Abstract
Introduction
The aim of the study was to evaluate the frequency of occurrence of Chlamydia trachomatis (C.t.) DNA in the prostate material in the group of individuals with the chronic prostatitis.
Material and methods
The study included 65 males aged between 47 and 68 years of age, reporting for transrectal prostate biopsy because of the elevated serum prostate-specific antigen concentration and/or abnormalities detected in prostate palpation per rectum. The urethral smear collection was performed in all the patients in order to detect C.t. DNA. After that, the transrectal prostate biopsy was performed (histopathology tests, C.t. DNA). Additionally, the levels of anti-C.t. IgG antibodies and anti-C.t. IgA antibodies were checked in the serum. The DNA isolation from prostate specimens was conducted with the use of the Chelex method, while the C.t. DNA detection – with the ligase chain reaction. Specific antibodies were detected with the use of the ELISA method.
Results
C.t. DNA in the prostate gland was found in 7 out of 65 men (10.8%). In urethral smear, C.t. was found in none of the individuals. Anti-C.t. IgA antibodies were detected in the serum of 16/65 (24.6%), while anti-C.t. IgG antibodies in 6/65 (9.2%) of the examined males. IgA antibodies were found in two and IgG in one out of the 7 men who had C.t. infection in the prostate.
Conclusions
The presence of C.t. DNA in the prostate gland may be indicative of the role of chlamydia in the development of chronic prostatitis.
Keywords: Chlamydia trachomatis, prostatitis, transrectal prostate biopsy, ligase chain reaction, anti-C. trachomatis IgG antibodies, anti-C. trachomatis IgA antibodies
INTRODUCTION
Chronic prostatitis is the most common urological disorder diagnosed in men younger than 50 years of age, and the third most common in older men [1, 2]. The frequency of this disease in the general population is estimated at 5–8.8% [1, 3]. The etiology, pathogenesis and pathophysiology of inflammatory conditions of the prostate gland remain unknown in the majority of cases [2, 4]. In some individuals, there is an accompanying infection of the prostate gland, which is manifested by the presence of microorganisms and leukocytes in EPS (expressed prostatic secretion) in the sample of urine collected after a massage or in semen. Despite a variety of research techniques, the etiologic factor is rarely detected [4]. One of the microorganisms that may play a role in etiopathogenesis of chronic prostatitis is Chlamydia trachomatis, a Gram-negative bacteria, having a characteristic intracellular growth cycle [5, 6, 7]. A special feature shown by the Chlamydiae is the ability to survive in host cells, which leads to occult or chronic inflammatory conditions [8]. Strains of oculogenital (D-K) Chlamydia trachomatis in males cause urethritis, conjunctivitis, acute epididymitis, epididymo-orchitis, reactive arthropathy and may also lead to fertility impairment [9–13]. The contribution of these bacteria to the etiology of chronic prostatitis has been the subject of numerous discussions and controversies for many years [2, 14]. Worldwide research provides very few reports on Chlamydia detection in prostate tissues, whilst in Poland such research has not been carried out yet [15].
The aim of this work was to evaluate the occurrence of genetic material of Chlamydia trachomatis collected by the transrectal biopsy in the prostate gland in the group of men with chronic prostatitis.
MATERIAL AND METHODS
Patients
The study included 65 men aged between 47 and 68 years of age (the average 60), the patients of the Department of Oncology and General Urology of The Jędrzej Śniadecki Memorial Integrated Hospital, Białystok. The patients were referred for the prostate core biopsy due to the elevated serum prostate-specific antigen (PSA) concentration – over 4 ng/ml and/or abnormalities in the rectal palpation of the prostate gland.
The further tests included only sexually active men with the diagnosed prostatitis and excluded prostate cancer based on histopathology (H-P) tests. Patients with the history of anal intercourses were excluded from the examination. The patients, who were qualified to the study group did not use any antibiotics or other chemotherapeutics within 3 months after the biopsy. None of the men in the study reported Chlamydia trachomatis infection.
The thorough medical history of the participants of the study was taken with reference to the course, nature, duration of the symptoms and the applied treatment. Most often, the patients complained about the symptoms connected with the phase of urine accumulation: nycturia, daytime pollakiuria, urinary urgency and painful urination. They did not tend to complain much about the symptoms, connected with the inflammatory syndromes of the prostate, such as testicle pain or pain in the groin or crotch, lower abdomen pain or other disfunctions of the genital system. In physical examination special attention was paid to the condition of the external urogenital organs, the prostate, groin and abdomen. Each patient underwent per-rectum examination to evaluate the condition of the prostate gland and tightness of the external sphincter.
