Group B (CALGB) 40601 study7 was lower and was similar to percentages of luminal A and luminal B tumors. Moreover, among patients with HR-positive, HER2-positive tumors, the luminal subtypes were found to be the most prevalent.7 The APT and PAMELA trials used the same nCounter platform to perform molecular profiling, whereas the RNA-seq method was used in the CALGB 40601 study. These data confirm that HER2-positive breast cancer is indeed a heterogeneous disease, not fully recapitulated by HR status, and that smaller HER2-positive tumors have a similar distribution of intrinsic subtypes as larger tumors. Therefore, the biology of these smaller tumors may not be dissimilar to larger tumors. Although it is known that intrinsic subtypes play a key role in determining response to anti-HER2 treatment,7,13 data from this study are not able to assess the association of subtype with long-term outcomes, given the low event rate. Interestingly, though, the majority (71%) of the tumor samples were categorized as high ROR (> 60). Previous work in HR-positive, HER2-negative breast cancer suggests high ROR is associated with higher risk of recurrence;14,15 however, given the low event rate seen in this study, these data may not be applicable to patients with small node-negative, HER2-positive tumors who receive therapy.
TIPN is an important toxicity that compromises paclitaxel treatment efficacy and patients’ quality of life. To date, there are only minimally effective treatments, and there are no clinically implemented predictive biomarkers available for TIPN. Previously, SNPs associated with TIPN were identified using two large phase III adjuvant breast cancer trials, E5103 and E1199.12 In the current study, we evaluated the same top 51 SNPs evaluated in the genome-wide association study from E510312 and found that rs3012437, which is in the uncharacterized LOC154449 region of chromosome 6, is associated with the risk of grade 2 or greater TIPN. Thus, this represents, to our knowledge, the first independent association between rs3012437 and the risk of TIPN warranting additional evaluation, because this might ultimately affect treatment decision making in this population at high risk of TIPN.
The APT study has several strengths and limitations. It is a phase II study with a large sample size and long-term follow-up that reaffirms the results reported earlier. We are aware, however, that a randomized trial would have been ideal. As previously mentioned, there were feasibility concerns about the most appropriate control arm, because no standard of treatment existed at that time in this population. The higher frequency of HR-positive tumors in our study (67%) than in the pivotal adjuvant trastuzumab trials (51% to 54%) could have implications for late recurrences, because the risk of recurrence in the first years after diagnosis is higher in HR-negative tumors than in HR-positive ones. It is of interest, however, that a high percentage of the HR-positive tumors were also HER2 enriched. Whether these HR-positive and HER2-enriched tumors are at higher risk of late recurrence is unknown. In any case, the long-term follow-up data in this updated report confirm that these patients continue to do well and uphold this treatment strategy as adequate for small HER2-positive breast cancers. It is important to note, however, that this study does not provide data to support the use of trastuzumab-based therapy in all patients with small HER2-positive tumors. There will be patients, particularly most patients with pT1a and some with pT1b, who may not require adjuvant trastuzumab-based chemotherapy, and a balance between a patient’s risk of recurrence and potential toxicities must be considered. Work is ongoing to see if a potentially less-toxic therapy, such as trastuzumab emtansine (T-DM1), may be an alternative treatment strategy for patients with small HER2-positive tumors. The ATEMPT trial (Adjuvant Trastuzumab Emtansine vs Paclitaxel/Trastuzumab) (ClinicalTrials.gov identifier: NCT01853748) is an ongoing randomized phase II trial of T-DM1 versus adjuvant paclitaxel and trastuzumab for patients with stage I HER2-positive breast cancer that is comparing clinically relevant toxicities between the two arms and assessing DFS for patients receiving T-DM1. This study has completed accrual, and results are pending.
In conclusion, after a median follow-up of 6.5 years, patients with small, HER2-positive breast cancer treated with adjuvant paclitaxel and trastuzumab continued to demonstrate excellent outcomes. These long-term data support the use of adjuvant paclitaxel and trastuzumab as a treatment option for patients with stage I, HER2-positive breast cancer. This regimen represents an important step forward in de-escalating therapy to preserve quality of life while achieving excellent outcomes for patients with HER2-positive breast cancer.
ACKNOWLEDGMENT
We thank all the patients and family members for participating in the study. We also thank Kaitlyn T. Bifolck for her editorial support.
Appendix
FIG A1.
