Abstract
Introduction
Chemohyperthermia is a feasible option in BCG (bacillus Calmette-Guérin) failure patients who desire bladder preservation. We aimed to assess outcomes and complications of chemohyperthermia using mitomycin C (MMC) or epirubicin (EPI).
Material and methods
From March 2017 to February 2020, 103 BCG failure or intolerance patients with high-risk NMIBC (non-muscle invasive bladder cancer) underwent a hyperthermic intravesical chemotherapy (HIVEC) regimen. Five patients did not complete at least 5 instillations and were excluded from analysis. MMC was used in 72 out of 98 patients (Group A) while EPI was used in 26 patients (Group B). Response to HIVEC, predictive factors for treatment outcome and the disease-free survival (DFS) were defined as primary endpoints. The complications of chemohyperthermia were assessed as a secondary endpoint.
Results
No significant differences were found in recurrence and progression after induction course between Groups A and B. Kaplan-Meier disease-free survival was 22.61 months in Group A and 21.93 in Group B. The log-rank test showed no statistically significant difference between the two curves (p = .627). In the multivariate analysis, patients with tumor size ≥3 cm (p = .029), recurrence rate >1/year (p = .034), concomitant carcinoma in situ (CIS) during transurethral resection of bladder (TURB) (p = .039) and BCG-unresponsive status (p = .048) were associated with a worse response to chemohyperthermia. The use of MMC or EPI did not influence the response to treatment (p = .157). A slightly significant higher rate of overall complications (p = .0488) was observed in Group B. A significantly higher rate of Grade 3 frequency/urgency (p = .0064) contributed to this difference. The use of EPI was the only independent factor associated with severe urinary frequency/urgency (p = .017). No patients experienced Grade 4/5 adverse events.
Conclusions
HIVEC can be considered a feasible option in BCG failure/intolerant NMIBC patients, avoiding or postponing radical cystectomy in some particular subclasses of patients.
Keywords: chemohyperthermia, BCG failure, bladder cancer, complications, mitomycin C, epirubicin
INTRODUCTION
Non-muscle invasive bladder cancer (NMIBC) is the most common category (about 75%) of diagnosed bladder carcinoma. BCG (bacillus Calmette-Guérin) is currently the gold standard adjuvant treatment for high-grade NMIBC (HG-NMIBC) but usually fails in 40% of cases [1]. According to EAU (European Association of Urology) guidelines, ‘BCG failure’ was defined as any disease occurrence following BCG therapy and can be categorized into: 1) Muscle-invasive disease detected during follow-up; 2) BCG refractory [A: T1G3/HG non-muscle invasive papillary tumor is present at three months; B: TaG3/HG non-muscle invasive papillary tumor or CIS (carcinoma in situ) is present at both three and six months (after a second induction course or the first maintenance course of BCG)]; 3) BCG relapsing (recurrence of G3/HG tumor after completion of BCG maintenance, despite an initial response); 4) BCG unresponsive (BCG refractory or T1Ta/HG BCG relapse within 6 months or development of CIS within 12 months of last BCG exposure) [2]. These classes of patients should undergo radical cystectomy as first-line treatment, which is a surgical procedure with high morbidity rates. [2]. Patients who desire bladder preservation or are unfit for radical surgery, can benefit from several bladder sparing strategies. These options include intravesical immunotherapy, chemotherapy or combined chemo-immunotherapy, device-assisted therapy or gene therapy [3]. Limited data and low-level evidence (LE) studies (LE:3) are available and all these techniques seem to be inferior in terms of bladder cancer specific mortality [2]. However, new bladder preservation approaches for high-risk NMIBC provide better quality of life (QoL). Hyperthermic intravesical chemotherapy (HIVEC) is a feasible option in BCG-unresponsive NMIBC cases and the treatment is commonly associated with a low rate of adverse events. In a cohort of 52 BCG unresponsive NMIBC patients, including 30 patients with concomitant CIS, 50% of the patients remained disease free after a median follow-up of 14.0 months [4]. The most common complications described were urinary frequency, haematuria and bladder spasms. Sometimes, allergic reactions were reported [5]. We aimed to assess the outcomes and complications of HIVEC treatment using two different drugs (Mitomycin-C [MMC] and Epirubicin [EPI]) that are commonly used for intravesical chemotherapy.
