Abstract
Introduction
The aim of this study was to find out if there are any conventional urodynamic (UDS) variables that would help to predict the necessity of overactive bladder (OAB) symptomatic therapy in women after transobturator tape surgery (TOT).
Material and methods
A total of 487 females after TOT were enrolled in this retrospective study. Inclusion criteria (UDS before surgery, follow-up visit within 2–6 month after TOT) were met in 169 women. Based on patient history, questionnaires and physical examination, two groups were distinguished: pure stress urinary incontinence (SUI) and stress-predominant mixed urinary incontinence (MixUI). A statistical analysis was performed including age and UDS variables. T-test was used for continuous data and Chi-squared test for categorical data. Combinations of these factors were analyzed using binary logistic regression and surgery outcome as the target variable.
Results
Significant correlations between the probability of a need for OAB therapy after TOT were observed with age (higher age increases OAB therapy necessity, p <0.001) and such UDS variables as cystometric capacity (CC) p <0.001; maximum flow rate (Qmax) p <0.001; detrusor contractility index (DCI) p <0.015 – higher value decreased the need for OAB therapy. Critical limit for these values: 60 years for age, 300 ml for CC, 15 ml/s for Qmax, but no specific value for DCI was observed. Binary logistic regression showed that the UI Group (p <0.01) and CC (p = 0.01) allow correctly classify 78.9% of TOT outcome (increased CC and SUI group are factors for TOT normal outcome).
Conclusions
UI group, age, CC, Qmax, DCI can help to predict the necessity of OAB symptomatic therapy in women after TOT.
Keywords: urinary incontinece, urodynamics, transobturator tape surgery, female, overactive bladder therapy
INTRODUCTION
To this day, one of the most common treatment options for women with stress urinary incontinence (SUI) is transvaginal tape surgery. There are two main types of urinary incontinence (UI) for which surgical treatment may be indicated: pure SUI and stress-predominant mixed UI (MixUI). Unfortunately, even surgical treatment does not always resolve the problem of UI. In addition, de novo urge or recurrent urgency UI episodes may occur, reducing the patient satisfaction with surgical treatment results and requiring continuous symptomatic overactive bladder (OAB) treatment [1]. Unfortunately, there is a lack of sensitive methods for identifying such patient groups prior to surgery.
The clinical significance of urodynamic variables (UDS) in uncomplicated SUI cases has been disputed even when surgical intervention is planned [1–6]. Some authors have asserted that UDS can provide essential clues about other types of lower urinary tract dysfunctions (LUTD), as well as the specific pathogenesis and severity of SUI, that may justify the inconvenience of UDS testing [5, 7–10]. However, it is still important to determine the most informative UDS parameters for the purpose of predicting postoperative outcomes for SUI patients.
There are also authors who concluded that UDS should not be indicated prior to surgical treatment of patients with pure SUI and stress predominant MixUI, because UDS had no effect on the outcome of surgery. This was also the conclusion of the large randomised ValUE and VUSIS-II studies [11, 12]. At the same time, there is no doubt about the benefit of UDS testing for women with recurrent or complicated SUI [10]. It is important to know that in the initial diagnostic algorithm, everything has been done to avoid complicated cases, and UDS can probably be of benefit for this purpose.
Taking into account the aforementioned considerations, the aim of our research was to establish whether specific UDS values may help to predict the outcomes of surgical treatment for SUI in women and to distinguish patient groups that have higher probability of a need for OAB symptomatic treatment after the transobturator tape surgery (TOT).
MATERIAL AND METHODS
Retrospective data analysis was used to evaluate the data from 487 women who underwent primary TOT surgery between April 2016 and January 2020. Statistical processing of data was performed for 169 women who had pre-operative UDS data: filling-cystometry followed by pressure-flow study (MCM) and urethral pressure profilometry (UPP), as well as information about a follow-up visit (within 2-6 months after TOT). The patients were divided into two preoperative groups: SUI group and MixUI group according to their patient history, patient questionnaires [Urogenital Distress Inventory short form questionnaire (UDI-6) and International Consultation on Incontinence Questionnaire short form (ICIQ-UI)], and the results of physical examination. Postoperative groups also were divided based on patient symptoms evaluated during a follow-up visit.