The first stage of the study involved the smear collection from the urethra after at least 3 hours from the last urination in order to detect Chlamydia trachomatis DNA. The urethral material was collected with the use of sterile dacron tip swabs (LCx STD Swab Specimen Collection System) which were inserted at the depth of 2–3 cm with a few circular motions performed to obtain more epithelial cells. The material was placed in a portable container and stored at the temperature of -70°C. After that, blood samples were taken from the participants of the study in order to check the anti - Chlamydia trachomatis IgG and IgA levels.
During the consecutive steps, transrectal core prostate biopsy was carried out under ultrasonography control with TOSHIBA CAPASEE equipped with a rectal probe with a linear transducer of 5.0 MHz frequency and a biopsy guide. The tissue samples were collected with automatic biopsy apparatus Magnum by BARD, equipped with sterile core biopsy needles 18 G. The collected tissues were used for histopathology tests and detection of the presence of Chlamydia trachomatis DNA. Therefore, the tissue sections of approximately 22 mm were placed in vials containing buffered formalin (H-P tests) and in Eppendorf probes (Chlamydia trachomatis DNA) and stored at the temperature of -70°C.
The control group, in the case of the urethra material collected for the detection of Chlamydia trachomatis infections, comprised 100 male patients aged between 22 and 68 years of age with no urogenital signs or symptoms. The participants were randomly selected from among the patients who registered to the urology outpatient clinic. The control group for checking the levels of the anti- Chlamydia trachomatis IgG antibodies comprised 103 men aged between 17–50, and for the anti- Chlamydia trachomatis IgA antibodies – 85 men aged between 40–60. The men in the control group for serological tests were blood donors and did not report any symptoms of genito-urinary diseases.
The patients who were diagnosed positive for the Chlamydia trachomatis infection underwent the 21-day-period of doxycycline antibiotic treatment, which also covered their sexual partners.
Methods
The DNA isolation from prostate specimens was performed at the Department of Forensic Medicine of Medical University of Białystok with the Chelex method [16]. The diagnostics of Chlamydia trachomatis infection involved the ligase chain reaction (LCR) with the use of a reagent set and an Abbott apparatus (Abbott LCx) [17]. Detection of Chlamydia trachomatis DNA was carried out in the Center for STD Research and Diagnostics in Białystok.
The levels of the specific IgA and IgG antibodies in serum were checked with the use of the ELISA method and the Trinity Biotech CaptiaTM Chlamydia IgA and the CaptiaTM Chlamydia IgG tests. The antigen used by the Trinity Biotech was the LPS LGV II biotype. The levels of the anti-Chlamydia trachomatis IgG and IgA antibodies were checked in accordance with the producer’s instruction. The IgA and IgG levels of 1.1 and above were considered positive, the levels of 0.91–1.09 were interpreted as doubtful and of 0.9 and below were negative. In the study, the results of 1.1–2.0 were considered weakly positive (+), of 2.1–3.0 were viewed as positive (++) and the results of >3.0 as highly positive (+++).
The statistical analysis was performed with the Fisher’s exact test and Fisher-Freeman-Halton’s test. The difference was considered statistically significant at p lower or equal to 0.05.
The study was approved by Bioethics Committee of Medical Academy of Białystok no R-I-003/21/2002 from 01. 02. 2002. a biopsy guide.
RESULTS
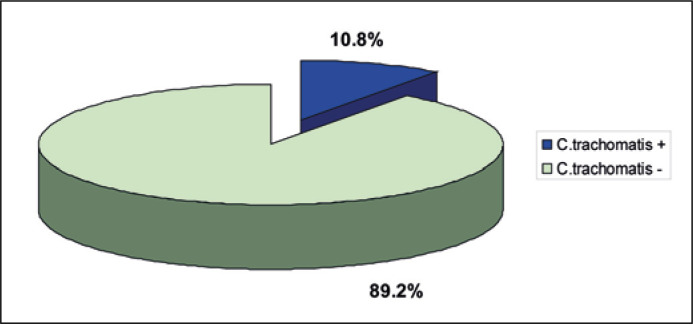
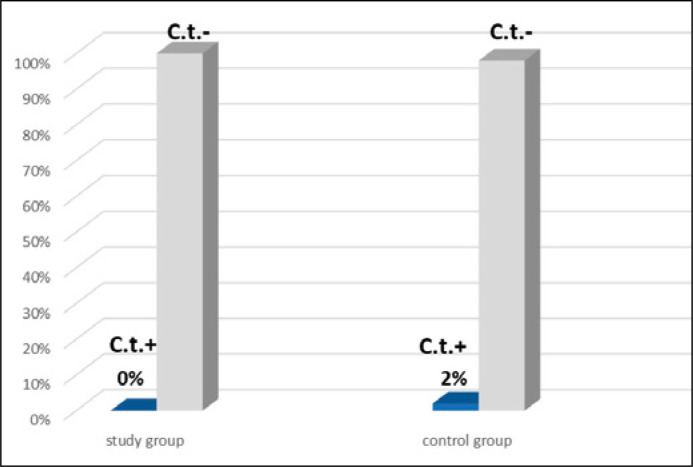
The Chlamydia trachomatis DNA in prostate tissues was detected in 7 patients out of 65, which accounted for 10.8% of all the participants (Figure 1). No Chlamydia trachomatis infection was found in the urethral samples in 65 examined patients. In the control group, the Chlamydia infection was detected in the urethral swabs in 2 out of 100 individuals, which amounted to 2% (p = 0.5) (Figure 2).
