Abstract
Common radiological findings of COVID -19 infection include bilateral ground-glass opacities in lower lobes with a peripheral distribution. Pleural effusion is considered a rare manifestation of COVID -19 infection. We present a 52 years old patient with a three-week history of right-sided pleuritic chest pain, fever, and dyspnea. Laboratory investigations revealed high C-reactive protein and ferritin levels and a positive COVID-polymerase chain reaction (PCR) from a nasopharyngeal swab. Chest X-ray and Computed tomography (CT) identified a moderate right-sided pleural effusion, which was exudative with mixed cellularity and high Lactate dehydrogenase (LDH). Histopathology of thoracoscopic pleural biopsy didn't reveal granulomas, malignancy, or any microbiological growth. We postulate that having ruled out any other cause the effusion was likely related to the Covid-19 infection. Our case highlights that COVID-19 can present with isolated pleural effusions, therefore it should be kept as an etiology of effusions especially if other possible causes have been ruled out.
Keywords: COVID-19, Pleural effusion, Lactate dehydrogenase, Pleural biopsy
1. Introduction
Coronaviruses are enveloped single-stranded RNA viruses that can affect the lung, liver, and neurological system [1].Its clinical spectrum ranges from asymptomatic to life-threatening acute respiratory distress syndrome. The disease was first identified in Wuhan and has been spreading rapidly worldwide resulting in a global pandemic [2]. A wide variety of clinical manifestations have been reported in patients infected with COVID-19. Common symptoms of Covid-19 include fever, cough, myalgia, fatigue, hemoptysis, headache, nausea, and diarrhea [3]. Chest imaging plays a vital role in diagnosing and assessing the severity and extent of the disease in COVID-19 pneumonia. Most patients with COVID-19 pneumonia have typical imaging features, such as ground-glass opacities alone or in combination with consolidation, vascular enlargement, pneumatocoeles, and traction bronchiectasis [4]. Isolated Pleural disease in the context of COVID-19 is rarely encountered in clinical practice. The common pleural abnormality detected in COVID 19 patients is pleural thickening (15%) followed by pleural effusion (4%) in a study comparing chest CTs from 219 patients with COVID-19 in China and 205 patients with other causes of viral pneumonia in the United States[5].
2. Case description
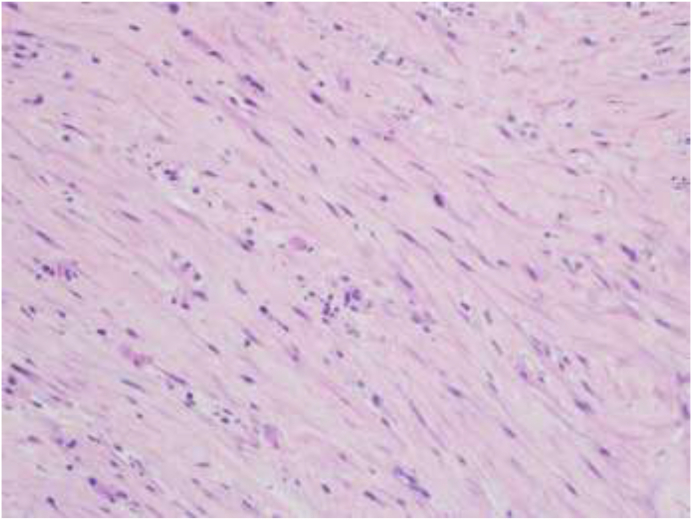