MATERIAL AND METHODS
From March 2017 to February 2020, 103 BCG failure or intolerance patients with high-risk NMIBC underwent HIVEC adjuvant regimen. The inclusion criteria were as follows: 1) histological diagnosis of high-grade papillary Ta/T1 NMIBC alone or in combination with CIS [WHO 2014 grading system]; 2) the criteria of BCG-refractory, BCG-relapse, BCG-unresponsive or BCG intolerance disease were determined according to EAU guidelines criteria [2]; 3) adequate BCG treatment which is defined as having had BCG 6 weekly induction instillations followed by at least one 3 week maintenance course or a second induction course of 6 BCG instillations [4]. Patients who were unsuitable to undergo radical cystectomy were excluded.
The surgical radical approach was offered as gold standard. The potential benefits and risks of early and delayed radical cystectomy were discussed with patients [6] and written informed consent was obtained. The study protocol was approved by the research ethics committee and all procedures performed in the study were in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Response to induction course of HIVEC, predictive factors for treatment outcome and the disease-free survival (DFS) were defined as primary endpoints of the study. The complications of the induction course using MMC or EPI were assessed as a secondary endpoint. Demographical, clinical and pathological data were collected for all patients. All HIVEC-related complications were classified according Common Terminology Criteria for Adverse Events (CTCAE) [7]. The HIVEC induction regimen consisted of six weekly intravesical instillations (MMC 40 mg or EPI 50 mg) diluted in 50 ml of distilled water. The target temperature was 43°C and the solution was repeatedly circulated inside the bladder at 200 ml/min for 60 minutes [8]. All instillations were performed with the CombatBRSsystem V2.0 (Combat Medical, Wheathampstead, UK). The response to treatment was assessed with a cytology and a flexible cystoscopy performed six weeks after the last instillation and subsequent transurethral resection of the bladder (TURB). ‘Non-responder patients’ were defined as all grades of bladder cancer recurrence or progression. Low-grade recurrent disease was managed with another six weekly HIVEC regimen while high-grade recurrences were managed with radical cystectomy. All patients with high-grade progression to muscle-invasive disease underwent consequent neoadjuvant chemotherapy plus radical cystectomy. All ‘responder patients’ underwent a maintenance course composed of once instillation per month for three months. Subsequently, the disease-free patients underwent another maintenance course (once monthly for six months). A computed tomography of the abdomen and pelvis was performed once a year.
All data were recorded in a prospectively maintained database and retrospectively examined. Yates's chi-squared (χ2) and Student's t-tests were used to compare the statistical significance of differences in proportions and means, respectively. DFS was assessed by Kaplan Meier analysis and the log-rank test was used to compare the survival distribution of the two groups. Subjects were assessed at the date of disease recurrence/progression or date of last cystoscopy. Logistic regression analysis was performed to evaluate independent predictors of failure to HIVEC and complications. Statistical analyses were performed using SPSS V23.0 (Armonk, NY: IBM Corp.), defining statistical significance at p <0.05.
RESULTS
MMC was used in 75 out of 103 patients (72.8%) [Group A] while EPI in 28 out of 103 (27.2%) [Group B], due to occasional shortage of MMC. We used the same chemotherapeutic agent for all courses of each patient. Five patients (3 patients in Group A and 2 patients in Group B) did not complete at least 5 instillations because of severe adverse events and they were excluded from outcome analysis.
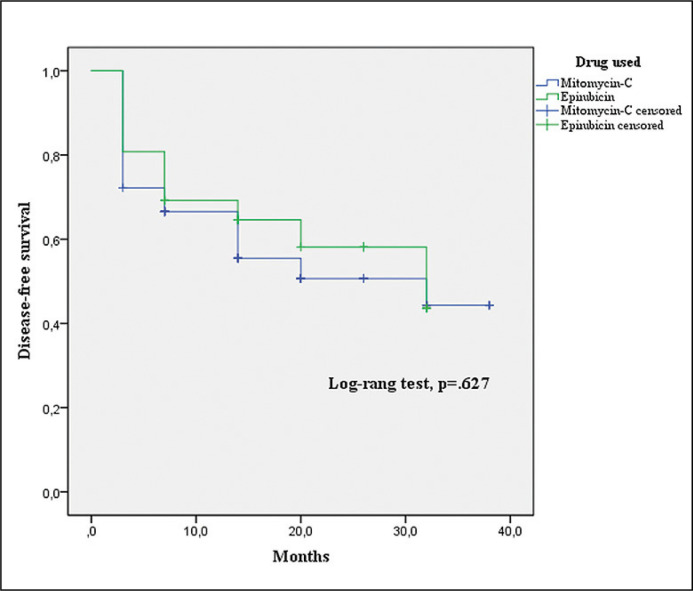
There were no significant differences in the demographics and baseline characteristics among the groups (Table 1). No significant differences were found in recurrence and progression after induction course between Group A and B (Table 2). In the multivariate analysis, patients with tumor size ≥3 cm (p = .029), recurrence rate >1/year (p = .034), concomitant CIS on TURB (p = .039) and BCG-unresponsive status (p = .048) were associated with a worse response to HIVEC regimen. The use of MMC or EPI did not influence the response to treatment (p = .157) (Table 3). The maximum follow-up was 38 months in Group A (median: 10.5; mean ±SD:13.42 ±10.55) and 32 months in Group B (median: 14; mean ±SD: 15.35 ±10.58). The overall mean DFS was 23.13 months (22.61 months in Group A and 21.93 in Group B). The log-rank test showed no statistically significant difference between the two curves (p = .627) (Figure 1).