Figure 1.
Detection of the Chlamydia trachomatis DNA in the prostate gland tissue in the examined patients (n = 65).
Figure 2.
Detection of Chlamydia trachomatis in the urethral smear in both the study (n = 65) and control (n = 100) groups.
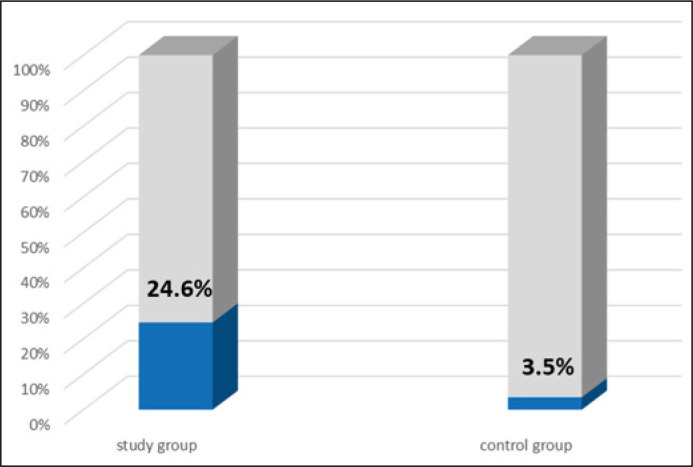
The anti- Chlamydia trachomatis IgA antibodies were identified in 16 out of 65 patients (24.6%), against 3 in 85 individuals (3.5%) (p = 0.0001) in the control group (Figure 3). Among the 16 patients with the specific IgA antibodies detected in serum, 2 (12.5%) had the Chlamydia trachomatis prostate infection (Table 1).
Figure 3.
Occurrence of anti-Chlamydia trachomatis IgA antibodies in serum in both the study (n = 65) and control (n = 85) groups.
Table 1.
Co-occurrence of specific IgA antibodies in the serum of the examined patients with the Chlamydia trachomatis infection of the prostate gland tissues (n = 65)
| Prostate gland tissues | anti-Chlamydia trachomatis IgA antibodies | |||
|---|---|---|---|---|
| (+) | (-) | |||
| n | % | n | % | |
| Chlamydia trachomatis (+) | 2 | 12.5 | 5 | 10.2 |
| Chlamydia trachomatis (-) | 14 | 87.5 | 44 | 89.8 |
| Total | 16 | 100 | 49 | 100 |
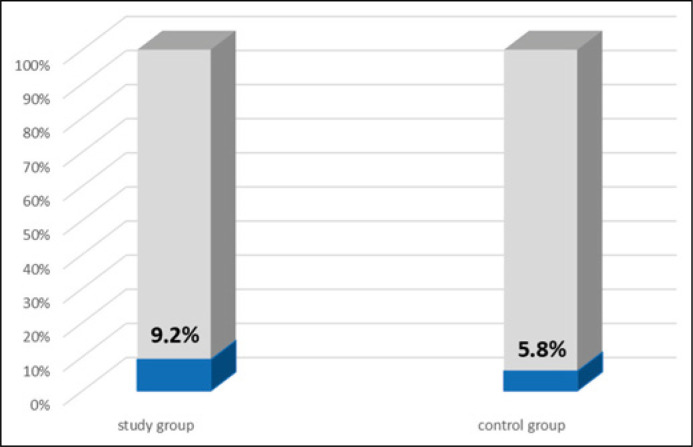
The anti-Chlamydia trachomatis IgG antibodies were found in 6 out of 65 patients (9.2%), against 6 out of 103 (5.8%) (p = 0.540) in the control group (Figure 4). Among the 6 patients with the specific IgG antibodies detected in serum, one (16.7%) had a chlamydial infection of the prostate tissue (Table 2).