A 52-year-old male patient, presented with a three weeks history of right-sided chest pain, dyspnea, and fever. The patient denied having night sweats, weight loss, and anorexia. He had no contact with sick patients and no previous history of any respiratory illness. He was non-smoker, with no family history of malignancies. Physical examination revealed decreased breath sounds on the right side. Laboratory results showed a high C-reactive protein, ferritin, and liver enzymes along with hyponatremia (Table 1). Chest X-ray and CT scan demonstrated a moderate right-sided pleural effusion, pleural thickening, and normal lung parenchyma (Fig. 1, Fig. 2). A diagnostic pleural tap was performed. The pleural fluid analysis was consistent with an exudative effusion with a pleural fluid PH of 7.5, the glucose of 6.8 mmol/L, and a high LDH of 1185U/L. The pleural fluid differential white cell count showed 45% lymphocytes, 41% neutrophils, and 9% eosinophils. No organism was detected in the pleural fluid including TB on culture and PCR, and the cytology was negative for malignant cells (Table 2). Given the significant community spread of SARS-COV2, a COVID-19 PCR was performed which was positive. Nonetheless, the patient underwent medical thoracoscopy and pleural biopsy to rule out other pathologies as the cause for the effusion. At thoracoscopy visually the parietal pleura was found to be mildly inflamed with a few pleural adhesions (Fig. 3). Histopathological examination showed acute fibrinous exudate consisting of layers of fibrin mixed with abundant neutrophils (Fig. 4). Also seen were areas of dense fibrosis characterized by fascicles of spindle cells mixed with fewer numbers of lymphocytes, plasma cells as well as eosinophils associated with deposits of dense collagen (Fig. 5) with no evidence of granulomas or malignancy. Culture samples of the pleural biopsy were negative for TB. He clinically improved on hydroxychloroquine and antibiotics.
Table 1.
Relevant lab investigations including infection workup.
| Investigation | Result | Normal range |
|---|---|---|
| WBC count | 11.3 | 4–10 × 10^3/uL |
| Platelet count | 940 | 15–400 × 10^3/uL |
| Hb | 11 | 13-17 gm/dL |
| Lymphocyte count | 2 | 1–3 x10^3/uL |
| Creatinine | 79 | 62-106 μmol/L |
| Sodium | 126 | 136–145 mmol/L |
| Alanine aminotransferase | 71 | 0–41 U/L |
| C- Reactive protein | 240 | 0–5 mg/L |
| Procalcitonin | 0.17 | <0.5 ng/ml |
| Glucose | 5.3 | 3.3–5.5 mmol/L |
| Lactate dehydrogenase | 214 | 135–225 U/L |
| Total protein | 76 | 66-87 gm/L |
| Ferritin | 657 | 30-533 μg/L |
| Blood cultures | No growth | – |
| Urine culture | No growth | – |
| Common Viruses panel | Negative | – |
| Sputum AFB smear, PCR, and culture | Negative | – |
| SARS-Cov 2 PCR | Positive | – |
| HIV antigen/antibody ELISA | Non-reactive |
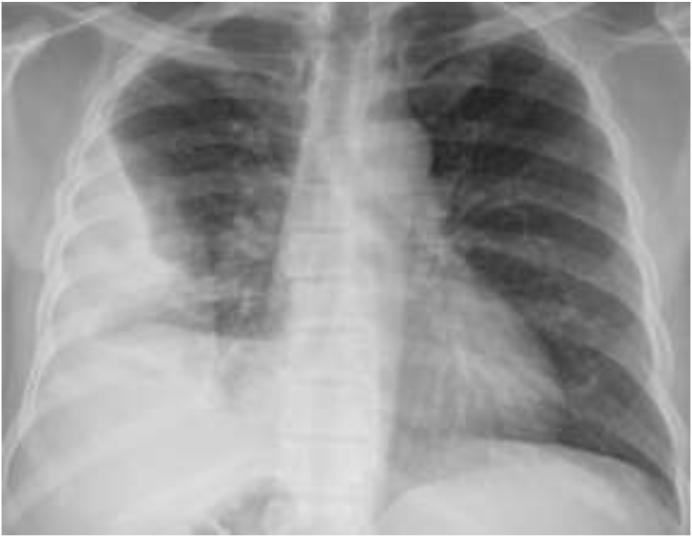
Fig. 1.