Table 1.
Demographics and baseline characteristics of patients who underwent HIVEC (hyperthermic intravesical chemotherapy) regimen with mitomycin C [Group A] (72 patients) and epirubicin [Group B] (26 patients)
| Variable | Group A | Group B | p-value |
|---|---|---|---|
| Mean ±SD | |||
| Age (years) | 67.54 ±7.96 | 64.35 ±8.56 | .089 |
| BMI (kg/m2) | 27.35 ±4.53 | 26.77 ±2.05 | .527 |
| ECOG performance status | 1.06 ±0.60 | 1.04 ±0.72 | .907 |
| N ±(%) | |||
| Gender Males Females |
47 (65.28%) 25 (34.72%) |
18 (69.23%) 8 (30.77%) |
.9017 .9017 |
| Smoking status Smoke Non-smoker Former smoker |
27 (37.50%) 30 (41.67%) 15 (20.83%) |
9 (34.62%) 14 (53.85%) 3 (11.54%) |
.9807 .4008 .4510 |
| Diabetes Diabetes (Yes) Diabetes (No) |
13 (18.06%) 59 (81.94%) |
5 (19.23%) 21 (80.77%) |
.8707 .8707 |
| Number of tumors Single Multiple |
21 (29.17%) 51 (70.83%) |
13 (50%) 13 (50%) |
.0944 .0944 |
| Tumor size < 3 cm ≥ 3 cm |
47 (65.28%) 25 (34.72%) |
19 (73.08%) 7 (26.92%) |
.6291 .6291 |
| Recurrence rate ≤1/year >1/year |
57 (20.83%) 15 (79.17%) |
15 (42.31%) 11 (57.69%) |
.0619 .0619 |
| Pathologic stage TaG3 T1G3 |
15 (79.17%) 57 (20.83%) |
11 (57.69%) 15 (42.31%) |
.0619 .0619 |
| Concomitant CIS | 11 (15.28%) | 5 (19.23%) | .8745 |
| Tumor on second TURB | 17 (23.61%) | 6 (23.08%) | .8299 |
| Prior history of UTUC | 7 (9.72%) | 2 (8.70%) | .7929 |
| Previously treated with MMC | 16 (22.22%) | 5 (19.23%) | .9682 |
| BCG faiilure group BCG intolerance BCG refractory BCG relapse BCG unresponsive |
13 (18.06%) 31 (43.06%) 16 (22.22%) 12 (16.67%) |
7 (26.92%) 11 (42.31%) 4 (15.38%) 4 (15.38%) |
.4979 .8688 .6472 .8745 |
BMI – Body Mass Index; ECOG – Eastern Cooperative Oncology Group; CIS – carcinoma in situ; TURB – transurethral resection of bladder; UTUC – upper urinary tract cell cancer; MMC – mitomycin C; BCG – bacillus Calmette-Guérin
Table 2.
Response to HIVEC regimen with mitomycin C [Group A] (72 patients) and epirubicin [Group B] (26 patients)
| Group A (MMC) | Group B (EPI) | p value | |
|---|---|---|---|
| N (%) | N (%) | ||
| HIVEC response | 51/72 (70.83%) | 21/26 (80.77%) | 0.4688 |
| HIVEC recurrence HG | 14/72 (19.44%) | 2/26 (7.69%) | 0.2801 |
| HIVEC recurrence LG | 3/72 (4.17%) | 1/26 (3.85%) | 0.6119 |
| HIVEC progression | 4/72 (5.56%) | 2/26 (7.69%) | 0.9302 |
HIVEC – hyperthermic intravesical chemotherapy; HG – high-grade; LG – low-grade; MMC – mitomycin C; EPI – epirubicin
Table 3.