Figure 4.
Occurrence of anti-Chlamydia trachomatis IgG antibodies in serum in both the study (n = 65) and control (n = 103) groups.
Table 2.
Co-occurrence of specific IgG antibodies in the serum of the examined patients with the Chlamydia trachomatis infection of the prostate gland tissues (n = 65)
| Prostate gland tissues | anti-Chlamydia trachomatis IgG antibodies | |||
|---|---|---|---|---|
| (+) | (-) | |||
| n | % | n | % | |
| Chlamydia trachomatis (+) | 1 | 16.7 | 6 | 10.2 |
| Chlamydia trachomatis (-) | 5 | 83.3 | 53 | 89.8 |
| Total | 6 | 100 | 59 | 100 |
In the group of 18 patients with the serological symptoms of chlamydial infection in serum only 4 (22.2%) were found to have the co-occurrence of antibodies of both classes of immunoglobulins (Table 3 and 4).
Table 3.
Co-occurrence of anti-Chlamydia trachomatis antibodies in the serum, depending on the class of immunoglobulins (IgA and IgG) (n = 65)
| anti-Chlamydia trachomatis IgG antibodies | anti-Chlamydia trachomatis IgA antibodies | ||
|---|---|---|---|
| (+) | (-) | total | |
| (+) | 4 | 2 | 6 |
| (-) | 12 | 47 | 59 |
| Total | 16 | 49 | 65 |
Table 4.
Occurrence of anti-Chlamydia trachomatis IgA and IgG antibodies in the serum of the examined patients with titers (n = 18)
| Patient | anti-Chlamydia trachomatis antibodies | |
|---|---|---|
| IgA (titre) | IgG (titre) | |
| 1 | + (1.71) | - |
| 2 | + (1.36) | - |
| 3 | - | + (1.11) |
| 4 | + (1.21) | - |
| 5 | + (1.15) | - |
| 6 | - | + (1.2) |
| 7 | + (1.22) | - |
| 8 | + (1.16) | ++ (2.5) |
| 9 | + (1.49) | - |
| 10 | + (1.22) | - |
| 11 | + (1.93) | - |
| 12 | + (1.48) | ++ (2.14) |
| 13 | +++ (6.1) | +++ (3.06) |
| 14 | + (1.14) | - |
| 15 | +++ (4.24) | +++ (3.23) |
| 16 | ++ (2.06) | - |
| 17 | + (1.19) | - |
| 18 | + (1.17) | - |
Table 5 presents the results of direct and serological tests of the patients’ serum identified to have the Chlamydia trachomatis DNA in the prostate tissue. None of the 7 men was diagnosed with the chlamydial infection of the urethra, while specific IgA antibodies were found in two and IgG antibodies in one of them.
Table 5.
Comparison of the results of the direct examination patients and the serology patients with the Chlamydia trachomatis infection in the prostate (n = 7)
| Patient | DNA Chlamydia trachomatis | anti-Chlamydia trachomatis antibodies | ||
|---|---|---|---|---|
| Urethra | Prostate gland | IgA | IgG | |
| 1 | (-) | (+) | (-) | (-) |
| 2 | (-) | (+) | (-) | (-) |
| 3 | (-) | (+) | (-) | (-) |
| 4 | (-) | (+) | (-) | (-) |
| 5 | (-) | (+) | (+) 1.93 | (-) |
| 6 | (-) | (+) | (+) 1.48 | (++) 2.14 |
| 7 | (-) | (+) | (-) | |
DISCUSSION
Prostatitis poses a great challenge to both patients and health professionals. Despite its high incidence and significant negative impact on quality of patients’ lives, little is known about its etiology and, therefore, face great difficulties in both diagnosing and providing effective treatment of the disease [2, 4]. Similarly, we have little data on the natural history of the prostatitis development as well as on the factors causing the change of incidental acute pelvic pain syndromes into chronic ones. It has been shown in rodent models that Chlamydia may persist in the prostate gland, avoiding the host immune system [8]. In the case of over 90% of patients, the etiology of prostatitis remains unknown [1, 4, 18]. The role of Chlamydia trachomatis in this disorder is still the subject of numerous discussions [2]. One of the factors leading to this situation is diagnostic difficulties of Chlamydia infection, namely – obtaining the proper testing material as well as the choice of method for detecting these microorganisms. An introduction of molecular methods (PCR, LCR) for the detection of Chlamydia trachomatis infection provided a wider range of diagnostic possibilities of prostatitis syndromes [15, 19]. The difficulty in detecting Chlamydia trachomatis in the prostate gland with non-molecular methods may result from, among others, the presence of the prostate antibacterial factor in semen, discharge and prostate tissues, which inhibits the growth of Chlamydia but does not impede other bacteria (Escherichia coli, Mycoplasma) [20].