An MMS Solar Silver system with 9 Fr triple lumen water-filled catheter was used for UDS. The MCM was performed in the sitting position with a filling rate of 50 ml/min. Upon reaching the cystometric capacity, the patient urinated in privacy. The UPP was performed in the lithotomy position, with a catheter pulling rate of 2 mm/s, filling rate of 2 ml/min. During the UPP, rest and cough stress profiles were acquired.
The TOT surgery was performed using the ‘Swing-bend’ urinary incontinence reconstruction mesh, inside-out transobturator system.
The taking of patient medical history, the UDS testing, and surgery were performed by a team of two doctors.
RESULTS
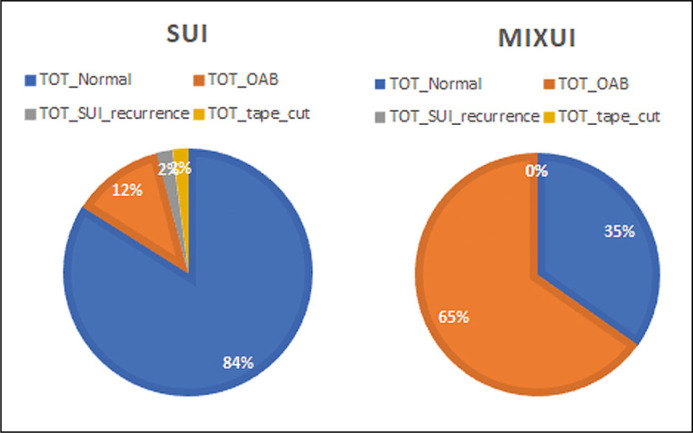
A total of 169 women after the TOT surgery were included in the analysed dataset. The preoperative diagnosis for 100 of the patients was SUI and for 69 patients – MixUI. The percentages of patients with no complaints about urinary incontinence after the surgery (TOT Normal group) were: 84 (84%) in the SUI group and 24 (34.8%) in the MixUI group. OAB therapy following TOT surgery was recommended for 42 (65.2%) of the patients in the MixUI group and for 12 (12%) of the patients in the SUI group, p <0.001. Furthermore, 2 (2%) of the patients in the SUI group suffered from a recurrence of SUI (TOT SUI recurrence group) and 2 (2%) required cutting of the TOT tape (TOT Tape cut group) due to urinary retention, while there were no such patients in the MixUI group. The results are shown in Figure 1.
Figure 1.
The transobturator (TOT) surgery outcome for stress (SUI) and mixed urinary incontinence (MIXUI) groups.
In order to establish the possible factors that can be useful for predicting the outcome of TOT surgery, a statistical analysis was performed including the age of the patients and such UDS parameters as detrusor overactivity (DO), urgency urinary incontinence (UUI), urodynamic stress urinary incontinence (SUI UDS), cystometric capacity (CC), opening detrusor pressure (p det open), maximum urinary flow (Qmax), detrusor pressure at Qmax (p det Qmax), detrusor contractility index (DCI), functional urethral length during rest profile (FUL rest), functional urethral length during stress profile (FUL stress), maximum urethral closure pressure at rest (MUCP rest), maximum urethral closure pressure at cough stress (MUCP stress), and the pressure transmission ratio (PTR).
These factors were analysed as differing factors in two main groups of postoperative outcomes: 1) TOT Normal; 2) TOT OAB. Significant differences were observed in age (the probability of a need for OAB therapy increased with age; p <0.001) and such UDS variables as cystometric capacity (CC) p <0.001; maximum flow rate (Qmax) p = 0.012; detrusor contractility index (DCI) p = 0.009 – as the value increases, the likelihood of requiring OAB therapy decreases.
There was no statistically significant difference in UPP values that would allow the prediction of the need for symptomatic OAB therapy after TOT. The results are shown in Table 1.
Table 1.