Logistic regression analysis evaluating independent predictors of failure to HIVEC
| Variables | B* | SE | Wald | p-value | OR [Exp(B)] | 95% CI |
|---|---|---|---|---|---|---|
| Age | -.017 | .037 | .213 | .644 | .983 | .914–1.057 |
| Gender Male (reference) Female |
– -.292 |
– .694 |
– .177 |
– .674 |
– .747 |
– .192–2.911 |
| Smoking status Non-smoker Smoker Ex-smoker |
– .583 -.275 |
– .661 .921 |
– .778 .089 |
– .378 .766 |
– 1.791 .760 |
– .491–6.539 .125–4.624 |
| BMI | .067 | .069 | .937 | .333 | 1.069 | .934–1.224 |
| Diabetes No (reference) Yes |
– -.041 |
– .858 |
– .002 |
– .962 |
– .959 |
– .179–5.153 |
| Number of tumors Single (reference) Multiple |
– .749 |
– .724 |
– 1.069 |
– .301 |
– 2.114 |
– .511–8.743 |
| Tumor size <3 cm (reference) ≥3 cm |
– 1.512 |
– .691 |
– 4.780 |
– .029 |
– 4.535 |
– 1.169–17.586 |
| Recurrence rate ≤1/year (reference) >1/year |
– 1.727 |
– .814 |
– 4.495 |
– .034 |
– 5.622 |
– 1.139–27.739 |
| Pathologic state Ta (HG) (reference) T1 (HG) |
– -.214 |
– .961 |
– .049 |
– .824 |
– .808 |
– .123–5.315 |
| Concomitant CIS No (reference) Yes |
– 2.407 |
– 1.164 |
– 4.278 |
– .039 |
– 11.097 |
– 1.134–108.568 |
| Tumor on RE-TURB No (reference) Yes |
– -1.289 |
– .956 |
– 1.820 |
– .177 |
– .276 |
– .042–1.793 |
| Previously treated with MMC No (reference) Yes |
– -1.259 |
– .895 |
– 1.979 |
– .159 |
– .284 |
– .049–1.641 |
| BCG classes BCG intolerance (reference) BCG refractory BCG relapse BCG unresponsive |
– 1.747 2.217 2.619 |
– 1.165 1.382 1.323 |
– 2.250 2.575 3.917 |
– .134 .109 .048 |
– 5.738 9.183 13.726 |
– .585–56.269 .612–137.778 1.026–183–696 |
| Drug used Mitomycin (reference) Epirubicin |
– -1.261 |
– .891 |
– 2.004 |
– .157 |
– .283 |
– .049–1.624 |
HIVEC – hyperthermic intravesical chemotherapy; RE-TURB – re-transurethral resection of bladder; MMC – mitomycin C; BCG – bacillus Calmette – Guérin
intercept;
SE – standard error; OR – odds ratio [exponentiation of the B coefficient]; CI – confidence interval; BMI – body mass index; HG – high-grade; CIS – carcinoma in situ
Figure 1.
Kaplan-Meier cancer-free survival curves for treatment with mitomycin C and epirubicin.
Table 4 lists the recurrence rate at 3, 7, 14, 20, 26, 32 and 38 months of follow-up.
Table 4.
Recurrence rate of patients who underwent HIVEC protocols (N = 98) at 3, 7, 14, 20, 26, 32 and 38 months of follow-up
| Follow-up months | N° of recurrent patients | Patients still under follow-up | Recurrence Rate |
|---|---|---|---|
| 3a | 26 | 98 | 26/98 (26.53%) |
| 7b | 7 | 72 | 7/72 (9.72%) |
| 14c | 7 | 65 | 7/65 (10.77%) |
| 20d | 3 | 58 | 3/58 (5.17%) |
| 26d | 0 | 55 | 0/55 (0%) |
| 32d | 2 | 55 | 2/55 (3.64%) |
| 38d | 0 | 53 | 0/53 (0%) |
HIVEC – hyperthermic intravesical chemotherapy;
– induction course;
– first maintenance course;
– second maintenance course;
– no treatment
Table 5 reports treatment complications. No patients had grade 4 or 5 adverse events. A slightly significant higher rate of overall complications (p = .0488) was observed in Group B. A significantly higher rate of Grade 3 frequency/urgency (p = .0064) contributed to this difference. The use of EPI was the only independent factor of severe urinary frequency/urgency (p = .017) (Table 6).
Table 5.