In the study, the tissue samples collected by the rectal biopsy were used for the diagnostics of Chlamydia infections in the course of acute prostatitis. Also, the urethral smear collection was performed, which allowed to exclude the possibility of contamination with microorganisms from the urethral epithelium. The study included only heterosexual patients in order to exclude the possibility of infecting with the material from the rectal epithelium. A rectal infection in men develops as a result of an anal intercourse [21]. In our study, Chlamydia trachomatis infections were detected with the amplification method, which is currently regarded to as a ‘golden mean’ in the diagnostics of chlamydia infections.
According to the presented study, the presence of Chlamydia trachomatis DNA in prostate tissues was observed in 7 out of 65 examined male patients, which accounted for 10.8%. As no similar studies have been carried out in Poland so far, it is impossible to compare the outcomes of the present study to the national ones on the subject.
Few reports on detecting Chlamydia infections in the prostate gland can be found in the literature worldwide. The results obtained by the authors may differ from other reports due to, among others, the use of different methods in collecting the study material, the choice of diagnostic methods as well as the selection of the study group. The number of patients undergoing different examinations was varied and ranged from 6 [22] to 135 [23]. Among a dozen or so reports on Chlamydia detection in the prostate gland, in 7 cases the samples were collected by the transperineal or transrectal prostate biopsy [22–28], while in 6 cases by the transurethral resection of the prostate (TURP) or prostatectomy [29–34]. The culture method as a diagnostic method was used in 5 studies [22, 27, 28, 31, 35], out of which in 2 [28, 35] no Chlamydia presence in the prostate gland was found. Poletti et al. and Kojima et al. observed Chlamydia trachomatis infection in the group of 33.3% of the examined individuals (Poletti et al. in 10 out of 30, while Kojima et al. in 2 out of 6 patients) [22, 27]. Dan et al. examined 100 men with no symptoms of urethra and prostate inflammation and found Chlamydia in 3 (3%) of individuals in the tissue samples collected by the transurethral resection of the prostate [31]. In one of those individuals, the authors observed a non-specific inflammatory condition. They imply that the inflammation caused by Chlamydia may have an asymptomatic course for many years. The culture method was also used by Berger et al. [36] and Lee et al. [37], who detected Chlamydia in the biopsy material in 1 out of 88 and 1 out of 60 patients, respectively. Lee et al. observed a Chlamydia infection in one patient from the control group while the results in the study group (120 persons) were negative [37]. Shurbaji et al. showed Chlamydia presence in the tissues collected by the prostate resection in 5 out of 16 patients (31.3%), while Kobayashi et al. – in one man tested by the prostate biopsy [24, 33]. In both studies, the immunoperoxidase method was used to detect the Chlamydia infection.
Other studies involved an in-situ hybridization method [26, 29]. Maruta et al. found a Chlamydia infection in the biopsy material in 2 out of 7 examined patients (28.6%) [26]. According to other authors, the Chlamydia DNA was detected in the prostate resection material, in 7/23 (30.4%) [29], 3/11 (27.3%) [32], 9/20 (45%) [30] and 4/10 (40%) men [34]. Krieger et al. examined the highest number of patients – 135 individuals with chronic prostatitis. The Chlamydia infection in the biopsy material was found in 4 men [23]. The samples were collected by the transperineal prostate biopsy, while the studies involved the polymerase chain reaction. The Chlamydia trachomatis infection had an isolated nature in 3 cases, while it co-existed with the Mycoplasma genital infection in 1 patient.
In our study, no case of a Chlamydia infection was found in the urethral epithelium in the tested material of the 65 examined individuals. The infection with Chlamydia trachomatis DNA in the prostate tissue was observed in 7 men, in that case the prostate alone was infected. No possibility of contamination with the urethral epithelium microorganisms was present in the conducted research, since the samples were collected by the rectal biopsy of the prostate. According to many authors, credibility of the positive results of bacteriological tests of the prostate samples is doubtful [20, 38]. In the case of prostate discharge collected after a prostate massage, the contamination is related to the expressed prostatic secretions (EPS) crossing through the urethra and thus, a secondary infection by the material from the urethra epithelium. During the transrectal biopsy or the prostate gland resection (TURP or surgery) the contamination with the material from the prostate part of the urethra is possible [20, 38]. According to Wagenlehner et al. only the transperineal biopsy of peripheral prostate lobes excludes the possibility of contamination [38]. The biopsy is also related to as a possibility of overlooking an inflammatory prostate lesion in the case of the focal nature of prostatitis [22].