The mean values of factors for different transobturator tape (TOT) surgery outcome groups and the corresponding p-values
| TOT Normal Mean | TOT OAB Mean | p-value | |
|---|---|---|---|
| Age | 57 | 64 | <0.001 |
| Cystometric capacity | 422.96 | 310.13 | <0.001 |
| Maximum flow rate (Qmax) | 25.71 | 21.50 | 0.012 |
| Detrusor contractility index | 152.32 | 130.80 | 0.009 |
| Opening detrusor pressure | 19.38 | 21.19 | 0.415 |
| Detrusor pressure at Qmax | 25.15 | 24.31 | 0.729 |
| Functional urethral length during rest profile | 32.75 | 32.96 | 0.905 |
| Functional urethral length during stress profile | 32.86 | 31.17 | 0.463 |
| Maximum urethral closure pressure at rest | 61.87 | 62.42 | 0.910 |
| Maximum urethral closure pressure at cough stress | 77.40 | 75.08 | 0.713 |
| Pressure transmission ratio | 61.40 | 63.96 | 0.659 |
| Detrusor overactivity | Yes/No | Yes/No | 0.062 |
| Urodynamic urgency urinary incontinence | Yes/No | Yes/No | 0.41 |
| Urodynamic stress urinary incontinence | Yes/No | Yes/No | 0.189 |
A focussed analysis of the age factor revealed that the patients in the MixUI group were significantly older than in the SUI group (p <0.001), with the average ages of 65.5 years and 54.67 years, respectively. Analysing age difference between the subgroups: TOT normal (mean age 56.69) and TOT OAB (mean age 63.9), patients in the TOT OAB group were significantly older, p <0.001. However, the age factor did not produce statistically significant differences during separate analyses of pure SUI group: TOT normal/TOT OAB (p = 0.654) and MixUI group: TOT normal/TOT OAB (p = 0.950). It can be concluded that age is a risk factor for the MixUI group, which is further associated with increased probability of a need for OAB therapy after the TOT surgery.
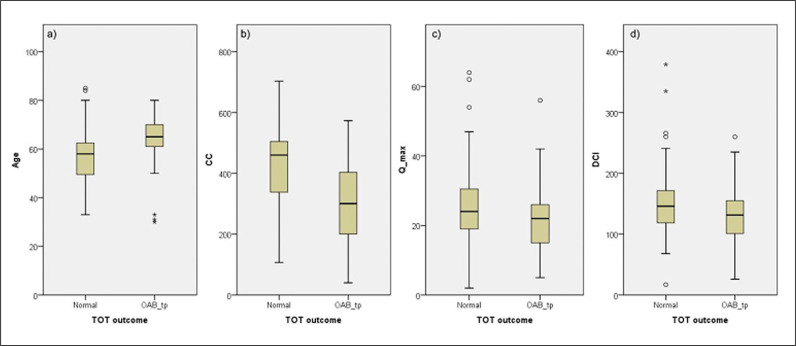
The factors that showed statistical significance for the outcome of TOT were subjected to additional analysis, in order to determine the critical limits of TOT normal and TOT OAB. Thus, each factor was divided into certain ranges. For the factor of age, the ranges were ≤50, 51–60, 61–70, and 70+. The critical limit was 60 years of age: for patients at or below 60 years the predominant outcome was TOT normal, while over 60 years of age the probability of TOT OAB was greater (p <0.005).
The factor of CC was divided into the ranges of 1–99, 100–199, 200–299, 300–399, 400–499, and 500+ ml. The critical limit was at CC 300 ml: for the CC values of 300 ml and greater the predominant probability was that of TOT normal, while CC of less than 300 ml favoured the outcome of TOT OAB (p <0.005).
The factor of Qmax was divided into the ranges of ≤15, 16–20, 21–25, 26–30, and 31+. The critical limit was at Qmax 15 ml/s: Qmax values of 15 ml/s or greater were associated with greater probability of TOT normal, while the patients with Qmax below 15 ml/s were more likely to experience TOT OAB (p = 0.034).
The factor of DCI was divided into the ranges of <100, 101–120, 121–140, 141–160, and 161+. None of these ranges were associated with statistically significant differences between TOT normal and TOT OAB (p = 0.163).
The results are shown in Figure 2.
Figure 2.