Tumor sample analyses. (*) Tumors less than 4 mm were excluded (insufficient invasive tissue). (†) NanoString set a predefined threshold to maintain the integrity of the subtyping call. A 100-count Geomean for the housekeeping genes (HKs) is the minimal amount needed to generate a confident subtype call. The algorithm requires data above the background to make a reasonable call of subtype, and HK was used as a marker for the rest of the targets. Cases with borderline HK Geomean were excluded from the risk of recurrence (ROR) score evaluation. (‡) Five cases previously incorrectly marked as failed subtyping were corrected and included in the ROR scoring. Therefore, there is a total discrepancy of 14 cases between the number of cases with PAM50 (Prosigna) results and number of cases with ROR scores. QNS, quantity not sufficient.
TABLE A1.
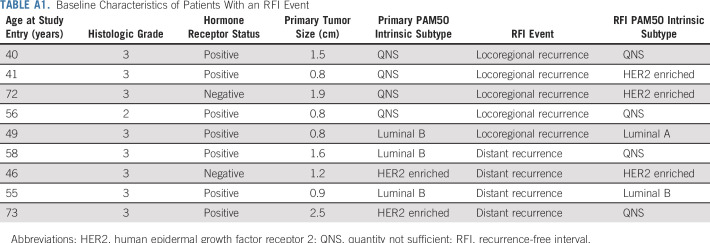
Baseline Characteristics of Patients With an RFI Event
TABLE A2.
ROR Scores on the Basis of the Intrinsic Subtype (N = 281)
Footnotes
Processed as a Rapid Communication manuscript.
Clinical trial information: NCT00542451.
Presented at the ASCO Annual Meeting, Chicago, IL, June 2-6, 2017.
Supported by Genentech.
AUTHOR CONTRIBUTIONS
Conception and design: Sara M. Tolaney, Harold J. Burstein, Eric P. Winer, William T. Barry
Provision of study materials or patients: Sara M. Tolaney, Ian E. Krop, Eric P. Winer, Harold J. Burstein, Chau T. Dang, Clifford A. Hudis, Denise A. Yardley, Beverly Moy, P. Kelly Marcom, Kathy S. Albain, Hope S. Rugo, Matthew J. Ellis, Iuliana Shapira, Lisa A. Carey, Beth Overmoyer, Ann H. Partridge, Antonio C. Wolf
Collection and assembly of data: Sara M. Tolaney, Hao Guo, Sonia Pernas, William T. Barry, Deborah A. Dillon, Lauren Ritterhouse, Kit Fuhrman, Fei Shen, Michele Baltay, Chau T. Dang, Denise A. Yardley, P. Kelly Marcom, Hope S. Rugo, Matthew J. Ellis, Iuliana Shapira, Antonio C. Wolff, Lisa A. Carey, Beth Overmoyer, Ann H. Partridge, Brian P. Schneider
Data analysis and interpretation: Sara M. Tolaney, Hao Guo, William T. Barry, Sonia Pernas, Eric P. Winer, Kit Fuhrman, Bryan P. Schneider, Chau T. Dang, Denise A. Yardley, Beverly Moy, Kathy S. Albain, Hope S. Rugo, Antonio C. Wolff, Lisa A. Carey, Beth Overmoyer, Ann H. Partridge, Clifford A. Hudis, Ian E. Krop, Harold J. Burstein, Matthew J. Ellis
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Seven-Year Follow-Up Analysis of Adjuvant Paclitaxel and Trastuzumab Trial for Node-Negative, Human Epidermal Growth Factor Receptor 2–Positive Breast Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Sara M. Tolaney
Consulting or Advisory Role: Novartis, Pfizer, Merck, Eli Lilly, Nektar Therapeutics, NanoString Technologies, AstraZeneca, Puma Biotechnology, Genentech, Eisai, Immunomedics, Sanofi, Tesaro, Celldex Therapeutics
Research Funding: Genentech (Inst), Merck (Inst), Exelixis (Inst), Pfizer (Inst), Eli Lilly (Inst), Novartis (Inst), Bristol-Myers Squibb (Inst), Eisai (Inst), AstraZeneca (Inst), Novartis (Inst), NanoString Technologies (Inst), Cyclacel (Inst), Nektar Therapeutics (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Eli Lilly, Merck, Nektar Therapeutics, Novartis, Pfizer, Genentech, Immunomedics, Eisai, NanoString Technologies, Puma Biotechnology, Celldex Therapeutics