In the aforementioned reports on Chlamydia detection in the prostate tissue, the authors applied various techniques of material collection. The transrectal prostate biopsy was performed by Poletti et al. [27], while the transperineal biopsy by, among others, Doble et al. [35], Berger et al. [36], and Lee et al. [37]. Also, the transurethral resection of the prostate was performed [30, 31], as well as tissue removal during the open surgical procedure [30, 34].
The specific anti-chlamydial IgA antibodies in the serum of the study group were detected more frequently (24.6%) than IgG (9.2%). The two patients with identified specific IgA antibodies and one with IgG were also diagnosed with Chlamydia in the prostate tissue. Only 4/18 (22.2%) of the patients were identified to have the co-occurrence of antibodies of both classes in the serum. The specific anti-Chlamydia trachomatis antibodies were detected by inter alia Motrich et al. (IgA – 2.5%, IgG – 15%) and Mazzoli et.al (IgA – 36%, IgG – 42%) [39, 40]. The infection spread into the prostate from urethra, thereby chlamydial prostatitis should be considered as a complication of the chlamydial urethritis that had not been totally cured before. This may explain the mismatch between the serological results (IgG especially) and the results obtained by LCR after the biopsy as well as after the urinalysis. Moreover, IgG should not be considered as a marker of the present chlamydial infection, while IgA – could [41]. The discrepancies could have also been caused by a variety of the applied diagnostic methods or a group selection method. Our present research shows that the presence of Chlamydia in the prostate is not always accompanied by the appearance of specific antibodies in the serum. Therefore, the diagnosis may not be based on the results of the serological tests only.
CONCLUSIONS
The following conclusions have been drawn on the basis of the obtained outcomes:
1. The presence of Chlamydia trachomatis DNA in the prostate gland tissue may be indicative of the etiopathogenetic role of chlamydia in the development of the chronic prostatitis.
2. The urethral samples are not a reliable material for the diagnosis of the chlamydial infection in the chronic prostatitis. A direct examination of the urethra in the case of a Chlamydia prostate infection shows a limited diagnostic value.
3. There was no correlation observed between the presence of the chlamydial DNA in the prostate and the serological test results.
4. The role of the Chlamydia trachomatis infection should be considered in microbiological diagnostics of the chronic prostatitis.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
References
- 1.Videcnik Zorman J, Maticic M, Jeverica S, Smrkolj T. Diagnosis and treatment of bacterial prostatitis. Acta Dermatovenerol Alp Pannonica Adriat. 2015;24:25–29. doi: 10.15570/actaapa.2015.8. [DOI] [PubMed] [Google Scholar]
- 2.Rees J, Abrahams M, Doble A, Cooper A, Prostatitis Expert Reference Group (PERG) Diagnosis and treatment of bacterial prostatitis and chronic prostatitis/chronic pelvic pain syndrome: a consensus guideline. BJU Int. 2015;116:509–525. doi: 10.1111/bju.13101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collins MM, Stafford RS, O’Leary MP, Barry MJ. How common is prostatitis? A national survey of physician visits. J Urol. 1998;159:1224–1228. [PubMed] [Google Scholar]
- 4.Vahlensieck W, Ludwig M, Wagenlehner FM, Naber K, Fabry W. Prostatitis - diagnostic and therapy. Aktuelle Urol. 2013;44:117–123. doi: 10.1055/s-0033-1337934. [DOI] [PubMed] [Google Scholar]
- 5.Choi YS, Kim KS, Choi SW, et al. Microbiological etiology of bacterial prostatitis in general hospital and primary care clinic in Korea. Prostate Int. 2013;1:133–138. doi: 10.12954/PI.13023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiao J, Ren L, Lv H, et al. Atypical microorganisms in expressed prostatic secretion from patients with chronic prostatitis/chronic pelvic pain syndrome: microbiological results from a case-control study. Urol Int. 2013;91:410–416. doi: 10.1159/000350934. [DOI] [PubMed] [Google Scholar]