Median values and quartiles of significant factors affecting transobturator tape (TOT) surgery outcome: age, cystometric capacity (CC), maximum flow rate (Qmax), detrusor contractility index (DCI).
We also additionally analysed the 34.8% of patients from the MixUI group who did not indicate problems with UI after the TOT surgery, and were not assigned any additional therapy. Taking into account this information, our subsequent goal was to identify any UDS variables that would help to distinguish the MixUI group patients who are most likely to be cured by TOT surgery from those who will probably need further OAB therapy after the surgery. For this purpose, two new groups were distinguished among the MixUI group patients: 1) MixUI normal and 2) MixUI OAB, and statistically significant differences of any UDS values were investigated among these two groups. None of the UDS values showed differences with a significance of p <0.05, but CC had a correlation with p=0.058, where higher CC values increased the probability that OAB is not needed after the surgery.
Similarly, in order to identify any UDS values that statistically significantly point to the probability of OAB symptoms and the need for symptomatic therapy in pure SUI patients after the surgery, the initial SUI group was divided into two subgroups: 1) SUI normal and 2) SUI OAB. The factors of Qmax and DCI showed statistically significant correlation – the probability of a need for OAB therapy increases with decreasing values of Qmax and DCI (p = 0.033).
Even though none of the UPP values showed significant association with the outcome of TOT surgery, we additionally analysed the possible presence of linear correlations between such variables of urethral function as FUL rest and MUCP rest – lower values of FUL rest correlated with lower MUCP rest values. However, this hypothesis was also rejected both for the combined patient population (p = 0.249), as well as separately for the SUI group (p = 0.659) and the MixUI group (p = 0.268).
Binary logistic regression was used to identify significant predictive factors, using conditional forward selection. It showed that UI Group (p <0.01) and CC (p = 0.01) are significant factors, which allow correctly classifying 78.9% of TOT outcome groups (increased CC and SUI group are factors for TOT normal outcome), AUC = 0.824. Controlling for age did not have any significant impact on factor significance. Excluding the UI group from the factor list in order to find type-independent models did not yield any satisfactory results [the best one included age (p = 0.01), CC (p <0.01) and p det open (p = 0.05)], and showed 69% correct classification of outcomes (AUC = 0.773).
DISCUSSION
Two opposite opinions are still encountered in the literature about the role of UDS in managing UI in female patients. A turning point in the application of UDS for women with SUI occurred in 2012, when two randomised trials ValUE and VUSIS-II were reported, which reached the conclusion that UDS is not indicated prior to surgical treatment of patients with pure SUI, as it does not improve the outcome of the surgery. One group of experts favoured this assumption, and as a result, the use of UDS in female SUI patients was reduced from 70 to 40% [11, 12, 13]. However, such a conclusion also led to questions about the study design, data interpretation, and the methodological basis of the conclusions [14]. Indeed, how can we be sure that pure SUI is indeed ‘pure’? A study by Digesu analysed the data of 3428 women with SUI and concluded that “only 8.9% of the patients were classified as having pure SUI… UDS provides useful information in the assessment of women with pure SUI” [10]. Also, McGuire emphasized individual differences between the patients with SUI and that the effectiveness of therapy directly depended on precise pathophysiological diagnosis, therefore UDS should be recommended for all female patients [15]. Our data also pointed to the clinical utility of UDS, helping to differentiate patients who with high probability will need to continue symptomatic therapy also after the TOT surgery (women over 60 years of age, with CC of less than 300 ml, and with Qmax below 15 ml/s). Such findings would be useful to the doctor in planning the appropriate treatment, as well as better explain to the patient her specific case. The demographic realities of an aging population have a direct impact on the UDS values, which deteriorate with age, thus increasing the risks and variety of LUTD [16]. The main findings by Choudhury et al. from UDS in postmenopausal women were incontinence, storage symptoms, and bladder outlet obstruction [17]. For these reasons, UDS should be considered for every aging woman, prior to undergoing surgery for SUI. Our results showed a similar situation: the risk of MixUI increased with age, thus OAB therapy was more frequently required after TOT. At the same time, age as an isolated factor did not increase the risk of recurrent OAB symptoms after TOT in women with MixUI and age should definitely not be the reason for refusing surgical correction of complaints about SUI.
