FIG 9.
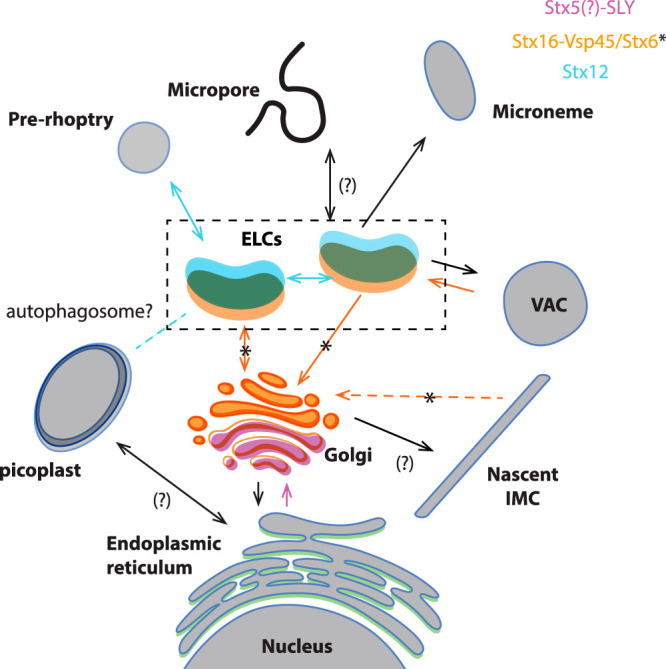
Model of vesicle trafficking in apicomplexan parasites. The model presented in this figure represents a combined adaptation of the trafficking models presented in references 11 and 21. Organelles are colored depending on the localization of proteins studied here. Putative fusion events involving these proteins are indicated with colors in the arrows. The directionality of the transport has been deducted considering that q-SNARES usually localize on the target membranes (2, 3). Proteins transported through the secretory pathway are synthesized in the rough ER. Vesicles shed from the ER are transported and fuse with the cis-Golgi, likely through the action of SLY1. Stx5 is likely an interactor of SLY1, as demonstrated in model organisms (64), mediating the fusion of these vesicles. The IMC is produced from the trans-Golgi compartment, while recycling of trafficking molecules needed for IMC biogenesis likely involves Vsp45 (likely mediated by its interacting partner Stx16) and Stx6. Transport between the ELC to the trans-Golgi involves Vsp45, likely its interacting partner Stx16, and Stx6. Endocytosis is proposed to occur in the micropore or the cytostome (17–19) and transported into subcompartments of the ELC (21). SNARE proteins involved in this process are unknown. Endocytosis and VAC-mediated digestion of internalized host proteins involve Vsp45 and likely Stx16. Microneme and rhoptry proteins are transported to the proper compartment in a temporally regulated manner (21) involving the action of Stx12. Apicoplast transport from the ER is accomplished by unknown SNARE proteins. Interestingly, homeostasis of the apicoplast depends on the ELC compartment and Stx12. A putative role of auto-phagocytosis in this process remains to be addressed.