Sonia Pernas
Consulting or Advisory Role: Polyphor
Speakers' Bureau: Roche
Travel, Accommodations, Expenses: Roche
William T. Barry
Research Funding: Pfizer (Inst)
Other Relationship: ARMO BioSciences
Deborah A. Dillon
Consulting or Advisory Role: Oncology Analytics, Novartis
Travel, Accommodations, Expenses: Novartis
Lauren Ritterhouse
Honoraria: Bristol-Myers Squibb, AbbVie
Research Funding: AbbVie
Travel, Accommodations, Expenses: Bristol-Myers Squibb
Kit Fuhrman
Employment: NanoString Technologies
Stock and Other Ownership Interests: NanoString Technologies
Chau T. Dang
Research Funding: Genentech (Inst), Puma Biotechnology (Inst)
Travel, Accommodations, Expenses: Genentech
Denise A. Yardley
Consulting or Advisory Role: Novartis (Inst), Genentech (Inst), Daiichi Sankyo/Eli Lilly (Inst), Eisai (Inst), Celgene (Inst), Biotheranostics (Inst), NanoString Technologies (Inst), Bristol-Myers Squibb (Inst)
Speakers' Bureau: Novartis, Genentech
Research Funding: AstraZeneca (Inst), Genentech (Inst), Syndax Pharmaceuticals (Inst), Novartis (Inst), MedImmune (Inst), Eli Lilly (Inst), Medivation (Inst), Pfizer (Inst), Eisai (Inst), Tesaro (Inst), MacroGenics (Inst), AbbVie (Inst), Immunomedics (Inst), Daiichi Sankyo (Inst), Merck (Inst), Clovis Oncology (Inst), Oncothyreon (Inst), InventisBio (Inst)
Travel, Accommodations, Expenses: Novartis, Genentech
Beverly Moy
Consulting or Advisory Role: MOTUS (I), Remedy Partners (I), Dark Canyon (I)
Research Funding: Puma Biotechnology (Inst)
P. Kelly Marcom
Consulting or Advisory Role: Genentech, Merck, Celltrion, Immunomedics
Speakers' Bureau: Catamount Medical Education, Clinical Care Options
Research Funding: AbbVie (Inst), Novartis (Inst), Genentech (Inst), Veridex (Inst), Innocrin Pharmaceuticals (Inst), AstraZeneca (Inst), Verily (Inst)
Kathy S. Albain
Consulting or Advisory Role: Novartis, Pfizer, Myriad Genetics, Genomic Health, Agendia, Genentech,
Research Funding: Seattle Genetics, Seattle Genetics (Inst)
Other Relationship: Puma Biotechnology
Hope S. Rugo
Research Funding: MacroGenics (Inst), OBI Pharma (Inst), Eisai (Inst), Pfizer (Inst), Novartis (Inst), Eli Lilly (Inst), Genentech (Inst), Merck (Inst), Immunomedics (Inst), Odonate Therapeutics (Inst), Daiichi Sankyo (Inst), Seattle Genetics (Inst)
Travel, Accommodations, Expenses: Novartis, Genentech, OBI Pharma, Pfizer, Puma Biotechnology, Mylan, Amgen, Sanofi
Matthew J. Ellis
Employment: Bioclassifier
Leadership: Bioclassifier
Stock and Other Ownership Interests: Bioclassifier
Consulting or Advisory Role: Novartis, Pfizer, Celgene, AstraZeneca, NanoString Technologies
Patents, Royalties, Other Intellectual Property: Patent on PAM50, which generates royalties on the license to Bioclassifier/Prosigna/NanoString
Travel, Accommodations, Expenses: Pfizer, AstraZeneca
Antonio C. Wolff
Research Funding: Myriad Genetics (Inst), Pfizer (Inst), BioMarin Pharmaceutical (Inst), Celldex Therapeutics (Inst)
Patents, Royalties, Other Intellectual Property: Antonio Wolff has been named as inventor on one or more issued patents or pending patent applications relating to methylation in breast cancer, and has assigned his rights to Johns Hopkins University, and participates in a royalty sharing agreement with Johns Hopkins University.