There are also discussions about surgical correction of SUI in women who have been diagnosed with DO during UDS. The findings of DO during UDS have been noted in several studies as an independent risk factor for poorer outcomes of anti-incontinence surgery [1, 12], however, our data were in agreement with the observations of those authors [1] who could not confirm this correlation. In our study, the registration of DO showed no significant correlation with increased need for OAB therapy after TOT. These findings meant that the registration of DO during UDS was not a contraindication for surgical correction of SUI.
The appearance of de novo symptoms during micturition cycle after anti-incontinence surgery is known but not fully understood. International Urogynecological Association (IUGA) guidelines report that: “the incidence of de novo detrusor overactivity and urge urinary incontinence varies depending upon the anti-incontinence procedure with rates as high as 33%” [7]. Another research group observed that low Qmax appeared as an independent risk factor for urinary retention after transvaginal tape surgery [12]. According to our data, 12% of women noted that the primary OAB symptoms appeared only after TOT surgery. None of these women had clinically significant residual urine volume, but decreased Qmax and decreased DCI during preoperative UDS helped to identify this patient group.
Finally, the role of UPP needs to be discussed. The most common assumption about the pathogenesis of SUI is associated with dysfunction at the level of urethral sphincter and urethral support [15, 18]. Thus, it can be expected that UPP should have a key role in the diagnostic algorithm for these patients. Several publications have pointed out that low MUCP may predict not only poorer outcomes of SUI correction, but also a higher DO risk after the surgery [1, 5, 7, 19]. Another research group recognized UPP as a substantial factor for determining the severity of SUI [20]. In our previous studies, when we analysed the role of UDS in the algorithm for the diagnostics of specific type of UI, we concluded that the p det open and PTR parameters describing urethral function may help to differentiate between different types of UI [21]. None of the functional parameters for urethra showed an effect on the postoperative outcome during this analysis. However, it should be taken into account that the central complaint of all patients during this study was SUI. At the same time, it should not be denied that low MUCP is indicative of ISD, which could require a specific surgical approach while informing the patient about lower risk for correcting specifically the SUI component [7].
Finally, a multidisciplinary pan-European working group performed a literature analysis in 2017–2018 regarding the application of UDS for women with UI. When the experts were asked about the reasons for performing UDS on their patients with SUI, the answers included the goal of avoiding recurring problems, desire to resolve the causes of UI, as well as the need to obtain information also about the voiding phase. On the other hand, the main reasons against UDS were associated with patient embarrassment, discomfort, and the economic aspect. Obviously, none of those is a valid clinical reason for a doctor to refuse performing UDS [14].
CONCLUSIONS
First, we conclude that the patient age and such UDS parameters as CC, Qmax, and DCI can help to recognize patients who are more likely to need symptomatic OAB therapy after the TOT surgery. Second, the TOT surgery in 35% of the cases can entirely prevent the problem of UI also for women with MixUI. Finally, UDS gives confidence to doctors, helps to correctly interpret every specific case, and improves the quality of patient information prior to SUI surgery, while managing the patient expectations regarding postoperative results as accurately as possible.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
References