Complications of HIVEC treatment with mitomycin C [Group A] (75 patients) and epirubicin [Group B] (28 patients)
| Grade | Group A, N (%) | Group B, N (%) | p value | |
|---|---|---|---|---|
| Facial swelling | 1 | 1/75 (1.33%) | 1/28 (3.57%) | .9441 |
| Facial swelling* | 2 | 1/75 (1.33%) | 0/28 (0%) | .6063 |
| Hand urticaria | 1 | 2/75 (2.67%) | 1/28 (3.57%) | .6777 |
| Total body urticaria* | 2 | 0/75 (0%) | 1/28 (3.57%) | .6063 |
| Bladder spasm | 1 | 3/75 (4%) | 1/28 (3.57%) | .6362 |
| Frequency/urgency | 1 | 3/75 (4%) | 1/28 (3.57%) | .6362 |
| Frequency/urgency | 2 | 2/75 (2.67%) | 1/28 (3.57%) | .6777 |
| Frequency/urgency | 3 | 2/75 (2.67%) | 5/28 (17.86%) | .0064 |
| Urinary tract pain | 1 | 4/75 (5.33%) | 2/28 (7.14%) | .9014 |
| Urinary tract pain | 2 | 3/75 (4%) | 1/28 (3.57%) | .6362 |
| Urinary tract pain* | 3 | 2/75 (2.67%) | 1/28 (3.57%) | .6777 |
| Abdominal pain | 1 | 1/75 (1.33%) | 1/28 (3.57%) | .9441 |
| Haematuria | 1 | 1/75 (1.33%) | 0/28 (0%) | .6063 |
| Overall complications | 25/75 (33.3%) | 16/28 (57.14%) | .0488 | |
| *Therapy discontinuation | 3/75 (4%) | 2/28 (7.14%) | .8847 |
HIVEC – hyperthermic intravesical chemotherapy
Table 6.
Logistic regression analysis evaluating independent predictors of grade 3 frequency/urgency
| Variables | B* | SE | Wald | p-value | OR [Exp(B)] | 95% CI |
|---|---|---|---|---|---|---|
| Gender Male (reference) Female |
– -1.067 |
– 1.310 |
– .663 |
– .416 |
– .344 |
– .026–4.489 |
| Diabetes No (reference) Yes |
– 1.877 |
– 1.107 |
– 2.875 |
– .090 |
– 6.535 |
– .746–57.229 |
| Number of tumors Single (reference) Multiple |
– 1.629 |
– 1.233 |
– 1.747 |
– .186 |
– 5.100 |
– .455–57.131 |
| Tumor size <3 cm (reference) ≥3 cm |
– -2.201 |
– 1.552 |
– 2.009 |
– .156 |
– .111 |
– .005–2.321 |
| Recurrence rate ≤1/year (reference) >1/year |
– .234 |
– 1.169 |
– .040 |
– .842 |
– 1.263 |
– .128–12.495 |
| Previously treated with MMC No (reference) Yes |
– 1.466 |
– 1.208 |
– 1.471 |
– .225 |
– 4.330 |
– .405–46.247 |
| Drug used Mitomycin (reference) Epirubicin |
– 2.756 |
– 1.156 |
– 5.682 |
– .017 |
– 15.738 |
– 1.632–151.759 |
| HIVEC response (induction course) Yes (reference) No |
– 1.988 |
– 1.228 |
– 2.622 |
– .105 |
– 7.299 |
– .658–80.933 |
– intercept; SE – standard error; OR – odds ratio [exponentiation of the B coefficient]; Cl – confidence interval; MMC – mitomycin C; HIVEC – hyperthermic intravesical chemotherapy
DISCUSSION
Radical cystectomy is the gold standard for BCG failure NMIBC patients who are unlikely to respond to a further BCG cycle [9]. Immediate radical cystectomy should be offered to subjects with highest risk of tumor progression, but it is however possible to consider the role of ‘bladder sparing’ strategies [10, 11], in particular for those unfit for or unwilling to undergo radical surgery [12].
Chemohyperthermia seems to be more effective on the treatment of bladder cancer than passive chemotherapy due to higher penetration of the drug into the bladder wall and a direct toxic effect of heat [13]. Moreover, chemohyperthermia induces an immune response stimulating cancer cells to release heat shock proteins that activate a T-cell response [14]. Synergism of chemotherapy and hyperthermia was clearly demonstrated for EPI, MMC and to a lesser extent gemcitabine (GEM). Indeed, a synergistic effect on decreased cell proliferation was demonstrated in all cell lines and chemotherapeutic agents used (GEM, EPI, MMC), although each one had a maximum effect at a different chemotherapy concentration and to a different extent [15]. HIVEC treatment with MMC is safe, effective and capable of obtaining good success rates in neoadjuvant and adjuvant settings for intermediate to high-risk patients who have contraindications for standard therapies [16]. In the case of occasional shortage of MMC, several other intravesical chemotherapeutic agents including GEM and EPI can be used [17].