Chest X-ray showing right-sided moderate effusion with thickening.
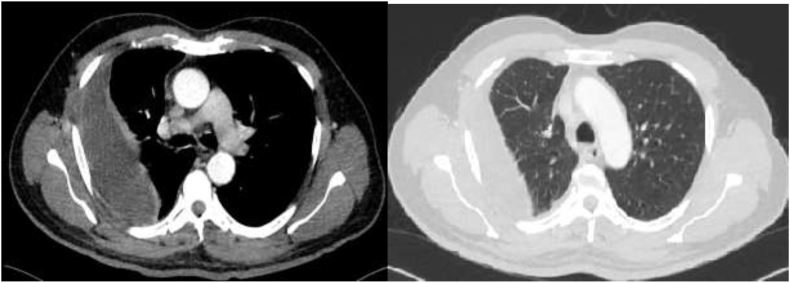
Fig. 2.
Computed tomography of the chest showing moderate right-sided effusion with normal lung parenchyma.
Table 2.
Pleural fluid characteristics.
| Parameter | Results |
|---|---|
| Appearance | Turbid |
| Color | Orange |
| PH | 7.5 |
| Total protein | 60 g/dL |
| Lactate dehydrogenase | 1185 U/L |
| Glucose | 6.8 mmol/L |
| WBC count | 2450/mcl (45% Lymphocytes, cells,41% neutrophils and 9% eosinophils) |
| Microbiology | Negative |
| Cytology | Few mesothelial cells, numerous inflammatory cells, mainly lymphocytes |
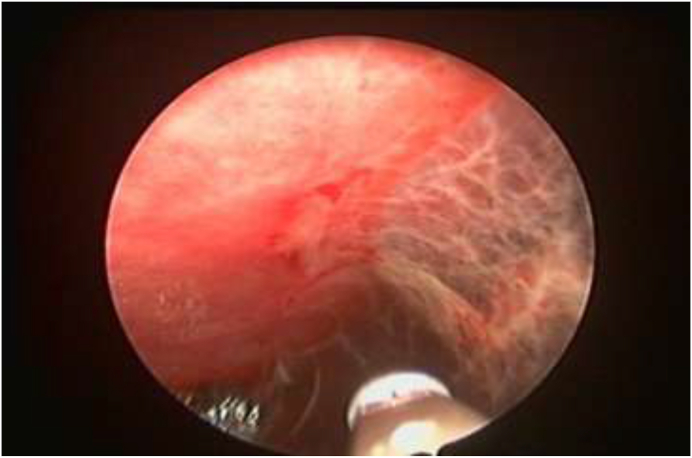
Fig. 3.
Medical thoracoscopy view, showing inflamed parietal pleura with few adhesions.
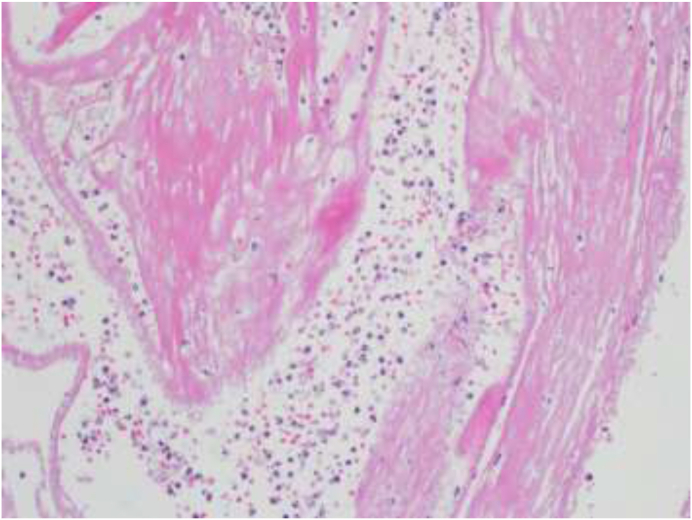
Fig. 4.