- 1.Fletcher SG, Lemack GE. Clarifying the role of urodynamics in the preoperative evaluation of stress urinary incontinence. Sci World J. 2008;8:1259–1268. doi: 10.1100/tsw.2008.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dillon BE, Zimmern PE. When are urodynamics indicated in patients with stress urinary incontinence? Curr Urol Rep. 2012;13:379–384. doi: 10.1007/s11934-012-0270-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosier PF. The evidence for urodynamic investigation of patients with symptoms of urinary incontinence. F1000Prime Rep. 2013;5:8. doi: 10.12703/P5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hickling DR, Steele SS. The role of preoperative urodynamics in stress urinary incontinence surgery. Can Urol Assoc J. 2017;11(6 Suppl 2):S113–S115. doi: 10.5489/cuaj.4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamanishi T, Sakakibara R, Uchiyama T, Hirata K. Role of urodynamic studies in the diagnosis and treatment of lower urinary tract symptoms. Urol Sci. 2011;22:120–128. [Google Scholar]
- 6.Yamanishi T, Sakakibara R, Uchiyama T, Hirata K. Practical urodynamics role of urodynamic studies in the diagnosis and treatment of lower urinary tract symptoms q CME credits. Urol Sci. 2011;22:120–128. [Google Scholar]
- 7.Ghoniem G, Stanford E, Kenton K, et al. Evaluation and outcome measures in the treatment of female urinary stress incontinence. Int Urogynecol J. 2008;19:5–33. doi: 10.1007/s00192-007-0495-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winters JC, Dmochowski RR, Goldman HB, et al. Title Urodynamic studies in adults: AUA/SUFU guideline. J Urol. 2012;188(6 Suppl):2464–2472. doi: 10.1016/j.juro.2012.09.081. [DOI] [PubMed] [Google Scholar]
- 9.Haddad JM, Gonzales Monaco HEM, Kwon C, et al. Predictive value of clinical history compared with urodynamic study in 1, 179 women. Rev Assoc Med Bras. 1992;62:54–58. doi: 10.1590/1806-9282.62.01.54. [DOI] [PubMed] [Google Scholar]
- 10.Al Mousa RT, Al Dossary N, Hashim H. The role of urodynamics in females with lower urinary tract symptoms. Arab J Urol. 2019;17:2–9. doi: 10.1080/2090598X.2019.1589931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nager CW, Brubaker L, Litman HJ, et al. A randomized trial of urodynamic testing before stress-incontinence surgery. N Engl J Med. 2012;366:1987–1997. doi: 10.1056/NEJMoa1113595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giarenis I, Cardozo L, Cardozo L. What is the value of urodynamic studies before stress incontinence surgery? BJOG. 2013;120:130–132. doi: 10.1111/1471-0528.12102. [DOI] [PubMed] [Google Scholar]
- 13.Lloyd JC, Dielubanza E, Goldman HB. Trends in urodynamic testing prior to midurethral sling placement-What was the value of the VALUE trial? Neurourol Urodyn. 2018;37:1046–1052. doi: 10.1002/nau.23398. [DOI] [PubMed] [Google Scholar]
- 14.Finazzi-Agro E, Gammie A, Kessler TM, et al. Urodynamics useless in female stress urinary incontinence? Time for some sense - A European expert consensus. Eur Urol Focus. 2020;6:137–145. doi: 10.1016/j.euf.2018.07.031. [DOI] [PubMed] [Google Scholar]
- 15.Mcguire EJ. Pathophysiology of Stress Urinary Incontinence. Rev Urol. 2004;6(Suppl 5):11–17. [PMC free article] [PubMed] [Google Scholar]
- 16.Shin YS, On JW, Kim MK. Effect of aging on urodynamic parameters in women with stress urinary incontinence. Korean J Urol. 2015;56:393–397. doi: 10.4111/kju.2015.56.5.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choudhury S, Das SK, Jana D, Pal DK. Is urodynamic study is a necessity for evaluation of lower urinary tract symptoms in postmenopausal female patients? Result of a prospective observational study. Urol Ann. 2017;9:239–243. doi: 10.4103/UA.UA_170_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delancey JOL. Why do women have stress urinary incontinence? Neurourol Urodyn. 2010;29(Suppl 1):S13–S17. doi: 10.1002/nau.20888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petros P. The female pelvic floor: function, dysfunction, and management according to the integral theory. Springer; 2010. [Google Scholar]
- 20.Pizzoferrato A-C, Fauconnier A, Fritel X, Bader G, Dompeyre P. Urethral closure pressure at stress: a predictive measure for the diagnosis and severity of urinary incontinence in women. Int Neurourol J. 2017;21:121–127. doi: 10.5213/inj.1732686.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pilsetniece Z, Vjaters E. The role of conventional urodynamic in diagnosing specific types of urinary incontinence in women. Turkish J Urol. 2020;46:134–139. doi: 10.5152/tud.2020.19218. [DOI] [PMC free article] [PubMed] [Google Scholar]