According to EAU guidelines [2], recurrence rate and number of tumors are the most important prognostic factors for bladder cancer recurrence. Moreover, about 54% of patients with CIS experience bladder cancer progression [18]. In BCG failure setting, BCG-relapse group has better outcomes [19] while BCG-unresponsive group has higher risk of progression [20].
In our experience, tumor size, recurrence rate, concomitant CIS and BCG unresponsive class are independent predictors of failure to HIVEC regimen at first follow-up and may contribute to identify patients that could benefit from conservative treatment. Moreover, MMC versus EPI showed no differences in recurrence and progression rate after the induction course and in disease-free survival at 38 months.
There is scarce literature available regarding the use of HIVEC in the setting of BCG failure. In a cohort of 52 BCG unresponsive patients, de Jong JJ et al. [4] previously showed a median DSF of 17.7 months. A total of 26 out of 52 patients remained disease-free, 22 experienced a recurrence and 4 a progression to muscle-invasive or metastatic bladder cancer. Marquette et al. showed a recurrence in 27.3% of BCG unresponsive patients during the first year [21].
HIVEC demonstrated similar outcomes compared to other intravesical agents. Shore et al. showed a 1-year DFS of 35% in high-grade NMIBC using recombinant adenovirus (rAd)–IFNα-2b [22]. Moreover, a 1-year DFS of 34.8% with Mycobacterium phlei cell wall-nucleic acid complex (MCNA) was observed [23].
Synergo HT® system was used in treatment of bladder cancer routinely since 2001. It provides chemohyperthermia irradiating the urothelium and bladder wall through a microwave applicator mounted on a 20 Fr three-way catheter. This regimen also showed good results in terms of DFS [24] but it was associated with a high rate of side effects with up to 38% of drop-out [25].
The HIVEC system was usually well tolerated due to the use of a soft 16 Fr three-way Foley catheter. Moreover, the drug was heated by an aluminium heat exchanger and then injected in the bladder. The most common adverse events were urinary frequency, pelvic pain, haematuria and urinary urgency [26]. As described in literature, in our analysis most adverse events were mild and self-limiting, rarely leading to therapy discontinuation. The rate of drop-out was similar among the groups. Despite this, a significant difference has been observed in the complications rate. Particularly, a higher rate of Grade 3 frequency/urgency contributed to this difference.
The direct irritative effect of chemotherapeutic drugs on bladder mucosa and changes in vesical capacity and bladder wall compliance [27] could explain the voiding lower urinary tract symptoms (LUTS) encountered in these patients. However, changes in bladder wall characteristics were not only related to the toxic effect of intravesical chemotherapy, but also to multiple resections and others demographic and pathologic characteristics. In our experience, the use of EPI was the unique negative prognostic factor for severe urinary frequency/urgency.
In vivo, cold EPI has a similar side-effect profile to cold MMC [28]. However, the existing literature provides no data about comparison of complications of these drugs in chemohyperthermia regimens. Two patients (one for each group) did not complete the whole induction course due to Grade 2 allergic reactions. Hypersensitivity reactions can be related to both contact and systemic allergy [29].
Some limitations of this study include the small cohort of patients and its retrospective non-randomized nature. Additionally, this study is limited by its short follow-up time; this may have determined that some patients might be falsely deemed as responders due to short follow-up alone. Obviously, the results of this study have to be confirmed in large-scale randomized prospective studies [30, 31].