Lisa A. Carey
Research Funding: Innocrin Pharmaceuticals (Inst), Syndax Pharmaceuticals (Inst), Immunomedics (Inst)
Patents, Royalties, Other Intellectual Property: Royalty-sharing agreement, investorship interest in licensed IP to startup company, Falcon Therapeutics, that is designing neural stem cell-based therapy for glioblastoma multiforme (I)
Beth Overmoyer
Research Funding: GTx (Inst), Genentech (Inst), Incyte (Inst), Eisai (Inst)
Travel, Accommodations, Expenses: Novartis
Clifford A. Hudis
Leadership: Alliance Foundation
Consulting or Advisory Role: Columbia University External Scientific Advisory Board, Alliance Foundation
Ian E. Krop
Employment: AMAG Pharmaceuticals (I)
Leadership: AMAG Pharmaceuticals (I)
Stock and Other Ownership Interests: AMAG Pharmaceuticals (I)
Honoraria: Genentech
Consulting or Advisory Role: Genentech, Daiichi Sankyo, Context Therapeutics, MacroGenics, Taiho Pharmaceutical
Research Funding: Genentech (Inst), Seattle Genetics (Inst), Pfizer (Inst), Daiichi Sankyo (Inst)
Eric P. Winer
Stock and Other Ownership Interests: Verastem
Honoraria: Genentech, Tesaro, Eli Lilly, Carrick Therapeutics, GlaxoSmithKline, Jounce Therapeutics, InfiniteMD
Consulting or Advisory Role: Leap Therapeutics, InfiniteMD
Research Funding: Genentech (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Perez EA, Romond EH, Suman VJ, et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: Planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol. 2014;32:3744–3752. doi: 10.1200/JCO.2014.55.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cameron D, Piccart-Gebhart MJ, Gelber RD, et al. 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: Final analysis of the HERceptin Adjuvant (HERA) trial. Lancet. 2017;389:1195–1205. doi: 10.1016/S0140-6736(16)32616-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Slamon DJ, Eiermann W, Robert NJ, et al: Ten year follow-up of the BCIRG-006 trial comparing doxorubicin plus cyclophosphamide followed by docetaxel (AC->T) with doxorubicin plus cyclophosphamide followed by docetaxel and trastuzumab (AC->TH) with docetaxel, carboplatin and trastuzumab (TCH) in HER2+ early breast cancer patients. Cancer Res 76, 2015 (4 suppl; abstr S5-04)
- 4.Vaz-Luis I, Ottesen RA, Hughes ME, et al. Outcomes by tumor subtype and treatment pattern in women with small, node-negative breast cancer: A multi-institutional study. J Clin Oncol. 2014;32:2142–2150. doi: 10.1200/JCO.2013.53.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gonzalez-Angulo AM, Litton JK, Broglio KR, et al. High risk of recurrence for patients with breast cancer who have human epidermal growth factor receptor 2-positive, node-negative tumors 1 cm or smaller. J Clin Oncol. 2009;27:5700–5706. doi: 10.1200/JCO.2009.23.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tolaney SM, Barry WT, Dang CT, et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N Engl J Med. 2015;372:134–141. doi: 10.1056/NEJMoa1406281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prat A, Carey LA, Adamo B, et al. Molecular features and survival outcomes of the intrinsic subtypes within HER2-positive breast cancer. J Natl Cancer Inst. 2014;106:dju152. doi: 10.1093/jnci/dju152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carey LA, Berry DA, Cirrincione CT, et al. Molecular heterogeneity and response to neoadjuvant human epidermal growth factor receptor 2 targeting in CALGB 40601, a randomized phase III trial of paclitaxel plus trastuzumab with or without lapatinib. J Clin Oncol. 2016;34:542–549. doi: 10.1200/JCO.2015.62.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hudis CA, Barlow WE, Costantino JP, et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: The STEEP system. J Clin Oncol. 2007;25:2127–2132. doi: 10.1200/JCO.2006.10.3523. [DOI] [PubMed] [Google Scholar]
- 10.Parker JS, Mullins M, Cheang MC, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009;27:1160–1167. doi: 10.1200/JCO.2008.18.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nielsen T, Wallden B, Schaper C, et al. Analytical validation of the PAM50-based Prosigna Breast Cancer Prognostic Gene Signature Assay and nCounter Analysis System using formalin-fixed paraffin-embedded breast tumor specimens. BMC Cancer. 2014;14:177. doi: 10.1186/1471-2407-14-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schneider BP, Li L, Radovich M, et al. Genome-wide association studies for taxane-induced peripheral neuropathy in ECOG-5103 and ECOG-1199. Clin Cancer Res. 2015;21:5082–5091. doi: 10.1158/1078-0432.CCR-15-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pernas S, Barroso-Sousa R, Tolaney SM. Optimal treatment of early stage HER2-positive breast cancer. Cancer. 2018;124:4455–4466. doi: 10.1002/cncr.31657. [DOI] [PubMed] [Google Scholar]
- 14.Dowsett M, Sestak I, Lopez-Knowles E, et al. Comparison of PAM50 risk of recurrence score with oncotype DX and IHC4 for predicting risk of distant recurrence after endocrine therapy. J Clin Oncol. 2013;31:2783–2790. doi: 10.1200/JCO.2012.46.1558. [DOI] [PubMed] [Google Scholar]
- 15.Gnant M, Filipits M, Greil R, et al. Predicting distant recurrence in receptor-positive breast cancer patients with limited clinicopathological risk: Using the PAM50 Risk of Recurrence score in 1478 postmenopausal patients of the ABCSG-8 trial treated with adjuvant endocrine therapy alone. Ann Oncol. 2014;25:339–345. doi: 10.1093/annonc/mdt494. [DOI] [PubMed] [Google Scholar]