- 7.Magri V, Boltri M, Cai T, et al. Multidisciplinary approach to prostatitis. Arch Ital Urol Androl. 2019;90:227–248. doi: 10.4081/aiua.2018.4.227. [DOI] [PubMed] [Google Scholar]
- 8.Mackern Oberti JP, Motrich RD, Breser ML, et al. Male rodent genital tract infection with Chlamydia muridarum: persistence in the prostate gland that tiggers self-immune reactions in genetically susceptible hosts. J Urol. 2011;186:1100–1006. doi: 10.1016/j.juro.2011.04.086. [DOI] [PubMed] [Google Scholar]
- 9.Banyra O, Nikitin O, Ventskivska I. Acute epididymo-orchitis: relevance of local classification and partner's follow-up. Cent European J Urol. 2019;72:324–329. doi: 10.5173/ceju.2019.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Connell CM, Ferone ME. Chlamydia trachomatis genital infection. Microb Cell. 2016;3:390–403. doi: 10.15698/mic2016.09.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kotake S, Nanke Y. Chlamydia-associated arthritis and enteropathic arthritis - two important spondyloarthritides. Nihon Rinsho Meneki Gakkai Kaishi. 2011;34:121–130. doi: 10.2177/jsci.34.121. [DOI] [PubMed] [Google Scholar]
- 12.Mackern Oberti JP, Motrich RD, Breser ML, Sanchez LR, Cuffini C, Rivero VE. Chlamydia trachomatis infection of the male genital tract: an update. J Reprod Immunol. 2013;100:37–53. doi: 10.1016/j.jri.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Motrich RD, Salazar FC, Breser ML, et al. Implications of prostate inflammation on male fertility. Andrologia. 2018;50:e13093. doi: 10.1111/and.13093. [DOI] [PubMed] [Google Scholar]
- 14.Ouzounova-Raykova V, Ouzounova I, Mitov IG. May Chlamydia trachomatis be an aetiological agent of chronic prostatic infection? Andrologia. 2010;42:176–181. doi: 10.1111/j.1439-0272.2009.00973.x. [DOI] [PubMed] [Google Scholar]
- 15.Cai T, Pisano F, Nesi G, et al. Chlamydia trachomatis versus common uropathogens as a cause of chronic bacterial prostatitis: Is there any difference? Results of a prospective parallel-cohort study. Investig Clin Urol. 2017;58:460–467. doi: 10.4111/icu.2017.58.6.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walsh S, Metzger DA, Higucki R. Chelex - 100 as a medium for simple extraction of DNA for PCR - based typing from forensic material. Bio Techniques. 1991;10:506–513. [PubMed] [Google Scholar]
- 17.Ostaszewska I, Zdrodowska-Stefanow B, Badyda J, Puciło K, Trybuła J, Bułhak V. Chlamydia trachomatis probable cause of prostatitis. Int J STD AIDS. 1998;9:350–353. doi: 10.1258/0956462981922395. [DOI] [PubMed] [Google Scholar]
- 18.Papes D, Pasini M, Jeroncic A, et al. Detection of sexually transmitted pathogens in patients with chronic prostatitis/chronic pelvic paIn: a prospective clinical study. Int J STD AIDS. 2017;28:613–615. doi: 10.1177/0956462417691440. [DOI] [PubMed] [Google Scholar]
- 19.Krieger JN, Riley DE. Bacteria in the chronic prostatitis - chronic pelvic pain syndrome: molecular approaches to critical research questions. J Urol. 2002;167:2574–2583. [PubMed] [Google Scholar]
- 20.Weidner W, Ludwig M. Common organisms in urogenital infections with special impact on prostatitis. In: Schaeffer AJ, Weidner W, editors. International Consensus Conference on Advances in the Diagnosis and Treatment of Prostatitis. Vol. 2. Eur Urol Suppl; 2003. pp. 15–18. [Google Scholar]
- 21.Cornelisse VJ, Sherman CJ, Hocking JS, et al. Concordance of chlamydia infections of the rectum and urethra in same-sex male partnerships: a cross-sectional analysis. BMC Infect Dis. 2017;17:22. doi: 10.1186/s12879-016-2141-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kojima H. Chlamydial infection in urological field. Akt Urol. 1990;21(Suppl):137–141. [Google Scholar]
- 23.Krieger JN, Riley DE. Chronic prostatitis: Charlottesville to Seattle. J Urol. 2004;172:2557–2560. doi: 10.1097/01.ju.0000144291.05839.a0. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi TK, Araki H, Sawaragi I. Immunocytochemical detection of chlamydial antigen in both the urethral scraping and prostatic aspirate in a case of abacterial prostatitis. Acta Cytol. 1988;32:270–272. [PubMed] [Google Scholar]