CONCLUSIONS
HIVEC treatment shows impressive results on disease-free survival and is well tolerated, considering a high possibility of Grade 3 adverse event using EPI. It can be considered a feasible option in BCG failure or intolerant NMIBC patients, theoretically avoiding or postponing radical cystectomy in some particular subclasses of patients. Pre-treatment recurrence rate, tumor size, concomitant CIS and BCG unresponsive class can predict the likelihood of success of HIVEC treatment providing some information in conservative decision making for urologists.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
References
- 1.Zlotta AR, Fleshner NE, Jewett MA. The management of BCG failure in non-muscle-invasive bladder cancer: an update. Can Urol Assoc J. 2009;3(6 Suppl 4):S199–205. doi: 10.5489/cuaj.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babjuk M, Böhle A, Burger M, et al. EAU Guidelines on Non-Muscle-invasive Urothelial Carcinoma of the Bladder: Update 2016. Eur Urol. 2017;71:447–461. doi: 10.1016/j.eururo.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 3.Shen PL, Lin ME, Hong YK, He XJ. Bladder Preservation Approach Versus Radical Cystectomy for High-Grade Non-Muscle-Invasive Bladder Cancer: A Meta-Analysis of Cohort Studies. World J Surg Oncol. 2018;16:197. doi: 10.1186/s12957-018-1497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Jong JJ, Hendricksen K, Rosier M, Mostafid H, Boormans JL. Hyperthermic Intravesical Chemotherapy for BCG Unresponsive Non-Muscle Invasive Bladder Cancer Patients. Bladder Cancer. 2018;4:395–401. doi: 10.3233/BLC-180191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan WS, Palou J, Kelly J. Safety and tolerability analysis of hyperthermic intravesical mitomycin to mitomycin alone in HIVEC I and HIVEC II: An interim analysis of 307 patients. Eur Urol Suppl. 2017;16:e1150. [Google Scholar]
- 6.Barski D. The arguments for an early cystectomy in patients with urothelial carcinoma. Cent European J Urol. 2014;67:333–334. doi: 10.5173/ceju.2014.04.art3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176–181. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 8.Chiancone F, Carrino M, Fedelini M, Meccariello C, Fedelini P. Intravesical thermo-chemotherapy using the combined antineoplastic thermotherapy bladder recirculation system for BCG-failure patients: A single center experience. Eur Urol Supp. 2019;18:e3248. [Google Scholar]
- 9.Raj GV, Herr H, Serio AM, et al. Treatment paradigm shift may improve survival of patients with high risk superficial bladder cancer. J Urol. 2007;177:1283–1286. doi: 10.1016/j.juro.2006.11.090. [DOI] [PubMed] [Google Scholar]
- 10.Nativ O, Witjes JA, Hendricksen K, et al. Combined thermo-chemotherapy for recurrent bladder cancer after bacillus Calmette-Guerin. J Urol. 2009;182:1313–1317. doi: 10.1016/j.juro.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 11.Racioppi M, Di Gianfrancesco L, Ragonese M, Palermo G, Sacco E, Bassi PF. Can Neutrophil-to-Lymphocyte ratio predict the response to BCG in high-risk non muscle invasive bladder cancer? Int Braz J Urol. 2019;45:315–324. doi: 10.1590/S1677-5538.IBJU.2018.0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sari Motlagh R, Pradere B, Mori K, Miura N, Abufaraj M, Shariat SF. Bladder-preserving strategies for Bacillus Calmette-Guérin unresponsive non-muscle invasive bladder cancer; where are we and what will be expected? Curr Opin Urol. 2020;30:584–593. doi: 10.1097/MOU.0000000000000792. [DOI] [PubMed] [Google Scholar]
- 13.Teicher BA, Kowal CD, Kennedy KA, Sartorelli AC. Enhancement by hyperthermia of the in vitro cytotoxicity of mitomycin C toward hypoxic tumor cells. Cancer Res. 1981;41:1096–1099. [PubMed] [Google Scholar]
- 14.Multhoff G, Habl G, Combs SE. Rationale of hyperthermia for radio(chemo)therapy and immune responses in patients with bladder cancer: Biological concepts, clinical data, interdisciplinary treatment decisions and biological tumour imaging. Int J Hyperthermia. 2016;32:455–463. doi: 10.3109/02656736.2016.1152632. [DOI] [PubMed] [Google Scholar]
- 15.van der Heijden AG, Verhaegh G, Jansen CF, Schalken JA, Witjes JA. Effect of hyperthermia on the cytotoxicity of 4 chemotherapeutic agents currently used for the treatment of transitional cell carcinoma of the bladder: An in vitro study. J Urol. 2005;173:1375–1380. doi: 10.1097/01.ju.0000146274.85012.e1. [DOI] [PubMed] [Google Scholar]