Microscopic view showing acute fibrinous exudate (H&E × 200).
Fig. 5.
Microscopic view revealing dense fibroblastic reaction characterized by fascicles of spindle cells mixed with fewer numbers of lymphocytes, plasma cells, and eosinophils associated with deposits of dense collagen (H&E × 200).
3. Discussion
COVID-19 has been regarded as a public health emergency of global concern for more than 6 months. With many countries now in the grip of the second wave of COVID-19, unusual presentations of COVID-19 infection are still being reported. Although mortality due to COVID-19 is comparable to MERS and SARS-CoV, its spread and virulence are significantly higher than these viruses [4]. SARS-CoV2 like MERS and SARS-CoV, has a particular affinity for the respiratory tract epithelium.[6] Review of the literature reveals that pleural effusions were seen in one-third of cases with MERS, but were rarelyreported in SARS-CoV [[7], [8]].
Chest imaging is essential for both the initial diagnosis and follow-up. The hallmark radiological findings in COVID-19 patients constitute bilateral peripherally located ground-glass opacities while atypical features include bronchial wall thickening, pleural effusions, and lymphadenopathy [9]. In a systematic review and meta-analysis, the commonest pleural abnormality was pleural thickening (52%) while pleural effusion occurred in only 6% of the cases.10 In our case, the patient developed the pleural effusion and pleural thickening in the absence of any parenchymal lung pathology which is a rare phenomenon. To our knowledge, no relationship has been established between pleural effusions and COVID- 19. However; pleural effusion is rarely encountered in the early phase of COVID-19 infection [10]. Mostly pleural effusions develop after recurrent pneumonia or after the third week of pneumonia [11].
Limited information is available about the characteristic of pleural fluid in COVID-19 patients, due to infection control protocols limiting invasive procedures during the current pandemic. A case series from Singapore describes lymphocytic exudative pleural effusions in three patients with SARS-CoV2. However, one of the patients had a positive pleural fluid culture for Mycobacterium Tuberculosis while the other two were also treated with anti-TB medications as well given a high clinical suspicion and elevated pleural Adenosine deaminase levels[12]. In another recently published study, the pleural fluid characteristics of four patients with COVID-19 pneumonia were reviewed. Two patients had neutrophil predominant exudative effusion while two had lymphocytic exudative effusion with a variable LDH level (284–3,651 U/L) and no evidence of malignancy or tuberculosis.[13] Elevated pleural fluid LDH levels (greater than 1000 IU/L) suggest empyema, malignant effusion, rheumatoid effusion, or pleural paragonimiasis[13,14].
The significantly elevated level of LDH in pleural fluid can be either due to the presence of hemolyzed red blood cells in the pleural fluid or a heightened immune response observed in many SARS-CoV-2 patients with extremely elevated inflammatory markers causing a high cell turnover [15]. In our case although the initial CRP was high other acute phase/Inflammatory reactants like ferritin and LDH were not significantly raised.
Pleural biopsy is often needed when pleural fluid analysis yields inconclusive results to identify the etiology of the pleural effusion. In our case, pleural biopsies were taken given a high index of suspicion of pleural TB. Biopsies have a higher diagnostic yield for TB and other pathologies and the diagnosis are often made, in the correct clinical context [13]. We attributed the pleural effusion in our case to COVID-19 as no other cause for the pleural effusion was identified despite extensive investigations and the patient improved post thoracoscopy and drainage of fluid with no recurrence to date.