- 25.Doble A, Thomas BJ, Furr PM, et al. A search for infectious agents in chronic abacterial prostatitis using ultrasound guided biopsy. Br J Urol. 1989;64:279–301. doi: 10.1111/j.1464-410x.1989.tb06017.x. [DOI] [PubMed] [Google Scholar]
- 26.Maruta N. Study of Chlamydia trachomatis in chronic prostatitis. Acta Urol Jap. 1992;38:297–304. [PubMed] [Google Scholar]
- 27.Poletti F, Medici MC, Alinovi A, et al. Isolation of Chlamydia trachomatis from the prostatic cells in patients affected by nonacute abacterial prostatitis. J Urol. 1985;134:691–693. doi: 10.1016/s0022-5347(17)47387-3. [DOI] [PubMed] [Google Scholar]
- 28.Weidner W, Schiefer HG, Krauss H, Jantos C, Friedrich HJ, Altmannsberger M. Chronic prostatitis: a thorough search for etiologically involved microorganisms in 1461 patients. Infection. 1991;19(Suppl 3):119–125. doi: 10.1007/BF01643680. [DOI] [PubMed] [Google Scholar]
- 29.Abdelatif OM, Chandler FW, McGuire BS. Chlamydia trachomatis in chronic abacterial prostatitis: demonstration by colorimetric in situ hybridization. Hum Pathol. 1991;22:41–44. doi: 10.1016/0046-8177(91)90059-x. [DOI] [PubMed] [Google Scholar]
- 30.Corradi GY, Bucsek M, Panovics J, et al. Detection of Chlamydia trachomatis in the prostate by in-situ hybridization and by transmission electron microscopy. Int J Androl. 1996;19:109–112. doi: 10.1111/j.1365-2605.1996.tb00446.x. [DOI] [PubMed] [Google Scholar]
- 31.Dan M, Samra Z, Siegel YI, Korczak D, Lindner A. Isolation of Chlamydia trachomatis from prostatic tissue of patients undergoing transurethral prostatectomy. Infection. 1991;19:162–163. doi: 10.1007/BF01643241. [DOI] [PubMed] [Google Scholar]
- 32.Kadar A, Buscek M, Kardos M, Corradi G. Detection of Chlamydia trachomatis in chronic prostatitis by in situ hybridization (preliminary methodical report) Orvosi Hetilap. 1995;136:659–662. [PubMed] [Google Scholar]
- 33.Shurbaji MS, Gupta PK, Myers J. Immunohistochemical demonstration of chlamydial antigens in association with prostatitis. Mod Pathol. 1988;1:348–351. [PubMed] [Google Scholar]
- 34.Toth M, Patton DL, Campbell LA, et al. Detection of chlamydial antigenic material in ovarian, prostatic, ectopic pregnancy and semen samples of culture-negative subjects. AJRI. 2000;43:218–222. doi: 10.1111/j.8755-8920.2000.430406.x. [DOI] [PubMed] [Google Scholar]
- 35.Doble A, Thomas BJ, Walker MM, Harris JR, Witherow RO, Taylor-Robinson D. The role of Chlamydia trachomatis in chronic abacterial prostatitis: a study using ultrasound guided biopsy. J Urol. 1989;141:332–333. doi: 10.1016/s0022-5347(17)40758-0. [DOI] [PubMed] [Google Scholar]
- 36.Berger RE, Krieger JN, Rothman I, Muller CH, Hillier SL. Bacteria in the prostatic tissue of man with idiopathic prostatic inflammation. J Urol. 1997;157:863–865. [PubMed] [Google Scholar]
- 37.Lee JC, Muller CH, Rothman I, et al. Prostate biopsy culture findings of men with chronic pelvic pain syndrome do not differ from those of healthy controls. J Urol. 2003;169:584–587. doi: 10.1097/01.ju.0000045673.02542.7a. [DOI] [PubMed] [Google Scholar]
- 38.Wagenlehner FME, Naber KG, Weidner W. Chlamydial infections and prostatitis in men. BJU Int. 2006;97:687–690. doi: 10.1111/j.1464-410X.2006.06007.x. [DOI] [PubMed] [Google Scholar]
- 39.Motrich RD, Cuffini C, Mackern Oberti JP, Maccioni M, Rivero VE. Chlamydia trachomatis occurence and its impact on sperm quality in chronic prostatitis patients. J Infect. 2006;53:175–183. doi: 10.1016/j.jinf.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 40.Mazzoli S, Cai T, Addonisio P, Bechi A, Mondaini N, Bartoletti R. Chlamydia trachomatis infection is related to poor semen quality in young prostatitis patients. Eur Urol. 2010;57:708–714. doi: 10.1016/j.eururo.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 41.Cai T, Verze P, Mazzoli S, et al. Chlamydial infections in urological disease: A challenging management. World J Clin Urol. 2014;3:38–43. [Google Scholar]