- 16.Sousa A, Piñeiro I, Rodríguez S, et al. Recirculant hyperthermic IntraVEsical chemotherapy (HIVEC) in intermediate-high-risk non-muscle-invasive bladder cancer. Int J Hyperthermia. 2016;32:374–380. doi: 10.3109/02656736.2016.1142618. [DOI] [PubMed] [Google Scholar]
- 17.Veeratterapillay R, Heer R, Johnson MI, Persad R, Bach C. High-Risk Non-Muscle-Invasive Bladder Cancer-Therapy Options During Intravesical BCG Shortage. Curr Urol Rep. 2016;17:68. doi: 10.1007/s11934-016-0625-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lamm DL. Carcinoma in situ. Urol Clin North Am. 1992;19:499. [PubMed] [Google Scholar]
- 19.Herr HW, Milan TN, Dalbagni G. BCG-refractory vs. BCG-relapsing non-muscle-invasive bladder cancer: a prospective cohort outcomes study. Urol Oncol. 2015;33:108.e1-108–e1084. doi: 10.1016/j.urolonc.2014.02.020. [DOI] [PubMed] [Google Scholar]
- 20.Kamat AM, Sylvester RJ, Böhle A, et al. Definitions, End Points, and Clinical Trial Designs for Non-Muscle-Invasive Bladder Cancer: Recommendations From the International Bladder Cancer Group. J Clin Oncol. 2016;34:1935–1944. doi: 10.1200/JCO.2015.64.4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marquette T, Walz J, Rybikowski S, et al. Tolérance de la thermo-chimiothérapie par HIVEC® chez les patients réfractaires au BCG [Safety of Hyperthermic IntraVEsical Chemotherapy (HIVEC) for BCG Unresponsive Non-Muscle Invasive Bladder Cancer Patients] Prog Urol. 2020;30:35–40. doi: 10.1016/j.purol.2019.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Shore ND, Boorjian SA, Canter DJ, et al. Intravesical rAd-IFNalpha/Syn3 for Patients With High-Grade, Bacillus Calmette-Guerin-Refractory or Relapsed Non-Muscle-Invasive Bladder Cancer: A Phase II Randomized Study. J Clin Oncol. 2017;35:3410–3416. doi: 10.1200/JCO.2017.72.3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li R, Amrhein J, Cohen Z, Champagne M, Kamat AM. Efficacy of Mycobacterium Phlei Cell Wall-Nucleic Acid Complex (MCNA) in BCG-Unresponsive Patients. Bladder Cancer. 2017;3:65–71. doi: 10.3233/BLC-160084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nativ O, Witjes JA, Hendricksen K, et al. Combined thermo-chemotherapy for recurrent bladder cancer after bacillus Calmette-Guerin. J Urol. 2009;182:1313–1317. doi: 10.1016/j.juro.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 25.Kiss B, Schneider S, Thalmann GN, Roth B. Is thermochemotherapy with the Synergo system a viable treatment option in patients with recurrent non-muscle-invasive bladder cancer? Int J Urol. 2015;22:158–162. doi: 10.1111/iju.12639. [DOI] [PubMed] [Google Scholar]
- 26.Tan WS, Kelly JD. Intravesical device-assisted therapies for non-muscle-invasive bladder cancer. Nat Rev Urol. 2018;15:667–685. doi: 10.1038/s41585-018-0092-z. [DOI] [PubMed] [Google Scholar]
- 27.Michielsen D, Amy JJ, Coomans D, Storme G, Wyndaele JJ. Mitomycin C and epirubicIn: functional bladder damage in rats after repeat intravesical instillations. J Urol. 2005;173:2166–2170. doi: 10.1097/01.ju.0000158123.33273.f9. [DOI] [PubMed] [Google Scholar]
- 28.Ali-el-Dein B, el-Baz M, Aly AN, Shamaa S, Ashamallah A. Intravesical epirubicin versus doxorubicin for superficial bladder tumors (stages pTa and pT1): a randomized prospective study. J Urol. 1997;158:68–74. doi: 10.1097/00005392-199707000-00018. [DOI] [PubMed] [Google Scholar]
- 29.de Groot AC, Conemans JM. Systemic allergic contact dermatitis from intravesical instillation of the antitumor antibiotic mitomycin C. Contact Dermatitis. 1991;24:201–209. doi: 10.1111/j.1600-0536.1991.tb01699.x. [DOI] [PubMed] [Google Scholar]
- 30.Walczak R, Bar K, Walczak J. The value of EORTC risk tables in evaluating recurrent non-muscle-invasive bladder cancer in everyday practice. Cent European J Urol. 2014;66:418–422. doi: 10.5173/ceju.2013.04.art6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lebentrau S, May M, Wick AK, Roiner M, Mathew M, Gilfrich C, et al. Non-muscle invasive bladder cancer: Are epicrises the 'Bermuda Triangle' of information transfer? Cent European J Urol. 2017;70:245–251. doi: 10.5173/ceju.2017.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]