4. Conclusion
COVID-19 exhibits a diverse range of clinical presentations and our knowledge regarding COVID-19 is constantly evolving. Clinicians need to familiarize themselves with both typical and atypical radiological manifestations of COVID-19. Pleural effusion in COVID-19 infected patients has been reported and may show a high pleural LDH but this finding needs to be confirmed in other studies. Covid-19 should be thought of as a cause of effusions especially in the absence of any other identifiable etiology.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
Open Access funding provided by the Qatar National Library
References
- 1.Weiss S.R., Leibowitz J.L. Coronavirus pathogenesis. Adv. Virus Res. 2011;81:85–164. doi: 10.1016/B978-0-12-385885-6.00009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hui D.S., Azhar E.I., Madani T.A., Ntoumi F., Kock R., Dar O. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health – the latest 2019 novel coronavirus outbreak in Wuhan, China. Int. J. Infect. Dis. 2020;91:264–266. doi: 10.1016/j.ijid.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao Wei, Zheng Zhong, Xie Xingzhi, Yu Qizhi, Liu Jun. Relation between chest CT findings and clinical conditions of coronavirus disease (COVID-19) pneumonia: a multicenter study. Am. J. Roentgenol. 2020;214:1072–1077. doi: 10.2214/AJR.20.22976. [DOI] [PubMed] [Google Scholar]
- 5.Bai H.X. Performance of radiologists in differentiating COVID-19 from viral pneumonia on chest CT. Radiology. 2020:200823. doi: 10.1148/radiol.2020200823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zou L. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N. Engl. J. Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hosseiny M., Kooraki S., Gholamrezanezhad A., Reddy S., Myers L. Radiology perspective of Coronavirus disease 2019 (COVID-19): lessons from severe acute respiratory syndrome and the Middle East respiratory syndrome. AJR Am. J. Roentgenol. 2020;214(5):1078–1082. doi: 10.2214/AJR.20.22969. [DOI] [PubMed] [Google Scholar]
- 8.Ketai L., Paul N.S., Wong K.T. Radiology of severe acute respiratory syndrome (SARS): the emerging pathologic-radiologic correlates of an emerging disease. J. Thorac. Imag. 2006;21(4):276–283. doi: 10.1097/01.rti.0000213581.14225.f1. [DOI] [PubMed] [Google Scholar]
- 9.Salehi S., Abedi A., Balakrishnan S., Gholamrezanezhad A. Coronavirus disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients. AJR Am. J. Roentgenol. 2020;14(March):1–7. doi: 10.2214/AJR.20.23034. [DOI] [PubMed] [Google Scholar]
- 10.Bao C., Liu X., Zhang H., Li Y., Liu J. Coronavirus disease 2019 (COVID-19) CT findings: a systematic review and meta-analysis. J. Am. Coll. Radiol. 2020 Jun;17(6):701–709. doi: 10.1016/j.jacr.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi H., Han X., Jiang N., Cao Y., Alwalid O., Gu J., Fan Y., Zheng C. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: adescriptive study. Lancet Infect. Dis. 2020;20(4):425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tham S.M., Lim W.Y., Lee C.K., Loh J., Premkumar A., Yan B., Kee A., Chai L., Tambyah P.A., Yan G. Four patients with COVID-19 and tuberculosis, Singapore, april-may 2020. Emerg. Infect. Dis. 2020 Jul 15;26(11) doi: 10.3201/eid2611.202752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mercer R.M., Corcoran J.P., Porcel J.M., Rahman N.M., Psallidas I. Interpreting pleural fluid results. Clin. Med. 2019;19(3):213–217. doi: 10.7861/clinmedicine.19-3-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verma A., Phua C.K., Sim W.Y. Pleural LDH as a prognostic marker in adenocarcinoma lung with malignant pleural effusion. Medicine (Baltim.) 2016;95(26) doi: 10.1097/MD.0000000000003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chong WH, Huggins JT, Chopra A. Characteristics of Pleural Effusion in Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Pneumonia. Am J Med Sci. 2020 Sep 11 doi: 10.1016/j.amjms.2020.09.008. Epub ahead of print. PMCID: PMC7485456. [DOI] [PMC free article] [PubMed] [Google Scholar]